Published online Jun 16, 2022. doi: 10.4253/wjge.v14.i6.376
Peer-review started: January 28, 2022
First decision: April 10, 2022
Revised: April 23, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 16, 2022
Processing time: 135 Days and 18.1 Hours
About 10%-30% of acute pancreatitis remain idiopathic (IAP) even after clinical and imaging tests, including abdominal ultrasound (US), contrast-enhanced computed tomography (CECT) and magnetic resonance cholangiopancreatography (MRCP). This is a relevant issue, as up to 20% of patients with IAP have recurrent episodes and 26% of them develop chronic pancreatitis. Few data are available on the role of EUS in clarifying the etiology of IAP after failure of one or more cross-sectional techniques.
To evaluate the diagnostic gain after failure of one or more previous cross-sectional exams.
We retrospectively collected data about consecutive patients with AP and at least one negative test between US, CECT and MRCP, who underwent linear EUS between January 2017 and December 2020. We investigated the EUS diagnostic yield and the EUS diagnostic gain over different combinations of these cross-sectional imaging techniques for the etiologic diagnosis of AP. Types and frequency of EUS diagnosis were also analyzed, and EUS diagnosis was compared with the clinical parameters. After EUS, patients were followed-up for a median of 31.5 mo to detect cases of pancreatitis recurrence.
We enrolled 81 patients (63% males, mean age 61 ± 18, 23% with previous cholecystectomy, 17% with recurrent pancreatitis). Overall EUS diagnostic yield for AP etiological diagnosis was 79% (20% lithiasis, 31% acute on chronic pancreatitis, 14% pancreatic solid or cystic lesions, 5% pancreas divisum, 5% autoimmune pancreatitis, 5% ductal abnormalities), while 21% remained idiopathic. US, CECT and MRCP, taken alone or in combination, led to AP etiological diagnosis in 16 (20%) patients; among the remaining 65 patients, 49 (75%) obtained a diagnosis at EUS, with an overall EUS diagnostic gain of 61%. Sixty-eight patients had negative US; among them, EUS allowed etiological diagnosis in 59 (87%). Sixty-three patients had a negative CECT; among them, 47 (74%) obtained diagnosis with EUS. Twenty-four had a negative MRCP; among them, 20 (83%) had EUS diagnosis. Twenty-one had negative CT + MRCP, of which 17 (81%) had EUS diagnosis, with a EUS diagnostic gain of 63%. Patients with biliary etiology and without previous cholecystectomy had higher median values of alanine aminotransferase (154 vs 25, P = 0.010), aspartate aminotransferase (95 vs 29, P = 0.018), direct bilirubin (1.2 vs 0.6, P = 0.015), gamma-glutamyl transpeptidase (180 vs 48, P = 0.006) and alkaline phosphatase (150 vs 72, P = 0.015) Chronic pancreatitis diagnosis was more frequent in patients with recurrent pancreatitis at baseline (82% vs 21%, P < 0.001). During the follow-up, AP recurred in 3 patients, one of which remained idiopathic.
EUS is a good test to define AP etiology. It showed a 63% diagnostic gain over CECT + MRCP. In suitable patients, EUS should always be performed in cases of IAP. Further prospective studies are needed.
Core Tip: Acute pancreatitis (AP) is a common and potentially severe disease. Imaging techniques allow an etiological diagnosis in most cases. However, about 20% of cases remain idiopathic, with negative consequences on patients’ outcomes. Endoscopic ultrasound (EUS) has emerged as a valid technique for the assessment of AP etiology. We share our experience with EUS in the identification of idiopathic AP etiology, after failure of one or more cross-sectional imaging techniques. We found a superiority of EUS over the standard cross-sectional imaging techniques. We therefore suggest the use of EUS to define idiopathic AP etiology in all suitable patients.
-
Citation: Mazza S, Elvo B, Conti CB, Drago A, Verga MC, Soro S, De Silvestri A, Cereatti F, Grassia R. Endoscopic ultrasound diagnostic gain over computed tomography and magnetic resonance cholang
iopancreatography in defining etiology of idiopathic acute pancreatitis. World J Gastrointest Endosc 2022; 14(6): 376-386 - URL: https://www.wjgnet.com/1948-5190/full/v14/i6/376.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i6.376
Acute pancreatitis (AP) is an inflammatory disorder characterized by the abnormal activation of digestive enzymes within the pancreatic gland. AP leads to the acute injury of the pancreas and may involve remote organs and systems. AP is one of the most common causes of hospitalization in the United States and Europe[1]. In most cases (about 80%), the prognosis is rapidly favorable[2]. Nevertheless, acute necrotizing pancreatitis may develop in up to 20% of cases, and it is associated with significant rates of early organ failure (38%), need for intervention (38%) and death (15%)[3].
The most common AP etiologies are common bile duct stones and alcohol abuse, accounting for about 60%-70% of all the cases[4]. Other etiologies include functional or anatomic lesions (pancreas divisum, pancreatic duct strictures/tumors, ampullary stenosis or sphincter of Oddi dysfunction), drugs, metabolic causes (hypertriglyceridemia, hypercalcemia), autoimmune disease, mechanical injury (e.g., blunt abdominal trauma, postoperative), infections, ischemia, hereditary conditions and toxins[5].
AP etiology can be found in most cases by combining cross-sectional abdominal imaging techniques, such as ultrasound (US), contrast-enhanced computed tomography (CECT) and magnetic resonance cholangiopancreatography (MRCP). However, 10%-30% of AP remains idiopathic (IAP) after clinical, laboratory and imaging tests[6,7]. This is a relevant issue, as 20% of patients with IAP have recurrent episodes, and 20%-30% of them develop chronic pancreatitis[6]. In recent years, endoscopic US (EUS) has emerged as a useful tool for the etiological diagnosis of AP. A recent systematic review and meta-analysis demonstrated that EUS is able to identify a potential etiology in the majority of patients with IAP[8].
EUS has shown high diagnostic accuracy for the identification of microlithiasis missed at CECT scan or MRCP[9,10]. Moreover, in a smaller but relevant percentage of cases, EUS detected small pancreatic or ampullary lesions that were not identified at CECT or magnetic resonance imaging[11-13]. To date, few data are available about the role of EUS after failure of multiple cross-sectional imaging techniques and specifically evaluating the diagnostic gain of EUS in this setting. The present study aimed to evaluate the role of EUS in the assessment of IAP etiology when US, CECT and MRCP failed.
We performed a retrospective, single-center study. We analyzed a database of consecutive adult patients prospectively enrolled between January 2017 and December 2020 to the Ospedale Maggiore of Cremona with a diagnosis of AP. The diagnosis of AP was made when 2 of 3 of the following criteria were met: abdominal pain consistent with pancreatitis; increased serum amylase or lipase levels, by at least 3 times the upper normal of limit; and characteristic findings on conventional radiologic methods (transabdominal US and/or CECT scan). MRCP was performed as a second-line technique after a negative US and/or CECT.
A thorough medical history and complete blood tests were collected for each patient at the clinical presentation. For final inclusion in the study analyses, the following criteria were ruled out: (1) History of alcohol or other toxic substance abuse; (2) Recent abdominal trauma; (3) Medications potentially related to AP; (4) Metabolic disorder like hypertriglyceridemia (≥ 1000 mg/dL) or hypercalcemia; (5) Clear etiology of AP identified at US, CECT or MRCP, without the need for further investigations; and (6) In the case of recurrent pancreatitis (i.e. ≥ 2 episodes of AP), a genetic cause was ruled out by testing for CFTR, SPINK-1 and PRSS1 mutations.
Therefore, the patients included in final analysis were those diagnosed with idiopathic acute pancreatitis (IAP), according to the American College of Gastroenterology guidelines[14].
All patients included in the study had undergone EUS after at least one US, CECT or MRCP test. Specifically, EUS was performed after a negative cross-sectional technique to investigate the AP etiology and after a positive exam to confirm a suspected diagnosis, to better characterize a lesion or to obtain biopsies.
After EUS examination, patients were followed up for at least 12 mo (median 31.5 mo, range 12-55), and recurrent episodes of acute pancreatitis were recorded.
The primary aim of the study was to evaluate the diagnostic gain of EUS in the identification of IAP etiology after failure of one or more previous cross-sectional exams. The secondary aims were: to assess the overall EUS diagnostic yield for IAP etiology; to compare the baseline clinical features with the IAP diagnosis; and to analyze the frequency and types of AP recurrence during the follow-up.
EUS examination was performed by 2 experienced operators (≥ 250 exams per year) using a linear echoendoscope (Pentax Medical EG3870UTK and EG38-J10UT), after informed consent had been obtained, with the patient in a left-side position under conscious sedation. EUS was mainly performed during admission after the acute phase of pancreatitis was clinically resolved, unless conditions such as persistent biliary obstruction required earlier evaluation. EUS was performed as an outpatient procedure in cases of mild pancreatitis with early patient discharge.
The examination was considered diagnostic with the following findings: biliary stones, criteria for chronic pancreatitis, presence of solid or cystic pancreatic lesions, pancreatobiliary duct abnormality, pancreas divisum, and features of autoimmune pancreatitis.
In detail: (1) Biliary etiology was diagnosed if stones or microlithiasis/biliary sludge were seen inside the gallbladder or the common bile duct. Biliary stones were defined as hyperechoic structures with an acoustic shadow, microlithiasis was defined as hyperechoic structures of 3 mm or less in diameter, and biliary sludge was defined as a hyperechoic material without an acoustic shadow[15]; (2) Chronic pancreatitis was defined according to the Rosemont criteria[16]; (3) Duct abnormality was diagnosed if a long pancreatobiliary junction (> 15 mm) was identified[17]; (4) Pancreas divisum was described in the presence of a dominant dorsal duct with or without evidence of communication between the ventral and dorsal ducts, or if the main pancreatic duct could not be traced from the major papilla[18]; (5) Solid or cystic pancreatic lesions were considered as the cause of AP if obstruction of the pancreatic duct was seen at EUS examination; and (6) The diagnosis of autoimmune pancreatitis was made when parenchymal or ductal features were seen (e.g., diffuse pancreas enlargement with delayed enhancement), and the International Consensus Diagnostic Criteria were met[19].
The categorical variables were described as absolute frequency and percentage. The continuous variables with normal distribution were described as mean ± SD, whereas the continuous variables without normal distribution were given as median and range. Mann-Whitney test and 2 or Fisher’s exact tests were used to associate baseline clinical and biochemical variables with biliary pancreatitis. Diagnostic yield of EUS was calculated as the overall percentage of etiological diagnosis obtained through EUS examination. EUS diagnostic gain was calculated as the percentage of additional diagnoses obtained at EUS over the total number of patients undergoing US, CECT and/or MRCP. All the analyses were carried out by computer software IBM SPSS Statistics (release 25; IBM Corporation, United States).
Between March 2017 and December 2020, a total of 81 patients underwent EUS for IAP (38% female, mean age at enrollment 61 ± 18 years). Fifteen (23%) patients had previous cholecystectomy, whereas 49 (77%) had an intact gallbladder. First episode of AP was the indication of EUS in 52 (81%) patients, while 12 (19%) patients had recurrent pancreatitis (58% with one episode, 42% with 2 or more episodes). The median time interval between patient admission and EUS was 5 d (range, 2-27). All patients’ demographic and clinical characteristics are summarized in Table 1.
| Parameter | n = 81 | EUS diagnosis, n = 64 | Missed EUS diagnosis, n = 17 | P value |
| Male, n (%) | 51 (63) | 43 (67) | 8 (46) | 0.208 |
| Age at enrollment, mean ± SD, yr | 61 ± 18 | 62 ± 18 | 59 ± 16 | |
| Previous cholecystectomy, n (%) | 19 (23) | 18 (28) | 0 | 0.028 |
| Recurrent pancreatitis, n (%) | 14 (17) | 14 (22) | 0 | 0.101 |
| One episode, n (%) | 7 (9) | |||
| ≥ 2 episodes, n (%) | 6 (7) | |||
| Amylase, median (range) | 468 (107-4988) | 465 (123-4988) | 500 (107-4753) | 0.861 |
| Lipase, median (range) | 777 (87-23840) | 774 (87-23840) | 780 (96-12800) | 0.914 |
| Gamma-glutamyl transpeptidase, median (range) | 70 (9-1665) | 70 (9-1665) | 125 (11-640) | 0.707 |
| Alkaline phosphatase, median (range) | 78 (32877) | 78 (32-877) | 90 (32-185) | 0.707 |
| Direct bilirubin, median (range) | 0.7 (0.2-8.5) | 0.4 (0.2-3) | 0.7 (0.2-8.5) | 0.933 |
| Alanine aminotransferase, median (range) | 34 (6-793) | 34 (6-793) | 33 (7-596) | 0.488 |
| Aspartate aminotransferase, median (range) | 38 (11-704) | 34 (11-704) | 33 (15-301) | 0.732 |
| Abdominal US, n (%) | 72 (89) | 63 (98) | 9 (54) | < 0.001 |
| Abdominal CECT, n (%) | 72 (89) | 56 (88) | 16 (94) | 1.000 |
| MRCP, n (%) | 32 (39) | 28 (44) | 4 (24) | 0.220 |
| EUS findings, n (%) | NA | NA | NA | |
| Normal (final IAP diagnosis) | 17 (21) | |||
| Biliary | 16 (20) | |||
| Microlithiasis / biliary sludge | 9 (11) | |||
| Acute on chronic pancreatitis | 25 (31) | |||
| Solid or cystic lesions | 11 (14) | |||
| Pancreatic adenocarcinoma | 4 (5) | |||
| Ampullary adenoma | 2 (3) | |||
| BD-IPMN with high-risk stigmata or worrisome features | 5 (6) | |||
| Pancreas divisum | 4 (5) | |||
| Ductal anomaly | 4 (5) | |||
| Autoimmune criteria | 4 (5) |
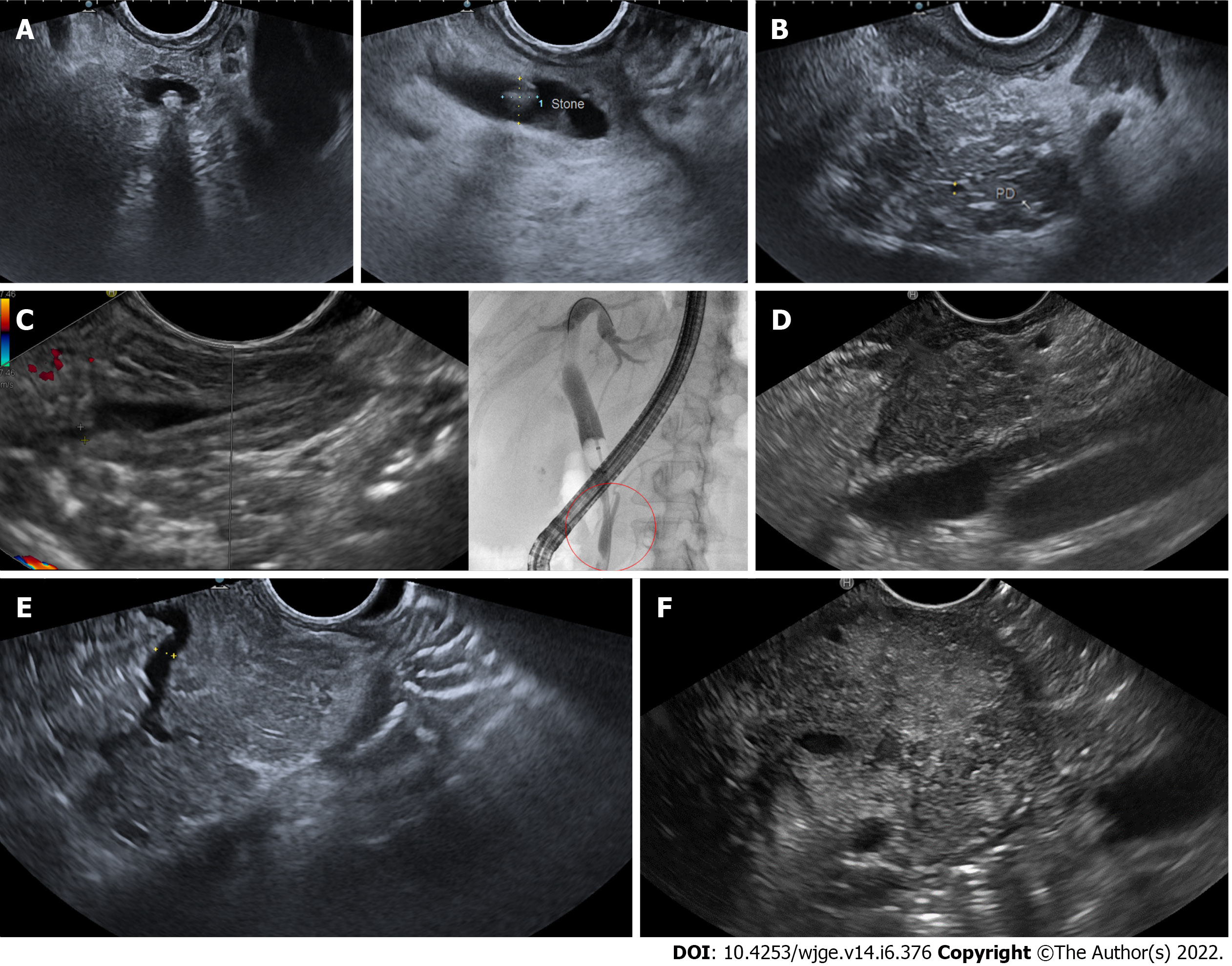
Overall, EUS led to an etiological diagnosis in 64 (79%) of the 81 patients. The diagnoses were as follows: 16 gallstone diseases, 25 acute on chronic pancreatitis, 4 pancreas divisum, 4 pancreatic duct anomalies, 11 solid or cystic lesions (4 pancreatic carcinomas with a maximum diameter of 15, 18, 20 and 24 mm; 2 ampullary adenomas of 8 and 13 mm; 5 branch-duct intraductal papillary mucinous neoplasms with high-risk stigmata or worrisome features) and 4 with criteria of autoimmune conditions. Example images of the main diagnosis obtained by EUS are shown in Figure 1. All patients underwent EUS and at least one exam with US, CECT and MRCP. The three cross-sectional techniques, alone or in combination, led to AP etiological diagnosis in 16 (20%) of the 81 patients. All diagnoses were confirmed at the following EUS. Among the remaining 65 patients, 49 (75%) obtained a diagnosis at EUS, with an overall EUS diagnostic gain of 61%.
US and EUS: Seventy-two (89%) patients underwent US, which allowed an etiological diagnosis in 4 (6%) cases. Among the 68 patients with a negative US, EUS allowed an etiological diagnosis in 59 (87%): 14 biliary pancreatitis, 25 acute on chronic pancreatitis, 2 pancreas divisum, 4 pancreatic duct anomalies, 10 solid or cystic lesions and 4 autoimmune conditions.
CECT and EUS: CECT scan was performed in 72 patients (89%), 9 of which (13%) resulted with an etiological diagnosis. Forty-seven (74%) out of the 63 patients with negative CECT obtained an etiological diagnosis at EUS: 10 lithiasis, 18 acute on chronic, 4 pancreas divisum, 4 duct anomalies, 9 solid/cystic lesions and 2 autoimmune pancreatitis.
MRCP and EUS: MRCP was performed in 32 patients, among which 8 (24%) obtained an etiological diagnosis. EUS allowed a diagnosis in 20 (83%) of the 24 patients with negative MRCP: 4 biliary etiology, 9 acute on chronic pancreatitis, 1 pancreas divisum, 1 pancreatic duct anomaly, 4 solid or cystic lesions and 1 autoimmune pancreatitis.
US + CECT: A combination of US and CECT was performed in 63 patients (78%); of the 54 patients with missed diagnosis at both US and CECT, 45 (83%) received a diagnosis at EUS: 10 biliary etiology, 17 acute on chronic pancreatitis, 3 pancreas divisum, 4 pancreatic duct anomalies, 8 solid or cystic lesions and 3 autoimmune conditions. EUS diagnostic gain over US + CECT was 71%.
US + MRCP: A combination of US and MRCP was performed in 31 patients (38%); of the 23 US + MRCP missed diagnosis, 20 (87%) were identified at EUS: 4 biliary etiology, 9 acute flares on chronic pancreatitis, 1 pancreas divisum, 1 pancreatic duct anomalies, 4 solid or cystic lesions and 1 inflammatory-autoimmune condition. EUS diagnostic gain over US + MRCP was 65%.
CECT + MRCP: CECT and MRCP were both performed in 27 patients; of the 21 CECT + MRCP missed diagnoses, 17 (81%) were identified at EUS: 3 gallstone disease, 7 acute on chronic pancreatitis, 1 pancreas divisum, 1 pancreatic duct anomalies, 4 solid or cystic lesions and 1 autoimmune condition. EUS diagnostic gain over CECT + MRCP was 63%.
US + CECT + MRCP: Finally, 25 patients (31%) received all 3 cross-sectional techniques, without obtaining the AP etiological diagnosis in 19 cases; among them, EUS allowed a diagnosis in 17 (89%) cases: 3 gallstone disease, 7 acute on chronic pancreatitis, 1 pancreas divisum, 1 pancreatic duct anomalies, 4 solid or cystic lesions and 1 autoimmune condition. EUS diagnostic gain over US + CECT + MRCP was 68%.
The percentage of types of EUS diagnosis after the different exam combinations are shown in Table 2.
| Type of previous negative exam/s | |||||||
| Type of AP etiology at EUS | US | CECT | MRCP | US + CECT | US + MRCP | CECT + MRCP | US + CECT + MRCP |
| Biliary; microlithiasis/biliary sludge | 20%; 10% | 16%; 5% | 17%; 17% | 19%; 7% | 18%; 18% | 14%; 14% | 16%; 16% |
| Acute on chronic | 37% | 29% | 38% | 32% | 39% | 33% | 37% |
| Solid or cystic lesions | 15% | 14% | 17% | 15% | 18% | 19% | 21% |
| Pancreas divisum | 3% | 6% | 4% | 5% | 4% | 5% | 5% |
| Anomalous pancreaticobiliary junction | 6% | 6% | 4% | 7% | 4% | 5% | 5% |
| Autoimmune criteria | 6% | 3% | 4% | 5% | 4% | 5% | 5% |
| Idiopathic | 13% | 26% | 16% | 17% | 3% | 9% | 11% |
All patients without etiological diagnosis at EUS had no previous cholecystectomy compared to 28% with EUS diagnosis (P = 0.028). Patients with a final diagnosis of biliary pancreatitis had higher baseline median values of alanine aminotransferase (median value 154 vs 25, P = 0.010), aspartate aminotransferase (median value 95 vs 29, P = 0.018), direct bilirubin (median value 1.2 vs 0.6, P = 0.015), gamma-glutamyl transpeptidase (median value 180 vs 48, P = 0.006) and alkaline phosphatase (median value 150 vs 72, P = 0.015) compared to patients with non-biliary diagnosis. After differentiating between patients with or without previous cholecystectomy, these associations were maintained only for the non-cholecystectomy group. Noteworthy, when differentiating between first-episode and recurrent pancreatitis, chronic pancreatitis was the diagnosis at EUS in 21% and 82% of cases, respectively, a difference that was statistically significant (P < 0.001).
During the follow-up, 12 out of the 16 patients diagnosed with biliary pancreatitis had evidence of choledocholithiasis; all of them underwent successful stone removal by endoscopic retrograde cholangiopancreatography (ERCP). Five out of the 25 patients with chronic pancreatitis underwent ERCP with pancreatic sphincterotomy (5/5) and pancreatic duct stenting (2/5) because of the evidence of Wirsung’s duct stenosis. Among the 11 patients with solid or cystic lesions as the cause of IAP, 4 were treated surgically, while the others were evaluated for a neoadjuvant or palliative approach. The 4 patients with features of autoimmune pancreatitis began steroid therapy with a good response.
During the follow-up time, a further episode of acute pancreatitis was observed in 3 patients (3.7%). Genetic tests for CFTR, SPINK-1 and PRSS1 mutations tested negative. All patients underwent EUS at recurrence. Two of these already had an EUS diagnosis of pancreas divisum and anomalous pancreatobiliary junction that were confirmed. The other had been initially diagnosed as idiopathic pancreatitis, which remained idiopathic even after the EUS examination performed after recurrence.
Our study investigated the role of EUS in the etiological diagnosis of IAP. Overall, the diagnostic yield of EUS for the identification of AP etiology was 80%, with 20% of patients with a final IAP diagnosis, which is in line with previous literature data[20,21]. This result is in keeping with two previous published meta-analyses reporting that EUS can detect a cause in most patients with IAP[8,22]. We found a high diagnostic gain of EUS after all combinations of previous negative cross-sectional techniques; interestingly, diagnostic gain remained remarkably high even after the combination of CECT and MRCP. This result supports EUS as the technique of choice after a negative CECT if the patient is suitable for endoscopic examination, while MRCP could be reserved for patients at elevated risk for invasive procedures.
The most common etiologies identified at EUS were lithiasis, acute on chronic pancreatitis and solid or cystic lesions. All the lithiasis identified at EUS after MRCP were microlithiasis/biliary sludge of gallbladder or common bile duct compared with about half after CECT; this finding confirms the superiority of EUS over MRCP in the identification of lithiasis of small size, as reported previously[9,21-24]. An increase in transaminases is known to have a high positive predictive value for gallstone pancreatitis[25]. Interestingly, in our study, patients with biliary pancreatitis showed higher levels of liver enzymes as compared to other types of diagnosis but only in the group without previous cholecystectomy, while patients with previous cholecystectomy showed similar median values of liver enzymes. This result seems to identify patients without prior cholecystectomy and with increased transaminases as those at greatest risk of biliary pancreatitis and suggests that these patients could benefit from EUS as the first diagnostic test, eventually followed by ERCP in the same session if the diagnosis is confirmed[26-28].
Chronic pancreatitis was the most frequent diagnosis overall, with similar frequencies after all combinations of previous cross-sectional imaging techniques. This data is in line with the current evidence that EUS has the highest diagnostic performance in the identification of chronic pancreatitis features[29,30]. This is especially true in the setting of early chronic pancreatitis where thanks to the high resolution, EUS may detect subtle parenchymal and ductal changes such as irregular ductal contour, side branch ectasia ≥1 mm and parenchymal lobularity, which are minor diagnostic criteria according to the Rosemont criteria[31-34]. When differentiating between single episode or recurrent pancreatitis at baseline, diagnosis of chronic pancreatitis was much more frequent in patients with recurrent forms; this result supports the use of EUS as the first diagnostic technique for the identification of AP etiology in this subgroup of patients.
Regarding solid lesions, all pancreatic carcinomas missed at CECT were 25 mm or less in size. This data agrees with previous evidence showing a superiority of EUS over CECT for the diagnosis of small pancreatic lesions[35-38]. Interestingly, the percentage of solid lesions identified at EUS was similar in groups with or without previous MRCP, suggesting that this technique does not improve the ability to diagnose small pancreatic lesions. The identification of solid pancreatic lesions, as well as cholelithiasis or choledocholithiasis, not seen at previous examinations is of paramount importance since it significantly changes the patient management and particularly the referral to surgery or ERCP. This is especially true for small pancreatic cancers, which may be suitable for curative treatment. Most cystic lesions were instead diagnosed after US and/or CECT failure. Indeed, as already demonstrated, MRCP and EUS have comparable diagnostic accuracy for the assessment of cystic lesions[39], although EUS can better identify some high-risk or worrisome features such as enhancing mural nodules or thickened or enhancing cyst walls[40].
Pancreatic duct anomalies, including pancreas divisum and anomalous pancreaticobiliary junction, were diagnosed at EUS in about 10% of cases. This percentage was the same even after the combination of CECT and MRCP, corroborating a high sensitivity of EUS in obtaining a detailed study of the distal portion of the pancreatic duct, as already reported in the literature[41,42]. In the meta-analysis by Wan et al[22], EUS and MRCP were equally effective in identifying pancreas divisum, while MRCP after secretin stimulation was superior to both techniques. However, due to increased costs and practical issues, secretin-enhanced MRCP has failed to gain widespread United States use across radiology practices[43] and is not routinely performed in our center.
Incidence of further AP episodes during the follow-up was low (3%) and related to non-modifiable causes (one idiopathic form and one pancreatic duct anomaly). The endoscopic treatment of all choledocholithiasis, followed by cholecystectomy when necessary, and of chronic pancreatitis when indicated may have contributed to reducing the risk of pancreatitis recurrence.
The strengths of the study were the homogeneity of the population, the availability of detailed clinical information and the availability of a long follow-up period after the treatment approach. The main limitations were the small sample size and the retrospective nature of the study, with the need of prospective, multicentric studies in order to delineate a diagnostic algorithm that optimizes the use of EUS in AP.
In conclusion, our study supports the role of EUS as the technique of choice in IAP after failure of one or more cross-sectional techniques including CECT and MRCP. We suggest the use of EUS as the first-level technique in patients presenting with increased liver enzymes and with no previous cholecystectomy and in the setting of recurrent pancreatitis. Given its high diagnostic yield, we also propose EUS as the first-line investigation in all suitable patients presenting with IAP. Finally, larger and prospective studies investigating not only the diagnostic but also the prognostic value of EUS in IAP are needed.
Idiopathic acute pancreatitis (IAP) is a common condition and represents a diagnostic challenge because up to 20% of patients with IAP have recurrent episodes and may evolve to chronic pancreatitis. Endoscopic ultrasound (EUS) is highly effective in the etiological diagnosis of IAP, even after failure of a previous imaging technique. A significant proportion of AP remains idiopathic even after multiple imaging techniques, mainly including abdominal US, contrast-enhanced computed tomography (CECT) and magnetic resonance cholangiopancreatography (MRCP).
The role of EUS in IAP has been established by multiple studies, including meta-analyses. However, limited data are currently available about the diagnostic gain of EUS in cases of failure of multiple previous imaging techniques.
The primary aim of the study was to evaluate the diagnostic gain of EUS after failure of US, CECT and MRCP and particularly after different combination of these techniques. The secondary aims were to assess the overall EUS diagnostic yield in IAP, to associate the baseline clinical features with the specific IAP diagnosis and to analyze the frequency and types of AP recurrence during the follow-up.
We performed a retrospective, single-center study. We enrolled all consecutive adult patients undergoing EUS for IAP over a 3-year period at the Ospedale Maggiore of Cremona. IAP was defined when a clear etiology could not be identified after a thorough medical history, complete blood tests and after performing at least one US, CECT or MRCP exam. The EUS diagnostic gain was calculated as the percentage of additional diagnoses obtained at EUS over the total number of patients undergoing US, CECT and/or MRCP.
Overall EUS diagnostic yield was 79%, with 21% of AP remaining idiopathic. This percentage is in line with the current literature. Gallstone disease and chronic pancreatitis were the most frequent diagnoses (20% and 31%, respectively). The EUS diagnostic gain over the associations of CECT + MRCP and US + CECT + MRCP was 63% and 68%, respectively. This is a relevant result that confirms the superiority of EUS in the etiological diagnosis of IAP, particularly in detecting microlithiasis and early signs of chronic pancreatitis. In patients without a previous cholecystectomy and with a final diagnosis of biliary pancreatitis, higher baseline median values of liver enzymes were found. Moreover, in patients with recurrent pancreatitis, chronic pancreatitis was the diagnosis in 82% of cases. These results suggest a high efficacy of EUS in the etiological diagnosis of IAP in patients without previous cholecystectomy and with recurrent pancreatitis. During a median follow-up of 31.5 mo, an additional episode of pancreatitis was observed in 3.7% of patients.
EUS has a high diagnostic yield in IAP. About two-thirds of patients with IAP without etiological diagnosis with various combinations of US, CECT and MRCP received a diagnosis at EUS. This finding confirms the superiority of EUS over these techniques and proposes EUS as the investigation of first choice in all suitable patients. EUS shows the highest diagnostic gain in the setting of increased liver enzymes with no previous cholecystectomy and in the setting of recurrent pancreatitis.
The role of EUS in the etiological diagnosis of IAP has been established by multiple studies including meta-analyses. Our study provided additional data supporting the high diagnostic gain of EUS in cases of failure of multiple previous imaging techniques. Future research should focus on the prognostic value of EUS in the setting of IAP, since patient management may change following the EUS diagnosis. Large multicentric and prospective studies addressing this issue are needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alali AA, Kuwait; Jin ZD, China; Kobayashi K, Japan A-Editor: Zhu JQ, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 522] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 2. | Singh VK, Bollen TL, Wu BU, Repas K, Maurer R, Yu S, Mortele KJ, Conwell DL, Banks PA. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 4. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 5. | Guda NM, Trikudanathan G, Freeman ML. Idiopathic recurrent acute pancreatitis. Lancet Gastroenterol Hepatol. 2018;3:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol. 2001;96:2540-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 597] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 8. | Umans DS, Rangkuti CK, Sperna Weiland CJ, Timmerhuis HC, Bouwense SAW, Fockens P, Besselink MG, Verdonk RC, van Hooft JE; Dutch Pancreatitis Study Group. Endoscopic ultrasonography can detect a cause in the majority of patients with idiopathic acute pancreatitis: a systematic review and meta-analysis. Endoscopy. 2020;52:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Kondo S, Isayama H, Akahane M, Toda N, Sasahira N, Nakai Y, Yamamoto N, Hirano K, Komatsu Y, Tada M, Yoshida H, Kawabe T, Ohtomo K, Omata M. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol. 2005;54:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Smith I, Ramesh J, Kyanam Kabir Baig KR, Mönkemüller K, Wilcox CM. Emerging Role of Endoscopic Ultrasound in the Diagnostic Evaluation of Idiopathic Pancreatitis. Am J Med Sci. 2015;350:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Valverde-López F, Ortega-Suazo EJ, Wilcox CM, Fernandez-Cano MC, Martínez-Cara JG, Redondo-Cerezo E. Endoscopic ultrasound as a diagnostic and predictive tool in idiopathic acute pancreatitis. Ann Gastroenterol. 2020;33:305-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Tepox-Padrón A, Bernal-Mendez RA, Duarte-Medrano G, Romano-Munive AF, Mairena-Valle M, Ramírez-Luna MÁ, Marroquin-Reyes JD, Uscanga L, Chan C, Domínguez-Rosado I, Hernandez-Calleros J, Pelaez-Luna M, Tellez-Avila F. Utility of endoscopic ultrasound in idiopathic acute recurrent pancreatitis. BMJ Open Gastroenterol. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Somani P, Sunkara T, Sharma M. Role of endoscopic ultrasound in idiopathic pancreatitis. World J Gastroenterol. 2017;23:6952-6961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Tenner S, Baillie J, DeWitt J, Vege SS, Gastroenterology ACo. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1415. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1377] [Article Influence: 114.8] [Reference Citation Analysis (3)] |
| 15. | Diehl AK, Holleman DR Jr, Chapman JB, Schwesinger WH, Kurtin WE. Gallstone size and risk of pancreatitis. Arch Intern Med. 1997;157:1674-1678. [PubMed] |
| 16. |
Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, et al EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification.
|
| 17. | Vila JJ. Endoscopic ultrasonography and idiopathic acute pancreatitis. World J Gastrointest Endosc. 2010;2:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Lai R, Freeman ML, Cass OW, Mallery S. Accurate diagnosis of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopy. 2004;36:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1055] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 20. | Norton SA, Alderson D. Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis. Br J Surg. 2000;87:1650-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Vila JJ, Vicuña M, Irisarri R, de la Higuera BG, Ruiz-Clavijo D, Rodríguez-Gutiérrez C, Urman JM, Bolado F, Jiménez FJ, Arín A. Diagnostic yield and reliability of endoscopic ultrasonography in patients with idiopathic acute pancreatitis. Scand J Gastroenterol. 2010;45:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Wan J, Ouyang Y, Yu C, Yang X, Xia L, Lu N. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:1180-1188.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Ortega AR, Gómez-Rodríguez R, Romero M, Fernández-Zapardiel S, Céspedes MeM, Carrobles JM. Prospective comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the etiological diagnosis of "idiopathic" acute pancreatitis. Pancreas. 2011;40:289-294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Meeralam Y, Al-Shammari K, Yaghoobi M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: a meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest Endosc. 2017;86:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 518] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 26. |
Vila JJ, Kutz M, Goñi S, Ostiz M, Amorena E, Prieto C, et al Endoscopic and anesthetic feasibility of EUS and ERCP combined in a single session vs two different sessions.
|
| 27. | Benjaminov F, Stein A, Lichtman G, Pomeranz I, Konikoff FM. Consecutive vs separate sessions of endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) for symptomatic choledocholithiasis. Surg Endosc. 2013;27:2117-2121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Fuccio L, Billi P, Maimone A, Ghersi S, Macchia S, Mwangemi C, Consolo P, Zirilli A, Eusebi LH, D'Imperio N. Single session vs separate session endoscopic ultrasonography plus endoscopic retrograde cholangiography in patients with low to moderate risk for choledocholithiasis. J Gastroenterol Hepatol. 2009;24:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Issa Y, Kempeneers MA, van Santvoort HC, Bollen TL, Bipat S, Boermeester MA. Diagnostic performance of imaging modalities in chronic pancreatitis: a systematic review and meta-analysis. Eur Radiol. 2017;27:3820-3844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Pungpapong S, Wallace MB, Woodward TA, Noh KW, Raimondo M. Accuracy of endoscopic ultrasonography and magnetic resonance cholangiopancreatography for the diagnosis of chronic pancreatitis: a prospective comparison study. J Clin Gastroenterol. 2007;41:88-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Stevens T, Parsi MA. Endoscopic ultrasound for the diagnosis of chronic pancreatitis. World J Gastroenterol. 2010;16:2841-2850. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 32. | Takasaki Y, Ishii S, Fujisawa T, Ushio M, Takahashi S, Yamagata W, Ito K, Suzuki A, Ochiai K, Tomishima K, Saito H, Isayama H. Endoscopic Ultrasonography Findings of Early and Suspected Early Chronic Pancreatitis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Dominguez-Munoz JE, Drewes AM, Lindkvist B, Ewald N, Czakó L, Rosendahl J, Löhr JM; HaPanEU/UEG Working Group. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology. 2018;18:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 34. | Kamat R, Gupta P, Rana S. Imaging in chronic pancreatitis: State of the art review. Indian J Radiol Imaging. 2019;29:201-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K, LeBlanc J, McHenry L, Madura J, Aisen A, Cramer H, Cummings O, Sherman S. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 332] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Michl P, Pauls S, Gress TM. Evidence-based diagnosis and staging of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:227-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Yasuda K, Mukai H, Nakajima M. Endoscopic ultrasonography diagnosis of pancreatic cancer. Gastrointest Endosc Clin N Am. 1995;5:699-712. [PubMed] |
| 38. | Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, Broemeling LD, Cleary KR, Charnsangavej C. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Kim YC, Choi JY, Chung YE, Bang S, Kim MJ, Park MS, Kim KW. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR Am J Roentgenol. 2010;195:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Lu X, Zhang S, Ma C, Peng C, Lv Y, Zou X. The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Endosc Ultrasound. 2015;4:324-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Shen Z, Munker S, Zhou B, Li L, Yu C, Li Y. The Accuracies of Diagnosing Pancreas Divisum by Magnetic Resonance Cholangiopancreatography and Endoscopic Ultrasound: A Systematic Review and Meta-analysis. Sci Rep. 2016;6:35389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. |
Kushnir VM, Wani SB, Fowler K, Menias C, Varma R, Narra V, et al Sensitivity of endoscopic ultrasound, multidetector computed tomography, and magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a tertiary center experience.
|
| 43. | Swensson J, Zaheer A, Conwell D, Sandrasegaran K, Manfredi R, Tirkes T. Secretin-Enhanced MRCP: How and Why-AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |