Published online Mar 16, 2023. doi: 10.4253/wjge.v15.i3.177
Peer-review started: November 3, 2022
First decision: December 11, 2022
Revised: January 12, 2023
Accepted: March 1, 2023
Article in press: March 1, 2023
Published online: March 16, 2023
Processing time: 132 Days and 9.9 Hours
Endoscopic radiofrequency ablation (ERFA), percutaneous radiofrequency ablation (PRFA), and photodynamic therapy (PDT), when used in conjunction with conventional biliary stenting, have demonstrated a survival benefit in patients with unresectable cholangiocarcinoma.
To compare pooled survival outcomes, adverse event rates, and mean stent patency for those undergoing these procedures.
A comprehensive literature review of published studies and abstracts from January 2011 to December 2020 was performed comparing survival outcomes in patients undergoing ERFA with stenting, biliary stenting alone, PRFA with stenting, and PDT with stenting for unresectable cholangiocarcinoma (CCA).
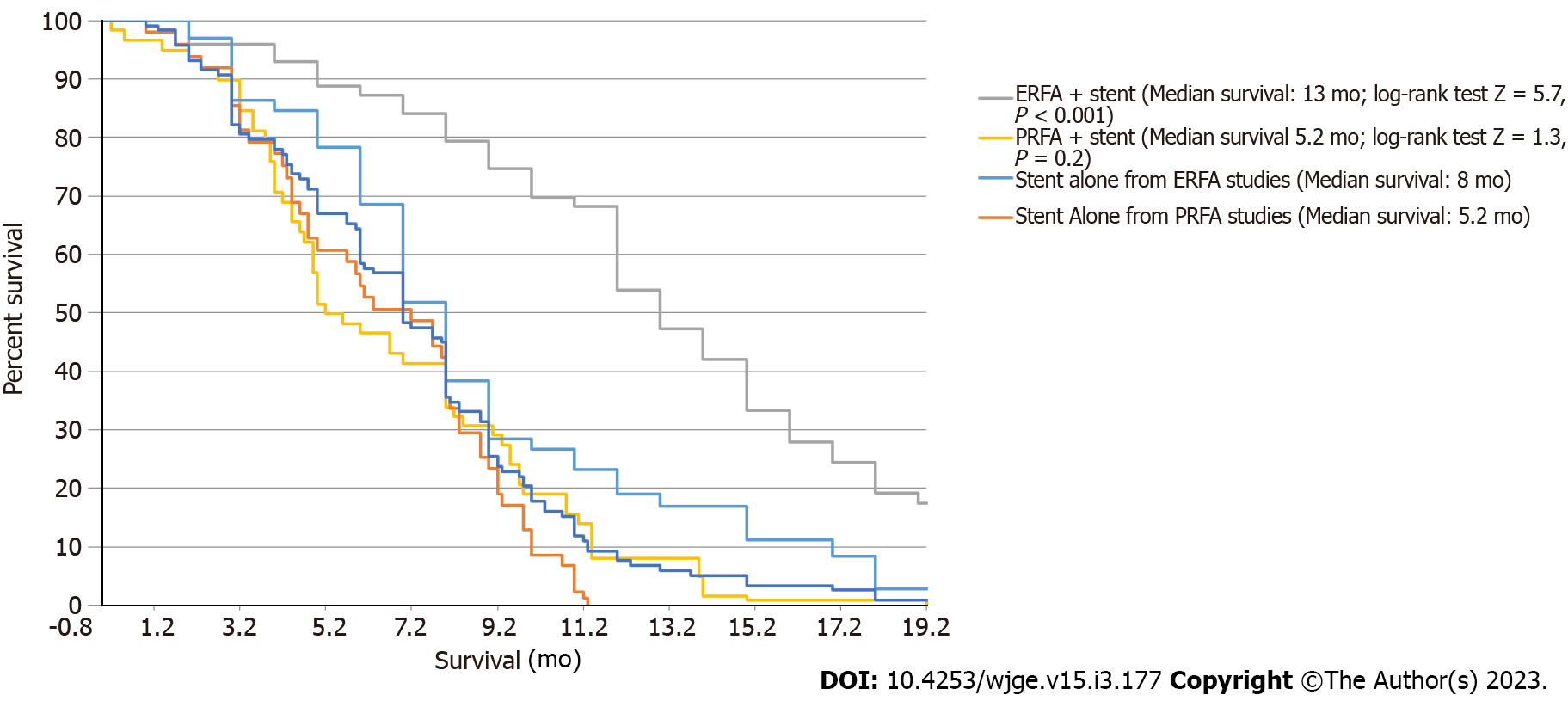
Data from four studies demonstrated a pooled mean survival favoring ERFA as compared to biliary stenting alone (12.0 ± 0.9 mo vs 6.8 ± 0.3 mo, P < 0.001) as well as statistically improved median survival time (13 mo vs 8 mo, P < 0.001). Both ERFA with stenting and PRFA with stenting groups demonstrated statistical superiority to biliary stenting alone (P < 0.001 and P = 0.004, respectively). However, when comparing ERFA to PRFA, pooled data demonstrated overall higher mean survival in the ERFA with stenting cohort as compared to PRFA with stent cohort (12.0 + 0.9 mo vs 8.1 + 2.1 mo, P < 0.0001). Data from two studies demonstrated a pooled median survival favoring ERFA with stenting as compared to PDT with stenting (11.3 mo vs 8.5 mo, P = 0.02).
While further prospective, randomized studies are needed to assess efficacy of ERFA, our meta-analysis demonstrated that this technique offers endoscopists a reasonable palliative method by which to treat patients with unresectable CCA that results in longer survival as compared to biliary stenting alone, percutaneous radiofrequency ablation with biliary stenting, and PDT with biliary stenting as well as an acceptable adverse event profile based on available published data.
Core Tip: Endoscopic radiofrequency ablation offers endoscopists a reasonable palliative method by which to treat patients with unresectable cholangiocarcinoma that results in longer survival as compared to biliary stenting alone, percutaneous radiofrequency ablation, with biliary stenting, and photodynamic therapy with biliary stenting.
- Citation: Rebhun J, Shin CM, Siddiqui UD, Villa E. Endoscopic biliary treatment of unresectable cholangiocarcinoma: A meta-analysis of survival outcomes and systematic review. World J Gastrointest Endosc 2023; 15(3): 177-190
- URL: https://www.wjgnet.com/1948-5190/full/v15/i3/177.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i3.177
Cholangiocarcinoma (CCA) is a primary cancer of the bile ducts accounting for 15% of primary hepatic malignancies and nearly 3% of malignant gastrointestinal tumors. 90% of CCA are extrahepatic (perihilar or main bile duct), while the remaining 10% are intrahepatic[1-3]. Due to location and delayed onset of symptoms, CCA has a poor prognosis with 5-year survival rates of 2%-25% and median survival of 3-6 mo for unresectable cancers[1,4]. 20%-30% of cholangiocarcinoma cases are surgically resectable, leaving the majority of CCA patients with only palliative options, namely, systemic chemotherapy and relief of biliary obstruction through surgical, percutaneous, and endoscopic approaches. The complex molecular landscape of cholangiocarcinoma, however, has limited the effectiveness of systemic chemotherapy in the treatment of unresectable cancer[5,6]. As a result of poor chemotherapeutic options, the mainstay of care for these patients with unresectable CCA revolves around endoscopic retrograde cholangiopancreatography (ERCP), interventional radiologic, or endoscopic ultrasound (EUS)-guided approaches for biliary decompression with biliary stenting and/or percutaneous drainage. While in the majority of cases these approaches are technically feasible and particularly effective at relieving biliary obstruction, the life-prolonging effects of these interventions remain poor, and adverse events, such as stent occlusion and cholangitis, limit their overall effectiveness[7,8].
Photodynamic therapy (PDT) is a well-studied, ablative technique resulting in cellular apoptosis or necrosis in cells that absorb a photosensitizer, an agent activated by a specific wavelength of light[9,10]. PDT protocols for CCA involve a two-stage treatment consisting of systemic administration of the photosensitizing agent (that is preferentially absorbed by pre-malignant and malignant tissue) followed 48 to 96 h later with transpapillary intra-biliary placement of a laser-emitting diode placed into the bile duct via cholangioscopy or ERCP. This diode, when activated, emits a wavelength of 630 nanometers (nm), and when directed towards cells that have absorbed the photosensitizer, results in cell death and necrosis of the target tissue. In a recent meta-analysis of ten studies assessing outcomes of PDT combined with biliary stenting compared to conventional biliary stenting alone, survival in the PDT group was 413 d, which was statistically superior to the 183 d for patients who underwent biliary stenting alone[10].
The limitations of this technology involve the two-stage approach and the resulting phototoxicity of the skin from the photosensitizer (lasting 4-6 wk in decreasing intensity), occurring in 0%-25% of patients undergoing PDT with meta-analytic data demonstrating a photosensitivity rate of 10.5%[9-15]. To minimize the risk of this adverse event, most protocols requires the patient to take significant measures to prevent any exposure to light following administration of the photosensitizer. Other reported adverse events reported include cholangitis and hepatic abscess.
Radiofrequency ablation (RFA) is a technology that delivers thermal energy via a catheter or probe to malignant tissue, resulting in locoregional coagulative necrosis and cellular death. RFA has been previously used successfully via percutaneous (PRFA) or intraoperative routes for the treatment of other solid organ tumors[16]. However, there is limited data available evaluating the role of endoscopic biliary RFA (ERFA) and PRFA as palliative measures in patients with unresectable cholangiocarcinoma. Our meta-analysis aims to evaluate survival outcomes of ERFA with biliary stenting compared with both the conventional stent-only approach and PRFA with stenting in the setting of unresectable CCA.
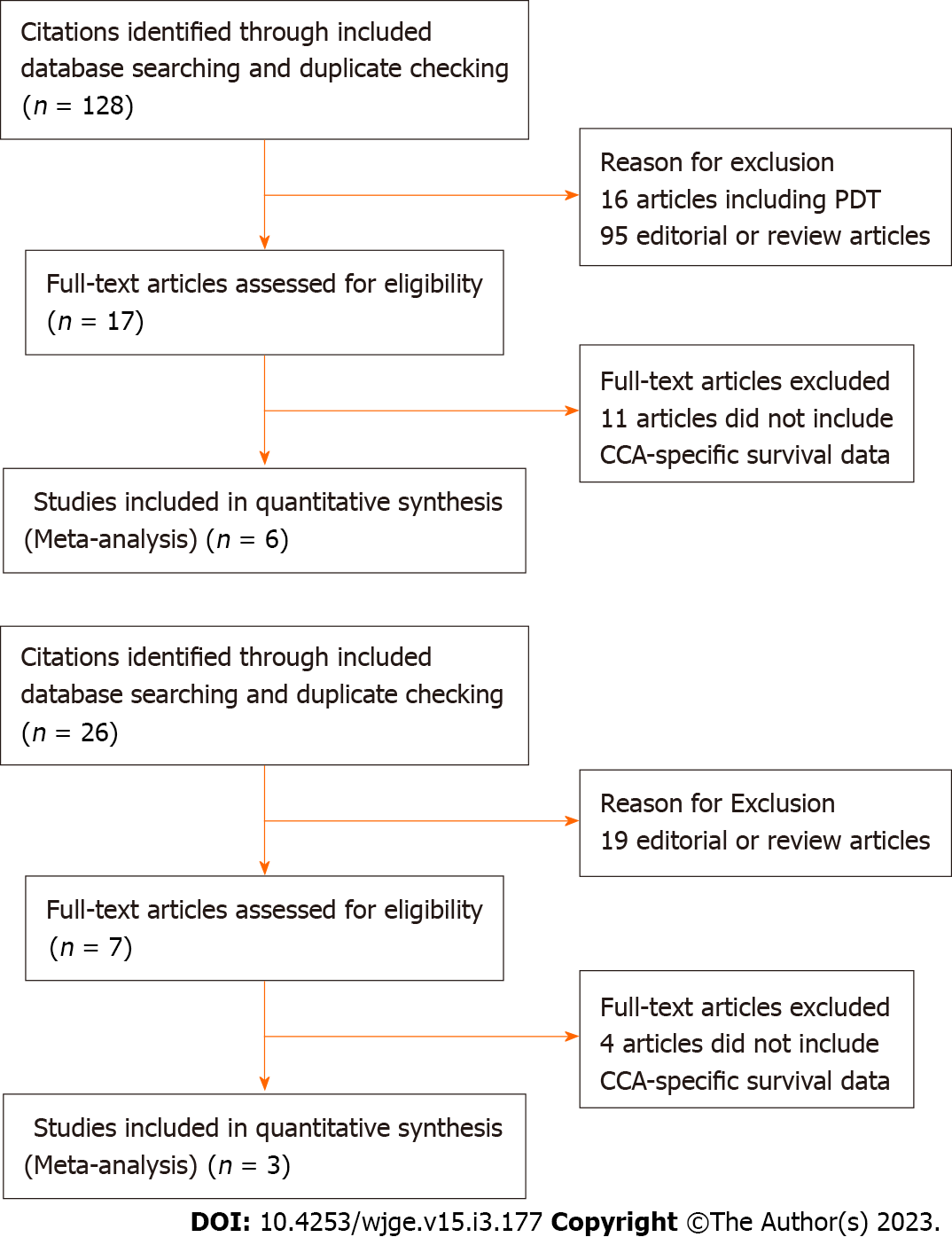
A comprehensive literature search was conducted querying the PubMed, EMBASE, and Cochrane databases from January 2011 to December 2020. Keywords in our search included: “endoscopic radiofrequency ablation” and “cholangiocarcinoma”. In compiling studies assessing percutaneous radiofrequency ablation, the keywords in our search included: “percutaneous radiofrequency ablation” and “cholangiocarcinoma”. In compiling studies assessing photodynamic therapy, the keywords in our search included: “cholangiocarcinoma” and “photodynamic therapy.” The connector word “AND” was used to capture articles that were pertinent to our study. Reference articles were analyzed multiple authors for use in our initial inclusion. Our study was limited to articles published after the 2011 pilot study documenting the initial use of endoscopic radiofrequency ablation in human subjects[17]. Articles eligible for inclusion were limited to published retrospective (case-control studies) or prospective studies (randomized controlled trials) in the English language, conducted on human subjects. Additionally, studies included must have assessed both populations of interest with the intervention provided under similar medical conditions. Exclusion criteria included: Systematic reviews and/or meta-analyses; opinion papers; editorials; studies in which a contingency of data could not be extrapolated to generate the targeted outcome of survival duration; studies in which the patients underwent previous surgical intervention; studies in which other malignancies resulting in biliary obstruction (namely, pancreatic adenocarcinoma or ampullary carcinoma) were included, particularly if a contingency of data could not be extrapolated to generate the targeted conclusions or outcomes in cholangiocarcinoma subgroups. PRISMA flow charts (Figures 1A and B) were compiled to illustrate the results of our literature search with an additional detailed search strategy included as Supple
Three authors (Rebhun J, Shin CM, and Villa E) independently reviewed each article yielded from the above search strategy. Full text of the articles was then assessed to determine if inclusion criteria were met. Any missing or unclear data resulted in an attempt to contact the original author with relevant questions. Data pulled from each article included the following: Author and year of the article; Origin of the study; Type of study conducted; Subgroup total population; Patient age and gender distribution; Mean survival in months; Median survival in months; Mean stent patency in months; Adverse Events; Chemotherapy status.
Data was extracted from articles meeting inclusion criteria and combined to perform a meta-analysis. The primary objective was to compare mortality outcomes in patients undergoing endoscopic RFA with biliary stenting (henceforth to be referred to as the “ERFA” subgroup) to those undergoing endoscopic stenting alone as well as to those undergoing percutaneous RFA with biliary stenting (henceforth to be referred to as the “PRFA” subgroup). Secondary outcomes included duration of stent patency and rates of adverse events between the treatment groups.
To better assess the quality of individual studies, we used the Newcastle-Ottawa scale (NOS) for retrospective case-control studies and the Cochrane tool for risk of bias for randomized controlled trials The NOS uses 3 domains: Selection, comparability, and ascertainment of outcome to award a maximum of 9 total points. A score > 7 indicates a study of good quality. The NOS has been shown to be a marker of individual study quality when using non-randomized studies in meta-analyses[18,19]. NOS scores are reported in the supplementary portion of the article. In order to best evaluate the quality of evidence for each outcome amenable to meta-analysis, we used the Grading of Recommendations, Assessment, Development, and Evaluation system to interpret the clinical implications of our findings.
Continuous variables were reported as mean ± standard deviation. Categorical variables were calculated as frequencies or percentages. Pooled survival data was used to generate Kaplan-Meier survival curves with log-rank test performed to assess for statistically significant differences in survival. Median days of survival was either reported in each study or extrapolated with use of study-specific survival tables and/or curves. Between-study heterogeneity was reported with the I2 statistic with values greater than 50 suggestive of substantial heterogeneity[20]. Categorical data underwent chi-square analysis to ascertain statistically significant differences. Mann-Whitney U-Test was performed to compare mean stent patency. If survival or stent patency was reported in number of days, conversion to number of months was made by dividing number of days by 30.42. Time in months was then rounded to the nearest tenth decimal place. P values were 2-sided and statistical significance was achieved with a P value of < 0.05. Data was analyzed using IBM SPSS Statistics for Macintosh, Version 27.0 (IBM Corp. Armonk, NY, United States). The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Our initial search returned 128 studies. After exclusion of studies that did not satisfy inclusion criteria and/or met no exclusion criteria, four studies[21-24] were included for quantitative and qualitative analyses. Summary of study characteristics (Table 1) as well as procedural and survival outcomes of each study (Table 2) are demonstrated in the corresponding tables.
| Ref. | Country | Study type | Total patients | Mean age | Female gender (%) | Chemotherapy |
| Sampath et al[23], 2016 | United States | Case-Control | 25 | 69.7 | 10 (40.0) | 19 (76) |
| Hu et al[21], 2016 | China | RCT | 63 | 71.4 | 32 (50.8) | - |
| Wu et al[26], 2017 | China | Case-Control | 71 | 57.9 | 28 (39.2) | 59 (83) |
| Cui et al[25], 2017 | China | Case-Control | 39 | 64.7 | 17 (43.5) | 2 (5) |
| Yang et al[22], 2018 | China | RCT | 65 | 63.2 | 32 (49.2) | - |
| Bokemeyer et al[24], 2019 | Germany | Case-Control | 44 | 67 | - | 13 (30) |
| Ref. | Total patients | Technical success | Major adverse events | Mean survival (mean ± SD) | P valuea | ||||
| Stent only | RFA-stent | Stent only | RFA-stent | Stent only | RFA-stent | Stent only | RFA-stent | ||
| Endoscopic | |||||||||
| Sampath et al[23], 2016 | 15 | 10 | - | 100 | 8 | 9 | 4.7 ± 5.5 | 12 ± 5.9 | 0.001 |
| Hu et al[21], 2016 | 31 | 32 | - | - | 22 | 26 | 5.7 ± 0.5 | 10.4 ± 1.2 | 0.001 |
| Yang et al[22], 2018 | 33 | 32 | 100 | 100 | 3 | 2 | 8.3 ± 0.5 | 13.2 ± 0.6 | < 0.001 |
| Bokemeyer et al[24], 2019 | 22 | 20 | 100 | 100 | 10 | 4 | 7.4 ± 0.9 | 11.4 ± 1.9 | 0.046 |
| Percutaneous | |||||||||
| Wu et al[26], 2017 | 36 | 35 | - | 100 | 5 | 0 | 6.5 ± 2.6 | 8.4 ± 2.3 | 0.80 |
| Cui et al[25], 2017 | 14 | 25 | - | - | - | - | 4.5 ± 2.1 | 6.7 ± 5.3 | 0.30 |
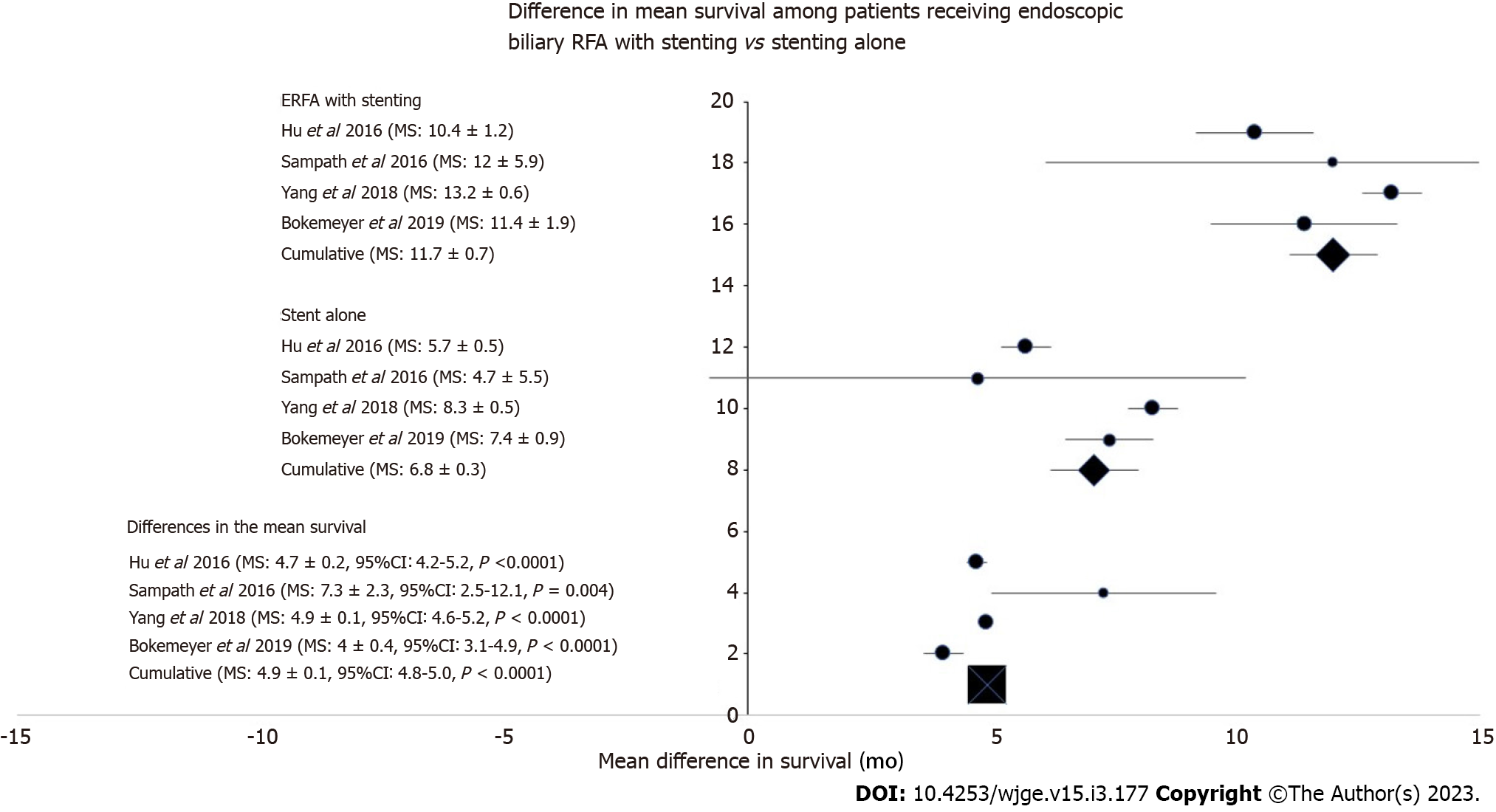
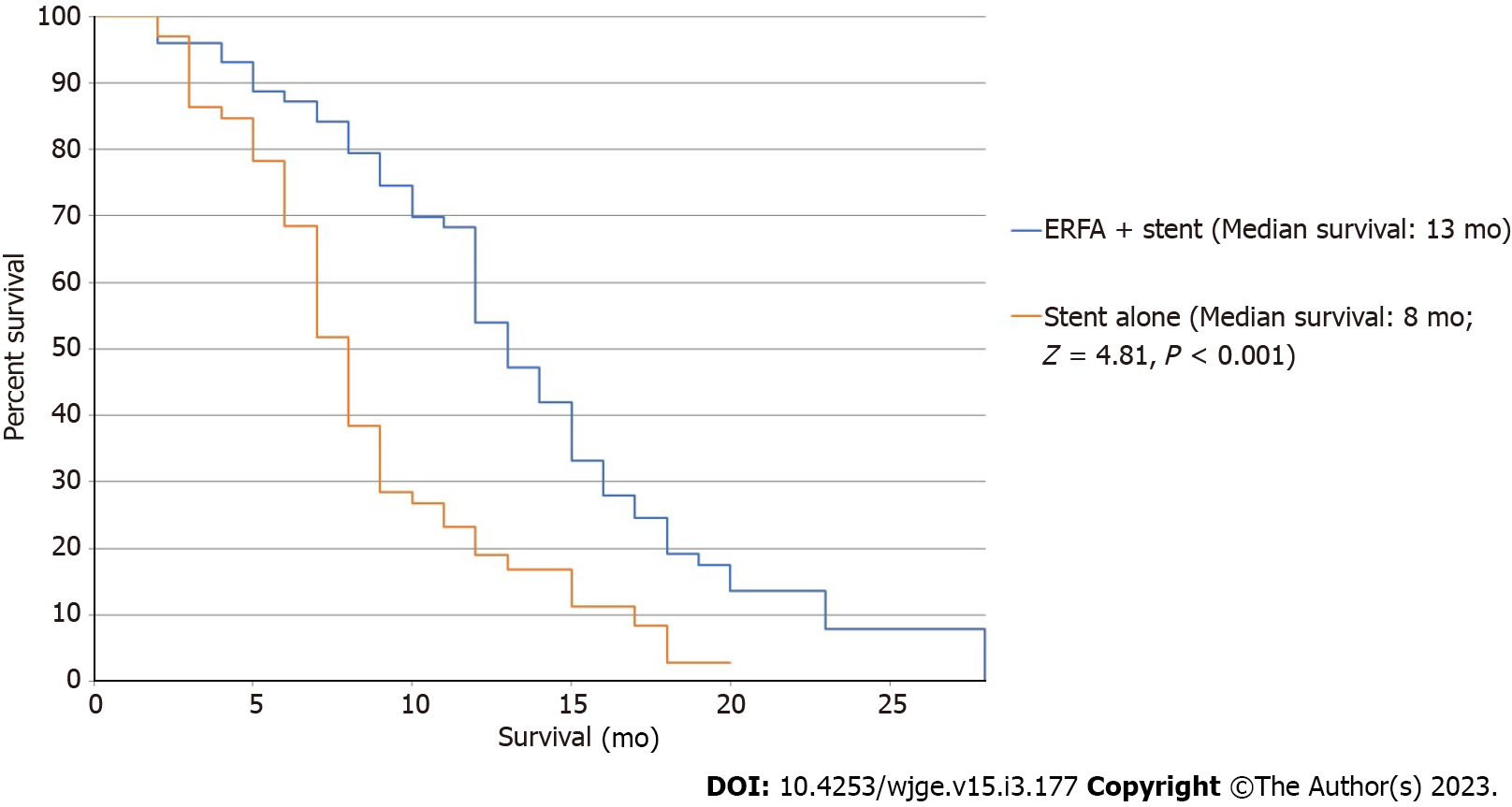
Patients in the ERFA cohort had a pooled mean survival time of 12.0 ± 0.9 mo (I2 = 37.0) while patients undergoing stenting alone had a mean survival time of 6.8 ± 0.3 mo (I2 = 78.4). Difference in survival was calculated to be 4.9 ± 0.1 mo and the analysis was associated with minimal heterogeneity (P < 0.001, I2 = 0) (Figure 2). Median survival of the ERFA cohort was calculated to be 13 mo while median survival of the stent only cohort totaled 8 mo with log-rank test performed to suggest a significant difference (P < 0.001, Figure 3).
Two of four studies reported data on stent patency[21,22] (Table 3). Stent patency was not found to be significantly different in the study by Hu et al[21] (P = 0.7); however, stent patency was significantly higher in the ERFA cohort in Yang et al[22] (P = 0.02)[19,20]. Both studies contributed similarly to the pooled analysis with only slightly more patients in the stent only treatment group being represented by Yang et al[22]. Pooled results of the two studies were calculated and demonstrated a mean stent patency in the ERFA with stent group to be 5.9 mo compared to 3.6 mo in the stent only group (P < 0.001). All four studies reported adverse event data and were used in our analysis (Table 4). Biliary stent occlusion was the most frequent adverse event that arose in both treatment groups, however there was no significant difference between ERFA (81%) and stent alone (67.3%, P = 0.148). Cholecystitis data was only reported in the Hu et al[21] and Bokemeyer et al[24] studies; however pooled analysis showed a 12.5% risk for cholecystitis in the ERFA cohort compared with 0% risk in the stent only cohort (P = 0.01). The frequency of hemobilia/bleeding was similar among the two groups (1.5% for both, P = 1.0).
| Adverse event | ERFA-stent n (%) | Stent alonen (%) | P value |
| Biliary stent occlusion | 34 (81.0) | 31 (67.3) | 0.148 |
| Cholangitis | 27 (25.5) | 15 (19.0) | 0.298 |
| Cholecystitis | 8 (12.5) | 0 (0) | 0.010 |
| Pancreatitis | 4 (4.2) | 3 (4.7) | 0.875 |
| Hemobilia/Bleeding | 1 (1.5) | 1 (1.5) | 1.000 |
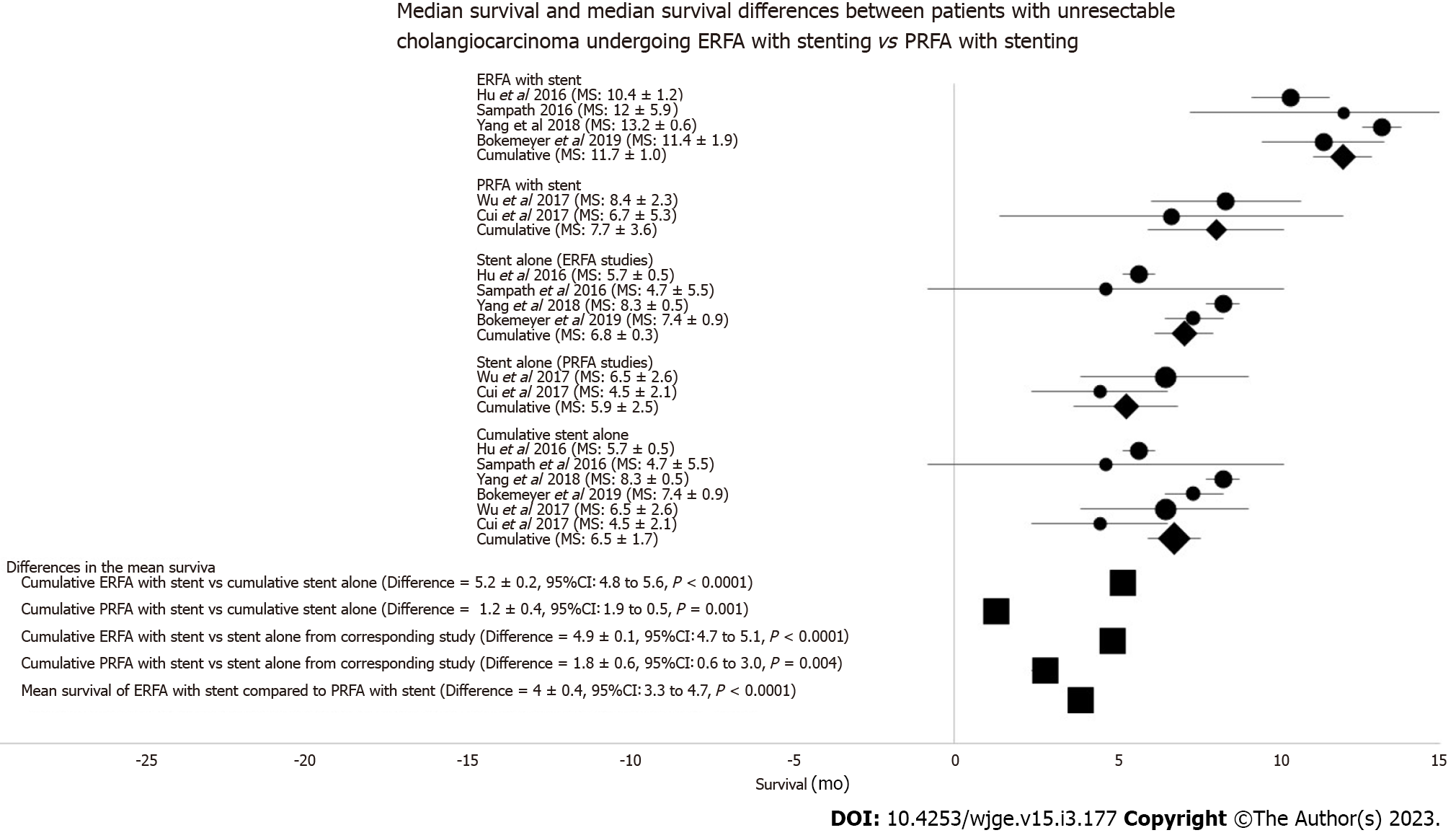
Of the 128 articles in our initial literature search, six studies were included for meta-analytic comparisons of survival between ERFA and PRFA groups[21-26]. From these studies, there were 106 patients that underwent ERFA with concomitant stenting, and 60 patients who underwent PRFA with stenting for unresectable CCA. Comparison control groups included 101 patients who underwent biliary stenting in the ERFA studies and 50 patients who underwent biliary stenting in the PRFA studies.
The ERFA with stent cohort had a mean survival of 12.0 + 0.9 mo (Q = 4.8, I = 37%, Figure 4). The PRFA with stent cohort had a mean survival of 8.1 + 2.1 mo (Q = 0.09, I = 0%, Figure 4). In both ERFA and PRFA studies, mean survival was significantly increased compared to biliary stent alone control groups (P < 0.001 and P = 0.004, respectively). The difference in mean survival among both biliary RFA groups favored ERFA with stenting by 3.9 + 0.2 mo (95%CI: 3.4-4.4, t-test =16.6, P < 0.0001; Figure 4).
The ERFA group had a median survival (Figure 5) of 13 mo compared to the PRFA group median survival of 5.2 mo (log-rank test Z = 5.3, P < 0.0001). Only patients undergoing ERFA with stenting had a significant difference in median survival as compared to the biliary stent alone control group (P < 0.001).
Adverse event data went unreported in the Cu et al[25] study, thus comparison of PRFA adverse event was limited to those of procedures reported by Wu et al[26]. In comparing this study to those of the ERFA cohort, the risk of cholangitis was increased in the ERFA with stent cohort (χ2 = 11.0, P = 0.001).
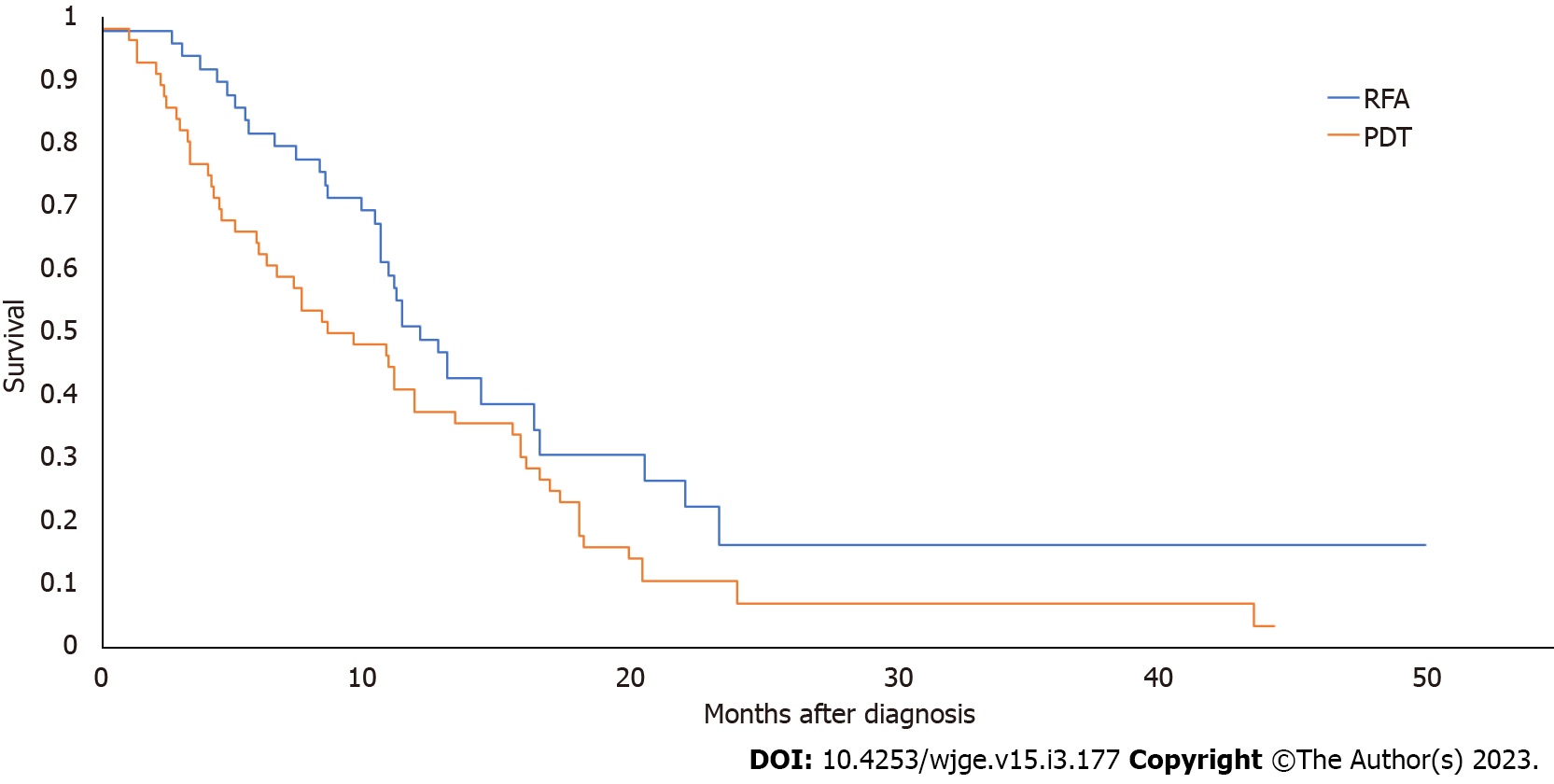
Of the 26 studies identified in our initial literature search, two studies provided data contingent for direct comparison of PDT and ERFA survival in patients with unresectable CCA (Table 5)[13,14]. From these studies, 49 patients underwent ERFA, and 56 underwent PDT (Table 5). All patients underwent concomitant biliary stenting whether via ERCP or via percutaneous transhepatic biliary drainage. Pooled median survival of the ERFA group was 11.3 mo, and median survival of the PDT group was 8.5 mo, a difference that was statistically significant (Figure 6; P = 0.02).
| Demographic data | |||||||||
| Strand et al[13], 2014 | Schmidt et al[15], 2016 | Gao et al[14], 2018 | |||||||
| Number of patients | RFA | 16 | P = 0.1 | RFA | 14 | NA | RFA | ||
| PDT | 32 | PDT | 20 | PDT | |||||
| Gender (male) | RFA | 10 | P = 1.0 | RFA | 8 | P = 0.1 | RFA | ||
| PDT | 19 | PDT | 6 | PDT | |||||
| Age (mean, yr) | RFA | 64.3 ± 11.9 | P = 0.1 | RFA | 73 ± 9 | P = 0.2 | RFA | ||
| PDT | 69.5 ± 13.6 | PDT | 70 ± 12 | PDT | |||||
| Number of treatments | RFA | 28 (mean: 1.2) | P = 0.02 | RFA | 31 | NA | RFA | ||
| PDT | 60 (mean: 2.1) | PDT | 36 | PDT | |||||
| Median survival (month) | RFA | 9.6 | P = 0.8 | RFA | NA | NA | RFA | ||
| PDT | 7.5 | PDT | NA | PDT | |||||
| Lead time to initial treatment (days) | RFA | NS | P = 0.6 | RFA | 300 ± 270 | NA | RFA | ||
| PDT | NS | PDT | 120 ± 90 | PDT | |||||
| Total bilirubin concentration (µmol/dL) | RFA | NA | NA | RFA | 3.3 ± 3.9 | P = 0.7 | RFA | ||
| PDT | NA | PDT | 4.1 ± 6.9 | PDT | |||||
| Tumor location | RFA | Intrahepatic | 1 | P = 0.1 | RFA | Intrahepatic | 1 | P = 0.5 | RFA |
| Hilar | 13 | Hilar | 11 | ||||||
| Distal/Extrahepatic | 2 | Distal/Extrahepatic | 1 | ||||||
| PDT | Intrahepatic | 0 | PDT | Intrahepatic | 3 | PDT | |||
| Hilar | 32 | Hilar | 15 | ||||||
| Distal/Extrahepatic | 0 | Distal/Extrahepatic | 1 | ||||||
| N1 staging | RFA | 7 | P = 0.8 | RFA | 3 | P = 0.4 | RFA | ||
| PDT | 12 | PDT | 2 | PDT | |||||
| M1 staging | RFA | 6 | P = 0.2 | RFA | 2 | P = 0.8 | RFA | ||
| PDT | 6 | PDT | 6 | PDT | |||||
| Stents placed | Total | RFA | 115 | NA | Total | RFA | 29 | NA | Total |
| PDT | 307 | PDT | 44 | ||||||
| Plastic | RFA | 69 | NA | Plastic | RFA | 26 | NA | Plastic | |
| PDT | 264 | PDT | 38 | ||||||
| Total metallic | RFA | 46 | NA | Total metallic | RFA | 3 | NA | Total metallic | |
| PDT | 43 | PDT | 6 | ||||||
| Fully covered | RFA | 17 | NA | Fully covered | RFA | NA | NA | Fully covered | |
| PDT | 14 | PDT | NA | ||||||
| Uncovered | RFA | 29 | NA | Uncovered | RFA | NA | NA | Uncovered | |
| PDT | 29 | PDT | NA | ||||||
| Number of ERCPs | RFA | 91 | NA | RFA | NA | NA | RFA | ||
| PDT | 170 | PDT | NA | PDT | |||||
| Percutaneous transhepatic biliary drainage (PTBD) | RFA | 2 | P = 0.2 | RFA | 2 | P = 0.3 | RFA | ||
| PDT | 10 | PDT | 6 | PDT | |||||
Of the 26 studies identified, three studies provided data contingent for direct comparison of PDT and ERFA adverse events (Table 5)[13-15]. With regard to pooled adverse events among 62 patients who underwent ERFA and 75 patients who underwent PDT, there were statistically higher rates of stent occlusions (22.6% vs 6.7%, P = 0.008) and cholangitis (74% vs 41.3%, P = 0.001) in the ERFA group (Table 6); however, there were increased rates of stent migration (16% vs 4.8%, P = 0.04), moderate or severe post-procedure pain (22.7% vs 4.8%, P = 0.003), and phototoxicity (2.7% vs 0%) in the pooled PDT cohort as compared to the pooled ERFA cohort (Table 6).
| Adverse events | |||
| RFA | PDT | P value | |
| Stent related complications | 17 | 17 | 0.7 |
| Stent occlusion | 14 | 5 | 0.008 |
| Stent migration | 3 | 12 | 0.04 |
| Cholangitis | 46 | 31 | 0.001 |
| Hepatic abscess | 4 | 3 | 0.5 |
| Bleeding | 1 | 1 | 0.9 |
| Moderate/Severe abdominal pain | 3 | 17 | 0.003 |
| Post-ERCP pancreatitis | 3 | 2 | 0.5 |
| Phototoxicity | 0 | 2 | NA |
The study by Strand et al[13] received a score of “9” out of 9 as confounders such as tumor stage, performance status, and number of procedures did not differ among cohorts. While described as a case series, the study from Schmidt and colleagues was largely retrospective and partly prospective. Designation of intervention in the prospective portion was determined by choice of the patient, thus losing a point in selection of the cohorts and receiving a score of “8” out of 9. The study performed by Wu et al[26] received a NOS score of “7” out of 9, as there were no cofounders corrected for. Additionally, the study by Cui et al[25] also received a score of “7” out of 9 because age significantly differed among study groups and was uncorrected for. The study by Bokemeyer et al[24] received a NOS score of “9” out of 9. In this case, confounders were adjusted for by age, extent of disease, the use of endoprostheses, and the application of systemic palliative chemotherapy. The study from Yang and colleagues was assessed using the Cochrane risk of bias tool. While subjects were randomized, patients and interventionalists could not be blinded. Additionally, there was some unclear risk for bias in this study as detailed in Supplementary Figure 1. Two studies that were published only as abstracts were not able to be assessed for bias. Detailed analysis of these scores can be seen in the appendix as Supplementary Table 3.
Although it remains a relatively rare disease, the incidence of CCA continues to increase worldwide. Surgical resection remains the only curative treatment option; however, resection is only an available option in up to 30% of patients diagnosed, likely due to a variety of factors, including delayed diagnosis, which is, in large part, due to late onset of symptoms[27]. As such, for many patients, palliative approaches become the mainstay treatment options.
Our study compiles pooled data from previous investigations to better describe the roles ERFA and PRFA with stenting have in the palliation of unresectable cholangiocarcinoma and ascertain the survival benefits, thereof, while identifying adverse events that could portend poor quality of remaining life.
The meta-analytic outcomes in our study demonstrated a statistically significant improvement in both mean and median survivals when comparing ERFA to endoscopic biliary stenting alone in this cohort of patients with unresectable cholangiocarcinoma. While percutaneous RFA (PRFA) performed by capable Interventional Radiologists leads to improvement in mean survival, median survival is not impacted. While there are no studies assessing direct comparisons between ERFA and PRFA, available data does suggest superiority of ERFA with regard to median survival in these CCA patients, arguing for more widespread implementation of this palliative technique.
Safety concerns have been raised, however, given risk of stent occlusion or migration-with resulting cholangitis or delays in chemotherapy due to ensuing hyperbilirubinemia-as well as the risk of hemobilia and cholecystitis. However, the pooled data of included studies did not reveal an increase in stent occlusion rates, cholangitis, or hemobilia as compared to biliary stenting alone but did demonstrate increased risk of cholecystitis. Subgroup analyses were insufficient to conclude whether reported cholecystitis occurred in those with plastic or metallic biliary stenting. As compared to PRFA, there was an increased risk of reported cholangitis cases. However, given the lack of PRFA adverse event data reported (only one study allowed for analysis), definitive conclusions are difficult to make.
While technically feasible with reasonable safety outcomes, ERFA is an appealing option for palliation in these patients. However, the technique is limited in certain respects to degree of stricture, as severe strictures make passage of the RFA probe difficult and mild strictures may not result in adequate contact of the RFA to achieve adequate ablation. There is also a lack of consensus with regard to the timing of repeat ablation, particularly in those with successful first ablations. Further studies are needed to ascertain the optimal period between procedures as well as endoluminal and clinical parameters that would otherwise warrant repeating or avoiding the procedure.
Given the paucity of comparative studies, this meta-analysis was restricted to a small number of published studies, which could potentially overstate the benefit of the approach. Thirteen articles in our literature review were excluded in this meta-analysis due to a lack of contingency of data to separate CCA patients from those studies with other malignant biliary obstructions (ampullary and pancreatic carcinomas), and another 15 articles were excluded for inclusion of other palliative endotherapies (photodynamic therapy) or included patients in whom a previous surgical intervention was undertaken.
To this point, a recent meta-analysis by Zheng and colleagues suggested that patients undergoing ERFA for malignant biliary obstruction had a pooled survival of 9.6 mo but included all patients with malignant biliary obstruction[28]. Similarly, a separate meta-analysis compared ERFA with biliary stenting and to biliary stenting alone for malignant biliary obstructions found a mean survival of 9.4 mo[29]. While the exact mechanism for prolonged survival is unknown, it has been postulated that the ablative process induces a systemic immune response which is then amplified by immune modulating agents resulting in improved clinical outcomes[30-32].
Our cohort of 94 cumulative patients with unresectable CCA receiving ERFA with stenting demonstrated a median survival of 13 mo. This difference may be explained by the exclusion of other etiologies for malignant biliary obstruction; technique advancement with the availability of improved cholangioscopic visualization of the malignant stricture; patient selection; or other confounders, such as stent selection.
PDT with biliary stenting is another endoscopic approach that has been well-studied as a palliative option for patients with unresectable CCA and has been shown to be superior to biliary stenting alone. While there is a paucity of studies, our meta-analysis demonstrated that in two comparative studies with available relevant contingency data, the median survival with ERFA is statistically superior than in PDT. This difference may be explained by lack of studies comparing the two modalities directly and the need for more study for adequate comparison of survival outcomes.
With lack of available studies, the direction of endoscopic palliative therapy is one that, at present, is largely center-dependent. PDT has the inconvenience of requiring two stages of intervention, one for administration of the photosensitizer and one for the delivery of therapy for tumor necrosis and cell death and also comes with the added inconvenience for the patient of avoiding direct exposure to light due to risk of skin photosensitivity. This is not the case with ERFA, which can be performed as a single procedure. It is worth noting, however, that increased rates of cholangitis and stent occlusion in ERFA cohorts would increase the need for subsequent interventions and increase costs related to repeat procedures, but this is an outcome that must also be studied further. In comparing ERFA with stenting compared to biliary stenting alone, however, there was no statistically significant difference in stent occlusion or cholangitis adverse events, so as a singular modality, safety outcomes are still comparable to biliary stenting alone while offering the benefit of longer survival as compared to biliary stenting. Interestingly, while PDT did have higher rate of stent migration, this may potentially reflect significant decrease in size of the obstructing tumor, which is a desirable outcome; this, however, was not quantified in the comparative studies and is an area for potential investigation.
In any event, endoscopic palliation of unresectable CCA with ERFA has shown significant promise in this patient population, but further studies are needed to assess our specific cohort of patients to further understand palliative, technical, and clinical outcomes, especially as they compare to other palliative therapies that extend beyond conventional biliary stenting alone.
Further prospective studies comparing all therapeutic modalities are needed to best understand their role in the treatment of unresectable cholangiocarcinoma.
Endoscopic radiofrequency ablation with biliary stenting is a promising palliative therapeutic option in patients presenting with unresectable cholangiocarcinoma.
Endoscopic radiofrequency ablation when used in conjunction with biliary stenting showed improved survival benefit when compared to alternative palliative therapies.
This is a comprehensive literature review of studies evaluating survival benefit and other clinical outcomes as it relates to the proposed therapeutic interventions.
To better understand, qualify, and quantify the survival outcomes of endoscopic radiofrequency ablation, percutaneous radiofrequency ablation, and photodynamic therapy in the treatment of unresectable cholangiocarcinoma as it compares to conventional therapy alone.
Our motivation for this study was to better understand alternative approaches to palliative endoscopic intervention for patients with unresectable cholangiocarcinoma.
There is limited data evaluating the clinical outcomes of endoscopic radiofrequency ablation and photodynamic therapy as interventions for unresectable cholangiocarcinoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koutsoumourakis A, Greece; Rahmati M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2055] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 2. | National Cancer Institute. "Cancer stat facts: liver and intrahepatic bile duct cancer." 2020. [DOI] [Full Text] |
| 3. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 4. | Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122:1349-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Massironi S, Pilla L, Elvevi A, Longarini R, Rossi RE, Bidoli P, Invernizzi P. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Rahnemai-Azar AA, Weisbrod AB, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: current management and emerging therapies. Expert Rev Gastroenterol Hepatol. 2017;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Chandrasegaram MD, Eslick GD, Mansfield CO, Liem H, Richardson M, Ahmed S, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc. 2012;26:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc. 2012;76:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Zoepf T. Photodynamic therapy of cholangiocarcinoma. HPB (Oxford). 2008;10:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, Uppu A, Bondalapati N, Duvvuri A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Gao DJ, Hu B, Ye X, Wu J, Wang TT, Xia MX, Sun B. Radiofrequency ablation has comparable overall survival to photodynamic therapy in the treatment of cholangiocarcinoma and ampullary carcinoma. Gastrointest Endosc. 2018;87:AB72 Abstract 340. [DOI] [Full Text] |
| 15. | Schmidt A, Bloechinger M, Weber A, Siveke J, von Delius S, Prinz C, Schmitt W, Schmid RM, Neu B. Short-term effects and adverse events of endoscopically applied radiofrequency ablation appear to be comparable with photodynamic therapy in hilar cholangiocarcinoma. United European Gastroenterol J. 2016;4:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Shah DR, Green S, Elliot A, McGahan JP, Khatri VP. Current oncologic applications of radiofrequency ablation therapies. World J Gastrointest Oncol. 2013;5:71-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses." 2000. [DOI] [Full Text] |
| 19. | Rahmati M, Keshvari M, Mirnasuri S, Yon DK, Lee SW, Il Shin J, Smith L. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis. J Med Virol. 2022;94:5112-5127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 20. | Rahmati M, Malakoutinia F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Physiotherapy. 2021;113:12-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Hu B, Gao DJ, Zhang X, Zhang YC. 121 Endobiliary radiofrequency ablation improve overall survival of Cholangiocarcinoma: A multi-center randomized control study. Gastrointest Endosc. 2016;83:AB126.. [DOI] [Full Text] |
| 22. | Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 23. | Sampath L. Gardner T, Gordon SR. Tu1526 The Effect of Endoscopic Radiofrequency Ablation on Survival in Patients with Unresectable Peri-Hilar Cholangiocarcinoma. Gastrointest Endosc. 2016;83:AB595. [DOI] [Full Text] |
| 24. | Bokemeyer A, Matern P, Bettenworth D, Cordes F, Nowacki TM, Heinzow H, Kabar I, Schmidt H, Ullerich H, Lenze F. Endoscopic Radiofrequency Ablation Prolongs Survival of Patients with Unresectable Hilar Cholangiocellular Carcinoma - A Case-Control Study. Sci Rep. 2019;9:13685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Cui W, Wang Y, Fan W, Lu M, Zhang Y, Yao W, Li J. Comparison of intraluminal radiofrequency ablation and stents vs. stents alone in the management of malignant biliary obstruction. Int J Hyperthermia. 2017;33:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Wu TT, Li WM, Li HC, Ao GK, Zheng F, Lin H. Percutaneous Intraductal Radiofrequency Ablation for Extrahepatic Distal Cholangiocarcinoma: A Method for Prolonging Stent Patency and Achieving Better Functional Status and Quality of Life. Cardiovasc Intervent Radiol. 2017;40:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 28. | Zheng X, Bo ZY, Wan W, Wu YC, Wang TT, Wu J, Gao DJ, Hu B. Endoscopic radiofrequency ablation may be preferable in the management of malignant biliary obstruction: A systematic review and meta-analysis. J Dig Dis. 2016;17:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Sofi AA, Khan MA, Das A, Sachdev M, Khuder S, Nawras A, Lee W. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:944-951.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, Strobel D. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006;12:3716-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Napoletano C, Taurino F, Biffoni M, De Majo A, Coscarella G, Bellati F, Rahimi H, Pauselli S, Pellicciotta I, Burchell JM, Gaspari LA, Ercoli L, Rossi P, Rughetti A. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int J Oncol. 2008;32:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Liu X, Qin S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist. 2019;24:S3-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |