Published online Apr 16, 2023. doi: 10.4253/wjge.v15.i4.195
Peer-review started: October 7, 2022
First decision: December 19, 2022
Revised: January 30, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: April 16, 2023
Processing time: 189 Days and 9.1 Hours
Endoscopic luminal stenting (ELS) represents a minimally invasive option for the management of malignant obstruction along the gastrointestinal tract. Previous studies have shown that ELS can provide rapid relief of symptoms related to esophageal, gastric, small intestinal, colorectal, biliary, and pancreatic neoplastic strictures without compromising cancer patients’ overall safety. As a result, in both palliative and neoadjuvant settings, ELS has largely surpassed radiotherapy and surgery as a first-line treatment modality. Following the abovementioned success, the indications for ELS have gradually expanded. To date, ELS is widely used in clinical practice by well-trained endoscopists in managing a wide variety of diseases and complications, such as relieving non-neoplastic obstructions, sealing iatrogenic and non-iatrogenic perforations, closing fistulae and treating post-sphincterotomy bleeding. The abovementioned development would not have been achieved without corresponding advances and innovations in stent technology. However, the technological landscape changes rapidly, making clinicians’ adaptation to new technologies a real challenge. In our mini-review article, by systematically reviewing the relevant literature, we discuss current developments in ELS with regard to stent design, accessories, techniques, and applications, expanding the research basis that was set by previous studies and highlighting areas that need to be further investigated.
Core Tip: Endoscopic luminal stenting (ELS) represents a well-established minimally invasive option for the management of malignant obstruction in the gastrointestinal tract. Following this successful application, recent advances in stent technology have gradually expanded the use of luminal stents in the management of various other disorders and complications. In this rapidly evolving field, clinicians are urgently required to learn new skills in order to advance their current practice. In an effort to facilitate this process, this article summarizes the current knowledge on ELS and highlights areas that need to be investigated in future studies.
- Citation: Moutzoukis M, Argyriou K, Kapsoritakis A, Christodoulou D. Endoscopic luminal stenting: Current applications and future perspectives. World J Gastrointest Endosc 2023; 15(4): 195-215
- URL: https://www.wjgnet.com/1948-5190/full/v15/i4/195.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i4.195
Gastrointestinal intraluminal stents (GISs) are short metal or plastic artificial tubes of different shapes that are endoscopically placed into the lumen of an anatomical channel to restore and maintain its patency. The medical procedure that leads to the endoscopic placement of GISs into the lumen of a hollow viscus of the gastrointestinal tract (esophagus, stomach, duodenum, intestinal, colorectal) is named endoscopic luminal stenting (ELS) whereas the inserted GISs are named endoprostheses[1].
Historically, the first use of GISs dates back to 1885 when Sir Charters Symonds, a British-Canadian surgeon, was the first to successfully place a boxwood endoprosthesis developed for blind oral insertion in a patient with esophageal cancer[2]. Thereafter, the technology of GISs was improved to improve stent elasticity and facilitate insertion, with different designs and materials, such as latex, silicone, and polyvinyl becoming popular in the mid-1970s. However, all these modifications were including semi-rigid tubes of a fixed diameter that required dilatation to be performed prior to their placement, compromising the safety of stenting. To overcome this drawback, a new type of GISs named self-expandable metal stents (SEMS) entered the global market in the early 1990s. This kind of softer GIS rapidly evolved into the gold-standard therapeutic option for the management of malignant esophageal obstruction, irreversibly displacing their more rigid predecessors[1,2]. Nowadays, growing experience and technical advancements progressively widened the indications and increased the use of GISs in the management of a variety of other diseases, making clinicians’ adaptation to new technologies a real challenge.
In this mini-review article, we provide a concise overview of the current knowledge on GI stenting, aiming to aid clinicians to advance their current practice and to highlight gaps that need investigation in future studies.
PubMed, Cochrane Library, Medline Scopus-clinical trial register, and Web of Science databases were initially searched by two of the authors (Moutzoukis MK and Kapsoritakis A) to retrieve studies reporting data on GIS from inception up to September 2022. The following Medical Subject Heading terms alone or matched by the logical operators “OR” or “AND” were used: ‘GI stents,’ ‘stents,’ ‘endoscopic retrograde cholangiopancreatography (ERCP),’ ‘ERCP’, GI malignancy,’ ‘Crohn Disease (CD),’ ‘CD,’ ‘inflammatory bowel disease (IBD),’ ‘IBD,’ ‘bariatric surgery.’ A manual search of the article reference lists and abstracts from Digestive Disease Week, United European Gastroenterology Week, and European Crohn’s Colitis Organization congresses were also conducted up to September 2022. A total of 3,988 references were retrieved. Old, repetitive, and non-English studies were excluded. After an initial title screen, each relevant article was subsequently reviewed and 161 representative scientific papers were finally quoted.
By systematically reviewing the relevant literature, it became evident that currently, there is a wide variety of stent types available, corresponding with the wide variety of tissue shapes and mechanical characteristics of the GI tract. With regards to the material used in the creation of a stent, GISs are grouped into three main categories: Metal, plastic, and biodegradable stents. Different stents serve different purposes according to their individual sets of physical characteristics, with the main advantages and disadvantages of the different available types of stents being shown in Table 1.
| Types of stents | Appropriate indications | Pros | Cons |
| Uncovered SEMS | Poor survival | Low risk of migration | High risk of tumor ingrowth |
| High risk of migration | Difficult removal | ||
| Gastroduodenal obstruction | |||
| Partially covered SEMS | Risk of migration | Difficult removal | |
| Fully covered SEMS | Risk of tumor in growth | Safe and easy removal | High risk of migration |
| Temporary measure | Low risk of tumor in growth | ||
| SEPS | Temporary measure | Safe and easy removal | Complex and stiff stent introducer |
| Esophageal stricture | No tumor in growth | High risk of stent failure |
Among the different types of GISs, the most common stents used in gastroenterology are SEMSs. SEMSs are made of stainless steel or nitinol and are cylindrical in shape with a funnel-like flange at one or both ends. These metal alloy compounds make the stent cylinder flexible, with nitinol, a nickel-titanium alloy, having shape memory and more hyper-elastic characteristics compared to its counterpart. These characteristics have given nitinol an advantage compared to stainless steel and allowed the development of different designs such as braided or knitted stents that increased the utility of metal stents[3].
Contemporary SEMSs are woven, knitted, or laser-cut nitinol mesh cylinders packed in a compressed form and constrained around a delivery device[4,5]. These cylinders gradually decompress over deployment, allowing the stents to self-expand to their maximum fixed diameter. Compared to the laser-cut, the braided or knitted SEMSs have a lower kinking effect when deployed and allow the placement of another stent across their mesh[6,7]. However, contrary to laser-cut SEMSs, they have a lower radial and axial force and a higher foreshortening property that make these stents less predictable after deployment and hence, less suitable for placement into channels that are straight, narrow, and tight such as those in the biliary tree[6,7]. Despite these advantages, some laser-cut stents have pointed struts at their distal end incurring tissue reaction from the direct piercing which can complicate their removal, limiting their use[6,7].
Early SEMSs were uncovered and carried a significant risk of obstruction secondary to tumor ingrowth[8]. This drawback reduced the popularity of uncovered SEMSs as epithelialization was found to occur from 3 to 6 wk after their placement in 13% of patients[9]. To overcome this drawback, partially covered SEMS (PCSEMSs) were developed[8,10]. PCSEMSs had a synthetic material cover around their body that was acting as a barrier to tissue ingrowth into the lumen of the stent; however, their ends remained uncovered, allowing the development of hypertrophic granulation at the exposed sites making difficult their reposition or removal. This drawback confined the use of PCSEMSs to patients with inoperable malignancy[11-13]. Fully covered SEMSs (FCSEMSs) were then designed to overcome the drawbacks of all other metal stents. Although FCSEMS successfully dealt with tumor ingrowth or overgrowth, they were predisposed to migration, because their outer cover prevented stents from being embedded into the tissue[14]. This led to the development of the covered Gianturco Z stent (Wilson Cook Medical, Letchworth, England), a polyethylene-coated stainless-steel stent, that was specifically designed to prevent migration. This stent had wide distal and proximal ends and strong hooks in the middle, allowing the stent to be held inside the tumor. Compared to FCSEMSs, the Gianturco Z stent was associated with lower migration rates but migration remained a significant problem for approximately 10% of cases. Subsequent developments led to other kinds of antimigratory stents characterized by the presence of a partial cover, flared ends, or a double stent design that managed to further reduce the migration rate of SEMSs to an average of 5%[15-17].
Plastic stents (PSs) represent the second broad category of GISs. GISs were initially used for the management of inoperable malignant GI obstruction. The first PSs were semi-rigid tubes that were composed of polyvinyl plastic. These stents were effective in more than 80% of cancer patients; however, they were associated with significant complications such as migration, food impaction, and perforation in up to 10% of them[18]. Conversely to the first PSs, contemporary PSs are made of polyethylene, which is a softer plastic with superior molding capabilities. Nowadays, PSs are exclusively used in the management of various pancreatic-biliary disorders and complications, such as strictures and fistulae, as first-line and cost-effective therapeutic options[19].
Self-expanding PSs (SEPSs) represent another category of PSs designed to increase their utility. SEPSs are devices with monofilament braid composed of polyester, with their mesh being fully coated by silicone. This coating is an inherent advantage of this kind of stents as it prevents impaction. However, it facilitates migration to a significant extent (6.7%-52.4%) which limits their popularity.
To overcome the drawbacks of metal and plastic stents, a novel type of stents named biodegradable stents (BDSs) were developed. BDSs are composed of biodegradable materials (i.e. polyesters, polycarbonates, bacterial-derived polymers, and corrodible metals) and after deployment exert a continuous radial tension for a defined period of time that ranges between 6 to 8 wk. After this period, BDSs undergo gradual hydrolysis-mediated self-degradation (8-12 wk), preventing tissue ingrowth and the necessity for second endoscopy for stent removal[20,21]. As yet, two types of BDS have been tested in humans. These stents were made of knitted poly-L-lactic acid monofilaments with the first being produced by Marui Textile Machinery of Osaka in Japan which is not currently available, and the second by ELLA-CS, s.r.o. from the Czech Republic which is still in the market[22-24].
Over time, there have been made significant changes in the way GISs are used in the GI tract. GISs were initially used only to treat malignant esophageal obstruction; however, in the past decade, their use was expanded to include various other GI pathologies, improving patients’ quality of life. The current list of the indications for ELS has expanded to include a variety of malignant strictures, obstructions, external compressions of the GI tract, malignant GI perforations and fistulae, and selected cases of benign strictures that are resistant to repeated balloon dilation or surgical bougienage that are not confined to the esophagus. However, the different applications of ELS are associated with different complications. In this section, we describe the most common applications of stenting and their associated complications per area of interest in the GI tract.
Malignant strictures: ELS plays a crucial role in the management of strictures associated with esophageal cancer as cancer patients are candidates for surgical resection in less than 50% of the cases due to advanced or disseminated disease. In these patients, obstructing cancer causes significant problems, with dysphagia being their major complaint. Several therapeutic modalities are used alone or in combination for the management of malignant dysphagia, including laser ablation, photodynamic therapy, argon plasma coagulation, intraluminal brachytherapy, external beam radiation therapy, chemotherapy, and stenting. Among the different available options, SEMSs are currently indicated for all cancer patients who are expected to live less than 3 mo and have poor performance status as they provide rapid relief from dysphagia within 1-2 d after their insertion[25]. Instead, for those patients expected to live longer, brachytherapy alone or in combination with SEMSs is indicated because brachytherapy has been found to be associated with better quality of life, better symptom control, and increased survival[25].
Among the different available types of SEMSs, no significant differences were found between FCSEMSs and PCSEMSs. However, special attention is required for cancer patients that develop dysphagia after radiotherapy because in these patients radiation-induced changes can increase the risk of stent-related adverse events. In this difficult patient population, the placement of SEMS cannot be excluded because the available evidence regarding the relationship between previous treatment and stent-related adverse events is controversial[25-27].
With regards to PSs, semi-rigid PSs and SEPSs are inferior to SEMSs in the management of dysphagia, because they are associated with more adverse outcomes such as perforation, bleeding, pain, migration, and fistulation. Therefore, plastic stents are currently excluded from the management of cancer-related dysphagia[25-27].
Conversely to the other stent types, the role of BDSs in the management of malignant dysphagia is not fully elucidated. Preliminary experience shows that BDSs can relieve dysphagia by restoring the patency of the lumen, with their short life span preventing patients from undergoing repeat endoscopy for stent removal. However, their use is associated with high-risk complications in up to 50% of the cases, such as severe retrosternal pain with or without vomiting, hematemesis, and dysphagia recurrence. In light of these findings, the use of BDS is not advised[28-30].
Extrinsic compression: Other than dysphagia associated with esophageal cancer, extrinsic esophageal compression can also cause dysphagia in patients with extraesophageal malignancies. The use of SEMSs in the management of this type of dysphagia shows encouraging results; however, their placement is associated with a lower success rate compared to their application for intraluminal pathologies[31].
Tracheo/broncho-esophageal fistula: Tracheo/broncho-esophageal fistula is a rare complication of esophageal cancer. It results from the infiltration of esophageal cancer to the respective neighboring anatomical structures[32]. This complication is associated with increased morbidity and mortality, with the main goal of treatment being to improve patients’ quality of life. Several modalities are used to treat this complication, including the use of feeding gastrostomy/jejunostomy tubes and SEMSs. Although all modalities are effective in the management of malignant fistulas, SEMSs are associated with better quality of life as they preserve oral nutrition. Taking this into account, together with the fact that SEMSs are placed with high clinical and technical success (up to 100%) in some series, the European Society of Gastrointestinal Endoscopy (ESGE) currently recommends SEMSs’ placement for the management of malignant fistulas. Based on available evidence, no recommendation can be made as per a specific category of SEMSs. However, FCSEMSs may be preferable as their migration rate is lower and their removal is easier compared to PCSEMSs[33].
Bridge to surgery: For operable esophageal cancer, the preoperative nutritional status interferes with the surgical outcomes and hence, patients’ prognosis. In an effort to prevent malnutrition and improve survival, the use of preoperative ELS is an interesting new concept. So far, among the different available stent types, only SEMSs were studied, with gathered evidence showing no significant benefit. Therefore, ESGE does not currently recommend SEMS placement as a bridge to surgery in patients with esophageal cancer[34].
Benign strictures: Following the high success rate of ELS in the management of malignant obstruction, the use of stents is expanded in the management of benign strictures. Benign strictures are relatively common in clinical practice and are commonly encountered after caustic substance intake, esophageal surgery, radiation, or advanced polypectomy techniques[35-38]. The current standard of therapy for benign strictures includes endoscopic dilation. However, all types of benign strictures are not amenable to dilation. Benign strictures that have lengths > 2 cm or diameters > 11 mm have irregular edges or are angulated represent a special kind of strictures that is called “complex” and do not respond to endoscopic dilation. For this kind of strictures as well as for strictures that do not respond to biweekly dilatations to reach a target diameter of 14 mm after 5 wk of treatment or to maintain the target diameter over a 4-wk period after the last dilation, ELS can offer an alternative solution to surgery. However, prior to stenting, it is essential to ensure that the stenosis is not malignant and to quantify the severity of dysphagia so that clinicians become able to assess the efficacy of their subsequent intervention[39,40].
Among the different available stent types, SEPSs, PCSEMSs, FCSEMSs, and BDSs have all been studied for the management of benign strictures that do not respond or recur after dilation. Available evidence on clinical success did not point out significant differences[36-40]. Therefore, the clinical decision of selecting a specific type of stents over the others is driven by weighing up the data on their safety. To date, enough evidence exists only for SEPSs and SEMSs, giving an advantage to FCSEMSs whereas for BDSs are scarce. Hence, it is of no surprise that ESGE currently recommends only the use of FCSEMSs in the treatment of refractory benign esophageal strictures[34].
Throughout the literature, the optimal duration for leaving a stent in situ remains elusive. Therefore, it is currently suggested that stents should remain positioned for at least 6 wk and for no more than 12 wk because this is the time period that is required for stricture remodeling and for avoiding stent embedment[41-43].
Anastomotic leaks: Esophagectomy plays a key role in the management of various malignant and benign conditions such as cancer, neuromotor dysfunction, scleroderma, acute perforations, and acute caustic injury. Esophagectomy is a high-risk procedure that is associated with increased morbidity and mortality; however, recent advances in surgical techniques and perioperative management improved patients’ outcomes. Despite advances, esophagectomy remains a complex procedure. Anastomotic leaks are rare but serious complications that implicate approximately 8.3% of esophagectomy cases even in specialized centers[44]. Anastomotic leaks lead to septic complications, such as abscesses, and fistulas that can be fatal in 30% to 40% of the patients postoperatively[27]. Their management requires immediate treatment in the intensive care unit with intravenous fluid administration, perianastomotic drainage, parenteral feeding, nasogastric decompression, and intravenous antibiotics[45].
For the management of patients with anastomotic leaks, ELS plays a crucial role and should be performed immediately to prevent mediastinal contamination and facilitate the transition from parenteral to enteral nutrition[46]. However, if this is not possible, delayed insertion should be also attempted as it contributes to the healing of the anastomosis. In general, for esophageal leaks, uncovered SEMS have no role in the management of anastomotic leaks. Instead, covered SEMSs and SEPSs achieve great outcomes, with success rates for leaks’ closure ranging from 60% to 100% and are preferred[46-49]. However, following successful deployment, a contrast agent should be injected to confirm that the leak is closed. Thereafter, the endoprosthesis should be left in situ for a period that ranges from 14 to 28 d, with removal being decided after radiologic confirmation of the complete leak healing and the absence of septic complications[50-52].
Common complications of esophageal stenting: Esophageal stenting is not a risk-free procedure with complications being divided into early and late complications. The early complication rate of esophageal stenting, defined as the rate of complications that occur within the 1st wk after successful deployment, is about 20% which can be subdivided into 14.6% for clinical complications (pain 12%, perforation 0.6%, bleeding 0.6%, and mortality 1.4%) and 5.3% for technical failures (misplacement 0.3%, expansion/deployment failure in 3.9/0.8%, and stent migration in 0.3%). Late complications include technical failures (stent migration in 7% of cases), lump complications (tissue ingrowth in 11.3% of cases), and clinical complications (gastroesophageal reflux in 3.7% of cases, recurrent dysphagia in 8.2%, esophageal fistula in 2.8% of cases, hemorrhage in 3.9% of cases, esophageal perforation in 0.8% of cases)[53-55]. However, the complication rate is not the same for all stent types and for all cases. For example, PSs have lower rates of migration and can also be removed easier whereas metal stents can cause dysphagia as the tumor progress. Likewise, stents that are placed near the gastroesophageal junction migrate more easily, whereas those extending to or above the level of the aortic arch are more likely to cause bleeding with the reason for this adverse event being debatable and pressure-induced necrosis of the tumor and the esophageal wall being the one plausible explanation[56,57]. An overview of the most common complications that complicate esophageal stenting is given in Table 2.
| Early complications | Long-term complications |
| Pain | Occlusion |
| Migration | Gastroesophageal reflux |
| Expansion/deployment failure | Dysphagia |
| Mispositioning | Fistula formation |
| Bleeding | Bleeding |
Similarly to the esophagus, in the gastroduodenal tract, advanced, metastatic, or inoperable cancer that causes obstruction, most of the time is treated with SEMSs, as curative resection is not possible in 40% of cases of gastric and 20% of cases of pancreatic cancer[58-61]. The same applies to cases where the obstruction is due to extrinsic compression from neighboring inoperable tumors[61,62]. In these cancer cases, stenting can offer an alternative therapeutic option to palliative surgery providing symptom relief in patients with short life expectancy, but with lower morbidity and cost compared to palliative surgery[63,64]. The superiority of stenting over palliative surgery was shown in a prospective randomized trial performed by Jeurnink et al[63]. In this trial, the authors found that duodenal stenting was better than surgical gastrojejunostomy for the treatment of malignant gastric outflow obstruction in terms of morbidity, surgical pain, hospital stay, and 30-day quality of life[63,65]. The results of this trial were later confirmed in two subsequent analyses performed by Rodríguez et al[66] and Ly et al[67], who reported the same mortality and morbidity rates for duodenal stents over palliative surgery. With technical success rates of 89% to 100% and clinical success rates ranging from 72% to 88%, the American Gastroenterology Association currently suggests SEMSs for patients with malignant obstruction who are poor surgical candidates whereas for patients who are fit for surgery with greater life expectancy than 2 mo suggests laparoscopic gastrojejunostomy[68]. However, the presence of multiple strictures that cannot be reached by endoscopists in situations like peritoneal carcinomatosis or in patients that had previous operations are contraindications to duodenal stenting.
With regards to other pathologies, in case of perforation or bleeding, duodenal stenting can rarely be of help, whereas it can be considered for cases of benign strictures that are resistant or refractory to endoscopic dilation in patients who are poor surgical candidates[67]. However, in these cases, BDSs and extractable stents are preferred.
Common complications of gastroduodenal stenting: Table 3 shows the most common complications associated with gastroduodenal stenting. These complications are classified as either early or delayed and require immediate intervention[68,69]. Early complications develop within 1 wk of stenting and mainly include stent migration, obstruction, cholangitis, pain, perforation, bleeding, and misplacement whereas late complications develop after this period of time and mainly include stent migration, obstruction, perforation, fistula formation, and stent fracture[70,71]. Overall, the complication rate of duodenal stenting ranges between 12% and 44%. More specifically, stent occlusion due to tumor ingrowth or overgrowth occurs in 9% to 26% of patients with time to stent occlusion being estimated at 1.6 mo for patients with pancreatic cancer and 4.3 mo for those with other malignancies. Likewise, cholangitis secondary to papilla compression from stenting complicates the procedure in 2% to 6% of patients with non-pancreatic cancer and in 59% of those with pancreatic cancer, whereas perforation due to the strong axial force exerted by metal stents in up to 14% of patients, with concurrent dilations, attempts to pass the stenosis with the endoscope and concurrent use of corticosteroid medication, chemotherapy, and radiation being the major predisposing risk factors. As for the other complications, stent migration complicates stenting in 2% to 10% of patients, pain in 2% to 8%, and bleeding in up to 6% of patients whereas fistula formation is a rare adverse outcome[72].
| Early complications | Long-term complications |
| Migration | Migration |
| Obstruction | Perforation |
| Biliary obstruction | Obstruction |
| Perforation | Fistula formation |
| Bleeding | Stent fracture |
| Mispositioning | None |
Regarding the applications of stenting in the large intestine, following the advances in stent technology, the indications for intraluminal stenting have increased over the last two decades. First, colonic stenting (CS) is used for palliation in all patients who are poor surgical candidates or have inoperable cancer, metastatic or fistulizing disease, with patients treated or who are to be treated with antiangiogenic drugs being excluded as these drugs carry a threefold increased risk of causing perforation. In cancer patients with short life expectancy, CS aims to improve quality of life by offering an alternative less morbid option to palliative surgery, whereas in those with longer life expectancy, aims to prolong survival by accelerating access to chemotherapy and by preventing the morbidity that is associated with surgery. Among the different available types of stents, SEMSs are currently the best option for palliation as they achieve sustained symptomatic relief in more than 70% of patients for a time period that ranges from 6 to 12 mo[73,74].
Except for palliation, this kind of stents is also used by clinicians as a temporary measure to relieve malignant obstruction in operable cancer patients, eliminating the need for an emergent operation which is associated with high morbidity (40%-60%) and mortality (8%-20%). The use of SEMSs as a bridge to surgery is a highly effective approach, with technical success, defined as the appropriate insertion of SEMSs across the stenosis, being achieved in 90%, and clinical success, defined as the resolution of malignant obstruction with symptomatic relief, in 70% of the cases. With this approach, clinicians do not only avoid emergent surgeries, but also acquire the time they need to accurately stage the disease, optimize patients’ condition, avoid emergent multiple-stage surgery with the creation of a temporary or permanent ostomy, give neoadjuvant therapy, and generally prepare their patients for elective surgery. The elective surgery is less morbid and is performed as a one-step procedure 8 to 10 d after the placement of SEMSs, with the presence of SEMSs not being a problem to surgeons since they can be removed together with obstructing cancer. This approach is useful for patients with curable left-sided colonic cancer who have an increased risk for postoperative mortality; however, it cannot be applied to those with rectal tumors because, in the rectum, the placement of SEMSs causes debilitating symptoms such as discomfort, pain, tenesmus, and incontinence[75,76].
Following the successful use of SEMSs in the management of malignant obstruction, their use expanded to the management of the obstruction caused by compression of the bowel from extraintestinal malignancies such as pelvic tumors and peritoneal carcinomatosis. In these cases, current literature shows that SEMSs could be used as alternative therapeutic options to surgery; however, their use is characterized by lower technical and clinical success rates compared to those achieved for colonic cancer[77].
Another application of CS is in the management of obstruction caused by various benign conditions including diverticular disease, Crohn’s disease, radiation therapy, and anastomotic strictures. In these cases, the placement of SEMSs is associated with an increased risk of complications and is not recommended. However, it can be useful in patients who are poor surgical candidates or who have strictures that are not amenable to other therapeutic modalities. No advice can be given with regard to other stent types[78-80].
A final indication for stenting in the large bowel is in the management of anastomotic leakage, which is a worrisome complication after colorectal surgery, especially in low colorectal or coloanal anastomosis. In the management of anastomotic leakage, SEMSs and BDSs are used. Both stent types are placed for 2 mo and are used in conjunction with percutaneous drainage of abscesses to promote the healing of the anastomosis. With this approach, it is obviated the need for surgical reintervention that is associated with prolonged hospitalization, increased morbidity, and death. The technical success of stenting reaches 100%, with a clinical success of 80%-100%. However, its application is restricted to anastomoses that are less than 5 cm above the anal verge because it is associated with an increased risk for migration. In these cases, alternative therapeutic options such as injection of fibrin sealant and clips should be considered[41,79-81]. All common applications of CS are listed in Table 4.
| Common applications |
| Colorectal carcinoma and bowel obstruction |
| In non-operative candidates |
| Bowel obstruction |
| In non-operative candidates |
| Local postoperative neoplastic recurrence |
| Preoperative decompression in obstructing resectable colorectal carcinoma (bridge-to-surgery) |
| Relief of large bowel obstruction from extracolonic malignancy, pelvic mass or peritoneal carcinomatosis |
| Malignant colorectal fistula, e.g., to the urinary bladder or vagina |
| Postoperative anastomotic leakage |
| Fistula formation |
Common complications of large intestinal stenting: CS is regarded as a low-risk procedure, with an associated morbidity of 20%, low mortality, and infrequent need for surgery and stoma[42,82]. However, the complication rate of CS increases in the presence of several predisposing factors such as SEMS caliber ≤ 22 mm, the presence of complete luminal obstruction, right colon malignancy, extraintestinal lesion, and positive history of radio-chemotherapy[83,84]. The most common complications associated with CS are classified as either early or late depending on the time period they present following the successful insertion of stents (Table 5). The early complications usually develop within the 1st wk after stenting and include perforation, bleeding, and stent malpositioning, whereas the late complications develop later and include pain, stent migration, obstruction recurrence, perforation, and fistula formation[85-87]. Every time clinicians have a suspicion of a stent-related complication, the most common modality that can be used to investigate patients is computed tomography (CT). Among the different complications, pelvic or rectal pain is a common complaint of patients with metal stents and is a complication that does not need CT imaging for establishing the diagnosis. For nearly all the other complications, CT imaging is particularly valued[83].
| Early complications | Long-term complications |
| Technical failure (malpositioning or incomplete expansion) | Stent migration |
| Stent misplacement/failed relief of obstruction | Obstruction recurrence |
| Bleeding | Chemotherapy-related perforation |
| Stent migration | Fistula formation |
| Pelvic or rectal pain | Bleeding |
| Ischemia |
Immediately after CS, patients with obstruction are expected to show clinical improvement. If this improvement does not occur and is associated with persistent pre-stenotic dilatation in radiological imaging, malpositioning or incomplete expansion of the stent should be suspected. Stent malpositioning occurs whenever a stent is deployed more distally and does not cover the full extent of the stricture and requires immediate treatment. This complication can be recognized both on a radiograph or CT by observing the discrepancy between the position of the stent and the site of the obstructing tumor. The differential diagnosis between malpositioning and incomplete expansion is relatively easy to be made since in the case of incomplete expansion the stent maintains its hourglass shape on CT[19,43,83,85,87]. Bleeding is a minor complication of CS that occurs in 5% of cases. Most of the time, it is of low severity and ceases spontaneously. CT imaging is not required for making the diagnosis but in cases where CT is requested for other reasons, the diagnostic hallmark is the presence of hyperdense content within the intestinal lumen[19,43,83,85,87]. Conversely to bleeding, perforation is a dreaded complication of CS because it is associated with an estimated mortality of 10%. It occurs in 5% of the procedures with previous irradiation, concurrent dilatation, and excessive manipulation of guidewires during endoscopy increasing the risk for this complication in the early postinterventional period. However, perforations can occur any time after stenting with some cases being reported even after 6 mo from stenting. Contrary to early perforations, those occurring late are caused by different factors such as systemic chemotherapy. CT imaging is of particular value in making the accurate diagnosis in all but especially in clinically silent cases[19,43,83,85,87]. Among the different complications of CS, migration is the most frequent. It occurs in up to 50% of the patients undergoing this procedure. Small and/or short and/or covered stents as well as concurrent chemotherapy, partial luminal obstruction, extraintestinal lesions, and rectal tumors are all significant factors that predispose to migration. Migration can be asymptomatic or lead to other complications such as obstruction and perforation. Endoscopic repositioning, removal, or replacement may be necessary for symptomatic patients[19,43,83,85,87]. Less frequently than migration, colonic stents can be occluded, leading to symptomatic recurrence. Stent occlusion is caused by tumor ingrowth or overgrowth or the impaction of fecal material. Depending on the etiology, the endoscopic or medical relief of the occlusion should be considered[83,85,87].
In the biliary system and pancreas, the placement of stents allows minimally invasive management of obstruction from benign to malignant causes with high technical and clinical success rates[19,88]. Initially, it was promoted the use of stents with a double-pigtail design to prevent upward migration, but over time, the use of straight stents with side flaps prevailed[89]. Nowadays, various plastic and metal stents with various shapes, diameters, and lengths are available for use in the biliopancreatic system. However, PSs are generally preferred for most of the benign indications that are outlined in the following paragraphs and metal stents for palliative treatment.
Benign biliary strictures: One common indication for stenting in the biliary system is the management of benign biliary strictures (BBSs). These strictures can be caused by post-operative injury (particularly after cholecystectomy), anastomotic injury following orthotopic liver transplantation (OLT), primary sclerosing cholangitis (PSC), post-endoscopic sphincterotomy (ES) and other less frequent conditions, such as radiation therapy, IgG4-related disease, and portal biliopathy[90,91].
Irrespective of the etiology, BBSs require immediate treatment with stenting playing a central role in their management. The choice of the type and number of stents that are to be used mainly depends on the etiology of BBSs. For a dominant bile duct stricture in PSC patients, defined as stenosis of the common bile duct with a diameter of ≤ 1.5 mm and/or stenosis of the hepatic duct with a diameter of ≤ 1.0 mm, a single plastic stent may be adequate, although, for the majority of other strictures, progressive dilation with the insertion of two or more plastic stents is the common practice[92,93]. Several clinical trials pointed out the high clinical success of the placement of PSs after progressive dilatations for relieving the obstruction in patients with either anastomotic or non-anastomotic post-OLT, or post-ES strictures[94-96]. However, a significant drawback of this approach is the requirement of repetitive endoscopic procedures over a short period of time, which compromises patients’ quality of life and concurrently increase healthcare costs. SEMSs overcome this drawback. This is because they can be positioned without dilation and permit the resolution of a benign stricture without the need for progressive stent switching[96]. However, among the different available SEMSs, uncovered SEMSs and PCSEMSs are not advised for the treatment of BBSs. This is because they allow tissue ingrowth and overgrowth that lead to the development of new strictures and/or to the premature occlusion and embedment of these stents into the biliary system. Instead, FCSEMs do not have these limitations. However, due to their full cover, they have increased risk for migration with reported rates approaching 45% in some series[97-100]. This led to the development of a novel type of FCSEMSs, named antimigratory stents that prevent this complication[19,101,102].
Biliary stones: ELS can be also used in cases where complete clearance of common bile duct (CBD) from stones is not achieved, and the placement of stents is required to maintain uninterrupted bile flow into the duodenum[103,104]. This approach entails the use of straight and double pigtail PSs that range in diameter from 2.3 to 3.3 mm (corresponding to 7 and 10 Fr respectively) and does not provide a definite therapeutic option. However, it serves as a temporary measure until complete clearance of CBD is achieved. In most cases, the use of ELS in the management of biliary stones reduces the size of the stones and increases their debris, facilitating complete clearance[105]. However, it is not a risk-free procedure and it is associated with several complications including occlusion, migration, and episodes of cholangitis. Conversely to plastic stents, metal stents have no role in the management of biliary stones.
Biliary leaks: Another common application for ELS is in the management of biliary leaks (BLs). BLs are often a consequence of surgery, such as open or laparoscopic cholecystectomy or hepatic resection, but they can be also caused due to trauma, or invasive procedures, such as liver biopsy and percutaneous transhepatic cholangiography[30].
Biliary stents are used to preserve the bile flow into the duodenum and concurrently cease its outflow from the leaking site. Several studies suggest the use of ES alone for the management of minor leaks and the insertion of PSs for major leaks[106]. However, in the clinical setting, the most common approach entails the placement of a plastic stent that has 2.3 or 3.3 mm in diameter with or without ES for a period of 4 to 6 wk[107].
PSs can be placed with or without ES with a high success rate (90%) for treating leaks at the peripheral duct but their success rate drops to approximately 60% when it comes to leaks at the hilar or the common bile duct. Metal stents are not regularly used for the management of BLs despite the fact that FCSEMSs were shown to lead to the resolution of the leak in approximately 70% of patients in some series. This is because their use was associated with the development of strictures after removal and with higher migration rates compared to PSs. In cases where endotherapy fails, surgery is the sole definite treatment but it is not preferred for high-risk patients with major co-morbidities[108].
Cholecystitis: Transpapillary gallbladder stenting (TGS) is useful for cases of acute calculous or acalculous cholecystitis when conventional therapies fail or cannot be performed. TGS is indicated for patients that are severely ill, with serious comorbidities that prohibit surgical cholecystectomy, and/or have contraindications to undergo percutaneous cholecystostomy (such as severe coagulopathy, ascites, or a bowel loop interference between the diaphragm and the liver preventing percutaneous access). TGS entails the selective cannulation of the cystic duct during ERCP and the placement of small-diameter stents. However, it cannot be performed in patients who cannot undergo ERCP for various reasons such as pregnancy[109,110]. Endoscopic ultrasound-guided gallbladder stenting allowed clinicians to overcome this problem, extending the use of gallbladder stenting. Gallbladder stents are placed with a technical success rate that ranges from 75% to 100%, and an adverse event rate of 0%-20%[37-40]. PSs are exclusively used to ensure continued gallbladder drainage. However, metal stents with large flares at their ends or with a "saddle" form and distal anchor flanges can be alternatively used[110-112].
Post-sphincterotomy /post-dilation bleeding and perforation: Epinephrine injection, thermal therapy, balloon tamponade, clips, and placement of large bore PSs have been used in order to control post-ERCP bleeding. PSs and currently FCSEMSs are effectively used to treat these complications as they exert direct pressure on the bleeding area[113]. Conversely to the management of bleeding, little is known regarding the efficacy of ELS in the management of post-ERCP perforations. This is because on the one hand, the incidence of this complication is very low and on the other hand, its clinical consequences are tremendous leading patients to emergent surgery. A few reports show PSs can be used for the management of post-ERCP perforations, but their small diameter may fail to fully seal the defect. FCSEMs can overcome this problem because they have a larger caliber but more research is currently required to make suggestions.
Malignant biliary diseases: Another common indication for ELS is in the palliative management of obstructive jaundice caused by biliopancreatic cancers that extrinsically compress or intraluminally obstruct the biliary tract such as cholangiocarcinoma, pancreatic cancer, tumors of the papilla of Vater or hilar lymph node compression. Among the different available stent types, PSs are preferred for cancer patients with short life expectancy. The PSs that are commonly selected for these patients have a large diameter that ranges from 2.8 to 3.3 mm and are left in place approximately for 3 mo with high efficiency. Instead, for patients with longer life expectancy, the use of metal stents is preferred because SEMSs are larger, they can be left in place for longer periods of time and their placement is easier. To date, uncovered, partially covered, and fully covered SEMS have been all successfully used for palliation in patients with inoperable cancer. However, the use of uncovered SEMSs was associated with an increased rate of stent occlusion whereas the covered ones with stent migration[91,114,115].
Applications in the pancreatic duct: In the pancreatic duct, the most common application of ELS is in the prevention of post-ERCP pancreatitis (PEP). PEP is a relatively common complication that occurs in up to 10% of patients undergoing ERCP. Multiple factors have been shown to affect the incidence of PEP such as patients’ comorbidities, operators’ experience, and the indication for ERCP with pancreatic stenting (PS) being found to reduce not only the frequency but also the severity of PEP. Nowadays, PS are used in various situations to prevent PEP. The common indications for placing PS include pancreatic sphincterotomy for sphincter of Oddi dysfunction, acute or recurrent pancreatitis, and ampullectomy. In addition, PS is highly recommended in cases of difficult biliary cannulation, instrumentation or injection of the pancreas, aggressive manipulation of the pancreatic duct (brush cytology, biopsies), balloon dilatation of an intact biliary sphincter (balloon sphincteroplasty), previous PEP and precut sphincterotomy starting at the papillary orifice[116-120].
Except for the prevention of PEP, PS is effectively used in the management of symptomatic main pancreatic duct strictures and after pancreatic sphincterotomy and fragmentation of pancreatic stones[121-124].
Common complications of biliary and PS: Biliary stenting is a relatively safe procedure with complication rates ranging between 8% and 10% and mortality of less than 1%. Migration is the most common complication associated with biliary stenting. It occurs in up to 10% of cases and is classified into proximal and distal. Distal stent migration into the bowel leads to spontaneous passage of the stent in more than 70% of the cases with the rest being easily retrieved endoscopically. Among the different available stents, SEMS migrate rarely compared to their plastic counterparts. Different stent designs have been used to prevent migration such as double pigtail stents, side flaps, and barbs. However, migration remains a problem with multiple factors being associated with increased migration rates including short or long stents, papillary stenosis, omission of sphincterotomy, and benign strictures. In rare cases where the migrated stents do not pass spontaneously, migration can lead to life-threatening complications including bowel obstruction, perforation, intra-abdominal sepsis, fistula formation, and necrotizing fasciitis[18].
Similar to biliary stenting, PS is not a risk-free procedure. The most common complication associated with PS is the development of morphological changes in the pancreatic duct. Morphologic changes are found in more than 50% of patients and resemble chronic pancreatitis. Although the consequences of this complication are unknown, ductal strictures can develop in a few patients requiring balloon dilatations. Stent occlusion represents another common complication associated with PS. The rate of pancreatic stent occlusion is similar to that of biliary stents with most stents being occluded within 3 mo after insertion. However, in most cases, stent occlusion is not associated with adverse outcomes since pancreatic juices can siphon across the sides of the stent. Other stent-related complications that are commonly reported to be associated with PS include acute pancreatitis, pancreatic infection, pseudocyst formation, duct injury, stone formation, and migration[18].
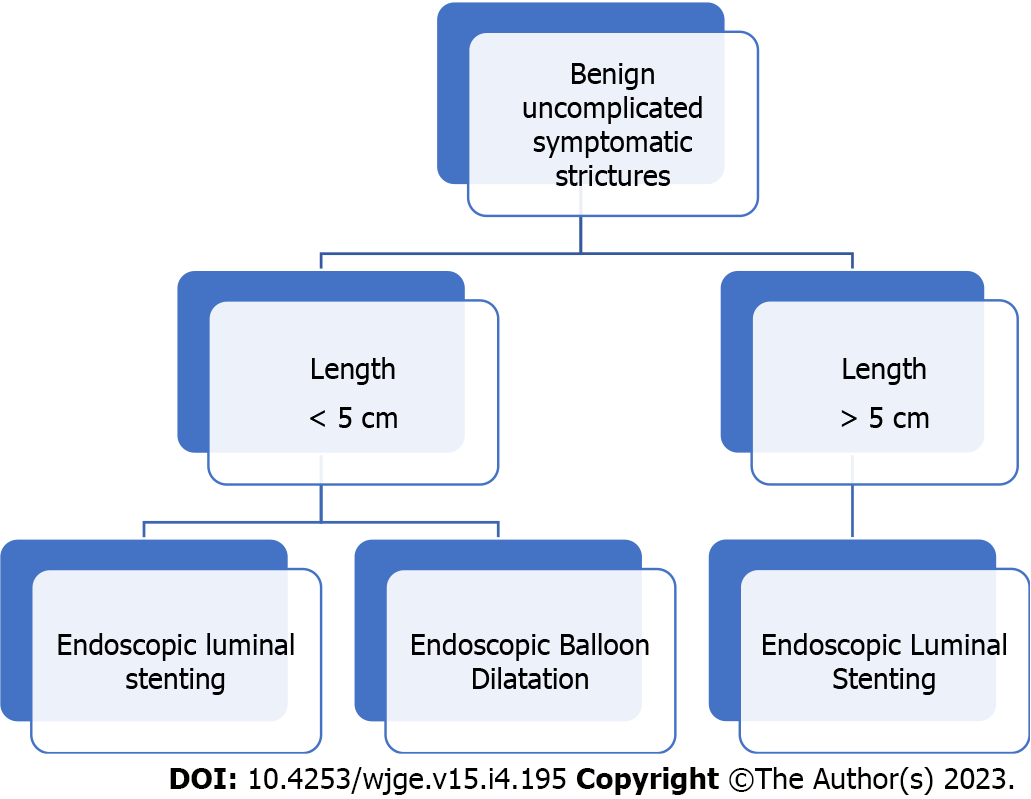
Following the successful application of ELS in the management of a variety of gastrointestinal and biliopancreatic disorders, the use of ELS has expanded to include the management of CD-related strictures. Strictures in CD occur in up to one-third of patients within 11 years of diagnosis secondary to chronic transmural inflammation, and in 50% of those undergoing ileocolic resection. Most strictures are located in the small intestine rather than in the colon (64% vs 5%, respectively)[125]. Endoscopic balloon dilation (EBD) is currently the preferred endoscopic therapy for CD strictures[126,127]. However, the use of EBD is confined to the management of primary or anastomotic strictures that do not exceed 5 cm because this is the maximum length of the largest balloon that is available for dilations. Longer strictures are not amenable to EBD. For these strictures, ELS can offer a minimally invasive alternative to surgery[126]. Among the different available stent types, FCSEMSs and PCSEMSs were used for this indication[128,129]. Preliminary experience has shown conflicting results with several studies reporting different clinical success rates that range between 36% to 100%. However, in all cases in that SEMSs were successfully applied, patients remained asymptomatic for a period of 10 to 12 mo of follow-up signifying a positive clinical outcome[129-133] (Figure 1).
Common complications of stenting in IBD: The use of SEMSs for the management of CD strictures can be challenging or impossible depending on the location and degree of the stenosis and is associated with several complications[134]. Migration is a common complication of ELS. It occurs in up to 52% of the cases with 70% of the episodes occurring within the first 3 d. It is classified into proximal or distal migration depending on the direction of the misplacement of the stent. Although migration is not considered a serious condition, it can lead to dreaded complications such as impaction and perforation, that require surgery. In the majority of cases, migrated stents are spontaneously expelled from the body, leaving a small minority for endoscopic retrieval. Except for migration, another common complication of ELS in IBD is stent occlusion. Stent occlusion develops secondary to the development of hyperplastic tissue at the exposed ends of several metal stents and occurs in up to 24% of some patient series. To prevent this complication, depending on the type of SEMSs (FCSEMS or PCSEMS) that was used, it is recommended to remove stents within 4 to 6 wk after their placement[134,135]. With regard to other complications associated with ELS in IBD, there is a theoretical risk for perforation due to mucosal erosion; however, this concern mainly stems from observations in cancer and not from IBD patients[134,135]. Nevertheless, more research is required in order to fully determine the risks associated with the use of ELS in IBD.
Obesity rates have significantly grown over the last decades constituting a global health problem. Obesity often contributes to the development of various chronic diseases such as type 2 diabetes, hypertension, heart disease, and cancer, adversely affecting patients’ quality of life and life expectancy[136-138]. Many treatments for obesity exist but bariatric surgeries are by far the most effective in achieving significant weight loss. Nowadays, the three most common types of performed bariatric surgeries include Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric band (LAGB), and sleeve gastrectomy (SG). However, all these operations are invasive therapeutic options with up to 12% of patients experiencing adverse events in the 1st 5 years after surgery. ELS represents a minimally invasive option that can be employed in the management of several post-bariatric complications including anastomotic strictures, anastomotic leaks, choledocholithiasis, sleeve stenosis, and band erosion[136-140].
In particular, in the management of post-bariatric strictures, available evidence suggests that ELS can be effective in up to 40% of the refractory cases presenting after RYGB whereas no suggestion can be made for the use of ELS in the management of strictures that develop after SG. In the management of anastomotic leaks, after RYGB, accumulated evidence suggests that FCSEMSs and PCSEMSs can lead to a high leak resolution rate (87.8%). However, these stents are poorly tolerated necessitating their removal after 6-8 wk. Alternatively to SEMSs, when the leak leads to an abscess cavity and persistent drainage is required until the resolution of the abscess, PSs with a double pigtail design can be a safe and effective solution. Likewise, FCSEMSs and PCSEMSs can be effectively used in the management of leaks associated with SG with a high success rate (72.8%). Regarding the use of PSs for the internal drainage of an abscess, their use after SG is a novel approach with preliminary evidence from retrospective studies showing a high efficacy (72.8% clinical success rate). As for the other bariatric complications, covered metal stents can be placed for a 2-wk period in order to promote migration if an eroded band after LAGB appears to be insufficiently migrated for endoscopic removal, facilitating its extraction whereas no differences exist as per the instruments that will be used for the management of RYGB-related choledocholithiasis between operated and non-operated patients[136-140].
Common complications of post-bariatric stenting
Reflux, discomfort, gastric ulcers, nausea/vomiting, and migration are common adverse events associated with ELS when used in the management of post-bariatric complications. Among these complications, the incidence rates of discomfort (6%), ulcer (4%), and nausea/vomiting (11%) are generally low[140]. Stent migration (39%) is the most common adverse event that can complicate ELS. Although in most cases migration is not associated with adverse clinical outcomes, it can have significant consequences when related to RYGB. This is because in RYGB cases, the migrated stent can be displaced outside of the range of a standard endoscope, making its retrieval challenging[138,141,142].
The placement of GISs is a minimally invasive option for treating various benign and malignant conditions with high technical and clinical success rates. Current stent technology has astonishingly progressed allowing the development of various stent types with cutting-edge designs and materials that expanded the indications of ELS and improve patients’ quality of life. However, there are still several drawbacks, such as stent occlusion and migration, that are difficult to overcome. Antimigratory, antireflux, shape-modified, irradiating, drug-eluting, and novel biodegradable stents specifically designed for various esophageal, gastroduodenal, biliary, and colonic indications are just a few of the significant advancements in the field of ELS. Current GISs innovations are outlined in the following paragraphs but more research is underway aiming to improve the properties of stents, reinforce their benefits, and lessen their drawbacks.
Migration constitutes a significant problem associated with FCSEMSs that compromise the safety of ELS. This led to the development of several stents with cutting-edge designs aiming to prevent this complication named antimigratory stents. This kind of stents includes anchoring components, such as flared ends, anchoring flaps, or serrated anchoring pins. These anchoring components were found to have been associated with lower migration rates compared to traditional FCSEMSs, with anchoring flaps being superior to the flared end with regard to stent migration. However, serrated anchoring pins are by far more effective for the prevention of migration but this design makes difficult the removal of the stent[143-145].
Tumor ingrowth and overgrowth are significant adverse events that complicate the use of GISs. Although PCSEMSs and FCSEMSs are designed to prevent these adverse events, their occlusion is inevitable because their cover is gradually degraded due to hydrolysis, oxidation, and contact with the luminal content GI. In an effort to prevent stent occlusion, a new kind of metal stents, named drug-eluting stents (DESs), was developed[146-150].
DESs are composed of three primary components that include a drug, a polymeric drug-delivery coating/carrier, and a stent platform. To date, various chemotherapeutic drugs such as paclitaxel have been trialed in different experimental models to evaluate the efficacy and safety of their local administration for different purposes. Paclitaxel is a chemotherapeutic agent that inhibits the proliferation of epithelial gallbladder cells, fibroblasts, and pancreatic carcinoma cells in a dose-dependent manner. In vitro trialing of paclitaxel in animal biliary ducts showed that it causes epithelial stripping and metaplasia, thickening of the bile duct, hypersecretion of mucus, and fibrosis, without significant complications. These positive outcomes allowed further testing in humans. However, accumulated evidence from human studies has shown conflicting results regarding the efficacy and safety of paclitaxel-eluting stents signifying a need for further improvement[146-150].
Another chemotherapeutic agent that was trialed for local delivery is gemcitabine. This agent is used in advanced pancreatic and biliary tract cancer. The hydrophilic properties of this agent make difficult its local delivery due to rapid degradation. A gemcitabine-eluting stent was designed to allow prolonged gemcitabine release by increasing the contact surface between the stent and the tumor. In this stent pullulan, a natural polysaccharide was used to increase the loading capacity of gemcitabine when loaded onto polytetrafluoroethylene, allowing a continuous release of the drug for 30 d[146-150].
With regards to other chemotherapeutic agents, Lee et al[146] developed a 5-Fluorouracil (5-FU)-eluting esophageal stent. Preliminary experience with this stent showed that the concentration of 5-FU that is achieved in the esophagus is higher than in other areas, with the highest levels being observed at the contact site in the mucosa. However, its use was associated with target and non-target organ dose-dependent toxicity. This adverse outcome led to ongoing research studies that aim to determine the ideal stent design and appropriate drug concentration, with their results being currently awaited[146-150].
In esophageal cancer, brachytherapy reduces dysphagia symptoms more slowly than stent implantation but offers longer patency and fewer complications. Patients with inoperable esophageal cancer may benefit from a palliative treatment approach consisting of stent implantation, combined with brachytherapy[151,152]. Radioactive stents that carry the advantages of traditional stents and brachytherapy were designed[30]. This kind of stents is loaded with iodine-125 seeds, with the interior being composed of standard metal, to facilitate insertion. Preliminary evidence suggests that these stents when used in cancer patients improve malignant dysphagia and prolong survival whereas their insertion is easy and safe. However, these results derive from small studies, with larger studies being needed prior to making any recommendation[153-155].
The use of SEMSs restores and maintains the patency of the obstructed lumen, relieving the symptoms associated with malignant obstruction in patients with inoperable cancer. Clinically approved SEMSs have a larger diameter than their plastic counterparts with reflux being a significant problem that adversely affect the quality of life of cancer patients[156,157]. In an effort to prevent reflux and improve patients’ quality of life, new stents with an attached antireflux valve are being developed. The preliminary experience did not show encouraging results but further improvement in the design of the antireflux valve is currently awaited.
One strategy to improve the efficacy and safety of ELS is to modify the stent’s design. Over the last 10 years, the stents’ design has undergone several changes aiming to reduce complication rates associated with ELS. One interesting modification refers to winged PSs with small central lumens. These stents were developed to prevent the occlusion associated with the placement of conventional SEMS. Despite expectations, these stents did not show positive results compared to conventional SEMS and hence, are not advised. Another interesting modification in the design of stents is the development of a dumbbell-shaped SEMS for minimizing the risk of migration and bile leakage associated with endoscopic ultrasound-guided gallbladder drainage. Preliminary experience with this stent showed that its use was associated with high clinical and technical success and no major complications, promoting its use[158,159].
BDSs have shown promising results in the management of benign strictures. Compared to other stent types, BDSs have several advantages such as their ability not to occlude, not to require repetitive endoscopies for removal, and being equipped with antibacterial or antitumor agents. However, BDSs have also disadvantages that can preclude their use such as the exertion of weaker radial forces than conventional metal stents and the need for balloon dilatation for their placement. Ongoing research in this field is awaited to reduce these drawbacks and enhance the use of BDSs in the future[19,160,161].
The role of stenting in the management of patients with malignant GI obstruction has expanded in recent years to include a variety of disorders in the esophagus, stomach, biliopancreatic system, small bowel, and colon. Recent advances in technology have improved stent efficacy and reduced stent-related complications, resulting in better clinical outcomes. However, GISs continue to undergo design improvements to address their limitations. Further research is required in order to enhance the use of ELS in the management of various conditions in the gastrointestinal tract.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Hellenic Society of Gastroenterology; British Society of Gastroenterology; United European Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anandan H, India; Velde LV, The Netherlands S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Roguin A. Stent: the man and word behind the coronary metal prosthesis. Circ Cardiovasc Interv. 2011;4:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Dua KS. History of the Use of Esophageal Stent in Management of Dysphagia and Its Improvement Over the Years. Dysphagia. 2017;32:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Castaño R, Lopes TL, Alvarez O, Calvo V, Luz LP, Artifon EL. Nitinol biliary stent versus surgery for palliation of distal malignant biliary obstruction. Surg Endosc. 2010;24:2092-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | ASGE Technology Committee; Varadarajulu S, Banerjee S, Barth B, Desilets D, Kaul V, Kethu S, Pedrosa M, Pfau P, Tokar J, Wang A, Song LM, Rodriguez S. Enteral stents. Gastrointest Endosc. 2011;74:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Schumacher B, Lübke H, Frieling T, Haussinger D, Niederau C. Palliative treatment of malignant esophageal stenosis: experience with plastic versus metal stents. Hepatogastroenterology. 1998;45:755-760. [PubMed] |
| 6. | Gupta K, Freeman ML. Enteral and colonic self-expanding metallic stents. Rev Gastroenterol Disord. 2008;8:83-97. [PubMed] |
| 7. | Domingo S, Puértolas S, Gracia-Villa L, Puértolas JA. Mechanical comparative analysis of stents for colorectal obstruction. Minim Invasive Ther Allied Technol. 2007;16:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Song HY, Jung HY, Park SI, Kim SB, Lee DH, Kang SG, Il Min Y. Covered retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience. Radiology. 2000;217:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Baerlocher MO, Asch MR, Dixon P, Kortan P, Myers A, Law C; Canadian Interventional Radiology Association. Interdisciplinary Canadian guidelines on the use of metal stents in the gastrointestinal tract for oncological indications. Can Assoc Radiol J. 2008;59:107-122. [PubMed] |
| 10. | Siersema PD, Hop WC, van Blankenstein M, Dees J. A new design metal stent (Flamingo stent) for palliation of malignant dysphagia: a prospective study. The Rotterdam Esophageal Tumor Study Group. Gastrointest Endosc. 2000;51:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Langer FB, Wenzl E, Prager G, Salat A, Miholic J, Mang T, Zacherl J. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg. 2005;79:398-403; discussion 404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Repici A, Conio M, De Angelis C, Battaglia E, Musso A, Pellicano R, Goss M, Venezia G, Rizzetto M, Saracco G. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc. 2004;60:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Radecke K, Gerken G, Treichel U. Impact of a self-expanding, plastic esophageal stent on various esophageal stenoses, fistulas, and leakages: a single-center experience in 39 patients. Gastrointest Endosc. 2005;61:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Sreedharan A, Harris K, Crellin A, Forman D, Everett SM. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2009;CD005048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Moss WJ, Pang J, Orosco RK, Weissbrod PA, Brumund KT, Weisman RA, Brigger MT, Coffey CS. Esophageal dilation in head and neck cancer patients: A systematic review and meta-analysis. Laryngoscope. 2018;128:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Cao M, Luo ZW. [Metallic stents for malignant esophageal neoplasms stenosis in advanced stage]. Zhongguo Yiliao Qixie Zazhi. 2000;24:347-348, 356. [PubMed] |
| 17. | Sabharwal T, Hamady MS, Chui S, Atkinson S, Mason R, Adam A. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003;52:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Mangiavillano B, Pagano N, Arena M, Miraglia S, Consolo P, Iabichino G, Virgilio C, Luigiano C. Role of stenting in gastrointestinal benign and malignant diseases. World J Gastrointest Endosc. 2015;7:460-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 19. | Mangiavillano B, Pagano N, Baron TH, Arena M, Iabichino G, Consolo P, Opocher E, Luigiano C. Biliary and pancreatic stenting: Devices and insertion techniques in therapeutic endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography. World J Gastrointest Endosc. 2016;8:143-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Lin M, Firoozi N, Tsai CT, Wallace MB, Kang Y. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater. 2019;83:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Yuan T, Yu J, Cao J, Gao F, Zhu Y, Cheng Y, Cui W. Fabrication of a Delaying Biodegradable Magnesium Alloy-Based Esophageal Stent via Coating Elastic Polymer. Materials (Basel). 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Patterson DJ, Graham DY, Smith JL, Schwartz JT, Alpert E, Lanza FL, Cain GD. Natural history of benign esophageal stricture treated by dilatation. Gastroenterology. 1983;85:346-350. [PubMed] |
| 23. | Hirdes MM, Vleggaar FP, Siersema PD. Stent placement for esophageal strictures: an update. Expert Rev Med Devices. 2011;8:733-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | van Boeckel PG, Vleggaar FP, Siersema PD. Biodegradable stent placement in the esophagus. Expert Rev Med Devices. 2013;10:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Dai Y, Li C, Xie Y, Liu X, Zhang J, Zhou J, Pan X, Yang S. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2014;2014:CD005048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | van Heel NC, Haringsma J, Spaander MC, Bruno MJ, Kuipers EJ. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol. 2010;105:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 395] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Siiki A, Vaalavuo Y, Antila A, Ukkonen M, Rinta-Kiikka I, Sand J, Laukkarinen J. Biodegradable biliary stents preferable to plastic stent therapy in post-cholecystectomy bile leak and avoid second endoscopy. Scand J Gastroenterol. 2018;53:1376-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Maishman T, Sheikh H, Boger P, Kelly J, Cozens K, Bateman A, Davies S, Fay M, Sharland D, Jackson A. A Phase II Study of Biodegradable Stents Plus Palliative Radiotherapy in Oesophageal Cancer. Clin Oncol (R Coll Radiol). 2021;33:e225-e231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hirdes MM, van Hooft JE, Wijrdeman HK, Hulshof MC, Fockens P, Reerink O, van Oijen MG, van der Tweel I, Vleggaar FP, Siersema PD. Combination of biodegradable stent placement and single-dose brachytherapy is associated with an unacceptably high complication rate in the treatment of dysphagia from esophageal cancer. Gastrointest Endosc. 2012;76:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Chen YH, Li SH, Chiu YC, Lu HI, Huang CH, Rau KM, Liu CT. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One. 2012;7:e42766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Hu Y, Zhao YF, Chen LQ, Zhu ZJ, Liu LX, Wang Y, Kou YL. Comparative study of different treatments for malignant tracheoesophageal/bronchoesophageal fistulae. Dis Esophagus. 2009;22:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Shin JH, Song HY, Ko GY, Lim JO, Yoon HK, Sung KB. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology. 2004;232:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Spaander MCW, van der Bogt RD, Baron TH, Albers D, Blero D, de Ceglie A, Conio M, Czakó L, Everett S, Garcia-Pagán JC, Ginès A, Jovani M, Repici A, Rodrigues-Pinto E, Siersema PD, Fuccio L, van Hooft JE. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 35. | Bethge N, Sommer A, Vakil N. Palliation of malignant esophageal obstruction due to intrinsic and extrinsic lesions with expandable metal stents. Am J Gastroenterol. 1998;93:1829-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:455-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 290] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med. 1985;145:1443-1446. [PubMed] |
| 38. | Isomoto H, Yamaguchi N, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Pereira-Lima JC, Ramires RP, Zamin I Jr, Cassal AP, Marroni CA, Mattos AA. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol. 1999;94:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Siersema PD. Stenting for benign esophageal strictures. Endoscopy. 2009;41:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Thomas MS, Margolin DA. Management of Colorectal Anastomotic Leak. Clin Colon Rectal Surg. 2016;29:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Elsberger B, Rourke K, Brush J, Glancy S, Collie M. Self-expanding metallic stent insertion in the proximal colon. Colorectal Dis. 2008;10:194-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Foo CC, Poon JT, Law WL. Self-expanding metallic stents for acute left-sided large-bowel obstruction: a review of 130 patients. Colorectal Dis. 2011;13:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Pucher PH, Allum WH, Bateman AC, Green M, Maynard N, Novelli M, Petty R, Underwood TJ, Gossage J. Consensus recommendations for the standardized histopathological evaluation and reporting after radical oesophago-gastrectomy (HERO consensus). Dis Esophagus. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N. Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001;88:1346-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Gelbmann CM, Ratiu NL, Rath HC, Rogler G, Lock G, Schölmerich J, Kullmann F. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy. 2004;36:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Hünerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg. 2004;240:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, Eder F, Lippert H, Pross M. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005;61:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Vakil N, Morris AI, Marcon N, Segalin A, Peracchia A, Bethge N, Zuccaro G, Bosco JJ, Jones WF. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol. 2001;96:1791-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Amrani L, Ménard C, Berdah S, Emungania O, Soune PA, Subtil C, Brunet C, Grimaud JC, Barthet M. From iatrogenic digestive perforation to complete anastomotic disunion: endoscopic stenting as a new concept of "stent-guided regeneration and re-epithelialization". Gastrointest Endosc. 2009;69:1282-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Mayoral W, Fleischer D, Salcedo J, Roy P, Al-Kawas F, Benjamin S. Nonmalignant obstruction is a common problem with metal stents in the treatment of esophageal cancer. Gastrointest Endosc. 2000;51:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Homs MY, Steyerberg EW, Kuipers EJ, van der Gaast A, Haringsma J, van Blankenstein M, Siersema PD. Causes and treatment of recurrent dysphagia after self-expanding metal stent placement for palliation of esophageal carcinoma. Endoscopy. 2004;36:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Ramirez FC, Dennert B, Zierer ST, Sanowski RA. Esophageal self-expandable metallic stents--indications, practice, techniques, and complications: results of a national survey. Gastrointest Endosc. 1997;45:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Saligram S, Lim D, Pena L, Friedman M, Harris C, Klapman J. Safety and feasibility of esophageal self- expandable metal stent placement without the aid of fluoroscopy. Dis Esophagus. 2017;30:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Mozafari H, Dong P, Zhao S, Bi Y, Han X, Gu L. Migration resistance of esophageal stents: The role of stent design. Comput Biol Med. 2018;100:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Zhan Y, Xu Z. Massive hemorrhage from an aortoesophageal fistula caused by esophageal stent implantation: A case report and literature review. Medicine (Baltimore). 2019;98:e18303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Tringali A, Costa D, Anderloni A, Carrara S, Repici A, Adler DG. Covered versus uncovered metal stents for malignant gastric outlet obstruction: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:1153-1163.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Diamantopoulos A, Sabharwal T, Katsanos K, Krokidis M, Adam A. Fluoroscopic-guided insertion of self-expanding metal stents for malignant gastroduodenal outlet obstruction: immediate results and clinical outcomes. Acta Radiol. 2015;56:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Tendler DA. Malignant gastric outlet obstruction: bridging another divide. Am J Gastroenterol. 2002;97:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Del Piano M, Ballarè M, Montino F, Todesco A, Orsello M, Magnani C, Garello E. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005;61:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Jang SH, Lee H, Min BH, Kim SM, Kim HS, Carriere KC, Min YW, Lee JH, Kim JJ. Palliative gastrojejunostomy vs endoscopic stent placement for gastric outlet obstruction in patients with unresectable gastric cancer: a propensity score-matched analysis. Surg Endosc. 2017;31:4217-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy vs duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 359] [Article Influence: 23.9] [Reference Citation Analysis (2)] |
| 65. | Tang CN, Siu WT, Ha JP, Li MK. Endo-laparoscopic approach in the management of obstructive jaundice and malignant gastric outflow obstruction. Hepatogastroenterology. 2005;52:128-134. [PubMed] |
| 66. | Rodríguez JI, Kutscher M, Lemus M, Crovari F, Pimentel F, Briceño E. Palliative gastrojejunostomy in unresectable cancer and gastric outlet obstruction: a retrospective cohort study. Ann R Coll Surg Engl. 2021;103:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Ly J, O'Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc. 2010;24:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Mosler P, Mergener KD, Brandabur JJ, Schembre DB, Kozarek RA. Palliation of gastric outlet obstruction and proximal small bowel obstruction with self-expandable metal stents: a single center series. J Clin Gastroenterol. 2005;39:124-128. [PubMed] |
| 69. | Masci E, Viale E, Mangiavillano B, Contin G, Lomazzi A, Buffoli F, Gatti M, Repaci G, Teruzzi V, Fasoli R, Ravelli P, Testoni PA. Enteral self-expandable metal stent for malignant luminal obstruction of the upper and lower gastrointestinal tract: a prospective multicentric study. J Clin Gastroenterol. 2008;42:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Vitale GC, Davis BR, Tran TC. The advancing art and science of endoscopy. Am J Surg. 2005;190:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Flug JA, Garnet DJ, Widmer J, Stavropoulos S, Gidwaney R, Katz DS, Abbas MA. Advanced gastrointestinal endoscopic procedures: indications, imaging findings, and implications for the radiologist. Clin Imaging. 2013;37:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Oh SY, Kozarek RA. Management of gastroduodenal stent-related complications. Int J Gastrointest Interv. 2015;4:89-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Baer C, Menon R, Bastawrous S, Bastawrous A. Emergency Presentations of Colorectal Cancer. Surg Clin North Am. 2017;97:529-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | van Hooft JE, Veld JV, Arnold D, Beets-Tan RGH, Everett S, Götz M, van Halsema EE, Hill J, Manes G, Meisner S, Rodrigues-Pinto E, Sabbagh C, Vandervoort J, Tanis PJ, Vanbiervliet G, Arezzo A. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:389-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 75. | Hong SP, Kim TI. Colorectal stenting: an advanced approach to malignant colorectal obstruction. World J Gastroenterol. 2014;20:16020-16028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Shimura T, Joh T. Evidence-based Clinical Management of Acute Malignant Colorectal Obstruction. J Clin Gastroenterol. 2016;50:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Kim MK. Is the ESGE guideline recommendation against the placement of stents for left-sided malignant colonic obstruction still open to debate? Endoscopy. 2016;48:199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Ahmed O, Lee JH, Thompson CC, Faulx A. AGA Clinical Practice Update on the Optimal Management of the Malignant Alimentary Tract Obstruction: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1780-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 79. | Shin JH, Song HY, Kim JH, Kim SB, Lee GH, Park SI, Han YM, Kang W. Comparison of temporary and permanent stent placement with concurrent radiation therapy in patients with esophageal carcinoma. J Vasc Interv Radiol. 2005;16:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Song HY, Lee DH, Seo TS, Kim SB, Jung HY, Kim JH, Park SI. Retrievable covered nitinol stents: experiences in 108 patients with malignant esophageal strictures. J Vasc Interv Radiol. 2002;13:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Ghassemi KF, Rodriguez HJ, Vesga L, Stewart L, McQuaid KR, Shah JN. Endoscopic treatment of Boerhaave syndrome using a removable self-expandable plastic stent. J Clin Gastroenterol. 2007;41:863-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Shim CS, Cho JY, Jung IS, Ryu CB, Hong SJ, Kim JO, Lee JS, Lee MS, Kim BS. Through-the-scope double colonic stenting in the management of inoperable proximal malignant colonic obstruction: a pilot study. Endoscopy. 2004;36:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Catalano O, De Bellis M, Sandomenico F, de Lutio di Castelguidone E, Delrio P, Petrillo A. Complications of biliary and gastrointestinal stents: MDCT of the cancer patient. AJR Am J Roentgenol. 2012;199:W187-W196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Cotton PB. Duodenoscopic placement of biliary prostheses to relieve malignant obstructive jaundice. Br J Surg. 1982;69:501-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | de Gregorio MA, Laborda A, Tejero E, Miguelena JM, Carnevale FC, de Blas I, Gimenez M, Maynar M, D'Agostino H. Ten-year retrospective study of treatment of malignant colonic obstructions with self-expandable stents. J Vasc Interv Radiol. 2011;22:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Cézé N, Charachon A, Locher C, Aparicio T, Mitry E, Barbieux JP, Landi B, Dorval E, Moussata D, Lecomte T. Safety and efficacy of palliative systemic chemotherapy combined with colorectal self-expandable metallic stents in advanced colorectal cancer: A multicenter study. Clin Res Hepatol Gastroenterol. 2016;40:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Bielawska B, Hookey LC, Jalink D. Large-diameter self-expanding metal stents appear to be safe and effective for malignant colonic obstruction with and without concurrent use of chemotherapy. Surg Endosc. 2010;24:2814-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 89. | Huibregtse K, Haverkamp HJ, Tytgat GN. Transpapillary positioning of a large 3.2 mm biliary endoprosthesis. Endoscopy. 1981;13:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Baron TH Sr, Davee T. Endoscopic management of benign bile duct strictures. Gastrointest Endosc Clin N Am. 2013;23:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Mangiavillano B, Pagano N, Baron TH, Luigiano C. Outcome of stenting in biliary and pancreatic benign and malignant diseases: A comprehensive review. World J Gastroenterol. 2015;21:9038-9054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 92. | Giri S, Jearth V, Sundaram S. Covered Self-Expanding Metal Stents Versus Multiple Plastic Stents for Benign Biliary Strictures: An Updated Meta-Analysis of Randomized Controlled Trials. Cureus. 2022;14:e24588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Pozsár J, Sahin P, László F, Forró G, Topa L. Medium-term results of endoscopic treatment of common bile duct strictures in chronic calcifying pancreatitis with increasing numbers of stents. J Clin Gastroenterol. 2004;38:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Costamagna G, Tringali A, Mutignani M, Perri V, Spada C, Pandolfi M, Galasso D. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Baillie J. Clinical trial report: endoscopic treatment of postoperative bile duct strictures using multiple stents: long-term results. Curr Gastroenterol Rep. 2011;13:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Rao HB, Prakash A, Sudhindran S, Venu RP. Biliary strictures complicating living donor liver transplantation: Problems, novel insights and solutions. World J Gastroenterol. 2018;24:2061-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 97. | Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, Sundaram V, Tokar J, Adams RB, Yeaton P. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? Gastrointest Endosc. 2008;67:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 98. | Sauer P, Chahoud F, Gotthardt D, Stremmel W, Weiss KH, Büchler M, Schemmer P, Weitz J, Schaible A. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Haapamäki C, Kylänpää L, Udd M, Lindström O, Grönroos J, Saarela A, Mustonen H, Halttunen J. Randomized multicenter study of multiple plastic stents vs. covered self-expandable metallic stent in the treatment of biliary stricture in chronic pancreatitis. Endoscopy. 2015;47:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 100. | Kaffes AJ, Liu K. Fully covered self-expandable metal stents for treatment of benign biliary strictures. Gastrointest Endosc. 2013;78:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Canena J, Liberato M, Meireles L, Marques I, Romão C, Coutinho AP, Neves BC, Veiga PM. A non-randomized study in consecutive patients with postcholecystectomy refractory biliary leaks who were managed endoscopically with the use of multiple plastic stents or fully covered self-expandable metal stents (with videos). Gastrointest Endosc. 2015;82:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Poley JW, Cahen DL, Metselaar HJ, van Buuren HR, Kazemier G, van Eijck CH, Haringsma J, Kuipers EJ, Bruno MJ. A prospective group sequential study evaluating a new type of fully covered self-expandable metal stent for the treatment of benign biliary strictures (with video). Gastrointest Endosc. 2012;75:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Lee DK, Jahng JH. Alternative methods in the endoscopic management of difficult common bile duct stones. Dig Endosc. 2010;22 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, Gyokeres T, Hafner M, Halttunen J, Hassan C, Lopes L, Papanikolaou IS, Tham TC, Tringali A, van Hooft J, Williams EJ. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 381] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 105. | Hong WD, Zhu QH, Huang QK. Endoscopic sphincterotomy plus endoprostheses in the treatment of large or multiple common bile duct stones. Dig Endosc. 2011;23:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Chinnery GE, Krige JE, Bornman PC, Bernon MM, Al-Harethi S, Hofmeyr S, Banderker MA, Burmeister S, Thomson SR. Endoscopic management of bile leaks after laparoscopic cholecystectomy. S Afr J Surg. 2013;51:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Jain V, Yeasted N, Pooran N. Necessity of a repeat cholangiogram during biliary stent removal after postcholecystectomy bile leak. Can J Gastroenterol. 2012;26:701-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, Talamini MA, Pitt HA, Coleman J, Sauter PA, Cameron JL. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786-92; discussion 793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 109. | Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010;71:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 110. | Salameh H, DiMaio CJ. Endoscopic Retrograde Cholangiopancreatography and Endoscopic Ultrasound-Guided Gallbladder Drainage. Gastrointest Endosc Clin N Am. 2019;29:293-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Jang JW, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc. 2011;74:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 112. | Ogura T, Nishioka N, Yamada M, Yamada T, Higuchi K. EUS-Guided Gallbladder Drainage Using an Improved Self-Expandable Covered Metal Stent with Anti-Stent Migration System (with Video). Dig Dis. 2021;39:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Simmons DT, Petersen BT, Gostout CJ, Levy MJ, Topazian MD, Baron TH. Risk of pancreatitis following endoscopically placed large-bore plastic biliary stents with and without biliary sphincterotomy for management of postoperative bile leaks. Surg Endosc. 2008;22:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | ASGE Technology Assessment Committee; Pfau PR, Pleskow DK, Banerjee S, Barth BA, Bhat YM, Desilets DJ, Gottlieb KT, Maple JT, Siddiqui UD, Tokar JL, Wang A, Song LM, Rodriguez SA. Pancreatic and biliary stents. Gastrointest Endosc. 2013;77:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 115. | ERCP Group; Chinese Society of Digestive Endoscopology; Biliopancreatic Group, Chinese Association of Gastroenterologist and Hepatologist; National Clinical Research Center for Digestive Diseases. [Chinese guidelines for ERCP (2018)]. Zhonghua Nei Ke Za Zhi. 2018;57:772-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 116. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, Shaw MJ, Snady HW, Erickson RV, Moore JP, Roel JP. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 835] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 117. | Costamagna G, Alevras P, Palladino F, Rainoldi F, Mutignani M, Morganti A. Endoscopic pancreatic stenting in pancreatic cancer. Can J Gastroenterol. 1999;13:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Somogyi L, Chuttani R, Croffie J, DiSario J, Liu J, Mishkin DS, Shah R, Tierney W, Wong Kee Song LM, Petersen BT. Biliary and pancreatic stents. Gastrointest Endosc. 2006;63:910-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2010;42:842-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 120. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 121. | Smithline A, Silverman W, Rogers D, Nisi R, Wiersema M, Jamidar P, Hawes R, Lehman G. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest Endosc. 1993;39:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | Delhaye M, Arvanitakis M, Bali M, Matos C, Devière J. Endoscopic therapy for chronic pancreatitis. Scand J Surg. 2005;94:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Cantù P, Parzanese I, Balassone V, Di Sario A, Soggiu F, Lombardi G, Barbaro F, Pisani A, Baldan A, Cariani G, Boarino V, Fasoli A, Bertani H, Forti E, Bulajic M, Ghinolfi D, Nadal E, Cerofolini A, Barresi L, Catalano G, Stroppa I, Traini S, Mazzaferro V, Cipolletta L, Tringali A, Costamagna G, Ravelli P, Bazzoli F, Merighi A, Parodi MC, Conigliaro R, Mutignani M, Zilli M, Filipponi F, Fantin A, Rodella L, Tarantino I, Traina M, Salizzoni M, Rosa R, Malinverno F, Invernizzi F, Manini MA, Donato MF, Colombo M, Conte D, Rossi G, Penagini R. Management of biliary anastomotic strictures after liver transplantation (BASALT study): A nationwide Italian survey. Liver Transpl. 2017;23:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Kapoor BS, Mauri G, Lorenz JM. Management of Biliary Strictures: State-of-the-Art Review. Radiology. 2018;289:590-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 125. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 975] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 126. | Rutgeerts P. Review article: recurrence of Crohn's disease after surgery - the need for treatment of new lesions. Aliment Pharmacol Ther. 2006;24 Suppl 3:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Singh VV, Draganov P, Valentine J. Efficacy and safety of endoscopic balloon dilation of symptomatic upper and lower gastrointestinal Crohn's disease strictures. J Clin Gastroenterol. 2005;39:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 128. | Thomas-Gibson S, Brooker JC, Hayward CM, Shah SG, Williams CB, Saunders BP. Colonoscopic balloon dilation of Crohn's strictures: a review of long-term outcomes. Eur J Gastroenterol Hepatol. 2003;15:485-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 129. | Thienpont C, D'Hoore A, Vermeire S, Demedts I, Bisschops R, Coremans G, Rutgeerts P, Van Assche G. Long-term outcome of endoscopic dilatation in patients with Crohn's disease is not affected by disease activity or medical therapy. Gut. 2010;59:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 130. | Suzuki N, Saunders BP, Thomas-Gibson S, Akle C, Marshall M, Halligan S. Colorectal stenting for malignant and benign disease: outcomes in colorectal stenting. Dis Colon Rectum. 2004;47:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 131. | Wholey MH, Levine EA, Ferral H, Castaneda-Zuniga W. Initial clinical experience with colonic stent placement. Am J Surg. 1998;175:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Branche J, Attar A, Vernier-Massouille G, Bulois P, Colombel JF, Bouhnik Y, Maunoury V. Extractible self-expandable metal stent in the treatment of Crohn's disease anastomotic strictures. Endoscopy. 2012;44 Suppl 2:E325-E326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Rodrigues C, Oliveira A, Santos L, Pires E, Deus J. Biodegradable stent for the treatment of a colonic stricture in Crohn's disease. World J Gastrointest Endosc. 2013;5:265-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 134. | Loras C, Pérez-Roldan F, Gornals JB, Barrio J, Igea F, González-Huix F, González-Carro P, Pérez-Miranda M, Espinós JC, Fernández-Bañares F, Esteve M. Endoscopic treatment with self-expanding metal stents for Crohn’s disease strictures. Aliment Pharmacol Ther. 2012;36:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 135. | van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A; European Society of Gastrointestinal Endoscopy. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46:990-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 136. | Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, Paciorek CJ, Farzadfar F, Riley L, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 598] [Cited by in RCA: 616] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 137. | Tsai YN, Wang HP, Huang CK, Chang PC, Lin IC, Tai CM. Endoluminal stenting for the management of leak following sleeve gastrectomy and loop duodenojejunal bypass with sleeve gastrectomy. Kaohsiung J Med Sci. 2018;34:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 138. | Puig CA, Waked TM, Baron TH Sr, Wong Kee Song LM, Gutierrez J, Sarr MG. The role of endoscopic stents in the management of chronic anastomotic and staple line leaks and chronic strictures after bariatric surgery. Surg Obes Relat Dis. 2014;10:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | Larsen M, Kozarek R. Therapeutic endoscopy for the treatment of post-bariatric surgery complications. World J Gastroenterol. 2022;28:199-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (5)] |
| 140. | Eisendrath P, Deviere J. Major complications of bariatric surgery: endoscopy as first-line treatment. Nat Rev Gastroenterol Hepatol. 2015;12:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 141. | Bhayani NH, Swanström LL. Endoscopic therapies for leaks and fistulas after bariatric surgery. Surg Innov. 2014;21:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 142. | Scavone G, Caltabiano G, Inì C, Castelli F, Falsaperla D, Basile A, Piazza L, Scavone A. Radiological stent placement of post sleeve gastrectomy leak: efficacy, imaging features and post-procedure complications. Heliyon. 2022;8:e08857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 143. | Moon SH, Kim MH, Park DH, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 144. | Lee TH, Moon JH, Lee YN, Jo SJ, Park JK, Yang JK, Cha SW, Cho YD, Park SH. Efficacy of a modified short fully covered self-expandable metal stent for perihilar benign biliary strictures. J Gastroenterol Hepatol. 2021;36:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 145. | Park DH, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, Park SH, Lee SK, Kim MH, Kim SJ. Anchoring flap vs flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011;73:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 146. | Lee DK, Kim HS, Kim KS, Lee WJ, Kim HK, Won YH, Byun YR, Kim MY, Baik SK, Kwon SO. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointest Endosc. 2005;61:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 147. | Jang SI, Kim JH, Kim M, Yang S, Jo EA, Lee JW, Na K, Kim JM, Jeong S, Lee DH, Lee DK. Porcine feasibility and safety study of a new paclitaxel-eluting biliary stent with a Pluronic-containing membrane. Endoscopy. 2012;44:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 148. | Suk KT, Kim JW, Kim HS, Baik SK, Oh SJ, Lee SJ, Kim HG, Lee DH, Won YH, Lee DK. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: multicenter pilot study. Gastrointest Endosc. 2007;66:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 149. | Moon S, Yang SG, Na K. An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of gastrointestinal cancer and related stenosis. Biomaterials. 2011;32:3603-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 150. | Arafat M, Song Y, Brewer K, Fouladian P, Parikh A, Albrecht H, Blencowe A, Garg S. Pharmaceutical Development of 5-Fluorouracil-Eluting Stents for the Potential Treatment of Gastrointestinal Cancers and Related Obstructions. Drug Des Devel Ther. 2021;15:1495-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 151. | Bergquist H, Johnsson E, Nyman J, Rylander H, Hammerlid E, Friesland S, Ejnell H, Lundell L, Ruth M. Combined stent insertion and single high-dose brachytherapy in patients with advanced esophageal cancer--results of a prospective safety study. Dis Esophagus. 2012;25:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 152. | Guo JH, Teng GJ, Zhu GY, He SC, Deng G, He J. Self-expandable stent loaded with 125I seeds: feasibility and safety in a rabbit model. Eur J Radiol. 2007;61:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 153. | Koo T, Park HJ, Kim K. Radiation therapy for extrahepatic bile duct cancer: Current evidences and future perspectives. World J Clin Cases. 2019;7:1242-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 154. | Liu Y, Liu JL, Cai ZZ, Lu Z, Gong YF, Wu HY, Man XH, Jin ZD, Li ZS. A novel approach for treatment of unresectable extrahepatic bile duct carcinoma: design of radioactive stents and an experimental trial in healthy pigs. Gastrointest Endosc. 2009;69:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 155. | Shim CS, Jung IS, Cheon YK, Ryu CB, Hong SJ, Kim JO, Cho JY, Lee JS, Lee MS, Kim BS. Management of malignant stricture of the esophagogastric junction with a newly designed self-expanding metal stent with an antireflux mechanism. Endoscopy. 2005;37:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 156. | Li XA, Chibani O, Greenwald B, Suntharalingam M. Radiotherapy dose perturbation of metallic esophageal stents. Int J Radiat Oncol Biol Phys. 2002;54:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 157. | Schoppmeyer K, Golsong J, Schiefke I, Mössner J, Caca K. Antireflux stents for palliation of malignant esophagocardial stenosis. Dis Esophagus. 2007;20:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 158. | Laasch HU, Marriott A, Wilbraham L, Tunnah S, England RE, Martin DF. Effectiveness of open versus antireflux stents for palliation of distal esophageal carcinoma and prevention of symptomatic gastroesophageal reflux. Radiology. 2002;225:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 159. | Schmidt A, Riecken B, Rische S, Klinger C, Jakobs R, Bechtler M, Kähler G, Dormann A, Caca K. Wing-shaped plastic stents vs. self-expandable metal stents for palliative drainage of malignant distal biliary obstruction: a randomized multicenter study. Endoscopy. 2015;47:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 160. | Vandenplas Y, Hauser B, Devreker T, Urbain D, Reynaert H. A degradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. Endoscopy. 2009;41 Suppl 2:E73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 161. | Mochizuki Y, Saito Y, Tanaka T, Nitta N, Yamada H, Tsujikawa T, Murata K, Fujiyama Y, Andoh A. Endoscopic submucosal dissection combined with the placement of biodegradable stents for recurrent esophageal cancer after chemoradiotherapy. J Gastrointest Cancer. 2012;43:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |