Published online Sep 16, 2022. doi: 10.4253/wjge.v14.i9.524
Peer-review started: May 24, 2022
First decision: June 27, 2022
Revised: July 15, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: September 16, 2022
Processing time: 113 Days and 2.9 Hours
Endoscopic ultrasound (EUS) can detect small lesions throughout the digestive tract; however, it remains challenging to accurately identify malignancies with this approach. EUS elastography measures tissue hardness, by which malignant and nonmalignant pancreatic masses (PMs) and lymph nodes (LNs) can be differentiated. However, there is currently little information regarding the strain ratio (SR) cutoff in Hispanic populations.
To determine the diagnostic accuracy of EUS elastography for PMs and LNs with an SR cutoff value in Hispanics.
A retrospective study of patients who underwent EUS elastography for PMs between December 2013 and December 2014. A qualitative (analysis of color maps) and quantitative (SR) analysis of PMs and their associated LNs was per
A sample of 121 patients was included, 45.4% of whom were female. 69 (57.0%) PMs were histologically malignant, with a median SR of 50.4 vs 33.0 for malignant vs nonmalignant masses (P < 0.001). EUS evaluation identified associated LNs in 43/121 patients (35.5%), in whom 22/43 (51.2%) patients had histologically confirmed malignant diagnosis, with a median SR of 30 vs 40 for malignant vs nonmalignant LNs (P = 0.7182). In detecting malignancy in PMs, an SR cutoff value of > 21.5 yielded a sensitivity of 94.2%, while a cutoff value of > 121 yielded a specificity of 96.2.2%. There were significant differences in the Giovannini scores, a previously established elastic score system, between the patients grouped by their final histology results (P < 0.001). For LNs, SR cutoff values of > 14.0 and > 155 yielded a sensitivity of 90.9% and a specificity of 95.2%, respectively, in detecting malignancy.
EUS elastography is a helpful technique for the diagnosis of solid PMs and their associated LNs. The proposed SR cutoff values have a high sensitivity and specificity for the detection of malignancy.
Core Tip: This single-center retrospective study aimed to determine the diagnostic accuracy of endoscopic ultrasound (EUS) elastography in the diagnosis of pancreatic masses (PMs) and associated lymph nodes (LNs) with a defined strain ratio (SR) cutoff value in a Hispanic population. In determining if PMs were malignant, an SR cutoff value > 21.5 had a sensitivity of 94.2%, while a cutoff value > 121 had a specificity of 96.2.2%. For diagnosing LNs, an SR cutoff value > 14.0 had a sensitivity of 90.9%, while a cutoff value > 155 had a specificity of 95.2% for malignancy. The proposed SR cutoff values have high sensitivity and specificity for malignancy detection during EUS elastography.
- Citation: Puga-Tejada M, Del Valle R, Oleas R, Egas-Izquierdo M, Arevalo-Mora M, Baquerizo-Burgos J, Ospina J, Soria-Alcivar M, Pitanga-Lukashok H, Robles-Medranda C. Endoscopic ultrasound elastography for malignant pancreatic masses and associated lymph nodes: Critical evaluation of strain ratio cutoff value. World J Gastrointest Endosc 2022; 14(9): 524-535
- URL: https://www.wjgnet.com/1948-5190/full/v14/i9/524.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i9.524
Pancreatic masses (PMs) include neoplastic and nonneoplastic lesions (i.e., anatomical variants, inflammatory lesions). One of the essential tasks during the assessment of PMs is identifying their benign or malignant nature. Along with the identification of malignant lesions, the presence of involved lymph nodes (LNs) is a prognostic factor of the disease. To date, one of the most sensitive methods for det
These shortcomings have been addressed with EUS elastography, an additional imaging technique used to determine tissue hardness. Malignant tissue is often more rigid than the normal surrounding tissue; thus, EUS elastography can differentiate between malignant and nonmalignant lesions. As a result, this technique has been applied in the diagnostic workup of PMs and their associated LNs[2-4]. EUS elastography is considered an accurate imaging technique for characterizing and detecting pancreatic lesions[2].
EUS elastography can be used to evaluate PMs and their associated LNs through qualitative and quantitative analyses; the former involves the analysis of color maps, while the latter is achieved by assessing the strain ratio (SR). However, previous studies, such as the one published by Altonbary et al[4], have reported differences in the SR cutoff value and the optimal internal sensitivity and specificity, suggesting a potential limitation of this technique[3,4]. The accuracy of this technique in differentiating malignant from nonmalignant lesions has only been assessed for masses consisting of solid tissue. The suitability of EUS elastography for solid-cystic lesions, which comprise an important percentage of pancreatic tumoral lesions, has not been reported.
Based on the above, through this retrospective study, we aim to determine the diagnostic accuracy of EUS elastography for diagnosing malignant PMs and LNs in a Hispanic cohort and define the SR cutoff values in this population, comparing the results with those obtained through FNA biopsy.
This was an observational, analytic, retrospective, case-control study performed at the Instituto Ecuatoriano de Enfermedades Digestivas (IECED, Guayaquil, Ecuador) from December 2013 to December 2014. Consecutive Hispanic patients (≥ 18 years old) were referred for the evaluation of suspected PMs using EUS following computed tomography (CT) or magnetic resonance imaging (MRI). Patients with incomplete clinical records were excluded. The patients were allocated into two groups (malignant or nonmalignant) according to the histological findings of biopsy samples and results from a 6-mo clinical follow-up (i.e., laboratory tests, imaging, and surgical findings). All participants or their legal guardians gave written informed consent before the procedure. The Institutional Review Board approved the use and management of the corresponding data, and the study was conducted in accordance with the Declaration of Helsinki.
All procedures were performed by two expert endoscopists (CRM and RV), who perform ≥ 300 EUS procedures per year. The patients were examined under general anesthesia using a 3.8 mm working-channel linear-array echoendoscope (EG3870UTK, Pentax Medical, Pentax, Hamburg, Germany) attached to a Hitachi AVIUS Ultrasound Console (Avius Hitachi, Tokyo, Japan).
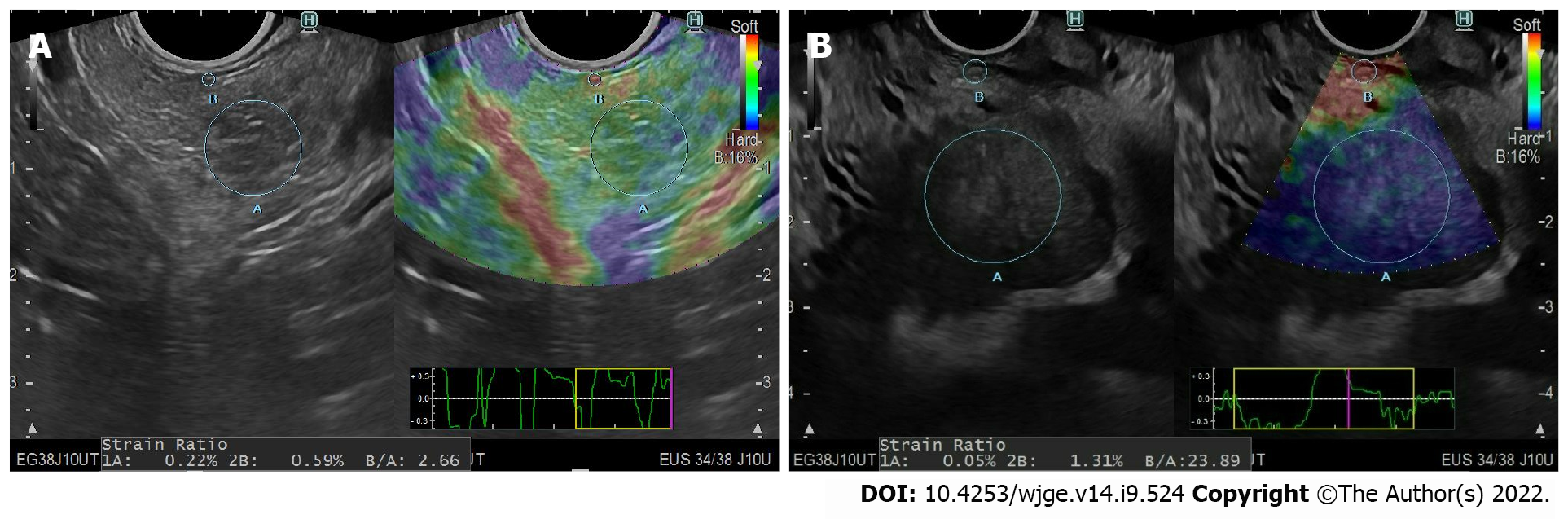
First, PMs or any associated LNs were examined under conventional B-mode scanning. Then, EUS elastography of the region of interest was performed using the ultrasound console. Tissue hardness was measured qualitatively and quantitatively in all regions of interest via EUS color maps and the SR, respectively. Subsequently, EUS-guided FNA was performed using a 22-gauge needle (Expect®, Boston Scientific, Marlborough, MA). A pathologist blinded to the EUS elastography results performed the histological analysis.
Two expert endoscopists (CRM and RV) performed the qualitative assessed by classifying the elas
The quantitative diagnosis was performed by calculating the semiquantitative proportion of tissue elasticity by measuring the SR of the region of interest. According to the method described by Iglesias-Garcia et al[6], at least three elasticity measurements for the mass lesion (A) and one for the surrounding area (B) were obtained. The corresponding SRs were then calculated by dividing B by each of the A values, and their mean was calculated[7]
Baseline data were extracted from medical records. The location, size, diameter, and color pattern of PMs and their associated LNs on EUS elastography, SR, and histological diagnosis were thoroughly described. Malignancy in solid and solid-cystic PMs was defined following the Fukuoka Consensus Guidelines, as detailed in Table 1[5].
Technical considerations: All statistical analyses were performed by an institutional GI attending and biostatistician (MPT) with 8 years of experience, sing R v4.0 (R Foundation for Statistical Computing; Vienna, Austria). A P value < 0.05 was considered statistically significant.
Sample size: The sample size was estimated considering a 100% specificity for an SR > 6.04 on EUS elastography in predicting malignancy in solid PMs, with a corresponding disease prevalence of 67.4%[5], δ = 10%, and α- and β-errors of 5% and 20%, respectively. Using these parameters, a sample size of twenty-four cases and eleven controls was estimated, with 80% statistical power. To respect the central limit theorem (in which thirty observations are necessary to reach a Gaussian distribution), we aimed to analyze no fewer than thirty patients with malignant PMs during the study period.
Comparisons of baseline data, EUS, and EUS elastography diagnostic outcomes: Quantitative var
EUS and EUS elastography qualitative analysis: The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of a Giovannini elastic score of 3 to 5 (cyan and dark blue) in predicting malignancy in PMs and their associated LNs were estimated. In the case of PMs, the subgroup analysis considered only solid PMs (excluding solid-cystic PMs). In the case of associated LNs, the sensitivity, specificity, PPV, NPV, and accuracy of conventional B-mode EUS criteria in predicting malignancy were also determined.
EUS elastography quantitative analysis: The sensitivity, specificity, PPV, NPV, and accuracy of SR measurements in predicting malignancy in PMs and their associated LNs were estimated. Subgroup analysis was also performed for only solid PMs (excluding solid-cystic PMs). In each situation, two internally derived SR cutoff values, one yielding the optimal sensitivity (and accuracy) and the other the optimal specificity, were calculated from the study data. We also calculated the corresponding areas under the receiver operating characteristic curve (AUROCs), in which AUROCs of 0.5 suggested a prediction of malignancy equivalent to chance, with values of 0.7 to 0.8 considered acceptable, 0.8 to 0.9 considered excellent, and more than 0.9 considered outstanding discriminability[6]. The corresponding ROC curves were also generated and compared using the roc.test function of the pROC (v1.16.2; Robin X, 2020) package when necessary.
A sample of 121 patients with previous CT or MRI scans for PMs underwent EUS evaluation and were enrolled in the study. In this cohort, 55/121 (45.5%) were female, and the median age was 67 years (13–99). There was a histologically confirmed diagnosis of malignancy in 69/121 (57%) patients who were allocated to the malignant group; the remaining patients were placed in the nonmalignant group. Additionally, 43/121 (35.5%) patients had associated LNs surrounding the gastrointestinal tract. The baseline data and EUS elastography diagnostic outcomes of the cohort are summarized in Table 2.
| Malignancy (n = 69) | Nonmalignancy (n = 52) | P value | |
| Age (yr), median (range) | 67 (13–93) | 68 (20–99) | 0.8907a |
| Sex (female), n (%) | 36 (52.2) | 19 (36.5) | 0.1271b |
| PM location, n (%) | 0.6891b | ||
| Head | 50 (72.5) | 35 (67.3) | |
| Neck | 3 (4.3) | 4 (7.7) | |
| Body | 13 (18.8) | 12 (23.1) | |
| Tail | 3 (4.3) | 1 (1.9) | |
| PM diameter (mm), median (range) | 37.0 (7.4–70.0) | 30 (10.0–60.0) | 0.0616a |
| Giovannini elastic score, n (%) | < 0.001b | ||
| Green (score 1 to 2) | - | 11 (21.2) | |
| Cyan (score 3) | 5 (7.2) | 11 (21.2) | |
| Dark blue (score 4 to 5) | 64 (92.8) | 30 (57.7) | |
| Strain ratio, median (range) | 50.4 (7.8–225.0) | 33.0 (2.6–321.0) | < 0.001a |
| Firmness/histopathology, n (%) | < 0.001b | ||
| Solid-cystic masses (n = 36) | 26/69 | 10/52 | < 0.001b |
| Serous cystadenoma | - | 10 (19.2) | |
| Mucinous cystadenoma | 5 (7.2) | - | |
| Mucinous cystadenocarcinoma | 3 (4.3) | - | |
| IPMN | 18 (26.1) | - | |
| Solid masses (n = 85) | 43/69 | 42/52 | < 0.001b |
| Normal | - | 4 (7.7) | |
| Acute pancreatitis | - | 10 (19.2) | |
| Chronic pancreatitis | - | 26 (50.0) | |
| Adenoma | - | 1 (1.9) | |
| Insulinoma | - | 1 (1.9) | |
| Adenocarcinoma | 33 (47.8) | - | |
| Lymphoma | 3 (4.3) | - | |
| PNETs | 6 (8.7) | - | |
| Pancreatoblastoma | 1 (1.4) | - |
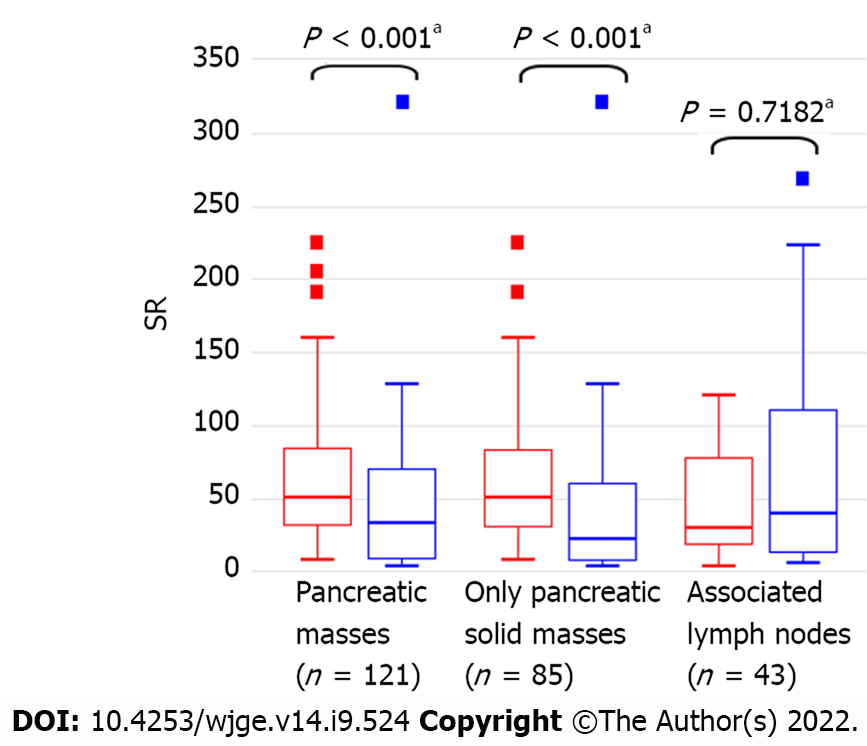
We compared both PM groups in terms of the variables obtained from the EUS elasticity qualitative and quantitative analyses. Regarding the qualitative outcomes, there were significant differences in the Giovannini scores between the patients grouped by their final histology results (P < 0.001). For the quantitative outcomes, there was a significant difference in the median SR between patients with malignant (50.4, range 7.8–22.5) and nonmalignant PMs (33.0, range 2.6–321.0) (P < 0.001). In the solid PM subgroup, the median SR values were 51.0 (7.8–225.0) and 21.9 (2.6–321.0), respectively (Figure 1). A proportionally significant association was demonstrated between a higher PM SR and a larger PM diameter (rho = 0.251, 95%CI: 0–0.481; P = 0.05).
In detecting malignancies among all PMs, a Giovannini elastic score of 3 to 5 had a sensitivity, specificity, PPV, NPV, and accuracy of 100.0%, 21.2%, 62.7%, 100.0%, and 66.1%, respectively. For the subgroup of solid PMs, the corresponding sensitivity, specificity, PPV, NPV, and accuracy were 100%, 23.8%, 57.3%, 100%, and 62.4%, respectively (Table 3).
| EUS-elastography qualitative analysis | EUS-elastography quantitative analysis | |||||
| All masses | Only solid pancreatic masses | All PMs | Only solid PMs | |||
| SR ≥ 21.51 | SR ≥ 121.02 | SR ≥ 21.51 | SR ≥ 121.02 | |||
| Sensitivity (%) | 100.0 | 100.0 | 94.2 | 14.5 | 90.7 | 14.0 |
| Specificity (%) | 21.2 | 23.8 | 40.4 | 96.2 | 47.6 | 95.4 |
| PPV (%) | 62.7 | 57.3 | 67.7 | 83.3 | 63.9 | 70.0 |
| NPV (%) | 100.0 | 100.0 | 84.0 | 45.9 | 83.3 | 52.0 |
| Accuracy (%) | 66.1 | 62.4 | 71.1 | 49.6 | 69.4 | 54.1 |
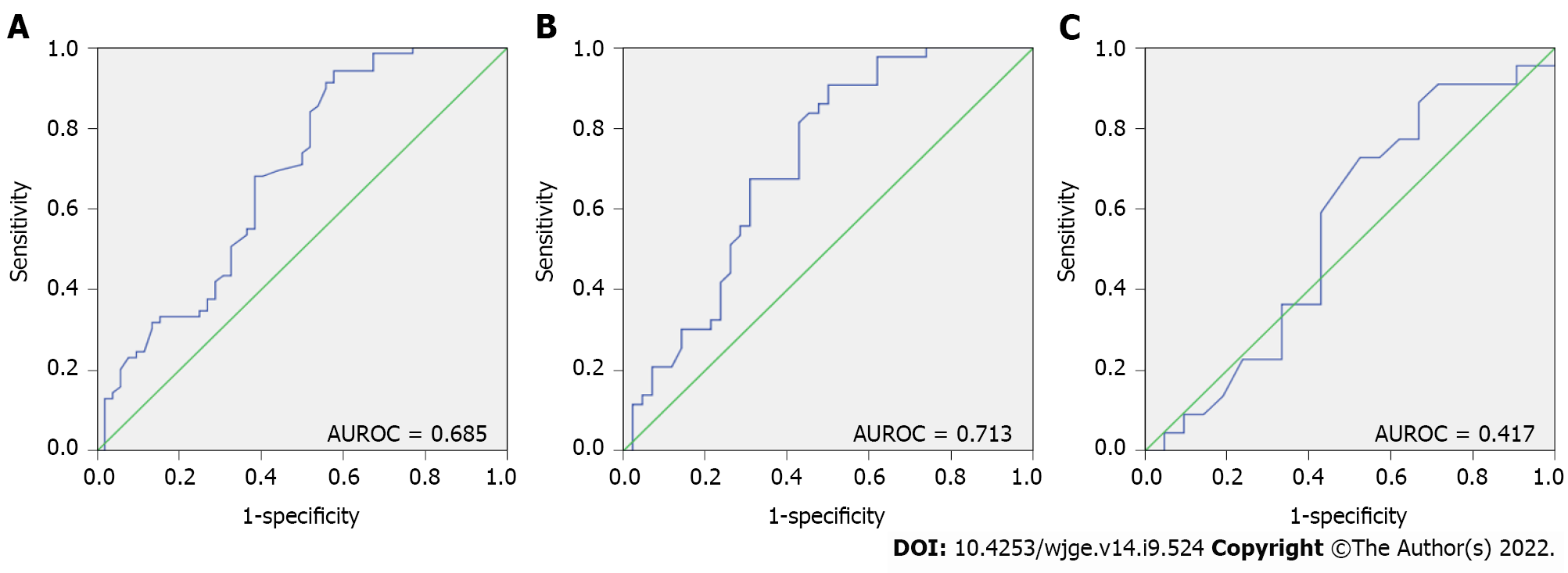
In the quantitative analysis, we found that optimal sensitivity and specificity values were obtained for SR cutoff values of 21.5 and 121.0, respectively, for both all PMs and solid PMs. The diagnostic accuracy parameters for both groups of PMs are shown in Table 3. Notably, in the overall PM analysis, the lower SR cutoff value (≥ 21.5) was associated with a higher sensitivity (94.2%) and NPV (84.0%), and the higher SR cutoff value (≥ 121.0) was associated with higher specificity (96.2%) and PPV (83.3%). A similar observation was made in the solid PM subgroup analysis; however, the SR cutoff value of ≥ 121.0 yielded higher accuracy in the subgroup analysis than in the overall PM analysis (54.1% vs 49.6%), while the SR cutoff of ≥ 21.5 yielded a lower accuracy (69.4% vs 71.1%). Additionally, the AUROC was slightly higher in the solid PM subgroup analysis (AUROC = 0.713) than in the overall PM analysis (AUROC = 0.685) (P = 0.7073) (Figure 2A and B).
Among the 43 patients with associated LNs, the median age was 67.5 (39–95) years, and 14/43 (32.6%) were female. Histology confirmed malignancy in 22/43 (51.2%) patients, who were subsequently placed in the malignant group. There were no significant differences between the malignant and nonmalignant LN groups in LN location, diameter, EUS characteristics, Giovannini elastic score, or SR (Table 4). Specifically, the average SR was 30.0 (3.0–120.0) for malignant LNs and 40.0 (5.0–269.0) for nonmalignant LNs (P = 0.7182) (Figure 1). There was no association between LN SR and diameter (rho = -0.017, 95%CI: -0.503–0.421; P = 0.937).
| Malignancy (n = 22) | Nonmalignancy (n = 21) | P value | |
| Age (yr), median (range) | 76 (57–95) | 65 (39–85) | 0.2037a |
| Sex (female), n (%) | 8 (36.4) | 6 (28.6) | 0.5860b |
| LN location, n (%) | 0.4250b | ||
| Esophagus | 13 (59.1) | 15 (71.4) | |
| Stomach | 2 (9.1) | 1 (4.8) | |
| Liver | 1 (4.5) | - | |
| Pancreas | 5 (22.7) | 5 (23.8) | |
| Kidney | 1 (4.5) | - | |
| LN diameter, median (range) | 20.0 (4.0–50.0) | 15.5 (7.0–21.6) | 0.2662a |
| EUS-LN characteristics, n (%) | |||
| Irregular shape | 11 (50.0) | 10 (47.6) | 0.8760b |
| Undefined border | 13 (59.1) | 8 (38.1) | 0.2730b |
| Anechoic density | 7 (31.8) | 3 (14.3) | 0.1740b |
| Giovannini elastic score, n (%) | 0.7970b | ||
| Green (score 1 to 2) | 1 (4.5) | 2 (9.5) | |
| Cyan (score 3) | 6 (27.3) | 6 (28.6) | |
| Dark blue (score 4 to 5) | 15 (68.2) | 13 (61.9) | |
| Strain ratio, median (range) | 30.0 (3.0–120.0) | 40.0 (5.0–269.0) | 0.7182a |
| Histopathology, n (%) | < 0.001b | ||
| Acute lymphadenitis | - | 10 (47.6) | |
| Chronic lymphadenitis | - | 11 (52.4) | |
| Lymphoma | 2 (9.1) | - | |
| Metastasis | 20 (90.9) | - |
Qualitative EUS elastography analysis yielded a sensitivity, specificity, PPV, NPV, and accuracy of 68.1%, 38.1%, 53.6%, 53.3%, and 53.5%, respectively; these values were lower than those obtained using the structural characteristics detected via conventional B-mode scanning (Table 5). For the PMs, we obtained two SR cutoff values by identifying the values that yielded optimal sensitivity and specificity. Specifically, an SR cutoff value of 14.0 yielded a sensitivity, specificity, PPV, NPV and accuracy of 90.0%, 28.6%, 51.4%, 75.0% and 60.4, respectively; the corresponding values for an SR cutoff value of 155.0 were 4.5%, 95.2%, 50.0%, 48.8% and 48.8% (Table 5). The use of SR for diagnosing malignancy yielded an AUROC of 0.417 (Figure 2C).
| Conventional B-mode EUS | EUS-elastography qualitative analysis | EUS-elastography quantitative analysis | |||||
| Size | Shape | Border | Density | SR ≥ 14.01 | SR ≥ 155.02 | ||
| Sensitivity (%) | 59.1 | 50.0 | 59.1 | 31.8 | 68.1 | 90.9 | 4.5 |
| Specificity (%) | 42.9 | 52.4 | 61.9 | 85.7 | 38.1 | 28.6 | 95.2 |
| PPV (%) | 52.0 | 52.4 | 61.9 | 70.0 | 53.6 | 51.4 | 50.0 |
| NPV (%) | 50.0 | 50.0 | 59.1 | 54.6 | 53.3 | 75.0 | 48.8 |
| Accuracy (%) | 51.2 | 51.2 | 60.5 | 58.1 | 53.5 | 60.4 | 48.8 |
In the present study, we found that qualitative EUS elastography analysis was highly sensitive for solid PMs. Moreover, in the quantitative assessment, an SR cutoff value of ≥ 21.5 had a 90% sensitivity for defining malignancy in solid PMs (Figure 3). In contrast, a cutoff value of ≥ 121.0 had a 95% specificity for malignant PMs. For the evaluation of associated LNs, an SR of ≥ 14.0 had a 91% sensitivity, whereas an SR of ≥ 155.0 had a 95% specificity.
Various studies have shown the ability of EUS to distinguish between malignant and nonmalignant lesions. Itokawa et al[8] proposed that a Giovannini elastic score of 5 during EUS elastography evaluation is a characteristic of pancreatic malignancy[8,9], with 98.6% of patients having a score of five and a confirmed pancreatic malignancy. However, our study found that 91.4% of patients with malignant PMs had a score of 4 to 5.
The qualitative elastic score had a high sensitivity of 100.0% in our study for solid and solid-cystic PMs. On the other hand, Itokawa et al[8] found that a considerable number of nonmalignant cases scored 5, decreasing the specificity of the elastic score to 64.3%[2]. Our study found a specificity of 21.15% for solid and solid-cystic PMs and 23.81% for solid masses alone. No malignant pancreatic lesions had an elastic score of 1 or 2 following Giovannini's classification. According to the qualitative analysis, our cases reported high sensitivity and NPV.
Iglesias-Garcia et al[6], in a prospective study of 86 patients, described one of the highest diagnostic accuracy values based on qualitative and quantitative EUS elastography analysis. For the qualitative measurements, the sensitivity, specificity, PPV, NPV, and overall accuracy were 100%, 71%, 87%, 100%, and 90%, respectively. For the quantitative values, a lower SR cutoff value of > 6.0 had a sensitivity, specificity, PPV, NPV, and overall accuracy of 100%, 92%, 96%, 100%, and 97%, respectively[6].
Dawwas et al[10] obtained a higher diagnostic accuracy for EUS elastography using an SR cutoff value of 4.65 to achieve a 100% sensitivity and a cutoff value of 59.25 to achieve a 100% specificity. Okasha et al[11] concluded that the best SR cutoff level was 7.8, which gave a sensitivity of 92%, a specificity of 77%, a PPV of 91%, an NPV of 80%, and an accuracy of 88%[11]. Our study achieved a higher sensitivity using a lower cutoff value. Actors such as tissue inflammation, fibrosis, necrosis, advanced age, or ethnicity may affect the hardness of tissue, explaining the difference in the cutoff values proposed in the literature[12-14]. Moreover, the size of the region of interest and tissue compression level could affect the quantitative evaluation of EUS elastography.
Additionally, a study published by Kongkam et al[15] showed that a cutoff SR level of 3.17 along with EUS-FNA provided a sensitivity, specificity, PPV, NPV and accuracy of 95.2%, 71.4%, 90.9%, 83.3%, and 89.3%, respectively, compared to the 90%, 100%, 100% 80% and 92.8% of EUS elastography alone. Based on these results, the authors raised the possibility of a future combination of both techniques for evaluating PMs[15].
Paterson et al[12] focused their research on the utility of quantitative EUS elastography analysis for defining malignancy in the LNs related to esophageal and gastric cancer and compared this approach to an analysis using conventional EUS LN features. Compared to our results, they found a lower diagnostic accuracy for conventional EUS but a higher diagnostic accuracy for EUS elastography[12].
The present study has several limitations, including its retrospective design and single-center nature, leading to a limited number of operators. A few patients from the malignant case group underwent surgery, limiting the histological description of this research. The nonmalignant control group was defined as patients with nonmalignant masses instead of a healthy population. However, this study has the advantage of using the qualitative elastic score proposed by Giovannini[3]. For the interpretation of PMs and their associated LNs, instead of the 4-score by Furukawa et al[16], and may be one of the first studies to evaluate the utility of EUS elastography in Hispanic patients. Future research on this topic will be designed as diagnostic trials, considering the Giovannini score for PMs and associated LN descriptions.
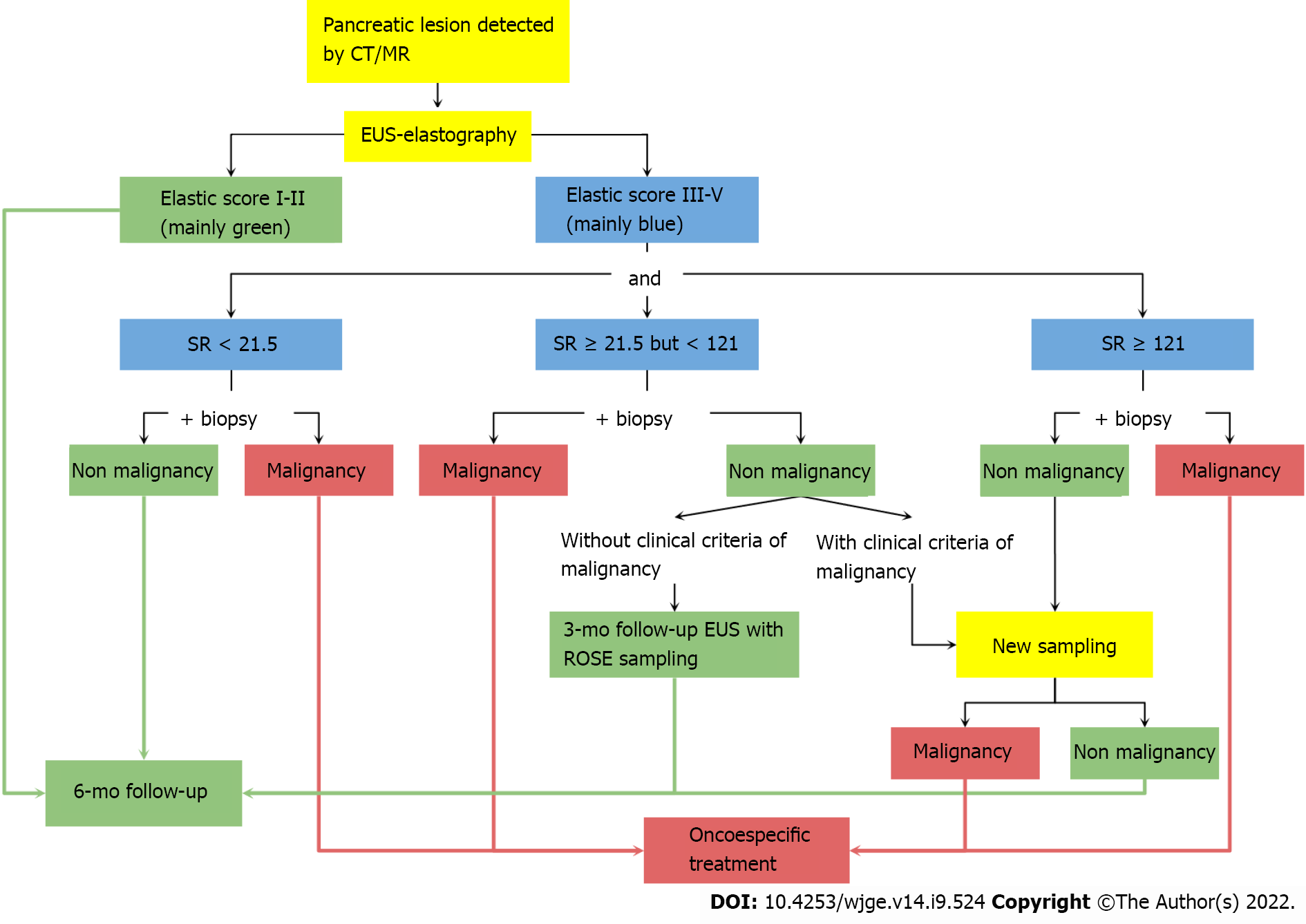
Finally, hard PMs are not necessarily malignant all the time, whereas soft lesions are not necessarily nonmalignant[2,17]. Therefore, a validated cutoff value for defining malignancy in PMs and their associated LNs is imperative for obtaining an appropriate diagnosis and providing management guidance. Based on our findings, we recommend an SR cutoff values of > 121.0 and > 155.0 as criteria for supporting the need for FNA sampling of pancreatic lesions or their associated LNs, respectively. In patients with SR values ranging from 21.5-121.0 and 14.0-155.0, sampling should be indicated if there is a high clinical suspicion of malignancy. Figure 4 shows a proposed clinical algorithm using EUS elastography evaluations. We recommend starting with a qualitative measurement. For those with a low risk of malignancy (elastic score I-II), a 6-mo follow-up is necessary. However, for those with an elastic score between 3 and 5, a quantitative evaluation is required to define the SR measurement and determine the necessity of FNA and whether a malignancy is suspected.
We found that EUS combined with qualitative and quantitative elastography analysis via SR is a helpful resource when assessing PMs and their associated LNs. This approach is more effective and convenient than limiting the evaluation to only conventional EUS-fine needle aspiration for the detection of malignancy. Although histological analysis is mandatory for a final diagnosis, elastography should be included in the diagnostic workup of PMs and their associated LNs. However, validating this recommendation through a prospective, multi-center, controlled trial is preferable.
Endoscopic ultrasound (EUS) elastography can be a useful technique for the evaluation of pancreatic masses (PMs) and their associated lymph nodes (LNs) through qualitative (analysis of color maps) and quantitative (assessing the strain ratio).
The accuracy of this technique in differentiating malignant from nonmalignant lesions has only been assessed for masses consisting of solid tissue. For the evaluation of solid-cystic lesions, the suitability of EUS-elastography has not been reported.
To determine the diagnostic accuracy of EUS elastography and the strain ratio (SR) cutoff value for malignant PMs and LNs in a Hispanic cohort.
A retrospective study of patients who underwent EUS elastography for PMs between December 2013 and December 2014. A qualitative and quantitative (SR) analysis of PMs and their associated LNs was performed. The accuracy of EUS elastography in identifying malignant PMs and LNs and cutoff value for SR were analyzed. A PM and/or its associated LNs were considered malignant based on histopathological findings from fine-needle aspiration biopsy samples.
Malignant PMs have a superior median SR compared to nonmalignant lesions (50.4 vs 33.0, respectively) (P < 0.001). When analyzing LNs, there was no statistical significance (SR 30.0 for PMs vs 40.0 for LNs) (P = 0.7182). An SR cutoff value > 21.5 in PMs yielded a 94.2% sensitivity. Meanwhile, an SR cutoff value > 14.0 yielded a 90.9% sensitivity.
The proposed EUS elastography SR cutoff values have a high sensitivity and specificity for the detection of malignancy.
Future research evaluating the utility of EUS elastography in Hispanic patients through a prospective, multi-center, controlled trial is necessary to validate our data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ecuador
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen SY, China; Chen C, China; Mahmoud MZ, Saudi Arabia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Woolf KM, Liang H, Sletten ZJ, Russell DK, Bonfiglio TA, Zhou Z. False-negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: one institution's experience. Cancer Cytopathol. 2013;121:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Xie J, Liu H, Liu WS, Li JW. Quantitative shear wave elastography for noninvasive assessment of solid pancreatic masses. Clin Hemorheol Microcirc. 2020;74:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Giovannini M. Endosonography: new developments in 2006. ScientificWorldJournal. 2007;7:341-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Altonbary AY, Hakim H, El-Shamy AM. Diagnostic Efficacy of Endoscopic Ultrasound Elastography in Differentiating Solid Pancreatic Lesions: A Single-Center Experience. Clin Endosc. 2019;52:360-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1154] [Article Influence: 144.3] [Reference Citation Analysis (1)] |
| 6. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 7. | Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 2549] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 8. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R, Yokoyama N, Moriyasu F, Kasuya K, Nagao T, Kamisawa T, Tsuchida A. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 10. | Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Okasha H, Elkholy S, El-Sayed R, Wifi MN, El-Nady M, El-Nabawi W, El-Dayem WA, Radwan MI, Farag A, El-Sherif Y, Al-Gemeie E, Salman A, El-Sherbiny M, El-Mazny A, Mahdy RE. Real time endoscopic ultrasound elastography and strain ratio in the diagnosis of solid pancreatic lesions. World J Gastroenterol. 2017;23:5962-5968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 12. | Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol. 2012;18:889-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Chantarojanasiri T, Kongkam P. Endoscopic ultrasound elastography for solid pancreatic lesions. World J Gastrointest Endosc. 2017;9:506-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Chacaltana Mendoza A, Jerez Lanza VF, Llatas Perez J, Li Salvatierra B, Vera Calderon A. [Usefulness of endoscopic ultrasound guided elastography in the assessment of solid pancreatic lesions]. Rev Gastroenterol Peru. 2019;39:38-44. [PubMed] |
| 15. | Kongkam P, Lakananurak N, Navicharern P, Chantarojanasiri T, Aye K, Ridtitid W, Kritisin K, Angsuwatcharakon P, Aniwan S, Pittayanon R, Sampatanukul P, Treeprasertsuk S, Kullavanijaya P, Rerknimitr R. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single-blinded study. J Gastroenterol Hepatol. 2015;30:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Furukawa MK, Kubota A, Hanamura H, Furukawa M. [Clinical application of real-time tissue elastography to head and neck cancer--evaluation of cervical lymph node metastasis with real-time tissue elastography]. Nihon Jibiinkoka Gakkai Kaiho. 2007;110:503-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Facciorusso A, Martina M, Buccino RV, Nacchiero MC, Muscatiello N. Diagnostic accuracy of fine-needle aspiration of solid pancreatic lesions guided by endoscopic ultrasound elastography. Ann Gastroenterol. 2018;31:513-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |