Published online Apr 16, 2023. doi: 10.4253/wjge.v15.i4.216
Peer-review started: November 17, 2022
First decision: December 26, 2022
Revised: January 9, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 16, 2023
Processing time: 147 Days and 12.7 Hours
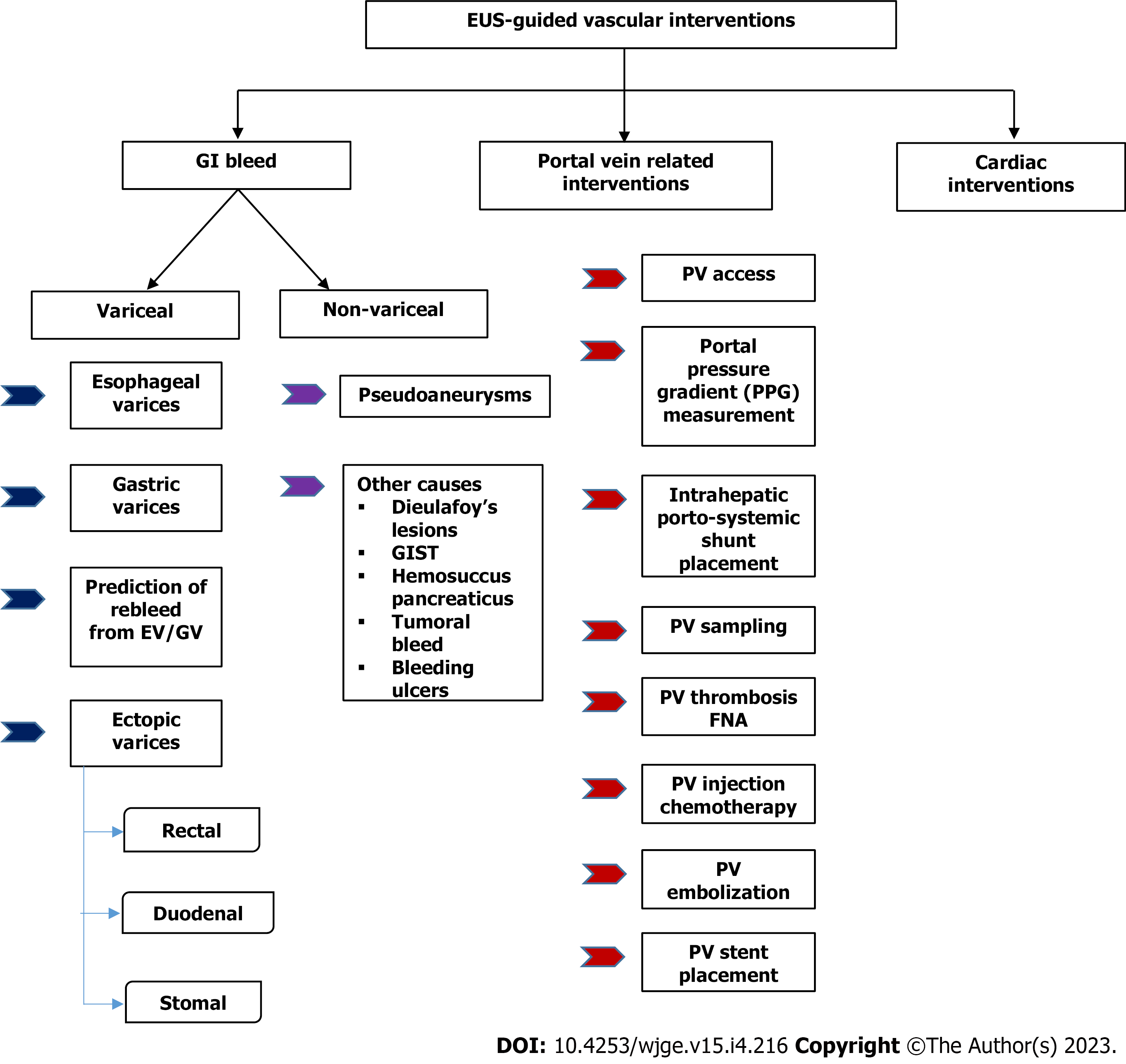
Endoscopic ultrasound (EUS) has expanded its arena from a mere diagnostic modality to an essential therapeutic tool in managing gastrointestinal (GI) diseases. The proximity of the GI tract to the vascular structures in the mediastinum and the abdomen has facilitated the growth of EUS in the field of vascular interventions. EUS provides important clinical and anatomical information related to the vessels' size, appearance and location. Its excellent spatial resolution, use of colour doppler with or without contrast enhancement and ability to provide images “real-time” helps in precision while intervening vascular structures. Additionally, structures such as venous collaterals or varices can be dealt with optimally using EUS. EUS-guided vascular therapy with coil and glue combination has revolutionized the management of portal hypertension. It also helps to avoid radiation exposure in addition to being minimally invasive. These advantages have led EUS to become an upcoming modality to complement traditional interventional radiology in the field of vascular interventions. EUS-guided portal vein (PV) access and therapy is a new kid on the block. EUS-guided portal pressure gradient measurement, injecting chemotherapy in PV and intrahepatic portosystemic shunt has expanded the horizons of endo-hepatology. Lastly, EUS has also forayed into cardiac interventions allowing pericardial fluid aspiration and tumour biopsy with experimental data on access to valvular apparatus. Herein, we provide a comprehensive review of the expanding paradigm of EUS-guided vascular interventions in GI bleeding, portal vein access and its related therapeutic interventions, cardiac access, and therapy. A synopsis of all the technical details involving each procedure and the available data has been tabulated, and the future trends in this area have been highlighted.
Core Tip: Therapeutic endoscopic ultrasound (EUS) has rapidly expanded into the field of vascular interventions. Published literature has shown that EUS-guided endovascular therapy is safe and scores over conventional endoscopic techniques achieving high obliteration rates with minimum re-intervention in variceal bleeding. EUS currently acts as a “rescue therapy” in cases of re-bleed or refractory bleeding from non-variceal sources, especially a pseudoaneurysm. In addition, portal vein access, portal pressure gradient measurement, and variceal assessment with liver biopsy have shown that EUS can act as a "one-stop-shop" for “Endo-hepatology”. This ever-expanding role of EUS-related vascular interventions has been thoroughly detailed in this comprehensive review.
- Citation: Dhar J, Samanta J. Endoscopic ultrasound-guided vascular interventions: An expanding paradigm. World J Gastrointest Endosc 2023; 15(4): 216-239
- URL: https://www.wjgnet.com/1948-5190/full/v15/i4/216.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i4.216
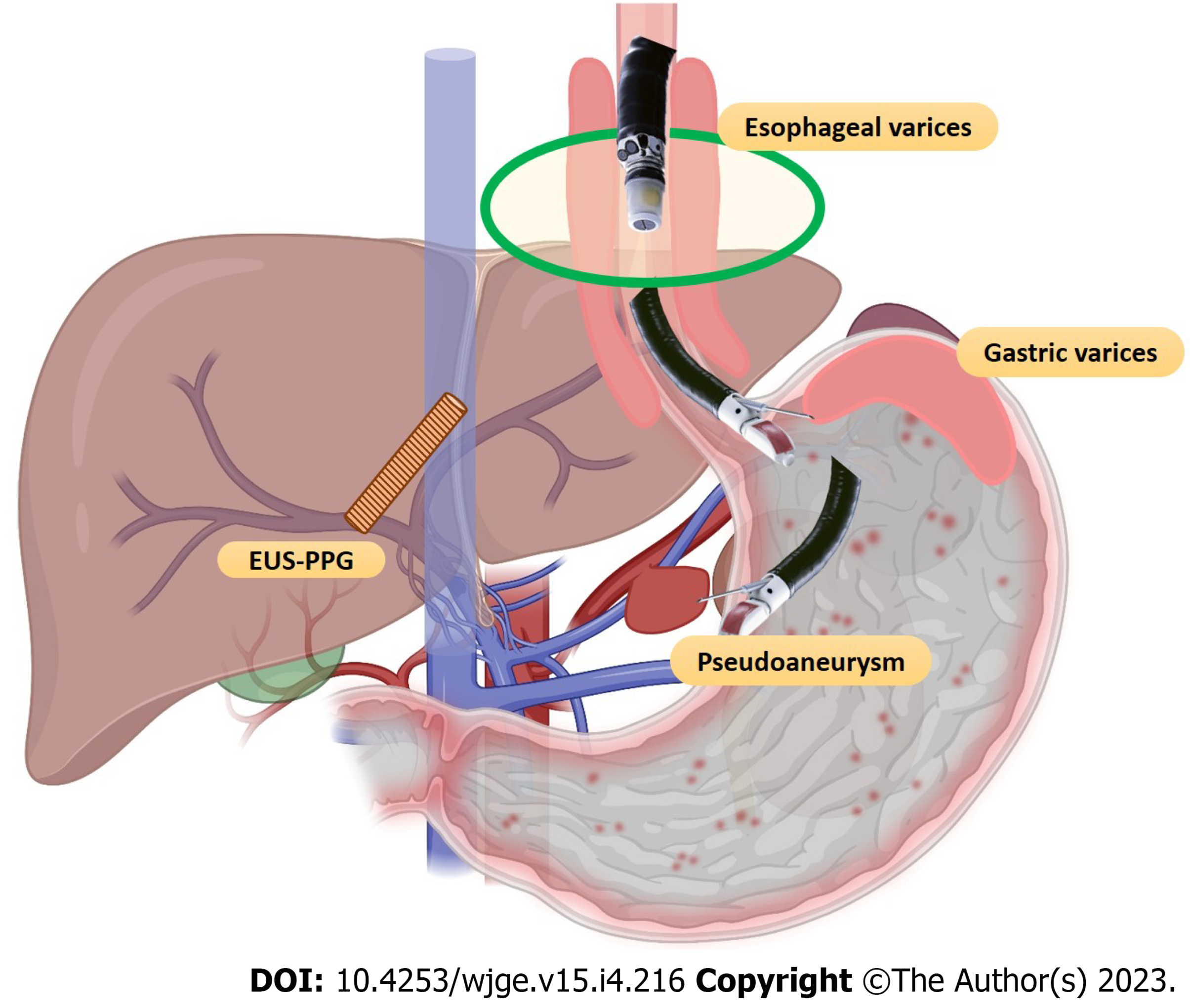
Therapeutic endoscopic ultrasound (EUS) procedures have come a long way using curvilinear array echo-endoscopes and various accessories. EUS, with its high spatial and contrast resolution, is constantly evolving and is currently one of the most commonly used minimally invasive techniques for diagnosing and managing various gastrointestinal (GI) disorders. The proximity of the GI tract to various vascular structures in the mediastinum and abdomen has allowed EUS to play a significant role in the field of vascular interventions. The necessity of developing a minimally invasive as well as a radiation-free alternative to interventional radiology (IR) or surgery has further strengthened its growth. The advantage of visualizing vascular structures in “real-time” has enabled access and delivery of targeted therapy[1]. EUS-guided vascular therapy has been found extremely useful in cases of variceal bleeding. EUS-guided injection of sclerosants, cyanoacrylate glue (CYA), thrombin, gelatin sponge and deployment of coils in gastric varices (GV) is safer and more effective over traditional endoscopic glue injection in terms of lower adverse events and reintervention rates.
Furthermore, EUS-guided portal vein (PV) access has opened the doors to experimental and clinical studies on portal pressure gradient (PPG) measurement, injection of chemotherapy, PV thrombus fine needle aspiration (FNA), and intrahepatic portosystemic shunt placement. This gamut of therapeutic options, combining EUS guided PPG (EUS-PPG) with variceal therapy and liver biopsy in a single session, represents an attractive option in the expanding field of “endo-hepatology”[2]. Therefore, this review focuses on elucidating the role of EUS-guided vascular interventions (Figures 1 and 2), a synopsis of the various available techniques, data on their safety and efficacy, and future advancements in this domain.
A detailed strategy, as outlined in Supplementary material, was performed in PubMed and Embase. All studies pertaining to applications of endoscopic ultrasound (EUS) in the field of vascular interventions (for example case series, review articles and clinical studies) were reviewed. Topics concerning GI bleeding (both variceal and non-variceal), PV-related interventions and cardiac access with therapy were looked into. Non-English language literature was excluded. EUS-guided liver biopsy and other aspects of Endo-hepatology are beyond this review’s scope and have been excluded.
GI bleeding secondary to gastro-esophageal varices is a well-known but one of the most lethal complications of portal hypertension (PHTN)[3,4]. The annual bleeding rate has been reported to be around 5%-15%, with a 20% 6-wk mortality rate[5]. In half of the cases, bleeding stops spontaneously but has a re-bleeding rate of 30%-40%[3,6]. The standard treatment options for gastro-esophageal varices have been conventional endoscopic band ligation (EBL) or CYA glue injection. For refractory bleed, transjugular intrahepatic portosystemic shunt (TIPS) and balloon-occluded retrograde transvenous obliteration (BRTO) are other options[7]. EUS-guided management of varices has recently become an additional tool in the armamentarium. EUS offers theoretical as well as practical advantages over the conventional techniques such as: (1) It helps to identify the actual size as well as the number of varices for precise vascular therapy; (2) It can locate feeders, perforators or shunts; (3) Enables real-time puncture of the varices under vision; (4) One need not have to “see” the endoscopic image while delivering targeted therapy. This is especially useful in cases of active bleed or when there are contents in the fundus, and (5) Objective obliteration of the varices can be confirmed by lack of flow in “real-time”.
EBL has been the first line of management for both primary and secondary prophylaxis of esophageal varices (EV)[4,8]. But high re-bleeding rates have been reported (15%-65%)[9,10], probably as a result of failure to obliterate the perforators or paraesophageal vessels that feed the EV[11,12]. Anecdotal case series exist on the use of EUS for EV management.
Existing literature: Lahoti et al[13] first described EUS-guide sclerotherapy for EV obliteration in 5 patients. Sodium morrhuate (sclerosant) was used to inject the perforators and feeder vessels until flow was obliterated using colour doppler, with no re-bleeding on a 15-mo follow-up period. One case had developed esophageal stricture, which was responsive to balloon dilatation. The only randomized controlled trial (RCT) comparing endoscopic vs EUS-guided sclerotherapy showed that there was no difference in the mean number of sessions needed for complete obliteration (4.3 vs 4.1) and re-bleeding rates (16.7% vs 4.2%). However, collaterals noted on EUS post-therapy were lower in the EUS arm (33.3% vs 0%)[14].
While EBL is still the preferred option, more data will be needed to define the role of EUS for EV management algorithms in clinical practice.
Future trends: Recently, a “jelly-filling” method has been found superior to the traditional water-filling method for EV visualization using EUS. The image quality score was significantly higher but with a longer procedure time using the former technique[15].
While EV account for a majority of the cases of GI bleeding in cirrhosis, GV can account for 20%-25% of them, with re-bleeding rates amounting to 65% in 2 years. Although GV bleeds less frequently, they are usually associated with an increased risk of uncontrolled bleeding, re-bleeding, more transfusion requirements and higher mortality. Described as per Sarin’s classification, varices along cardia (GOV2) or isolated GV in the fundus (IGV1) are the most difficult to treat[3,4,16]. Therefore, both endoscopic sclerotherapy and EBL are discouraged for GV. While the former leads to an unusually high incidence of adverse events (37%-53%) like ulceration, re-bleeding, or perforation, the latter is difficult to execute due to thick musculature of the gastric wall leading to possible catastrophic post-banding bleed[17,18].
Thus, the first line of therapy for managing bleeding GV is the endoscopic injection of acrylate polymers such as CYA under direct vision. First described by Soehendra et al[19] in 1986, this technique has success rates of 58%-100% with re-bleeding of 40%-65%. This technique, however, has its own set of complications, including the risk of systemic embolization, bleeding from needle site ulcers, peritonitis, needle impaction, scope damage and even death. On the other hand, EUS-guided management has some advantages over conventional glue injection, i.e., (1) Higher detection rate (6 times) over conventional endoscopy, as GV is located deep in the submucosa and commonly mistaken as thick gastric folds[20,21]; and (2) avoidance of inadvertent para-variceal injection (in up to 60%)[22].
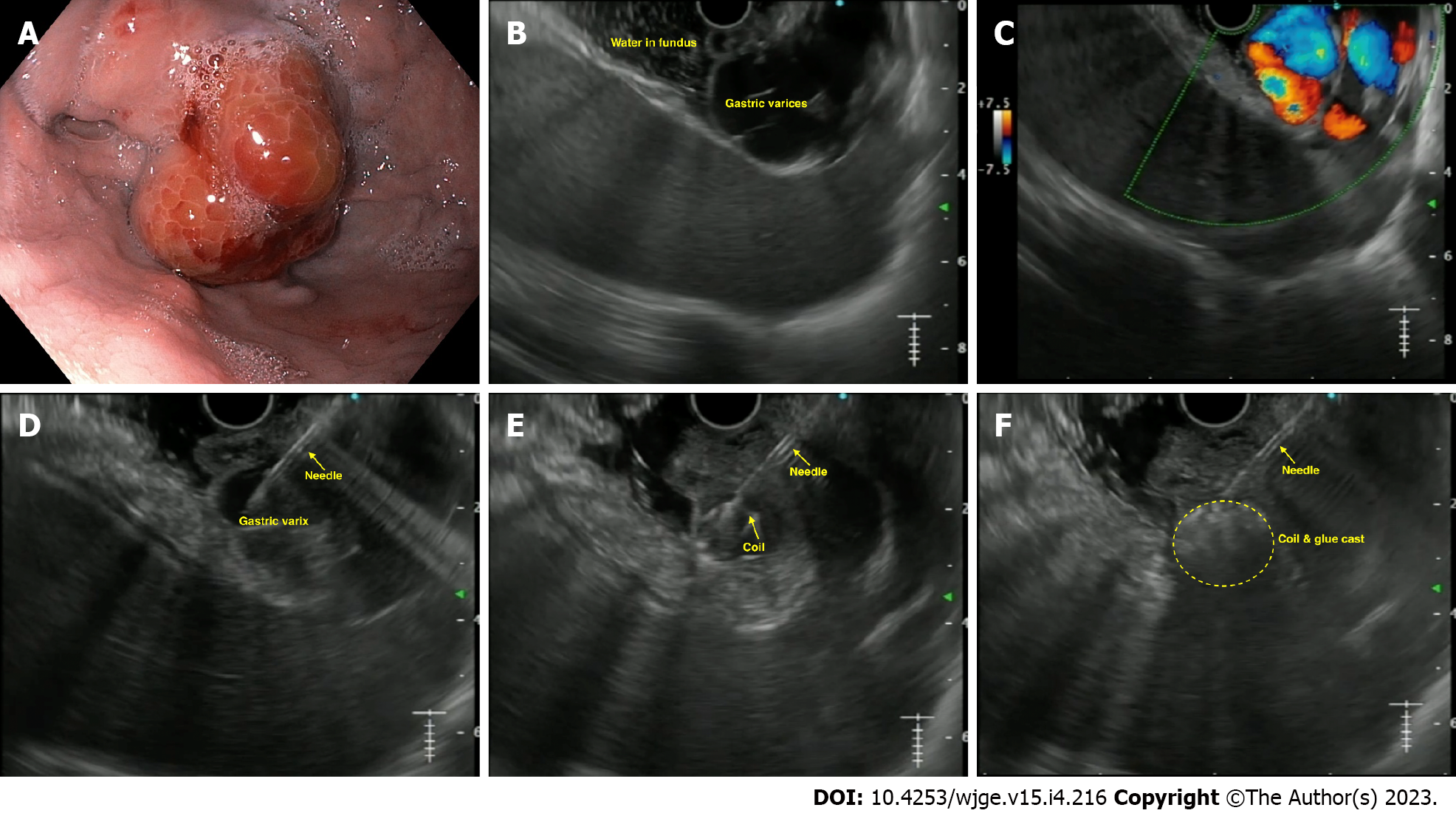
The technique of EUS-guided GV management: The most commonly used method is a combination of coil and CYA glue, as outlined in Table 1[2] and Figure 3.
| EUS-guided management of gastric varices using coil and glue combination |
| Pre-procedure requirements |
| All procedures are done under the cover of pre/peri-procedural antibiotics |
| Patient is usually kept fasting for 4-6 h before the procedure |
| Adequate resuscitation of the patient, in case of active bleeding is ensured, prior to the procedure |
| Informed consent prior to the procedure |
| What is needed prior to the procedure |
| Linear echoendoscope with at least a 3.7 mm working channel |
| Needle size: depends on the choice of the endoscopist; for > 10 mm coils, we need 0.035’ coil (19-G needle); can also use 0.018’ coil (22-G needle) |
| Diameter of the coils: 1.2-1.5 times the largest diameter of varix |
| Number of coils: depends on size of the varix |
| Amount of glue: depends on the size of the varix; but usually 2-4 mL is sufficient |
| Technical aspects |
| A proper diagnostic EUS is performed |
| The echoendoscope is usually positioned either in the distal esophagus or the gastric fundus |
| Saline is filled intra-luminally in the fundus to let the varices “float”. This enables a good acoustic coupling for better visualization of the gastric varices |
| Adequate examination of the fundus, the intramural varices and the feeder vessels is carried out |
| The approach can be trans-esophageal or trans-gastric, wherein the trans-esophageal route is given preference |
| Aim is to obliterate the intramucosal part of the varix |
| EUS-guided coil and glue embolization is usually performed using a 22-G/19-G (gauge) FNA needle |
| The size of the coil is determined by the short axis of the diameter of the varix |
| After puncture of the varix, blood is aspirated to confirm the location. This is followed by flushing of the needle with saline |
| The coils are then deployed into the varix using the stylet as a pusher. Once the coils are deployed, flushing of the needle is done with normal saline |
| After coil deployment, 1-2 mL of cyanoacrylate glue is injected followed by rapid flushing with saline |
| Once, the varix is obliterated, visualized by absence of flow on colour Doppler, the sheath of the needle is advanced beyond the endoscope tip for 2-3 cm before withdrawing the scope. This avoids contact of glue with the endoscope tip |
| Post procedure |
| The patients are kept under observation for 12 h |
| Repeat EUS can be done after 2 d to look for residual varices |
| Follow-up EUS to be performed at 1- and 3-mo intervals |
Existing literature: The options for EUS-guided GV therapy include: CYA glue, coils alone, a coil with glue combination, gelatin sponge and thrombin.
EUS-guided glue injection only: In their pilot study, Romero-Castro et al[23] evaluated the efficacy of CYA glue with lipiodol mixture in 5 cases of bleeding GV using a 22-G needle. Complete obliteration was achieved in all with no re-bleeding or complications.
EUS-guided coil injection only: A life-threatening complication of CYA injection is systemic embolization, the most common location being the lungs[24]. Coils can be used as an alternative to glue injection to mitigate this risk. Coils are made of a stainless-steel alloy with radially extending synthetic fibres that induce clot formation and hemostasis. The coils are usually 2-15 mm in length, and the loops are 2-20 mm in diameter. The choice of size would depend on the diameter of the varix.
The first report by Romero-Castro et al[25] demonstrated its efficacy in 4 cases. Complete obliteration was achieved in 75% of patients. Furthermore, the same group compared the EUS-guided coil (11 patients) vs CYA (19 cases). Though the obliteration rates were similar (91% vs 95%), the coil group needed fewer endoscopy sessions and had lower adverse event rates (9.1% vs 58%)[26].
EUS-guided coil with glue combination: This combination is based on the concept that use of coil with glue: (1) Achieves higher variceal obliteration rates with better hemostasis control; (2) decreases the amount of CYA needed; and (3) provides a framework or scaffold to hold the CYA glue within the varix, thus mitigating the risk of embolization. The largest data by Bhat et al[27] evaluated it in 152 cases of GV. The mean number of coils and glue used was 1.4 and 2 mL, respectively. On follow-up, complete obliteration was achieved in 93% of cases. Furthermore, mild post-procedure pain was seen in 3% of cases, with only one case of embolization. This data strongly supports the use of combination therapy for GVs. Recently, Kouanda et al[28] demonstrated its effectiveness in primary prophylaxis, with an obliteration rate of 96.7% with 2.5% re-bleed rates. A recent RCT and a meta-analysis have confirmed the superiority of EUS-guided coil with glue as the best modality for tackling GV[29,30].
Comparison of EUS-coil with CYA vs endoscopic glue injection: Limited data exist (retrospective and one RCT) comparing EUS combination therapy vs conventional endoscopic glue injection[31-34]. Robles-Medranda et al[31] compared the cost-effectiveness of the two procedures and found EUS therapy to be better.
The author’s experience of the largest multicenter study involving four centers to evaluate the effectiveness of EUS combination therapy (52 cases) vs endoscopic therapy (118 patients) showed that the EUS arm required a lower number of sessions for complete obliteration (1 vs 2), lower re-bleeding rates (15.4% vs 31.3%) and lower post-procedure abdominal pain (0% vs 13.9%)[34]. Currently, an RCT is recruiting patients for EUS-guided coil and glue vs endoscopic CYA therapy for GV[35].
Newer therapies: Isolated case series exists on the utilization of thrombin, a coil with an absorbable gelatin sponge and ethanolamine oleate, with good results[36-38].
Various studies published on EUS-guided vascular interventions in GV have been tabulated in Table 2[39-47]. Published literature strongly supports using EUS-guided vascular therapy for managing GV for primary and secondary prophylaxis. The combination strategy has definite advantages and may be preferred over conventional CYA therapy in certain situations.
| Ref. | Cases | Treatment used in EUS | EUS needle size | Number of coils (EUS only) | Use of Glue/others (mL) (EUS/endoscopic therapy) | Number of sessions (EUS/endoscopic) | Technical success (%) | Clinical success (%) | Adverse events (overall) (%) | Reintervention rates (%) | Rebleeding rates (%) | All-cause mortality (%) |
| Studies on only EUS-guided Glue injection | ||||||||||||
| Lee et al[39], 2000 | 54 | CYA (0.5 mL) with lipiodol (0.7 mL) | - | - | 3 (1-8) | 2.2 ± 1.7 | 52/54 (96.3%) | 43/54 (79.6%) | 22/54 (40.7%) | - | 19/54 (35.2%) | 28/54 (51.9%) |
| Romero-Castro et al[23], 2007 | 5 | CYA-lipiodol (1 mL; 1:1) | 22-G | - | 1.6 (1-2) | 2 cases: 1 each; 3 cases: 2 each | 100% | 100% | None | - | None | 20% |
| Gubler and Bauerfeind[40], 2014 | 40 | CYA-lipiodol (1 mL; 1:1) | 22-G | - | 1.9 (1-10) | 1.4 (1-7) | 40/40 (100%) | 36/36 (100%) | 2/40 (5%) | 6/40 (15%) | - | 6/40 (15%) |
| Studies on only EUS-guided coil injection | ||||||||||||
| Romero-Castro et al[25], 2010 | 4 | Coils | 19-G | Each case: 22; 7; 3; 2 | - | - | 100% | 3/4 (75%) | None | - | None | 25% |
| Khoury et al[41], 2018 | 10 | Coils | 19-G | 4.5 (mean) | - | 2.8 (mean) | 100% | complete (20%); near-complete (50%) | 5 cases (minimal self-limited bleeding); 1 case needing blood transfusion | 30% (3/10) | 1 case (10%) | None |
| Studies on only EUS-guided coil + glue injection | ||||||||||||
| Binmoeller et al[42], 2011 | 30 | Coil + 1 mL CYA | 19-G | - | 1.4 (1-4) | 1 | 30/30 (100%) | 23/24 (95.8%) | None | 1/30 (3.3%) | 4/24 (16/6%) | 1/30 (3.3%) |
| Bhat et al[27], 2015 | 152 | Coil + 1 mL CYA | 19/22-G | 1.4 (1-4) | 2 (0.5-6) | - | 151/152 (99.3%) | 93/100 (93%) | 9/124 (7%) | 7/125 (5.6%) | 20/125 (16%) | 3/151 (1.98%) |
| Kozieł et al[43], 2019 | 16 | Coil + CYA (1:1 with lipiodol) | 19-G | Total 21; mean 1.7 (1-3) | 2 (1-9) | - | 15/16 (94%) | Overall, 12/15 (75%) {coil+CYA (11/12 [92%]; only CYA [0%]} | 6/16 (37.5%) | 5/16 (31.3%) | 1/16 (6.25%) | None |
| Robles-Medranda et al[44], 2019 | 30 | Coil + CYA | 19-G | 2 (1-3) | 1.8 (1.2-2.4 mL) | Mean 1.1 | 100% | 96.6% | 2 cases (6.7%) | 3/27 (11.1%) | 5 (16.7%) | 4/30 (13.3%) |
| Kouanda et al[28], 2021 | 80 | Coil + CYA | - | 1.5 (1-3) | 2 (0.5-5) mL | Mean 1.4 | 100% | 60/62 (96.7%) | 4 (4.9%) | 6 (7.5%) | 17 (21.3%) | |
| Comparison of different treatment modalities for GV management | ||||||||||||
| Romero-Castro et al[26], 2013 | 30 | EUS-Coil (11) vs EUS-CYA (19) | 19/22-G | 5.8 (2-13) (overall 64 coils) | 1.5 (1-3) (overall 29 mL) | Overall, 1.4 ± 0.1 (14 vs 29) | Overall, 27/30 (90%): 10/11 (90.9%) vs 17/19 (89.5%) | Overall, 29/30 (96.7%): 10/11 (90.9%) vs 19/19 (100%) | Overall, 12/30 (40%): 1/11 (9.1%) vs 11/19 (57.9%) | 2/11 (18.1%) vs 9/19 (47.3%) | None (0 vs 0) | Overall, 6/30 (20%) |
| Bick et al[45], 2018 | 104 | EUS-CYA (64) vs endoscopic CYA (40) | 19/22-G | - | 2 (0.8) vs 3.3 (1.3) mL | 1 session (79% vs 75%); 2 sessions (21% vs 17.5%); 3 sessions (0% vs 7.5%) | 100% vs 100% | 49/64 (79%) vs 30/40 (75%) | 13/64 (20.3%) vs 7/40 (17.5%) | - | 5/57 (8.8%) vs 9/38 (23.7%) | - |
| Mukkada et al[32], 2018 | 81 | EUS-coil +/- CYA (30) vs endoscopic CYA (51) | 19-G | 2.36 (mean) (total 71) | 2 (1-10 mL) in 15 cases vs 3 ± 1.5 ml | Overall [42 vs 77] | 100% vs 100% | 8/20 (40%) vs (NA) | 0% vs 0% | 12/30 (40%) vs 26/51 (51%) | 6/30 (20%) vs 26/51 (51%) | 3/30 (10%) vs 2/51 (4%) |
| Robles-Medranda et al[29], 2019 | 60 | EUS-coil + CYA (30) vs EUS-coil (30) | 19-G | 2 (1-3) vs 3 (1-7) | 1.8 (1.2-2.4) vs - | - | 100% vs 100% | 30/30 (100%) vs 27/30 (90%) | 2 (6.7%) vs 1 (3.3%) | 5 (16.7%) vs 12 (40%) | 1 (3.3%) vs 6 (20%) | 9/30 (30%) vs 8/30 (26.7%) |
| Lôbo MRA et al[33], 2019 | 32 | EUS-coil + CYA (16) vs endoscopic CYA (16) | 19-G | Total 21 | 1.4 ± 0.74 vs 3.07 ± 1.94 | Overall, 20 vs 18 | 100% vs 100% | 11 (73.3%) vs 12 (75%) | 8 (50%) vs 10 (62.5%) | 4/15 (26.7%) vs 4/16 (25%) | 2 (12.5%) vs 2 (12.5%) | 0 (0%) vs 2 (12.5%) |
| Bazarbashi et al[46], 2020 | 40 | EUS-coil + AGS (10) vs EUS/endoscopic CYA/histocryl (30) | 19/22-G | 8 ± 2.9 | 1.7 ± 2.9 | - | 10/10 (100%) vs 29/30 (96.7%) | 100% vs 87% | 1/10 (10%) vs 5/30 (20%) | 1/10 (10%) vs 17/20 (56%) | 0% vs 38% | 1/10 (10%) vs 5/30 (16.6%) |
| Robles-Medranda et al[31], 2021 | 36 | EUS-coil + CYA (17) vs endoscopic CYA (19) | 19-G | 0 vs 2 (1-3) | 1.8 (1.2-2.4) vs 1.8 (0.6-6.6) | 1 vs 1 (1-4) | 17/17 (100%) vs 16/19 (84.2%) | - | 2/17 (11.8%) vs 3/19 (15.8%) | - | 0 vs 3/19 (15.8%) | - |
| Seven et al[47], 2022 | 28 | EUS-coil (19) vs EUS-coil + CYA (9) | 19-G | 5 (3-9) vs 5 (3-9) | - | 1 vs 1 | 19/19 (100%) vs 9/9 (100%) | 19/19 (100%) vs 8/9 (88.9%) | 1/19 (5.3%) vs 1/9 (11.1%) | 1/19 (5.3%) vs 0/9 (0%) | 1/19 (5.3%) vs 22.2%) | 6/28 (21.42%) |
| Samanta et al[34], 2022 (Author’s centre) | 170 | EUS-coil+CYA (52) vs endoscopic CYA (118) | 19-G | Median 2 | 2 (1) vs 2 (1) mL | 1 (0) vs 2 (2) | 52 (100%) vs 117 (99.2%) | - | 0% vs 13.9% | 7 (13.5%) vs 58 (49.6%) | 8 (15.4%) vs 36 (31.3%) | - |
| Studies on EUS-guided treatment of GV using agents other than glue | ||||||||||||
| Frost and Hebbar[36], 2017 | 8 | Thrombin (1000 IU/5 mL; 2500 IU/5 mL) | 22-G | - | For active bleeder: mean 7250 IU; for elective: mean 2520 IU | 1 for each case | 100% overall | Overall, 75% (active bleeder: 67%; elective cases: 80%) | None | None | None | 1 case |
| Bazarbashi et al[37], 2019 | 10 | Coil + AGS | 19/22-G | 8 ± 2.9 | AGS: 2.5 ± 0.7 | 1 each | 100% | 9/9 (100%) | None | None | 1/10 (10%) | None |
| Irisawa et al[38], 2020 | 8 | Coil + sclerosant [EO] | 19-G | 5.6 ± 2.9 | EO: 7.8 ± 6.7 mL | 1.9 ± 1 | 100% | 7/8 (87.5%) | None | - | - | - |
Future trends: Zhang et al[48] described a novel technique that can be incorporated into the EUS-hepatology toolbox. They described partial splenic embolization with endoscopic CYA for GV in cases with underlying hypersplenism with excellent results post-procedure.
EUS along with Doppler detects EV and GV with higher sensitivity, as compared to upper GI endoscopy, which helps in assessing the risk of bleeding, pre-procedure evaluation and predicting recurrence.
Predicting the risk of bleeding: The presence of hematocystic spots usually correlate with increased risk of esophageal variceal rupture. They can be identified as “saccular aneurysm” on EUS[49].
Preoperative evaluation: EUS-doppler can diagnose collateral veins, peri and para esophageal veins, and the perforators found adjacent to or outside the esophageal wall in patients with EV. The presence of the former is a strong indicator of a future occurrence of a re-bleed[50,51]. Intravariceal pressure can also be recorded in animal models by Miller et al[52] using a non-invasive EUS-based 20-MHz ultrasound transducer in a latex balloon catheter sheath.
Predicting recurrent bleed: The main factor predicting re-bleed for EV is the diameter of the paraesophageal vessels. Paraesophageal diameter before or after EVL is a better recurrence predictor (cut-off of 6.3 mm and 4 mm, respectively, having 60% and 70.6% sensitivity)[53]. Additionally, the velocity of hepatofugal blood flow in the left gastric vein and the branching pattern are associated with variceal recurrence after endoscopic treatments[54]. A cut-off of 0.45 cm2 on digital image analysis using EUS (which identifies distal esophageal cross-sectional area) has 83% sensitive in predicting the risk of re-bleeding[55]. EUS has also been shown to objectively assess response to propranolol to determine variceal recurrence post-EBL[56].
Ectopic varices account for 1%-5% of cases of variceal bleeding. However, the management of ectopic varices holds a diagnostic challenge because of the diverse clinical presentation and lack of defined guidelines for its management. The most frequent sites are the duodenum, small bowel, colon, rectum and, very rarely, parastomal varices or choledochal varices[57,58]. Commonly used management options include endoscopic CYA glue/sclerotherapy injection, TIPS or BRTO. In addition, EUS-guided therapy can be used as a salvage therapy in cases where the above mentioned methods fail or are not possible.
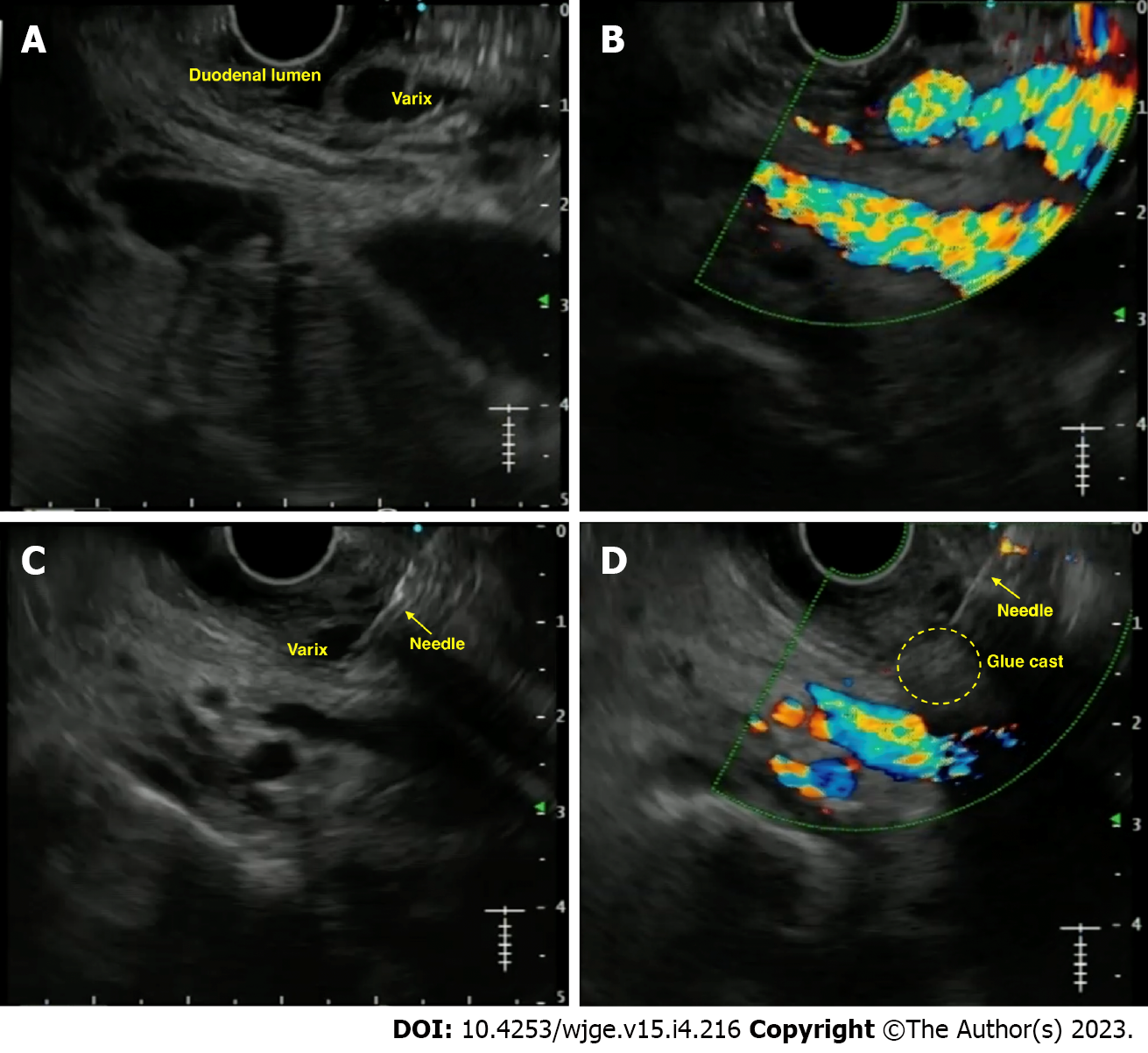
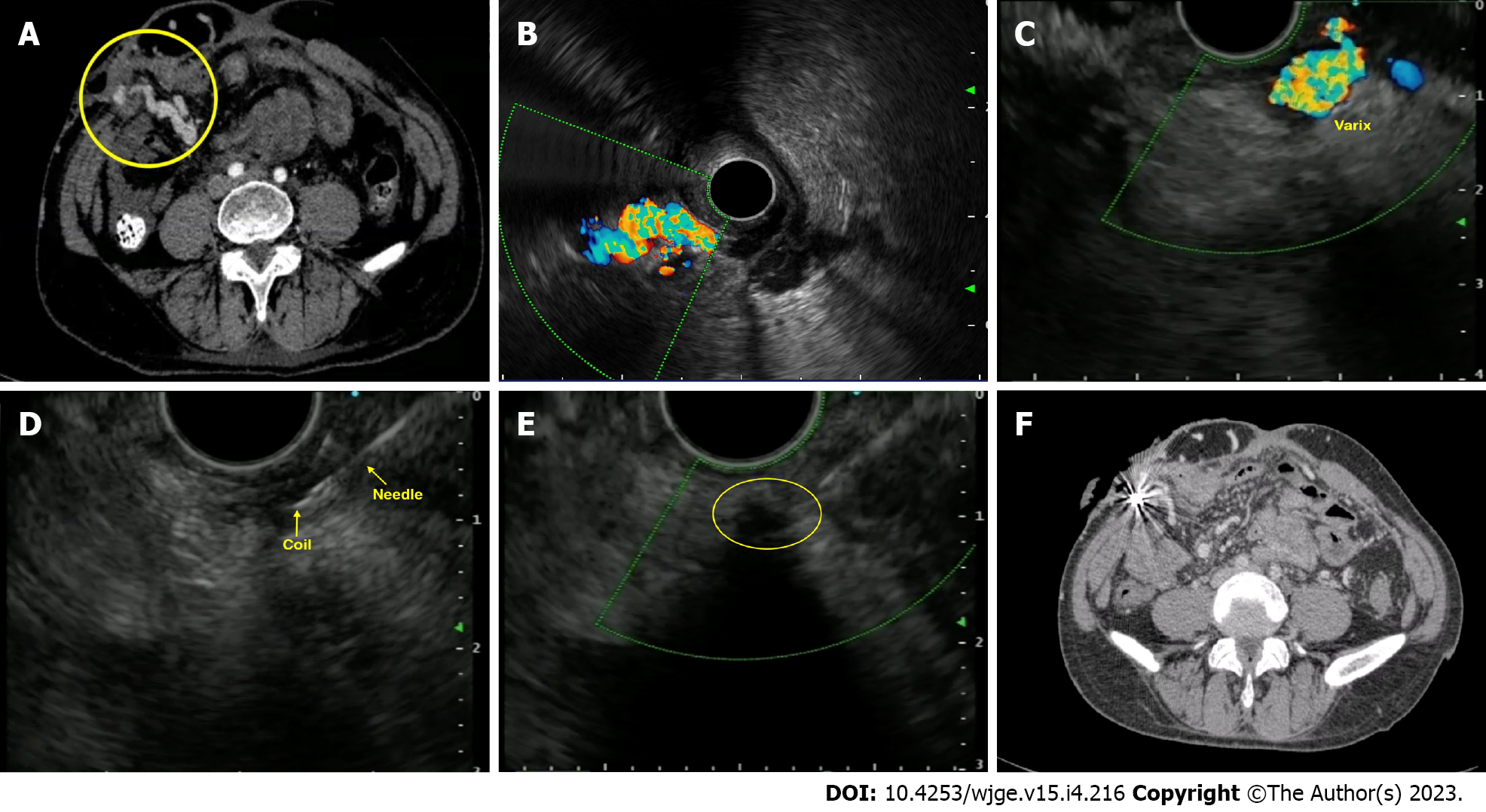
Duodenal varices: Duodenal varices (DV) is extremely rare (0.4% cases). They are isolated in the submucosa and are easily missed on routine EGD. EUS plays an important role in determining the exact site, size, and location necessitating targeted therapy. Unfortunately, few case reports exist on using EUS-guided vascular therapy for DV[59-61] (Figure 4).
Rectal varices: Rectal varices (RV) has been reported in up to 44%-89% of cases of cirrhosis[62,63]. Due to their 'deep submucosal' nature, EUS has a higher sensitivity in identifying them over endoscopy (75% vs 43.3%), including perirectal collateral veins and perforators[64,65]. Multiple case reports have been published using EUS for RV management[66,67].
Parastomal varices: Bleeding stomal varices account for only 5% of bleeding ectopic varices (1%-5% of all cases)[57]. EUS-guided angiotherapy can be used as an alternative in managing such cases[68,69]. The author's center has experience performing EUS-coil with glue injection for parastomal varices in a cirrhotic patient ineligible for TIPS[70] (Figure 5).
Choledochal varices: The first case of ectopic variceal bleeding was reported in a case of anastomotic choledocho-jejunal varices[71]. They are rare, and EUS may help diagnose such cases. EUS mini probe can identify pericholedochal varices in patients with extrahepatic venous obstruction and help differentiate from biliary stones or sludge (Figure 6).
Table 3 summarizes published literature on EUS-guided angiotherapy for ectopic variceal bleeding[72-79].
| Ref. | Cases | Underlying diagnosis | Age/sex | Size of varix | Any prior therapy given | EUS therapy (agent used) | EUS needle used | Coils | Glue | Post procedure EUS findings | Follow-up duration | Comments |
| Duodenal varices | ||||||||||||
| So et al[60], 2016 | 1 | PC/EHPVO | 65/F | 2 cm | - | Coil | 19-G FNA | 3 | - | Color Doppler: cessation of blood flow | 10 mo | No bleeding on F/U |
| Kimura et al[61], 2017 | 1 | PC | 76/F | - | - | CYA glue | 22-G FNA | - | 0.5 mL (3 sessions) | - (f/u CT: shows extinction of contrast enhancement in DV) | 6 mo | No bleeding on F/U |
| Kinzel et al[72], 2014 | 1 | Cirrhosis (Child C) | 31/M | 10 mm | Endoscopic ethanolamine oleate | Coil + CYA glue | 19-G (for coil) + 22-G (for glue) FNA | 1 | 2 mL | Near complete thrombosis of varix | 3 mo | No bleeding on F/U |
| Fujii-Lau et al[73], 2016 | 3 | PVT; SMV-T; SMV-T | 57/M; 46/F; 62/F | -; -; - | Glue; -; Clip + coil (IR) | Coil; Coil; Coil + CYA glue | 22-G FNA (for all) | 4; 4; 8 | -; -; 2 mL | dec. flow; dec. flow; no flow | 30 mo; 12 mo; 6 mo | No bleeding on F/U (all cases) |
| Bahdi et al[74], 2020 | 1 | Cirrhosis | 41/M | - | None | Coil + CYA glue | 22-G FNA | 8 | 2 mL | - | - | - |
| Rectal varices | ||||||||||||
| Messallam et al[66], 2014 | 1 | Cryptogenic cirrhosis | 78/M | 45 × 12 mm | None | Coil + CYA glue | 19-G FNA | 2 | 4 mL | No flow | 12 wk | No bleeding on F/U |
| Sharma et al[67], 2010 | 1 | PHTN | 68/M | 2.2 mm | None | Histocryl glue | - | - | 1 mL | Decreased flow | 6 mo | No bleeding on F/U |
| Mukkada et al[75], 2017 | 1 | PHTN | 65/M | 5.9 mm | Endoscopic sclerotherapy (tetradecyl sulphate 16 ml; CYA glue) | Coil | 19-G FNA | 2 | - | No flow | - | - |
| Bazarbashi et al[76], 2020 | 1 | Cirrhosis | 71/M | 4 mm | None | Coil | 19-G FNA | 1 | - | No flow | 6 mo | No bleeding on F/U |
| Philips et al[77], 2017 | 1 | Cirrhosis | 48/M | - | None | Coil + CYA glue | 22-G FNA | 1 | 1 mL | No flow | 1 mo | No bleeding on F/U |
| Weilert et al[78], 2012 | 1 | Cirrhosis | 60/F | > 3 cm | None | Coil + CYA glue | 19-G FNA | 5 | 4 mL | No flow | 12 mo | No bleeding on F/U |
| Jana et al[79], 2017 | 1 | Hepatitis C/PHTN | 54/M | - | None | Coil + CYA glue | 22-G FNA | 3 | 0.8 mL | No flow | 1 mo | No bleeding on F/U |
| Stomal varices | ||||||||||||
| Tabibian et al[68], 2016 | 1 | Cirrhosis PSC/post colectomy for UC | 70/F | 5 mm | Somatostatin/topical silver nitrate | Coil | 22-G FNA | 6 | - | No flow | 9 mo | No bleeding on F/U |
| Tsynman et al[69], 2014 | 1 | UC/post colectomy/cirrhosis | 74/F | - | TIPS | CYA glue with lipiodol | 22-G FNA | - | 0.5 mL | No flow | 8 mo | No bleeding on F/U |
| Samanta et al[70], 2022 | 1 | Alcohol cirrhosis/tubercular cocoon/ileostomy | 52/M | - | Endoscopic glue injection | Coil + CYA glue | 19-G FNA | 2 | 4 mL | No flow | 6 mo | No bleeding on F/U |
| Choledochal varices | ||||||||||||
| Levy et al[71], 2008 | 1 | CP/post total pancreatectomy | 50/F | 14 mm | - | Coil | 22-G FNA | 5 | - | No flow | 1 mo | No bleeding on F/u |
| Fujii-Lau et al[73], 2016 | 5 | Cirrhosis; SMV-T; PVT; PHTN; PVT | 61/M; 56/M; 27/M; 71/M; 50/F | -; -; -; -; - | None; None; None; None; None | Coil; Coil; Coil; Coil; Coil | 22-G FNA (for all) | 7; 9; 4; 5; 5 | -; -; -; -; - | dec. flow; dec. flow; dec. flow; dec. flow; dec. flow | 24 mo; 37 mo; 26 mo; 1 mo; 87 mo | Recurrent bleed in 3 cases; one case died due to underlying disease |
EUS-guided angiotherapy has theoretical benefits for variceal bleeding over the standard of care, primarily for GV. EUS offers additional benefits as a “rescue” modality for refractory/unsuccessfully treated cases. This management modality may be considered in the management algorithm of variceal bleed, albeit only in expert centers with adequate backup.
Treatment of non-variceal bleed (NVB) entails the standard use of well-established therapies categorized into injection (epinephrine), mechanical (clip/EBL) or thermal (argon plasma coagulation) or hemostatic agents[80-82]. Despite this, 10%-24% of cases re-bleed or are refractory to the standard treatment modalities. In these cases, EUS-guided angiotherapy can be beneficial by helping in directly visualizing the bleeding vessel, its feeders or perforators and help in targeted therapy. Currently, the role of EUS for the management of NVB is more of a rescue therapy. However, a recent systematic review reported a favourable outcome of EUS-guided therapy in 91.4% of cases[83]. In addition, EUS-angiotherapy is feasible and safe for managing Dieulafoy’s lesion, bleeding ulcer or tumour, GI stromal tumour (GIST) and sometimes, visceral artery pseudoaneurysms (PsA).
PsA is a rare vascular complication noted in various conditions, more commonly in acute or chronic pancreatitis, with an incidence of 0.05% and 0.03%, respectively. The splenic artery is the most common vessel involved (37.7%). The most frequent line of management is IR-guided endovascular therapy[84,85]. However, EUS-guided angiotherapy can be an exciting alternative to manage such cases. The proximity of PsA of splenic vessels or gastroduodenal artery to the GI wall enables them to be targeted and obliterated. Various agents like coil, CYA glue, a coil with glue combination and thrombin have been used.
The technique of performing EUS-guided angiotherapy in PsA: The technical details have been highlighted in Table 4.
| EUS-guided angioembolization of visceral artery pseudoaneurysm |
| Pre-procedure requirements |
| All procedures are done under the cover of pre/peri-procedural antibiotics |
| Patient is usually kept fasting for 4-6 h before the procedure |
| Adequate resuscitation of the patient, in case of active bleeding is ensured, prior to the procedure |
| Informed consent prior to the procedure |
| What is needed prior to the procedure |
| Linear echoendoscope with at least a 3.7 mm working channel |
| Needle size: depends on the choice of the endoscopist; usually a 19-G needle is used with 0.035’coil. However, a 22-G needle with 0.018’ coils may be used |
| Diameter of the coils: Smaller than the shortest diameter of the PsA |
| Number of coils: depends on size of the PsA |
| Amount of glue: depends on the size of the PsA |
| Technical aspects |
| A proper diagnostic EUS is performed |
| The echoendoscope is positioned optimally for a stable PsA access |
| Optimum examination of the PsA, the feeding vessel and the anatomy is delineated |
| The approach should always be through parenchyma, either pancreatic or hepatic. Bare puncture of the PsA without supporting parenchyma should not be performed |
| EUS-guided coil and glue embolization is usually performed using a 22-G/19-G (gauge) FNA needle |
| The size of the coil is determined by the short axis of the diameter of the PsA |
| After puncture of the varix, blood is aspirated to confirm the location. This is followed by flushing of the needle with saline. The pressure is high in the aneurysm, hence care should be taken to avoid creeping of blood along the hollow of the needle and causing needle block |
| The coils are then deployed into the varix using the stylet as a pusher. Packing with coils slows the flow inside the PsA, which can be visualized and further requirement of coils is assessed. Once the coils are deployed, flushing of the needle is done with normal saline |
| After coil deployment, cyanoacrylate glue is injected using the coils as scaffold |
| Once, the PsA is obliterated, visualized by absence of flow on colour Doppler, the sheath of the needle is advanced beyond the endoscope tip for 2-3 cm before withdrawing the scope. This avoids contact of glue with the endoscope tip |
| Post procedure |
| The patients are kept under observation for 12 h |
| Post embolization X-ray would help visualize the coils and also look for complications |
| Repeat EUS can be done after 48 hrs. to look for residual flow |
| Cross-sectional imaging is usually done after 72 h. to document success of therapy |
| Follow-up EUS may be performed at 1-mo |
Existing literature: Case reports: The use of thrombin in PsA was first described by Roach et al[86], wherein thrombin (500 IU, 1 mL) was injected in a PsA arising from a superior mesenteric artery under EUS guidance with no re-bleeding at 42 wk of follow-up. The use of CYA glue with lipiodol was described by Gonzalez et al[87], wherein a splenic artery PsA was tackled, and there was no re-bleed on a 2-mo follow-up. Similarly, the first use of coil was described by Robb et al[88] in superior mesenteric artery PsA using multiple Nester coils, achieving complete obliteration in one session. Rai et al[89] used coil with CYA glue combination in a 3 cm splenic artery PsA in a single sitting with no re-bleed in 1 mo. Giant PsA (> 5 cm) have also been reported to have been managed with EUS-angiotherapy. The author’s center reported a 6.5 cm splenic artery PsA using a coil and glue combination in 2 sessions achieving complete obliteration[90]. The case reports have been outlined in Supplementary Table 1.
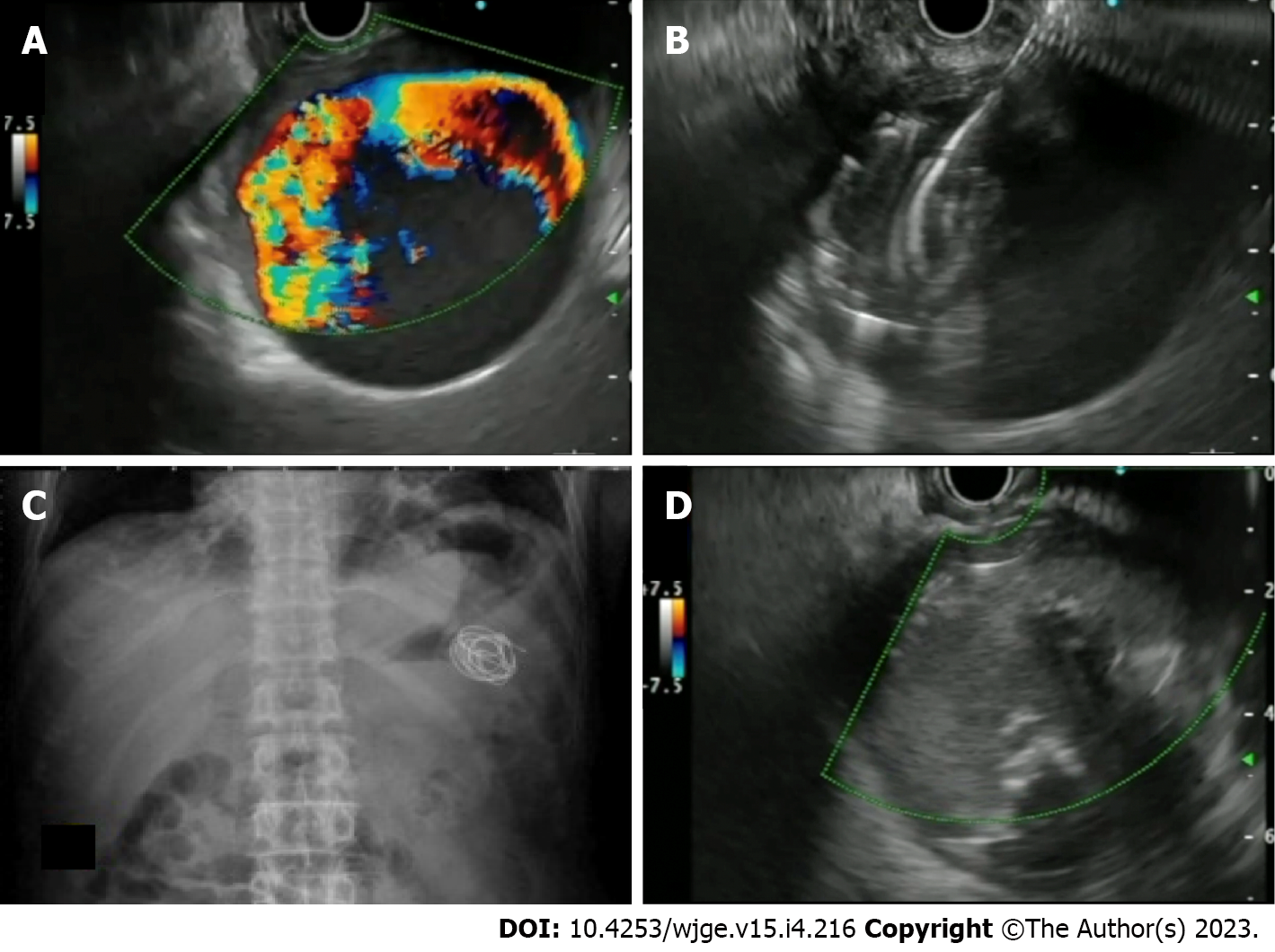
Case series: Only 5 case series (> 3 cases) have been reported, mainly from the Indian subcontinent and have been tabulated in Table 5. Three of them have utilized thrombin, while two have used coil with glue[91-95]. The author’s centre has reported the largest series of 16 cases of visceral artery PsA in 15 patients. The median size of the PSA was 2.8 cm (0.9-9.7 cm). A median of 2 coils (1-8) and 2 mL of CYA (1-5 mL) was used. Complete obliteration in the first session was achieved in 15 PSA (93.8 %)[95] (Figure 7).
| S.No. | Ref. | Cases | Age/sex | Chief complaints | Artery involved | PSA size (mm) | EUS needle used | Embolization agent used | EUS sessions needed | Technical/clinical success | Complications | Follow up and comments |
| 1 | Gamanagatti et al[91], 2015 | 3 | 56/M; 45/M; 30/M | Upper GI bleed (all 3) | GDA; Splenic; Splenic | - | 22-G | Thrombin (500 IU, 300 IU, 400 IU) | 1 each | Yes/yes | None | Imaging F/U: complete obliteration; no bleeding at 1 mo F/U |
| 2 | Jhajharia et al[92], 2018 | 3 | 43/M; 25/M; 55/M | Pain abdomen; hematemesis; Malena (respectively) | GDA; Right hepatic; splenic | 40 × 50; 30 × 22 × 27; 15 × 13 | 22-G | Thrombin (1000 IU; 1000 IU; 500 IU) | 1 each | Yes/yes | None | F/U at 1.5 years, 1 year and 3 mo: no bleeding (respectively) |
| 3 | Rai et al[93], 2018 | 6 | Median 36.7 years (19-60); 5 men | 3 asymptomatic; 3 upper GI bleed | All Splenic artery PSA | 25-65 (range) | 19-G | Coils (size 8, 14, 16; number 1-5) and glue (1-2 mL) | 3 cases needed 2 EUS sessions (size > 4 cm) | Yes/yes (all cases) | None | EUS (4 wk) and CT (3 mo): complete obliteration |
| 4 | Maharshi et al[94], 2020 | 8 | Median 34 years (27-58); all males | Malena (100%); hematemesis (75%) | Splenic (5); left hepatic (2); GDA (1) | Median 29 × 26 (range 18 × 19 – 40 × 50) | 22-G | Thrombin (200-500 IU) | 1 | Yes/87.5% clinical success (7/8 cases) | 2 cases post procedural pain | EUS (1 and 3 mo) and CT (1 mo): complete obliteration; only 1 case with PSA > 5 cm needed second EUS session after 6 wk |
| 5 | Samanta et al[95], 2022 | 16 PsA (in 15 patients) | Median 44 (17-56); males 14 (93.3%) | Malena/ incidental/ PCD bleed | Splenic (12); GDA (4) | Median 2.8 (0.9-9.7 cm) | 19-G | Coils (median 1[1-8]) with CYA glue (median 2 [1-5 mL]) | 1 session in 15 (93.8%) | Yes/yes | One case had splenic infarct (managed conservatively) | Follow-up at 6 mo: no rebleed; one case developed recurrent PsA at a site separate from first PsA (managed again with EUS) |
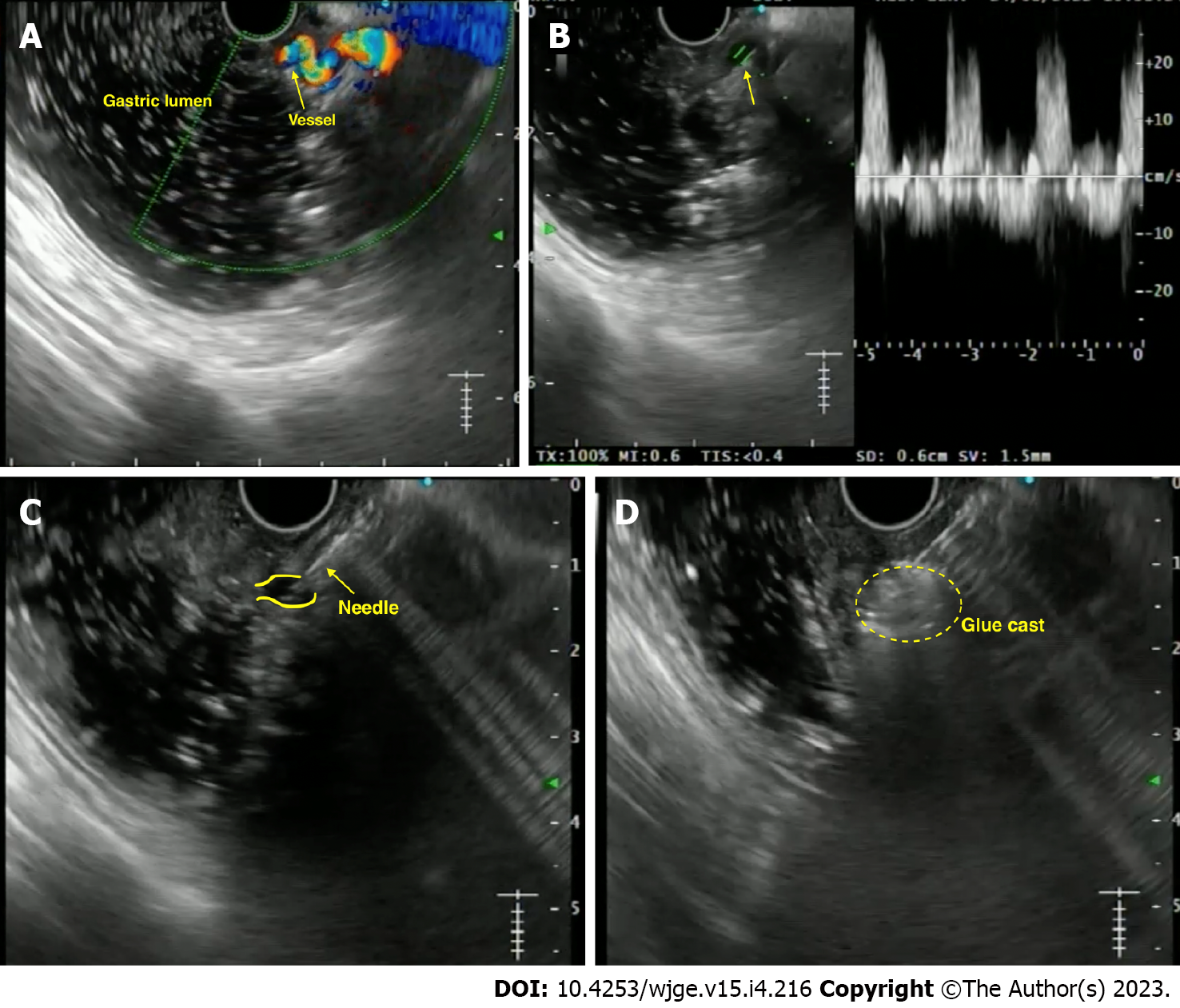
Anecdotal reports have been published on using EUS-guided angiotherapy to manage NVB (Supplementary Table 2). In 1996, the first report used EUS-guided epinephrine/polidocanol injection for managing bleeding dieulafoy’s lesion[96] (Figure 8). Levy et al[97] reported a series of 5 refractory NVBs, including dieulafoy’s lesion, hemosuccus pancreaticus, duodenal ulcer and GIST. The largest data of EUS-guided therapy reported to date involves a cohort of 17 cases using various agents. On a median 12-mo follow-up, 15/17 (88%) patients had no re-bleed[98].
The data on EUS-guided vascular interventions for NVB is limited and comparative studies are needed to establish its role in therapeutic algorithms. However, EUS-guided angiotherapy may be considered a second-line “rescue” treatment, especially in refractory/re-bleeding cases. The feasibility and safety data are encouraging, though larger multicentre data is required to define its role further.
PV dynamics are crucial for decision-making in chronic liver disease and PHTN cases. EUS-guided PV access is a viable option with a probable advantage over the percutaneous route owing to the relative difficulty experienced in the latter in patients with obesity, ascites, and overlying distended bowel[99]. In addition, there are various potential clinical applications of EUS-guided PV access that include angiography, measurement of the PPG, EUS-guided TIPS, and PV sampling for evaluation in GI cancer[1,99].
Access to the PV can be achieved on EUS via both, trans-gastric or trans-duodenal route. However, the most frequently targeted site is the intrahepatic PV through the hepatic parenchyma[1,2,99].
The technique: PV puncture is done using the standard EUS-FNA needle after confirming with colour doppler and pulse-wave verification. Some important points for consideration are: (1) 25-G needle is the least traumatic; (2) trans-gastric, trans-hepatic route on EUS is safer than accessing from duodenum; and (3) use of CO2 as a contrast agent is better than iodine, as it allows better visualization of needle as-well-as easier administration using small-caliber FNA needle. Following the puncture of PV, the needle is slightly withdrawn and the tract is monitored using colour-Doppler for any bleeding episodes. If positive signal is reported, the needle is kept in place until the bleeding has stopped[100].
Existing literature in animal models: Lai et al[101] proved the technical feasibility of the procedure by reporting the first case of PV access in 2004 using EUS guidance wherein extrahepatic PV was accessed using 22-G FNA needle, via duodenum, in 21 swine models. Subsequently, Magno et al[102] performed PV angiography in 2007 in 5 pigs, demonstrating that the 25-G needle showed no signs of injury. Subsequently, Giday et al[100,103] performed trans-hepatic PV access using a 25-G FNA needle under CO2 insufflation. Portal pressure measurements were also taken, indicating it to be technically feasible (Supplementary Table 3).
Once it is established that EUS-guided PV access is feasible, it paves the path for further interventions such as PPG measurement, PV sampling and even EUS-guided intrahepatic portosystemic shunt.
PPG measurement has been shown to correlate with the prognosis and complications of cirrhosis. In addition, PPG ≥ 10 mmHg and ≥ 12 mmHg are associated with the development of EV and bleeding, respectively. Currently, the standard practice is to measure hepatic venous pressure gradient (HVPG) via the percutaneous route. But, both direct PV access and HVPG measurement have high complication rates[104]. Moreover, HVPG correlated poorly with presinusoidal PHTN. Hence, the concept of EUS-PPG arose to overcome these difficulties, with the added benefit of assessment of varices and liver biopsy in the same setting, if required.
The technique of the procedure: This has been highlighted in Table 6[105].
| Procedural steps for measuring EUS-PPG |
| The measurement of PPG via EUS requires 4 components: 25-G FNA needle, non-compressible tubing, a compact digital manometer, and heparinized saline. The tubing is connected by a luer lock to the distal port and heparinized saline is connected the proximal port of the manometer |
| With the patient supine, the manometer is placed at the patient’s midaxillary line |
| The HV measurement is conducted first, in which middle HV is targeted most often (larger calibre and better alignment with the needle trajectory). Then PV measurement is taken (umbilical portion of left PV is the target) |
| Doppler flow is used to confirm the typical multiphasic waveform of hepatic venous flow and typical venous hum of the portal venous flow |
| Trans-gastric trans-hepatic route is taken for HV and PV puncture |
| Needle is flushed with heparinized saline (1 mL). The steadiest reading at equilibrium is recorded. Three measurements are taken and their mean is calculated (both HV and PV pressures) |
| The FNA needle is slowly withdrawn from the vein into the liver parenchyma and then back into the needle sheath with Doppler flow on to ensure there is no flow within the needle tract |
Existing literature and future trends: The first clinical report of the use of EUS-PPG was given by Fujii-Lau et al[106], wherein a 27-year-old man with recurrent GI bleed (post EUS-coil insertion in duodenal vessels) underwent this procedure. The first large-scale study in 28 cases was done by Huang et al[105], using a 25-G FNA needle with 100% technical success and no adverse events. PPG correlated with varices, thrombocytopenia, and notable clinical evidence of cirrhosis. Zhang et al[107] demonstrated its use in patients with acute or subacute PHTN, with an excellent correlation between EUS-PPG and HVPG (r = 0.923). Acting as a “one-stop-shop”, performing EUS-PPG with EUS-liver biopsy in the same sitting has shown to be technically feasible in a study of 24 cases, with good correlation with the non-invasive markers of fibrosis[108]. Table 7 highlights the published literature on the use of EUS-PPG[105-111].
| Ref. | Year | Number of cases | Approach | EUS-FNA needle | Technical success | Complications | Correlation between EUS and trans-hepatic PVP measurement |
| Fujii-Lau et al[106] | 2014 | 1 | Trans-gastric | 22-G | 1 | None | PPG 1 mmHg (excellent correlation with HVPG) |
| Huang et al[105] | 2017 | 28 | - | 25-G | 25/25 cases | None | Excellent correlation with varices (P = 0.0002), PHG (P = 0.007), and thrombocytopenia (P = 0.036); few of them also underwent liver biopsy in same setting |
| Zhang et al[107] | 2020 | 12 | - | 22-G | 11/12 cases (91.7%) | None | R = 0.923 |
| Shah et al[109] | 2021 | 1 | Trans-gastric | 25-G | 1 | None | NA (same session EUS-liver biopsy was done) |
| Hajifathalian et al[108] | 2021 | 24 | Trans-gastric | 25-G | 23/24 (96%) patients also underwent EUS-liver biopsy (TS: 24/24 [100%]) | One case of mild abdominal pain (resolved with analgesics) | NA; excellent correlation with fibrosis-4 score (P = 0.026) and transient elastography (P = 0.011) |
| Choi et al[110] | 2022 | 83 | Trans-gastric | 25-G | 100%; 71 cases underwent EUS-liver biopsy | No major events; minor abdominal pain (8 [9.6%] cases) | Correlation with clinical features of cirrhosis (9.46 vs 3.61 mmHg, P < 0.0001), EV/GV (13.88 vs 4.34 mmHg, P < 0.0001), and thrombocytopenia (9.25 vs 4.71 mmHg, P = 0.0022) |
| Choi et al[111] | 2022 | 64 | Trans-gastric | 25-G | 100% (concurrent EUS-LB in 43/64 [67.2%]) | 1 case (EUS-PPG alone); 5 cases (EUS-PPG + EUS-LB both) | EUS-PPG > 5 mmHg correlated with EUS-liver biopsy fibrosis stage ≥ 3 [LR 27] (P = 0.004) |
The benefits of trans-jugular intrahepatic portosystemic shunt (TIPS), as a pre-emptive or rescue procedure in cases of variceal bleeding or refractory ascites has been well established. Buscaglia et al[112] described the first case of EUS-TIPS in a live porcine model in 2009, wherein after sequential puncture of HV and PV, a metal stent was inserted with the distal end in PV and proximal end in HV with no complications on follow-up in 2 wk. Similarly, Binmoeller et al[113] and Schulman et al[114] have reported similar results in porcine models using lumen-apposing metal stent (LAMS). Poincloux et al[115] reported the largest series of 21 porcine models showing a technical success of 91% with 14.2% morbidity. EUS-guided TIPS is still in the pre-clinical stages, and many technical issues must be resolved before embarking on human trials.
“Liquid biopsy” for hepatobiliary malignancies is gaining popularity. The PV has been shown to harbour circulating tumour cells (CTCs) for the primary tumour, forerunners of future metastasis of solid organ cancers. This signifies tumor signature and can help in prognostication and also can be used for organoid formation for future studies. The first human study was reported by Catenacci et al[116] wherein CTCs were detected in 100% of cases of PV and 4/18 (22.2%) cases from peripheral blood. Zhang et al[117] reported that CTCs are more in PV than peripheral blood (97% vs 87%; 10 vs 6 cells per 5 mL). Further studies are needed to standardize this technique.
The presence of malignant PV thrombosis (PVT) is a poor prognostic sign and precludes curative resection. Usually, imaging (ultrasound/computed tomography) can help differentiate bland and malignant PVT, but definitive confirmation would require sampling. Performing the latter via the percutaneous route is difficult and may lead to serious vascular and biliary injury. This can be overcome by EUS-guided PV access. Trans-duodenal approach to extrahepatic PV using a 25-G FNA needle yields excellent results. Various case reports have been published on using EUS-FNA of PVT, especially in cases of hepatocellular carcinoma[118-122]. Rustagi et al[118] showed that in 17 patients, EUS-FNA of remote malignant thrombi upstaged the diagnosis by 37.5% and converted 25% to an unresectable stage. This underlines using EUS-FNA of PV thrombus as a cancer staging modality.
Systemic palliative or trans-arterial chemotherapy for diffuse liver metastasis is fraught with problems like suboptimal hepatic tissue levels and the possibility of secondary sclerosing cholangitis. However, Faigel et al[123,124] first reported the technical feasibility of EUS-guided PV injection of chemotherapy (EPIC) using drug-eluting microbeads and nanoparticle in 24 swine models. Although further studies are warranted, this study proved the feasibility of EPIC in an animal model.
Preoperative PV embolization (PVE) before liver resection has been practiced via IR[125]. In addition, preliminary studies in an animal model by Matthes et al[126] using EUS-guided ethylene-vinyl alcohol copolymer leading to PVE have been reported. Recently, Park et al[127] reported technical success of 88.9% and 87.5%, respectively, with coil and CYA glue embolization in 9 swine models with no evidence of organ damage. Although further studies are needed, this technique does show promise for future application.
The PV-stenting (for occlusion/thrombosis) is usually carried out by the percutaneous route (USG-guided catheter-directed thrombolysis). The use of EUS has opened up avenues of PV access and subsequent stent placement. This was first reported by Park et al[128] in 6 swine models, using uncovered stents, with 100% technical success.
The proximity of the posterior mediastinum to the esophagus has allowed EUS easy access to the heart and associated vascular structures. Like trans-esophageal echocardiography, EUS is technically feasible in animal models to sample the coronaries, atria, ventricles, and valvular apparatus. Fritscher-Ravens et al[129] demonstrated radiofrequency ablation of the aortic valve, pericardial fluid aspiration, and atrial mass biopsy in swine models with no major adverse events. Most isolated case reports exist on EUS-biopsy of intracardiac/pericardial tumours[130-132]. EUS-aspiration of pericardial fluid has been performed with no reported arrhythmias[133]. Even EUS-guided thrombolysis of pulmonary artery and mesenteric thrombi has been reported. Under EUS guidance, Tenecteplase was injected into the thrombus using a 25-G needle[134].
While the reports are exciting, these are anecdotal cases, and more data is warranted in the future to establish the safety and efficacy of such interventions.
EUS-guided vascular intervention is gradually becoming a promising new technique for managing vascular complications around the GI tract as a salvage and/or primary modality. While comprehensive data has established its safety and efficacy in managing conditions such as GV and measurement of PPG, its role for other applications such as management of visceral artery pseudoaneurysms and PV access for various therapies needs further validation. Nevertheless, proper selection of cases, adequate precautions and optimum backup can make EUS-guided angiotherapy an essential tool in the endoscopist’s armamentarium.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakamura K, Japan; Reihani H, Iran S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Levy I, Binmoeller KF. EUS-guided vascular interventions. Endosc Ultrasound. 2018;7:228-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Dhar J, Samanta J. Role of endoscopic ultrasound in the field of hepatology: Recent advances and future trends. World J Hepatol. 2021;13:1459-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 847] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 4. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1440] [Article Influence: 180.0] [Reference Citation Analysis (3)] |
| 5. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 6. | Oleas R, Robles-Medranda C. Insights into the role of endoscopic ultrasound-guided vascular therapy. Ther Adv Gastrointest Endosc. 2019;12:2631774519878282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Saad WE, Darcy MD. Transjugular Intrahepatic Portosystemic Shunt (TIPS) vs Balloon-occluded Retrograde Transvenous Obliteration (BRTO) for the Management of Gastric Varices. Semin Intervent Radiol. 2011;28:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 9. | Krige JE, Bornman PC, Goldberg PA, Terblanche J. Variceal rebleeding and recurrence after endoscopic injection sclerotherapy: a prospective evaluation in 204 patients. Arch Surg. 2000;135:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hou MC, Lin HC, Lee FY, Chang FY, Lee SD. Recurrence of esophageal varices following endoscopic treatment and its impact on rebleeding: comparison of sclerotherapy and ligation. J Hepatol. 2000;32:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Irisawa A, Obara K, Bhutani MS, Saito A, Shishido H, Shibukawa G, Takagi T, Yamamoto G, Seino O, Shishido F, Kasukawa R, Sato Y. Role of para-esophageal collateral veins in patients with portal hypertension based on the results of endoscopic ultrasonography and liver scintigraphy analysis. J Gastroenterol Hepatol. 2003;18:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Irisawa A, Saito A, Obara K, Shibukawa G, Takagi T, Shishido H, Sakamoto H, Sato Y, Kasukawa R. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: severe periesophageal collateral veins and large perforating veins. Gastrointest Endosc. 2001;53:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Lahoti S, Catalano MF, Alcocer E, Hogan WJ, Geenen JE. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc. 2000;51:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | de Paulo GA, Ardengh JC, Nakao FS, Ferrari AP. Treatment of esophageal varices: a randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest Endosc. 2006;63:396-402; quiz 463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Kato T, Hikichi T, Nakamura J, Takasumi M, Hashimoto M, Kobashi R, Yanagita T, Takagi T, Suzuki R, Sugimoto M, Sato Y, Irie H, Okubo Y, Kobayakawa M, Ohira H. Usefulness of Endoscopic Ultrasound with the Jelly-Filling Method for Esophageal Varices. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986;32:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 239] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Ríos Castellanos E, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue vs other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev. 2015;CD010180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Soehendra N, Nam VC, Grimm H, Kempeneers I. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy. 1986;18:25-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Boustière C, Dumas O, Jouffre C, Letard JC, Patouillard B, Etaix JP, Barthélémy C, Audigier JC. Endoscopic ultrasonography classification of gastric varices in patients with cirrhosis. Comparison with endoscopic findings. J Hepatol. 1993;19:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Iwase H, Suga S, Morise K, Kuroiwa A, Yamaguchi T, Horiuchi Y. Color Doppler endoscopic ultrasonography for the evaluation of gastric varices and endoscopic obliteration with cyanoacrylate glue. Gastrointest Endosc. 1995;41:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sarin SK, Kumar A. Sclerosants for variceal sclerotherapy: a critical appraisal. Am J Gastroenterol. 1990;85:641-649. [PubMed] |
| 23. | Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, Marcos-Sanchez F, Caunedo-Alvarez A, Ortiz-Moyano C, Gomez-Parra M, Herrerias-Gutierrez JM. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc. 2007;66:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Cameron R, Binmoeller KF. Cyanoacrylate applications in the GI tract. Gastrointest Endosc. 2013;77:846-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Romero-Castro R, Pellicer-Bautista F, Giovannini M, Marcos-Sánchez F, Caparros-Escudero C, Jiménez-Sáenz M, Gomez-Parra M, Arenzana-Seisdedos A, Leria-Yebenes V, Herrerias-Gutiérrez JM. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy. 2010;42 Suppl 2:E35-E36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, Subtil-Inigo JC, Junquera-Florez F, Gornals JB, Repiso-Ortega A, Vila-Costas J, Marcos-Sanchez F, Muñoz-Navas M, Romero-Gomez M, Brullet-Benedi E, Romero-Vazquez J, Caunedo-Alvarez A, Pellicer-Bautista F, Herrerias-Gutierrez JM, Fritscher-Ravens A. EUS-guided coil vs cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc. 2013;78:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, Binmoeller KF. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc. 2016;83:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Kouanda A, Binmoeller K, Hamerski C, Nett A, Bernabe J, Shah J, Bhat Y, Watson R. Safety and efficacy of EUS-guided coil and glue injection for the primary prophylaxis of gastric variceal hemorrhage. Gastrointest Endosc. 2021;94:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Robles-Medranda C, Oleas R, Valero M, Puga-Tejada M, Baquerizo-Burgos J, Ospina J, Pitanga-Lukashok H. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices vs coiling alone: a randomized trial. Endoscopy. 2020;52:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Ryou M. Combination therapy vs monotherapy for EUS-guided management of gastric varices: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 31. | Robles-Medranda C, Nebel JA, Puga-Tejada M, Oleas R, Baquerizo-Burgos J, Ospina-Arboleda J, Valero M, Pitanga-Lukashok H. Cost-effectiveness of endoscopic ultrasound-guided coils plus cyanoacrylate injection compared to endoscopic cyanoacrylate injection in the management of gastric varices. World J Gastrointest Endosc. 2021;13:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Mukkada RJ, Antony R, Chooracken MJ, Francis JV, Chettupuzha AP, Mathew PG, Augustine P, Koshy A. Endoscopic ultrasound-guided coil or glue injection in post-cyanoacrylate gastric variceal re-bleed. Indian J Gastroenterol. 2018;37:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Lôbo MRA, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol. 2019;56:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Samanta J, Nabi Z, Facciorusso A, Dhar J, Akbar W, Birda CL, Mangiavillano B, Auriemma F, Lakhtakia S, Kochhar R, Duvvur NR. EUS-guided treatment of gastric fundal varices with coil and glue injection is safe and fares better than endoscopic glue injection: an international mutli-centre experience. Gastrointest Endosc. 2022;95:AB518-AB519. [DOI] [Full Text] |
| 35. |
Ramchandani M.
Safety and efficacy of endoscopic conventional cyanoacrylate glue |
| 36. | Frost JW, Hebbar S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc Int Open. 2018;6:E664-E668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Bazarbashi AN, Wang TJ, Thompson CC, Ryou M. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable hemostatic gelatin sponge: a novel alternative to cyanoacrylate. Endosc Int Open. 2020;8:E221-E227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Irisawa A, Shibukawa G, Hoshi K, Yamabe A, Sato A, Maki T, Yoshida Y, Yamamoto S, Obara K. Endoscopic ultrasound-guided coil deployment with sclerotherapy for isolated gastric varices: Case series of feasibility, safety, and long-term follow-up. Dig Endosc. 2020;32:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, Chung SC, Sung JJ. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 40. | Gubler C, Bauerfeind P. Safe and successful endoscopic initial treatment and long-term eradication of gastric varices by endoscopic ultrasound-guided Histoacryl (N-butyl-2-cyanoacrylate) injection. Scand J Gastroenterol. 2014;49:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Khoury T, Massarwa M, Daher S, Benson AA, Hazou W, Israeli E, Jacob H, Epstein J, Safadi R. Endoscopic Ultrasound-Guided Angiotherapy for Gastric Varices: A Single Center Experience. Hepatol Commun. 2019;3:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Binmoeller KF, Weilert F, Shah JN, Kim J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest Endosc. 2011;74:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 43. | Kozieł S, Pawlak K, Błaszczyk Ł, Jagielski M, Wiechowska-Kozłowska A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Robles-Medranda C, Valero M, Nebel JA, de Britto Junior SR, Puga-Tejada M, Ospina J, Muñoz-Jurado G, Pitanga-Lukashok H. Endoscopic-ultrasound-guided coil and cyanoacrylate embolization for gastric varices and the roles of endoscopic Doppler and endosonographic varicealography in vascular targeting. Dig Endosc. 2019;31:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Bick BL, Al-Haddad M, Liangpunsakul S, Ghabril MS, DeWitt JM. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc. 2019;33:1837-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Bazarbashi AN, Wang TJ, Jirapinyo P, Thompson CC, Ryou M. Endoscopic Ultrasound-Guided Coil Embolization With Absorbable Gelatin Sponge Appears Superior to Traditional Cyanoacrylate Injection for the Treatment of Gastric Varices. Clin Transl Gastroenterol. 2020;11:e00175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Seven G, Musayeva G, Seven OO, Herdan E, Ince AT, Senturk H. Comparison of endoscopic ultrasound-guided coil deployment with and without cyanoacrylate injection for gastric varices. Arab J Gastroenterol. 2022;23:115-119. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Zhang ZG, Li Z, Yang Y, Cheng B, Yan W, Yuan Y, Chen M, Hou W, Yang M, Chen Q. Hemodynamic effect through a novel endoscopic intervention in management of varices and hypersplenism (with video). Gastrointest Endosc. 2022;95:172-183.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Schiano TD, Adrain AL, Vega KJ, Liu JB, Black M, Miller LS. High-resolution endoluminal sonography assessment of the hematocystic spots of esophageal varices. Gastrointest Endosc. 1999;49:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Caletti G, Brocchi E, Baraldini M, Ferrari A, Gibilaro M, Barbara L. Assessment of portal hypertension by endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S21-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Wiechowska-Kozłowska A, Zasada K, Milkiewicz M, Milkiewicz P. Correlation between Endosonographic and Doppler Ultrasound Features of Portal Hypertension in Patients with Cirrhosis. Gastroenterol Res Pract. 2012;2012:395345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Miller ES, Kim JK, Gandehok J, Hara M, Dai Q, Malik A, Miller A, Miller L. A new device for measuring esophageal variceal pressure. Gastrointest Endosc. 2002;56:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Carneiro FO, Retes FA, Matuguma SE, Albers DV, Chaves DM, Dos Santos ME, Herman P, Chaib E, Sakai P, Carneiro D'Albuquerque LA, Maluf Filho F. Role of EUS evaluation after endoscopic eradication of esophageal varices with band ligation. Gastrointest Endosc. 2016;84:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Kuramochi A, Imazu H, Kakutani H, Uchiyama Y, Hino S, Urashima M. Color Doppler endoscopic ultrasonography in identifying groups at a high-risk of recurrence of esophageal varices after endoscopic treatment. J Gastroenterol. 2007;42:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Miller L, Banson FL, Bazir K, Korimilli A, Liu Ji, Dewan R, Wolfson M, Panganamamula KV, Carrasquillo J, Schwartz J, Chaker AE, Black M. Risk of esophageal variceal bleeding based on endoscopic ultrasound evaluation of the sum of esophageal variceal cross-sectional surface area. Am J Gastroenterol. 2003;98:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Liao WC, Chen PH, Hou MC, Chang CJ, Su CW, Lin HC, Lee FY. Endoscopic ultrasonography assessment of para-esophageal varices predicts efficacy of propranolol in preventing recurrence of esophageal varices. J Gastroenterol. 2015;50:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Sarin SK, Kumar CKN. Ectopic varices. Clin Liver Dis (Hoboken). 2012;1:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (35)] |
| 58. | Watanabe N, Toyonaga A, Kojima S, Takashimizu S, Oho K, Kokubu S, Nakamura K, Hasumi A, Murashima N, Tajiri T. Current status of ectopic varices in Japan: Results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Lienhart I, Lesne A, Couchonnal E, Rivory J, Sosa-Valencia L, Ponchon T, Pioche M. Massive duodenal variceal bleed: endoscopic ultrasonography of ruptured varix and successful endoscopic clipping treatment. Endoscopy. 2016;48 Suppl 1 UCTN:E80-E81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | So H, Park do H, Jung K, Ko HK. Successful Endoscopic Ultrasound-Guided Coil Embolization For Severe Duodenal Bleeding. Am J Gastroenterol. 2016;111:925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Kimura G, Hashimoto Y, Ikeda M. Successful obliteration of bleeding duodenal varices by EUS-guided injection of N-butyl-2-cyanoacrylate. VideoGIE. 2017;2:317-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Hosking SW, Smart HL, Johnson AG, Triger DR. Anorectal varices, haemorrhoids, and portal hypertension. Lancet. 1989;1:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Chawla Y, Dilawari JB. Anorectal varices--their frequency in cirrhotic and non-cirrhotic portal hypertension. Gut. 1991;32:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Dhiman RK, Saraswat VA, Choudhuri G, Sharma BC, Pandey R, Naik SR. Endosonographic, endoscopic, and histologic evaluation of alterations in the rectal venous system in patients with portal hypertension. Gastrointest Endosc. 1999;49:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Dhiman RK, Choudhuri G, Saraswat VA, Mukhopadhyay DK, Khan EM, Pandey R, Naik SR. Endoscopic ultrasonographic evaluation of the rectum in cirrhotic portal hypertension. Gastrointest Endosc. 1993;39:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Messallam AA, Kumbhari V, Saxena P, Azola AM, Kalloo AN, Khashab MA. Large bleeding rectal varices treated with endoscopic ultrasound-guided coiling and cyanoacrylate injection. Endoscopy. 2014;46 Suppl 1 UCTN:E28-E29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Sharma M, Somasundaram A. Massive lower GI bleed from an endoscopically inevident rectal varices: diagnosis and management by EUS (with videos). Gastrointest Endosc. 2010;72:1106-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Tabibian JH, Abu Dayyeh BK, Gores GJ, Levy MJ. A novel, minimally invasive technique for management of peristomal varices. Hepatology. 2016;63:1398-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Tsynman DN, DeCross AJ, Maliakkal B, Ciufo N, Ullah A, Kaul V. Novel use of EUS to successfully treat bleeding parastomal varices with N-butyl-2-cyanoacrylate. Gastrointest Endosc. 2014;79:1007-8; discussion 1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Samanta J, Dhar J, Bharath P, Kumar A, Bhujade H, Gupta P, Kochhar R. Bleeding parastomal varices: endoscopic ultrasound to the rescue. Endoscopy. 2022;54:OP279V. [DOI] [Full Text] |
| 71. | Levy MJ, Wong Kee Song LM, Kendrick ML, Misra S, Gostout CJ. EUS-guided coil embolization for refractory ectopic variceal bleeding (with videos). Gastrointest Endosc. 2008;67:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Kinzel J, Pichetshote N, Dredar S, Aslanian H, Nagar A. Bleeding from a duodenal varix: a unique case of variceal hemostasis achieved using EUS-guided placement of an embolization coil and cyanoacrylate. J Clin Gastroenterol. 2014;48:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Fujii-Lau LL, Law R, Wong Kee Song LM, Gostout CJ, Kamath PS, Levy MJ. Endoscopic ultrasound (EUS)-guided coil injection therapy of esophagogastric and ectopic varices. Surg Endosc. 2016;30:1396-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Bahdi F, George R, Patel K. EUS-guided coiling and cyanoacrylate injection of ectopic duodenal varices. VideoGIE. 2021;6:35-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Mukkada RJ, Mathew PG, Francis Jose V, Paul Chettupuzha A, Antony R, Koshy A. EUS-guided coiling of rectal varices. VideoGIE. 2017;2:208-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Bazarbashi AN, Thompson CC, Ryou M. Targeting the perforator vein: EUS-guided coil embolization for the treatment of bleeding rectal varices. VideoGIE. 2020;5:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Philips CA, Augustine P. Endoscopic Ultrasound-Guided Management of Bleeding Rectal Varices. ACG Case Rep J. 2017;4:e101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Weilert F, Shah JN, Marson FP, Binmoeller KF. EUS-guided coil and glue for bleeding rectal varix. Gastrointest Endosc. 2012;76:915-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Jana T, Mistry T, Singhal S. Endoscopic ultrasound-guided hemostasis of rectal varices. Endoscopy. 2017;49:E136-E137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD. A prospective, randomized trial of large- vs small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Kanai M, Hamada A, Endo Y, Takeda Y, Yamakawa M, Nishikawa H, Torii A. Efficacy of argon plasma coagulation in nonvariceal upper gastrointestinal bleeding. Endoscopy. 2004;36:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Han C, Ling X, Liu J, Lin R, Ding Z. Management of non-variceal upper gastrointestinal bleeding: role of endoscopic ultrasound-guided treatments. Therap Adv Gastroenterol. 2022;15:17562848211056148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 83. | De Angelis CG, Cortegoso Valdivia P, Rizza S, Venezia L, Rizzi F, Gesualdo M, Saracco GM, Pellicano R. Endoscopic Ultrasound-Guided Treatments for Non-Variceal Upper GI Bleeding: A Review of the Literature. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Mallick B, Malik S, Gupta P, Gorsi U, Kochhar S, Gupta V, Yadav TD, Dhaka N, Sinha SK, Kochhar R. Arterial pseudoaneurysms in acute and chronic pancreatitis: Clinical profile and outcome. JGH Open. 2019;3:126-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Sagar S, Soundarajan R, Gupta P, Praveen Kumar M, Samanta J, Sharma V, Kochhar R. Efficacy of endovascular embolization of arterial pseudoaneurysms in pancreatitis: A systematic review and meta-analysis. Pancreatology. 2021;21:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Roach H, Roberts SA, Salter R, Williams IM, Wood AM. Endoscopic ultrasound-guided thrombin injection for the treatment of pancreatic pseudoaneurysm. Endoscopy. 2005;37:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Gonzalez JM, Ezzedine S, Vitton V, Grimaud JC, Barthet M. Endoscopic ultrasound treatment of vascular complications in acute pancreatitis. Endoscopy. 2009;41:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Robb PM, Yeaton P, Bishop T, Wessinger J. Endoscopic Ultrasound Guided Embolization of a Pancreatic Pseudoaneurysm. Gastroenterology Res. 2012;5:239-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Rai P, Bhera B, Sharma M. First report of successful treatment of splenic artery pseudoaneurysm with endoscopic ultrasound-guided coil and glue. Endoscopy. 2017;49:E179-E180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Shah J, Samanta J, Muktesh G, Kumar N, Gupta P, Kumar KH, Kochhar R. Primary EUS-guided therapy of a giant visceral artery pseudoaneurysm: Expanding horizons (with video). Endosc Ultrasound. 2021;10:307-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 91. | Gamanagatti S, Thingujam U, Garg P, Nongthombam S, Dash NR. Endoscopic ultrasound guided thrombin injection of angiographically occult pancreatitis associated visceral artery pseudoaneurysms: Case series. World J Gastrointest Endosc. 2015;7:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Jhajharia A, Wanjari S, Ashdhir P, Sharma D, Pokharna R, Nijhawan S. Endoscopic ultrasound-guided thrombin injection for management of visceral artery pseudoaneurysm: A novel approach. Indian J Gastroenterol. 2018;37:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Rai P, Kc H, Goel A, Aggarwal R, Sharma M. Endoscopic ultrasound-guided coil and glue for treatment of splenic artery pseudo-aneurysm: new kid on the block! Endosc Int Open. 2018;6:E821-E825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Maharshi S, Sharma SS, Sharma D, Sapra B, Nijhawan S. Endoscopic ultrasound-guided thrombin injection, a management approach for visceral artery pseudoaneurysms. Endosc Int Open. 2020;8:E407-E412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Samanta J, Dhar J, Birda CL, Mandavdhare HS, Gupta P, Gorsi U, Kumar KH, Gupta V, Kochhar R. Endoscopic ultrasound-guided angiotherapy using coil and cyanoacrylate glue for the management of visceral artery pseudoaneurysms in patients of pancreatitis: a safe and effective technique. Endoscopy. 2022;54:OP269. [DOI] [Full Text] |
| 96. | Fockens P, Meenan J, van Dullemen HM, Bolwerk CJ, Tytgat GN. Dieulafoy's disease: endosonographic detection and endosonography-guided treatment. Gastrointest Endosc. 1996;44:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Levy MJ, Wong Kee Song LM, Farnell MB, Misra S, Sarr MG, Gostout CJ. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008;103:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Law R, Fujii-Lau L, Wong Kee Song LM, Gostout CJ, Kamath PS, Abu Dayyeh BK, Gleeson FC, Rajan E, Topazian MD, Levy MJ. Efficacy of endoscopic ultrasound-guided hemostatic interventions for resistant nonvariceal bleeding. Clin Gastroenterol Hepatol. 2015;13:808-12.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Samarasena JB, Chang KJ. Endoscopic Ultrasound-Guided Interventions for the Measurement and Treatment of Portal Hypertension. Gastrointest Endosc Clin N Am. 2019;29:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Giday SA, Clarke JO, Buscaglia JM, Shin EJ, Ko CW, Magno P, Kantsevoy SV. EUS-guided portal vein catheterization: a promising novel approach for portal angiography and portal vein pressure measurements. Gastrointest Endosc. 2008;67:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Lai L, Poneros J, Santilli J, Brugge W. EUS-guided portal vein catheterization and pressure measurement in an animal model: a pilot study of feasibility. Gastrointest Endosc. 2004;59:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Magno P, Ko CW, Buscaglia JM, Giday SA, Jagannath SB, Clarke JO, Shin EJ, Kantsevoy SV. EUS-guided angiography: a novel approach to diagnostic and therapeutic interventions in the vascular system. Gastrointest Endosc. 2007;66:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Giday SA, Ko CW, Clarke JO, Shin EJ, Magno P, Jagannath SB, Buscaglia JM, Kantsevoy SV. EUS-guided portal vein carbon dioxide angiography: a pilot study in a porcine model. Gastrointest Endosc. 2007;66:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 105. | Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, Chen WP, Chang KJ. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 106. | Fujii-Lau LL, Leise MD, Kamath PS, Gleeson FC, Levy MJ. Endoscopic ultrasound-guided portal-systemic pressure gradient measurement. Endoscopy. 2014;46 Suppl 1 UCTN:E654-E656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Zhang W, Peng C, Zhang S, Huang S, Shen S, Xu G, Zhang F, Xiao J, Zhang M, Zhuge Y, Wang L, Zou X, Lv Y. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 108. | Hajifathalian K, Westerveld D, Kaplan A, Dawod E, Herr A, Ianelli M, Saggese A, Kumar S, Fortune BE, Sharaiha RZ. Simultaneous EUS-guided portosystemic pressure measurement and liver biopsy sampling correlate with clinically meaningful outcomes. Gastrointest Endosc. 2022;95:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 109. | Shah SL, Dawod Q, Kumar S, Fortune B, Sharaiha RZ. "One stop" liver-focused endoscopy: EUS-guided portal pressure gradient measurement technique. VideoGIE. 2020;5:658-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Choi AY, Kolb J, Shah S, Chahine A, Hashimoto R, Patel A, Tsujino T, Huang J, Hu KQ, Chang K, Samarasena JB. Endoscopic ultrasound-guided portal pressure gradient with liver biopsy: 6 years of endo-hepatology in practice. J Gastroenterol Hepatol. 2022;37:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 111. | Choi AY, Chang KJ, Samarasena JB, Lee JG, Li X, Guo W, Chandan VS, Hu KQ. Endoscopic Ultrasound-Guided Porto-systemic Pressure Gradient Measurement Correlates with Histological Hepatic Fibrosis. Dig Dis Sci. 2022;67:5685-5692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 112. | Buscaglia JM, Dray X, Shin EJ, Magno P, Chmura KM, Surti VC, Dillon TE, Ducharme RW, Donatelli G, Thuluvath PJ, Giday SA, Kantsevoy SV. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video). Gastrointest Endosc. 2009;69:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 113. | Binmoeller KF, Shah JN. Sa1428 EUS-guided transgastric intrahepatic portosystemic shunt using the axios stent. Gastrointest Endosc. 2011;73:AB167. [DOI] [Full Text] |
| 114. | Schulman AR, Ryou M, Aihara H, Abidi W, Chiang A, Jirapinyo P, Sakr A, Ajeje E, Ryan MB, Thompson CC. EUS-guided intrahepatic portosystemic shunt with direct portal pressure measurements: a novel alternative to transjugular intrahepatic portosystemic shunting. Gastrointest Endosc. 2017;85:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 115. | Poincloux L, Chabrot P, Mulliez A, Genes J, Boyer L, Abergel A. Interventional endoscopic ultrasound: A new promising way for intrahepatic portosystemic shunt with portal pressure gradient. Endosc Ultrasound. 2017;6:394-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015;149:1794-1803.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 117. | Zhang Y, Su H, Wang H, Xu C, Zhou S, Zhao J, Shen S, Xu G, Wang L, Zou X, Zhang S, Lv Y. Endoscopic Ultrasound-Guided Acquisition of Portal Venous Circulating Tumor Cells as a Potential Diagnostic and Prognostic Tool for Pancreatic Cancer. Cancer Manag Res. 2021;13:7649-7661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Rustagi T, Gleeson FC, Chari ST, Abu Dayyeh BK, Farnell MB, Iyer PG, Kendrick ML, Pearson RK, Petersen BT, Rajan E, Topazian MD, Truty MJ, Vege SS, Wang KK, Levy MJ. Remote malignant intravascular thrombi: EUS-guided FNA diagnosis and impact on cancer staging. Gastrointest Endosc. 2017;86:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Lai R, Stephens V, Bardales R. Diagnosis and staging of hepatocellular carcinoma by EUS-FNA of a portal vein thrombus. Gastrointest Endosc. 2004;59:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Storch I, Gomez C, Contreras F, Schiff E, Ribeiro A. Hepatocellular carcinoma (HCC) with portal vein invasion, masquerading as pancreatic mass, diagnosed by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Dig Dis Sci. 2007;52:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Michael H, Lenza C, Gupta M, Katz DS. Endoscopic Ultrasound -guided Fine-Needle Aspiration of a Portal Vein Thrombus to Aid in the Diagnosis and Staging of Hepatocellular Carcinoma. Gastroenterol Hepatol (N Y). 2011;7:124-129. [PubMed] |
| 122. | Moreno M, Gimeno-García AZ, Corriente MM, Nicolás-Pérez D, Brito-García A, García-Castro C, Quintero E. EUS-FNA of a portal vein thrombosis in a patient with a hidden hepatocellular carcinoma: confirmation technique after contrast-enhanced ultrasound. Endoscopy. 2014;46 Suppl 1 UCTN:E590-E591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Faigel DO, Lake DF, Landreth TL, Kelman CC, Marler RJ. EUS-guided portal injection chemotherapy for treatment of hepatic metastases: feasibility in the acute porcine model. Gastrointest Endosc. 2016;83:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 124. | Faigel D, Lake D, Landreth T, Kelman C, Marler R. Endoscopic ultrasonography-guided portal injection chemotherapy for hepatic metastases. Endosc Ultrasound. 2014;3:S1. [PubMed] |
| 125. | Loffroy R, Favelier S, Chevallier O, Estivalet L, Genson PY, Pottecher P, Gehin S, Krausé D, Cercueil JP. Preoperative portal vein embolization in liver cancer: indications, techniques and outcomes. Quant Imaging Med Surg. 2015;5:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 126. | Matthes K, Sahani D, Holalkere NS, Mino-Kenudson M, Brugge WR. Feasibility of endoscopic ultrasound-guided portal vein embolization with Enteryx. Acta Gastroenterol Belg. 2005;68:412-415. [PubMed] |
| 127. | Park TY, Seo DW, Kang HJ, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Feasibility and safety of EUS-guided selective portal vein embolization with a coil and cyanoacrylate in a live porcine model. Endosc Ultrasound. 2018;7:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Park TY, Seo DW, Kang HJ, Cho MK, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasonography-guided placement of a transhepatic portal vein stent in a live porcine model. Endosc Ultrasound. 2016;5:315-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Fritscher-Ravens A, Ganbari A, Mosse CA, Swain P, Koehler P, Patel K. Transesophageal endoscopic ultrasound-guided access to the heart. Endoscopy. 2007;39:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Romero-Castro R, Rios-Martin JJ, Gallego-Garcia de Vinuesa P, Castro-Fernandez AJ, Marques-Asin FJ, Caparros-Escudero C, Pellicer-Bautista F, Herrerias-Gutierrez JM. Pericardial tumor diagnosed by EUS-guided FNA (with video). Gastrointest Endosc. 2009;69:562-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Haja A, Lakhtakia S, Sekaran A, Reddy P, Shah S, Pratap A, Reddy N. Cardiac sarcoma diagnosed with EUS-FNB. Endosc Int Open. 2021;9:E152-E153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Gornals JB, de la Hera M, de Albert M, Claver E, Catala I. EUS cardiac puncture-guided right atrial tumor. Gastrointest Endosc. 2015;82:165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Larghi A, Stobinski M, Galasso D, Amato A, Familiari P, Costamagna G. EUS-guided drainage of a pericardial cyst: closer to the heart (with video). Gastrointest Endosc. 2009;70:1273-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 134. | Somani P, Talele R, Sharma M. Endoscopic Ultrasound-Guided Thrombolysis of Pulmonary Artery Thrombus and Mesenteric Vein Thrombus. Am J Gastroenterol. 2019;114:379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |