Published online Nov 16, 2023. doi: 10.4253/wjge.v15.i11.649
Peer-review started: September 5, 2023
First decision: September 13, 2023
Revised: September 21, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: November 16, 2023
Processing time: 65 Days and 15.5 Hours
Gas-related complications present a potential risk during transoral endoscopic resection of upper gastrointestinal submucosal lesions. Therefore, the identification of risk factors associated with these complications is essential.
To develop a nomogram to predict risk of gas-related complications following transoral endoscopic resection of the upper gastrointestinal submucosal lesions.
We collected patient data from the First Affiliated Hospital of the Army Medical University. Patients were randomly allocated to training and validation cohorts. Risk factors for gas-related complications were identified in the training cohort using univariate and multivariate analyses. We then constructed a nomogram and evaluated its predictive performance based on the area under the curve, decision curve analysis, and Hosmer-Lemeshow tests.
Gas-related complications developed in 39 of 353 patients who underwent transoral endoscopy at our institution. Diabetes, lesion origin, surgical resection method, and surgical duration were incorporated into the final nomogram. The predictive capability of the nomogram was excellent, with area under the curve values of 0.841 and 0.906 for the training and validation cohorts, respectively.
The ability of our four-variable nomogram to efficiently predict gas-related complications during transoral endoscopic resection enhanced postoperative assessments and surgical outcomes.
Core Tip: This is a retrospective study to create a nomogram that efficiently evaluates the risk of gas-related complications in patients undergoing transoral endoscopic resection of upper gastrointestinal submucosal lessions. Our study excluded upper gastrointestinal malignancies and explored risk factors for gas-related complications during transoral endoscopic resection. Predictive models were developed based on diabetes status, lesion origin layer, operative resection technique, and duration of the operation.
- Citation: Yang J, Chen ZG, Yi XL, Chen J, Chen L. Nomogram to predict gas-related complications during transoral endoscopic resection of upper gastrointestinal submucosal lesions. World J Gastrointest Endosc 2023; 15(11): 649-657
- URL: https://www.wjgnet.com/1948-5190/full/v15/i11/649.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i11.649
Submucosal gastrointestinal lesions, often referred to as subepithelial gastrointestinal lesions (SELs)[1], encompass a range of submucosal stromal tumors, leiomyomas, lipomas, and schwannomas. They also include non-neoplastic lesions such as heterotopic pancreas and cysts[2]. The incidence of SEL in the general population ranges between 0.76%-1.7%[3,4]. Although most lesions are benign[5] and frequently identified during health screens due to abdominal discomfort, vomiting, acid reflux, or anemia, some carry risks of bleeding, obstruction, and potential malignant transformation over time[6]. Hence, treatment approaches must be individualized.
The application of minimally invasive endoscopic techniques has recently increased along with enhanced operative skills among endoscopists. This has resulted in an uptick in the number of gastrointestinal submucosal lesions that are treated endoscopically[7,8]. The repertoire of endoscopic interventions includes high-frequency electrocoagulation resection, endoscopic mucosal resection (EMR), endoscopic submucosal excavation (ESE), endoscopic submucosal dissection (ESD), submucosal tunnelling endoscopic resection (STER), and endoscopic full-thickness resection (EFTR).
Despite many advantages, all endoscopic procedures run the risk of potential complications. A significant proportion of these complications involve the unintended escape of gas outside the digestive tract wall, resulting in gas-related complications such as subcutaneous emphysema, pneumothorax, pneumomediastinum, and pneumoperitoneum. Studies have designated such complications as critical issues in endoscopic surgery because they lead to extended hospital stays and increased socioeconomic burdens on patients[9]. Consequently, we aimed to identify risk factors associated with gas-related complications during transoral endoscopic resection and to develop and validate a clinically useful nomogram.
The First Affiliated Hospital Ethics Committee of the Army Medical University approved the study [Approval ID: (B) KY2023006], and all patients provided written informed consent.
This study included 353 patients [male, 163 (46.2%); female, 190 (53.8%); mean age, 48.12 ± 0.55 year; range, 17-76 year] who underwent transoral endoscopic resection of upper gastrointestinal submucosal lesions at the First Affiliated Hospital of the Army Medical University between July 2012 and June 2022. We randomized the patients into training (n = 247) and validation (n = 106) cohorts in a 7:3 ratio using R software version 4.1.2 (Foundation for Statistical Computing, Vienna, Austria).
The inclusion criteria comprised histologically confirmed diagnosis of upper gastrointestinal submucosal lesions, preoperative endoscopic ultrasonography (EUS) findings indicating the lesion origin layer, and having undergone transoral endoscopic resection at our institution. The exclusion criteria comprised intolerance to general anesthesia, intraoperative emergencies that halted the procedure, or undergoing concurrent endoscopic procedures. The patients were endotracheally intubated after general anesthesia and were grouped according to the presence or absence of gas-related complications.
We characterized subcutaneous emphysema as the finding of gas in subcutaneous tissues. Pneumothorax results from ruptures in the visceral or parietal pleura, leading to air entering the pleural space. Pneumomediastinum arises due to air leaking into the mediastinal space. Pneumoperitoneum can arise from gut perforation or gas entering the peritoneum via the diaphragmatic foramina. Our diagnosis of gas-related complications relied on clinical findings, and computed tomography or postoperative radiographic images acquired within 24 h. We treated subcutaneous emphysema and pneumomediastinum conservatively[10]. Thoracic drainage, laparotomy, perforation repair, or surgical treatment were considered to alleviate pronounced symptoms.
Clinical data comprised age, gender, body mass index, underlying conditions (diabetes, hypertension), disease duration, medical history, and EUS findings. Surgical data comprised the histological category of the lesion, lesion size, surgical duration, and resection method. We categorized the surgical duration as < 1, 1-2, or > 2 h and lesion size based on the largest lesion diameter as ≤ 2.0 or > 2.0 cm. The categories of histological lesions were leiomyomas, stromal tumors, schwannomas, heterotopic pancreas, cysts, and lipomas. The muscle layers where lesions originated were classified as non-intrinsic or intrinsic.
Procedures involved the use of an Olympus Q260-J gastroscope (Olympus Optical Co. Ltd., Tokyo, Japan), a high-frequency electrogenic generator (Erbe Elektromedizin GmbH, Tübingen, Germany) a range of specialized knives, titanium clamps, biopsy forceps, loopers, ligatures, and disposable endoscopic syringes. Patients undergoing transoral endoscopic resection were insufflated with CO2 at pressure of 1 MPa.
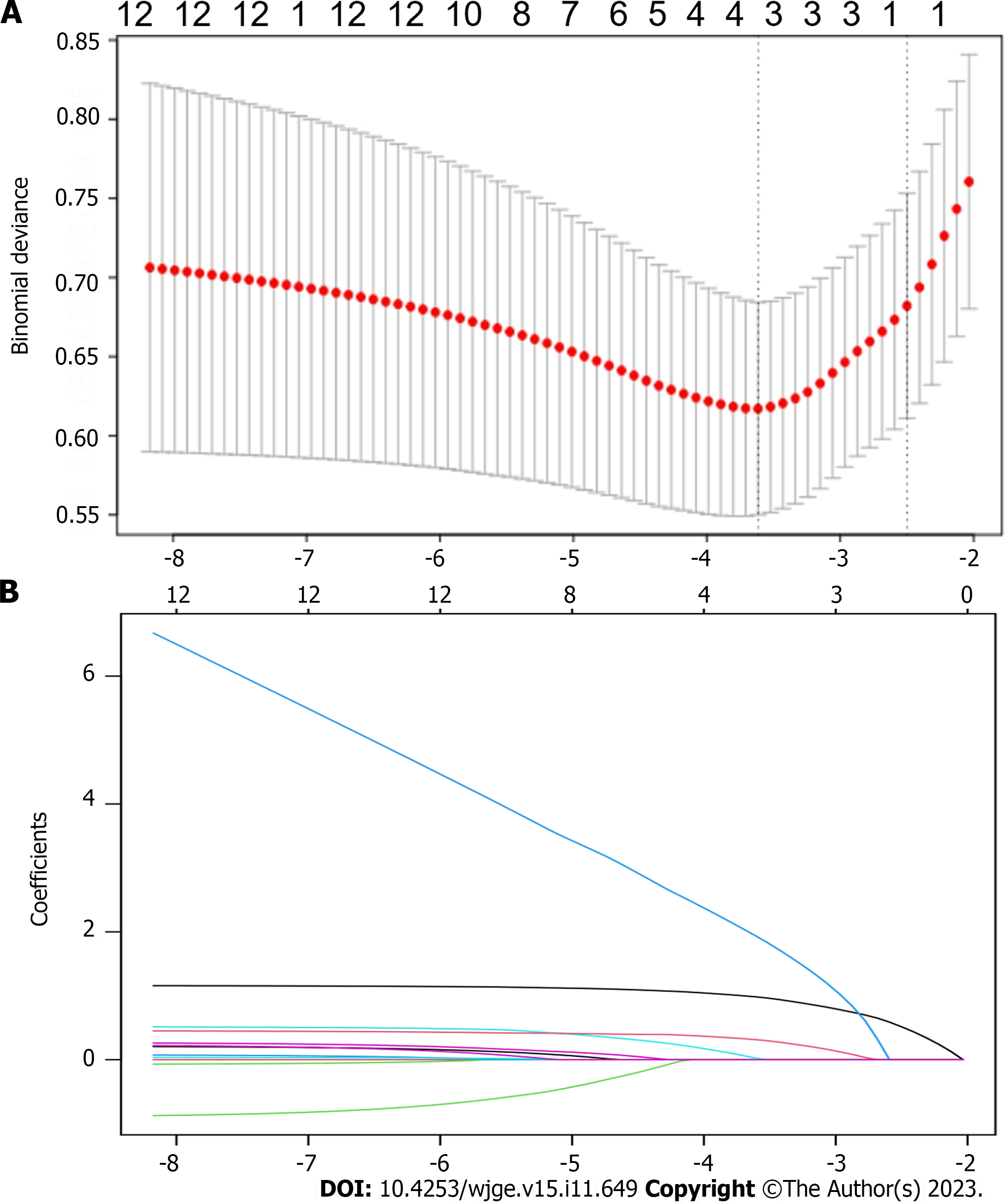
All data were statistically analyzed using R (version 4.1.2) and SPSS version 26.0 (IBM Corp., Armonk, NY, United States) software. Continuous data are presented as means ± SD and were compared between cohorts using ANOVA or t test. Categorical data are presented as frequencies and ratios (%) with a comparative approach using Fisher exact, and χ2 tests. We considered that values with P < 0.05 were statistically significant. Variables with a univariate analysis P < 0.05 were also included in the training cohort. We screened variables and identified factors influencing gas-related complications in transoral endoscopic resection using least absolute shrinkage and selection operator (LASSO) regression. These factors served as predictor variables to calculate risk scores and construct a nomogram. We plotted the receiver operating characteristic curves for the nomogram in the training and validation cohorts and calculated the area under the curve (AUC). We evaluated the predictive power of our model using calibration curves, decision curve analysis, and the Hosmer-Lemeshow test.
Gas-related complications arose in 39 (11.05%) of 353 patients, comprising 22 (6.20%) with subcutaneous emphysema and pneumomediastinum, 20 (5.67%) with pneumoperitoneum, and 4 (1.13%) with pneumothorax. Supplementary Table 1 shows the baseline demographics and characteristics of the patients. Symptoms that were mild in 29 patients with gas-related complications independently resolved within 3-5 d. Ten patients underwent thoracic drainage and perforation repair. Among these complications, 163 and 190 originated from the non-intrinsic and intrinsic muscular layers, respectively.
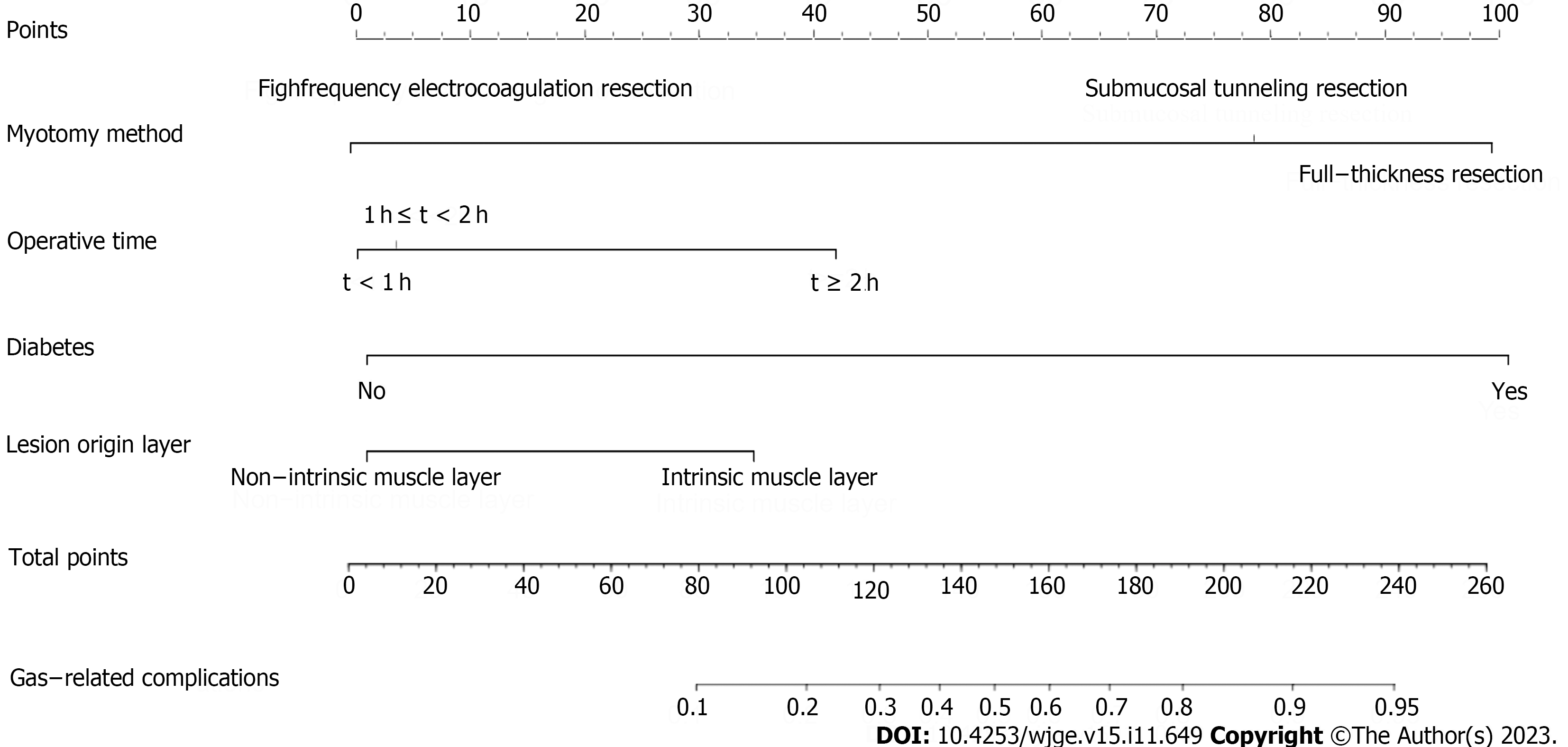
Univariate analysis revealed that histological type, lesion layer of origin, diabetes, lesion size, surgical duration, and resection method significantly influenced the development of gas-related complications (Table 1). The LASSO regression analysis selected the resection method, surgical duration, diabetes, and lesion layer of origin as independent risk factors for gas-related complications during surgery in the training cohort (Figure 1). We then constructed a model based on these variables (Figure 2). The risk scores of patients were calculated by summing the scores of each item. The sum total scores predicted the likelihood of gas-related complications.
| Variables | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Sex | 1.087 (0.512-2.310) | 0.827 | ||
| Age | 1.009 (0.975-1.045) | 0.598 | ||
| Hypertension | 0.267 (0.035-2.045) | 0.203 | ||
| Diabetes | 8.306 (1.135-60.774) | 0.037 | 11.043 (0.921-132.452) | 0.058 |
| Medical history | 1.032 (0.476-2.236) | 0.936 | ||
| Histological type | 0.019 | |||
| Largest lesion diameter | 3.120 (1.483-6.565) | 0.003 | ||
| Lesion origin layer | 5.011 (1.855-13.534) | 0.001 | 1.774 (0.583-5.394) | 0.028 |
| Resection method | < 0.001 | < 0.001 | ||
| Electrocoagulation | 11.326 (3.025-42.403) | 10.296 (2.714-39.059) | ||
| Full-thickness | 24.615 (6.802-89.074) | 23.167 (6.367-84.295) | ||
| Surgical duration | 2.828 (1.823-4.387) | < 0.001 | 2.085 (1.290-3.370) | 0.029 |
The training and validation cohorts yielded AUCs of 0.841 [95% confidence interval (CI): 0.774-0.908; Figure 3A] and 0.906 (95%CI: 0.845-0.966; Figure 3B), respectively. The discriminatory power of the model was excellent with a C-index of > 0.800 for both cohorts. Hosmer-Lemeshow tests demonstrated a good model fit, with P = 0.36 and 0.31 for the training and validation cohorts, respectively. The clinical decision curves derived from the model had a wide range of relative thresholds (5%-100%) and robust clinical applicability (Supplementary Figures 1 and 2), indicating that the model holds strong predictive validity.
The rise of gastroscopy has led to increased rates of detecting submucosal lesions in the gastrointestinal tract during health examinations. Symptoms of these lesions are linked to their size and location. While most lesions are benign, they still carry risk of malignancy[11]. The American Society for Gastrointestinal Endoscopy and the National Comprehensive Cancer Network guidelines suggest endoscopic monitoring for asymptomatic lesions < 2 cm in diameter[12]. However, larger lesions, or those causing significant symptoms, require immediate intervention.
The techniques of transoral endoscopic resection include high-frequency electrocoagulation, ESE, EMR, ESD, STER, and EFTR. High-frequency electrocoagulation resection is routinely applied in clinical practice due to its safety and simplicity. Although technically challenging[13], ESD is preferred for lesions originating from the superficial intrinsic muscular layer. It can also boost overall resection rates and decrease local recurrence rates compared with EMR[14]. A significantly higher incidence of perforation after ESD compared with EMR has been identified (3.6% vs 1.2%)[15]. STER is an extension of peroral endoscopic myotomy[16] that is typically used to resect lesions derived from the lamina propria of the esophagus or the cardia, or located in the body of the distal stomach[17]. Compared with ESD, STER helps to preserve the normal mucosal epithelium overlying the lesion surface that reduces the likelihood of gastrointestinal perforation to some extent[18]. One retrospective cohort study found overall STER and ESE resection rates of 70.2% and 67.5% respectively[19].
EUS is instrumental for diagnosing and localizing lesions; it uses a high-frequency probe and is frequently used to detect submucosal lesions in the gastrointestinal tract[20,21]. The layered structure of the upper gastrointestinal tract wall can be robustly visualized on EUS images[22], which helps to identifying the origins of lesions[23]. Distinct types of lesions with different ultrasonographic features can be discriminated by EUS[24-26]. Hence, EUS plays a pivotal role in directing the choice of endoscopic treatment.
Complications after transoral endoscopic resection are common, and those that are gas-related are the most frequent[27]. These specific issues arise due to the accumulation of gases in tunnels. During surgery, gas can leak continuously into the mediastinum, subcutaneous space, and thoracic or abdominal cavity due to the integrity of the digestive tract wall being disrupted. Being absorbed 150-fold faster than air in the digestive tract, CO2 can be eliminated through the pulmonary circulation, significantly reducing the occurrence of gas-related complications, air embolism, and other complications[28-30]. Insufflation with CO2 effectively diminishes patient discomfort and pain[31-33]. However, rapid CO2 absorption by the gastrointestinal tract, excessive surgical durations and injections of gas that exceed mucosal absorption capacity can still lead to gas-related complications. This was corroborated by our previous findings[34].
The probability of gas-related complications during transoral endoscopic resection significantly varies based on the surgical method of resection. Full-thickness resection inherently risks gastrointestinal tract perforation. Gas-related complications can arise if the gastrointestinal wall is not repaired during the procedure. Submucosal tunnelling resection that removes the mass by creating a tunnel between the mucosa and the submucosa is less likely to have gas-related complications compared with full-thickness resections. However, submucosal tunnelling is susceptible to gas-related complications when the plasma layer is damaged. Leaving the mucosal epithelium on the perforated surface intact and maintaining a distance from the tunnel opening can prevent gas-related complications if the tunnel opening is closed promptly. The present study did not find any gas-related complications due to high-frequency electrocoagulation resection.
Patients with diabetes mellitus (DM) often have compromised immunity. Prolonged hyperglycemia can harm the nervous system and slow peristalsis in the gastrointestinal tract. Prolonged hyperglycemia can also cause microangiopathy, that significantly slows blood flow to the gastric mucosa and weakens its defense mechanism. Anxiety and prolonged tension in some patients can result in sympathetic excitation and vasoconstriction of the gastrointestinal tract, further diminishing mucosal circulation. This can decrease the defensive function of the gastrointestinal mucosa and increase the incidence of gas-related complications during surgery[35].
Therefore, our nomogram can help to screen patients at elevated risk of gas-related complications. Controlling blood glucose levels, reducing surgical durations, and selecting the most appropriate method of surgical resection might positively affect the prognosis of high-risk patients.
However, our study has some limitations. We primarily relied on retrospective data that might not account for all factors such as infection with Helicobacter pylori that might be associated with gas-related complications. Furthermore, the data were sourced from a single center with a limited patient cohort. Prospective studies with larger patient cohorts at several institutions are crucial to enhance the predictive capacity of our model. External validation or future prospective trials might help to determine the applicability and generalizability of our model and guide the preoperative manage
Our nomogram incorporating surgical duration, method of surgical resection, DM, and the lesion layer of origin had excellent predictive efficacy. Its practical application in clinical settings can serve as a valuable guide for endoscopists.
With the popularity of endoscopy, more and more digestive tract lesions have been discovered. Some of these lesions affect the quality of life of patients, and are potentially fatal. Oral endoscopic resection is becoming the main treatment.
Gas-related complications are inevitable in endoscopic resection. The occurrence of gas-related complications during surgery may increase a patient’s burden and prolong their hospital stay.
The risk factors of gas-related complications were analyzed, and a corresponding prediction model was established.
The variables were screened by univariate and multivariate analysis.
Univariate analysis showed statistically significant differences in histological type, lesion layer of origin, diabetes, lesion size, surgical duration, and resection method. Diabetes, lesion origin, surgical resection method, and surgical duration were incorporated into the final nomogram.
Our nomogram had excellent predictive efficacy.
We hope to conduct a multi-center study with a larger sample size for verification in the future.
The authors sincerely appreciate all the patients who contributed to our study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho JY, South Korea S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | McCarty TR, Ryou M. Endoscopic diagnosis and management of gastric subepithelial lesions. Curr Opin Gastroenterol. 2020;36:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Chiu PWY, Yip HC, Chan SM, Ng SKK, Teoh AYB, Ng EKW. Endoscopic full-thickness resection (EFTR) compared to submucosal tunnel endoscopic resection (STER) for treatment of gastric gastrointestinal stromal tumors. Endosc Int Open. 2023;11:E179-E186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Lee JH, Lee HL, Ahn YW, Lee KN, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS. Prevalence of Gastric Subepithelial Tumors in Korea: A Single Center Experience. Korean J Gastroenterol. 2015;66:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Lim YJ, Son HJ, Lee JS, Byun YH, Suh HJ, Rhee PL, Kim JJ, Rhee JC. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010;16:439-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 5. | Longcroft-Wheaton G, Bhandari P. Endoscopic resection of submucosal tumors. Expert Rev Gastroenterol Hepatol. 2015;9:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017;31:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Huang K, Zhao X, Chen X, Gao Y, Yu J, Wu L. Analysis of Digestive Endoscopic Results During COVID-19. J Transl Int Med. 2021;9:38-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Wang J, Zhao Y, Li P, Zhang S. Advances in The Application of Regenerative Medicine in Prevention of Post-endoscopic Submucosal Dissection for Esophageal Stenosis. J Transl Int Med. 2022;10:28-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Kim M, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Seo HE, Chung YJ, Kwon JG, Jung JT, Kim EY, Jang BI, Lee SH, Kim KO, Yang CH; Daegu-Kyungpook Gastrointestinal Study Group (DGSG). Predictive risk factors of perforation in gastric endoscopic submucosal dissection for early gastric cancer: a large, multicenter study. Surg Endosc. 2013;27:1372-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Tay YBE, Loh WS. Extensive subcutaneous emphysema, pneumomediastinum, and pneumorrhachis following third molar surgery. Int J Oral Maxillofac Surg. 2018;47:1609-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Moon JS. Role of Endoscopic Ultrasonography in Guiding Treatment Plans for Upper Gastrointestinal Subepithelial Tumors. Clin Endosc. 2016;49:220-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 926] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 13. | Higuchi K, Katagiri A, Nakatani S, Kikuchi K, Fujiwara T, Gocho T, Inoki K, Konda K, Yamamura F, Yoshida H. Risk Factors Indicating Difficulty During Gastric Endoscopic Submucosal Dissection for Inexperienced Endoscopists: A Retrospective Study. Cureus. 2022;14:e32713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Zhao Y, Wang C. Long-Term Clinical Efficacy and Perioperative Safety of Endoscopic Submucosal Dissection versus Endoscopic Mucosal Resection for Early Gastric Cancer: An Updated Meta-Analysis. Biomed Res Int. 2018;2018:3152346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, Hamada T, Inoue H, Gotoda T, Yoshida S. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 16. | Tan Y, Huo J, Liu D. Current status of submucosal tunneling endoscopic resection for gastrointestinal submucosal tumors originating from the muscularis propria layer. Oncol Lett. 2017;14:5085-5090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Liu BR, Song JT. Submucosal Tunneling Endoscopic Resection (STER) and Other Novel Applications of Submucosal Tunneling in Humans. Gastrointest Endosc Clin N Am. 2016;26:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kim TW, Kim GH, Park DY, Ahn S, Lim W, Lee BE, Song GA. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc. 2017;31:1936-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Du C, Chai N, Linghu E, Gao Y, Li Z, Li L, Zhai Y, Lu Z, Meng J, Tang P. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc. 2018;32:4543-4551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Asai H, Furukawa K, Miyahara R, Funasaka K, Furune S, Nakamura M, Kawashima H, Ishigami M, Hirooka Y, Fujishiro M. Feasibility of endoscopic ultrasonography using a 60-MHz ultrasound miniature probe in the upper gastrointestinal tract. J Med Ultrason (2001). 2022;49:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Kamata K, Kurita A, Yasukawa S, Chiba Y, Nebiki H, Asada M, Yasuda H, Shiomi H, Ogura T, Takaoka M, Hoki N, Ashida R, Shigekawa M, Yanagisawa A, Kudo M, Kitano M. Utility of a 20G needle with a core trap in EUS-guided fine-needle biopsy for gastric submucosal tumors: A multicentric prospective trial. Endosc Ultrasound. 2021;10:134-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Muto M. Endoscopic diagnostic strategy of superficial esophageal squamous cell carcinoma. Dig Endosc. 2013;25 Suppl 1:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Azuma M, Kusano C, Gotoda T. Diagnostic potential of endoscopic ultrasonography-elastography for gastric submucosal tumors. Dig Endosc. 2015;27 Suppl 1:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Li S, Gao M, Tao L, Luo G, Gao Q, Qian K, Deng L. Clusters of malignant cysts in the gastric submucosal layer (with video). Endosc Ultrasound. 2022;11:518-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Reinecke J, Amanzada A, Elger F, Ghadimi M, Neesse A. A rare cause of upper gastrointestinal bleeding from a submucosal tumor. Endosc Ultrasound. 2021;10:75-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 28. | Yasumasa K, Nakajima K, Endo S, Ito T, Matsuda H, Nishida T. Carbon dioxide insufflation attenuates parietal blood flow obstruction in distended colon: potential advantages of carbon dioxide insufflated colonoscopy. Surg Endosc. 2006;20:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Hirai F, Beppu T, Nishimura T, Takatsu N, Ashizuka S, Seki T, Hisabe T, Nagahama T, Yao K, Matsui T, Nakashima R, Inada N, Tajiri E, Mitsuru H, Shigematsu H. Carbon dioxide insufflation compared with air insufflation in double-balloon enteroscopy: a prospective, randomized, double-blind trial. Gastrointest Endosc. 2011;73:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Yamano HO, Yoshikawa K, Kimura T, Yamamoto E, Harada E, Kudou T, Katou R, Hayashi Y, Satou K. Carbon dioxide insufflation for colonoscopy: evaluation of gas volume, abdominal pain, examination time and transcutaneous partial CO2 pressure. J Gastroenterol. 2010;45:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Suzuki T, Minami H, Komatsu T, Masusda R, Kobayashi Y, Sakamoto A, Sato Y, Inoue H, Serada K. Prolonged carbon dioxide insufflation under general anesthesia for endoscopic submucosal dissection. Endoscopy. 2010;42:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Lee IL, Wu CS, Tung SY, Lin PY, Shen CH, Wei KL, Chang TS. Endoscopic submucosal dissection for early gastric cancers: experience from a new endoscopic center in Taiwan. J Clin Gastroenterol. 2008;42:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Zhang S, Wen J, Du M, Liu Y, Zhang L, Chu X, Xue Z. Diabetes is an independent risk factor for delayed perforation after foreign bodies impacted in esophagus in adults. United European Gastroenterol J. 2018;6:1136-1143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |