Published online May 16, 2022. doi: 10.4253/wjge.v14.i5.335
Peer-review started: December 6, 2021
First decision: January 12, 2022
Revised: January 25, 2022
Accepted: April 26, 2022
Article in press: April 26, 2022
Published online: May 16, 2022
Processing time: 160 Days and 17 Hours
Endoscopic ultrasonography (EUS) has evolved in the last years making it not only a diagnostic modality but a therapeutic procedure. EUS is now used as an alternative technique to percutaneous and surgical drainage. Even though EUS is a challenging procedure and not always suitable compared to percutaneous drainage, there is a need for developing new therapeutic approaches to the liver for when percutaneous drainage is not feasible.
We present the case of a 82 years old male who developed an infected subcapsular hepatic hematoma (SHH) of the left lobe following percutaneous biliary drainage. After 2 failed attempts of percutaneous drainage of the SHH and because the patients couldn’t withstand surgery, we conducted a EUS drainage and debridement of the SHH. Using a lumen apposing metal stent (LAMS) by a transgastric approach, we were able to gain endoscopic access to the SHH. With our experience in the debridement of walled off pancreatic necrosis using this technique, we were confident it was the right approach. After four debridement sessions, the computed tomography scan showed a clear regression of the SHH.
To our knowledge, this is the first case of successful endoscopic debridement of a SHH using a LAMS which appear to be feasible and safe in this specific case.
Core Tip: We conducted an endoscopic ultrasonography drainage and debridement of a subcapsular hepatic hematoma (SHH). Using a lumen apposing metal stent (LAMS) with a transgastric approach, we were able to gain endoscopic access to the SHH. With our experience in the debridement of walled off pancreatic necrosis using this technique, we were confident it was the right approach. After four sessions of debridement, the computed tomography scan showed a clear regression of the SHH. To our knowledge, this is the first case of successful endoscopic debridement of a SHH using a LAMS which appear to be feasible and safe in this specific case.
- Citation: Doyon T, Maniere T, Désilets É. Endoscopic ultrasonography drainage and debridement of an infected subcapsular hepatic hematoma: A case report. World J Gastrointest Endosc 2022; 14(5): 335-341
- URL: https://www.wjgnet.com/1948-5190/full/v14/i5/335.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i5.335
Endoscopic ultrasonography (EUS) has evolved making it more and more a therapeutic procedure[1-3]. For instance, it is now used for drainage of abscesses or hematomas when the first line of treatment that is percutaneous drainage is not feasible or has failed[1,4-7] or for gallbladder drainage for cases of refractory acute cholecystitis in the elderly who can’t withstand surgery[8]. EUS is now used as an alternative technique to surgical drainage which is highly invasive, making EUS more favorable in term of procedural complications[1]. Percutaneous drainage, despite its high success rate also has its complications: Bleeding, perforation, peritonitis, fistula, sepsis and hematomas like subcapsular hepatic hematoma (SHH)[4,5,9]. Even though EUS is a challenging procedure and not always suitable compared to percutaneous drainage[5], there is a need for developing new therapeutic approaches to the liver when percutaneous drainage is not feasible[5] thus preventing the use of surgical drainage and its potential complications[1]. SHH can be a life-threatening situation[9-13]. SHH are traditionally managed conservatively with antibiotics and pain management[4,11,12,14]. However, when the SHH is persistent, becomes infected or worsens, it can be treated by percutaneous drainage and in case of failure by surgical drainage[4,5,13].
In walled off pancreatic necrosis (WOPN), debridement of the necrosis can be done surgically or by EUS which is less at risk of complications compared to conventional surgery[3,15,16]. The usual procedure for the drainage and debridement of WOPN is a puncture of the collection under EUS and dilation of the track using a cystotome or a balloon[15,16]. Endoscopic drainage of WOPN is then assured by the placement of multiple double pigtail stents or by installing a lumen apposing metal stent (LAMS) under EUS and use the stent as an access to get inside the necrosis for debridement of the WOPN[15]. Knowing that surgical drainage of SHH is an invasive and risky procedure, that the site of the hematoma can make percutaneous drainage difficult[1,4,5], that EUS drainage of a liver abscess is an effective and successful method to drain difficult to access abscess using a transgastric or transduodenal approach[4,5,7] and that EUS is used in debridement of WOPN[15,16]; we hypothesized that debridement of a SHH using EUS could be successful.
We report the case of a 82 years old male, known for a pancreatic cystic lesion under punctual surveillance by EUS.
The patient has a pancreatic cystic lesion under punctual surveillance by EUS.
The history of past illness are chronic kidney failure, hypertension, type 2 diabetes, dyslipidemia and coronary artery disease for which he took medication.
None personal or family history.
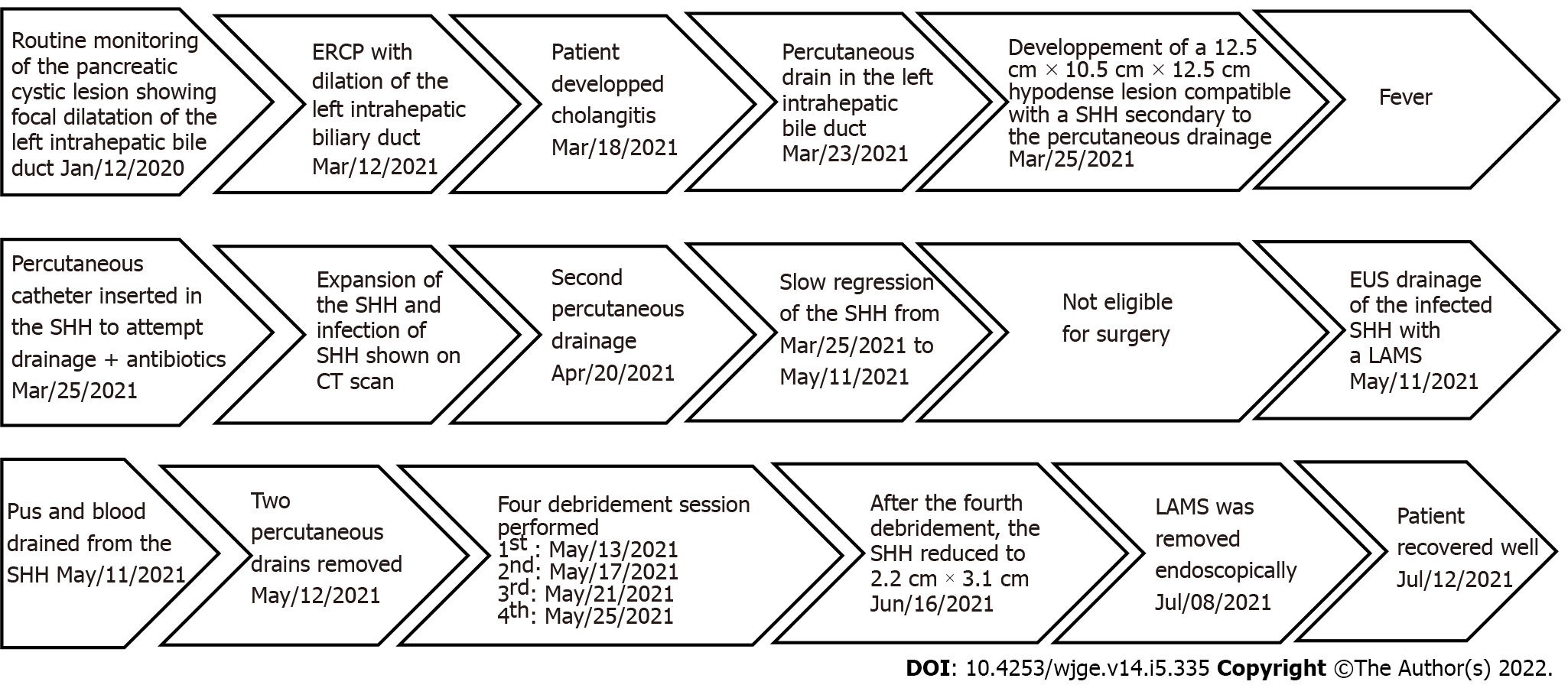
During a routine monitoring of the pancreatic cystic lesion, EUS revealed a focal dilatation of the left intrahepatic bile duct.
His laboratory tests showed white blood cells at 10.9 × 109/L, hemoglobin at 109 g/L, bilirubin at 23 μmol/L, alkaline phosphatase 231 U/L, aspartate aminotransferase 70 U/L, alanine aminotransferase 134 U/L and CA199 at 315 kU/L. Hours after the percutaneous drainage, the patient developed right upper quadrant pain and the hemoglobin level went down to 62 g/L.
Sequential endoscopic retrograde cholangiopancreatography was performed with cytology brushing and dilatation of a left intrahepatic biliary stricture followed by deployment of a 15 cm 8.5 Fr plastic stent in that area. A percutaneous drain in the left intrahepatic bile duct was then added in radiology.
The patient developed cholangitis.
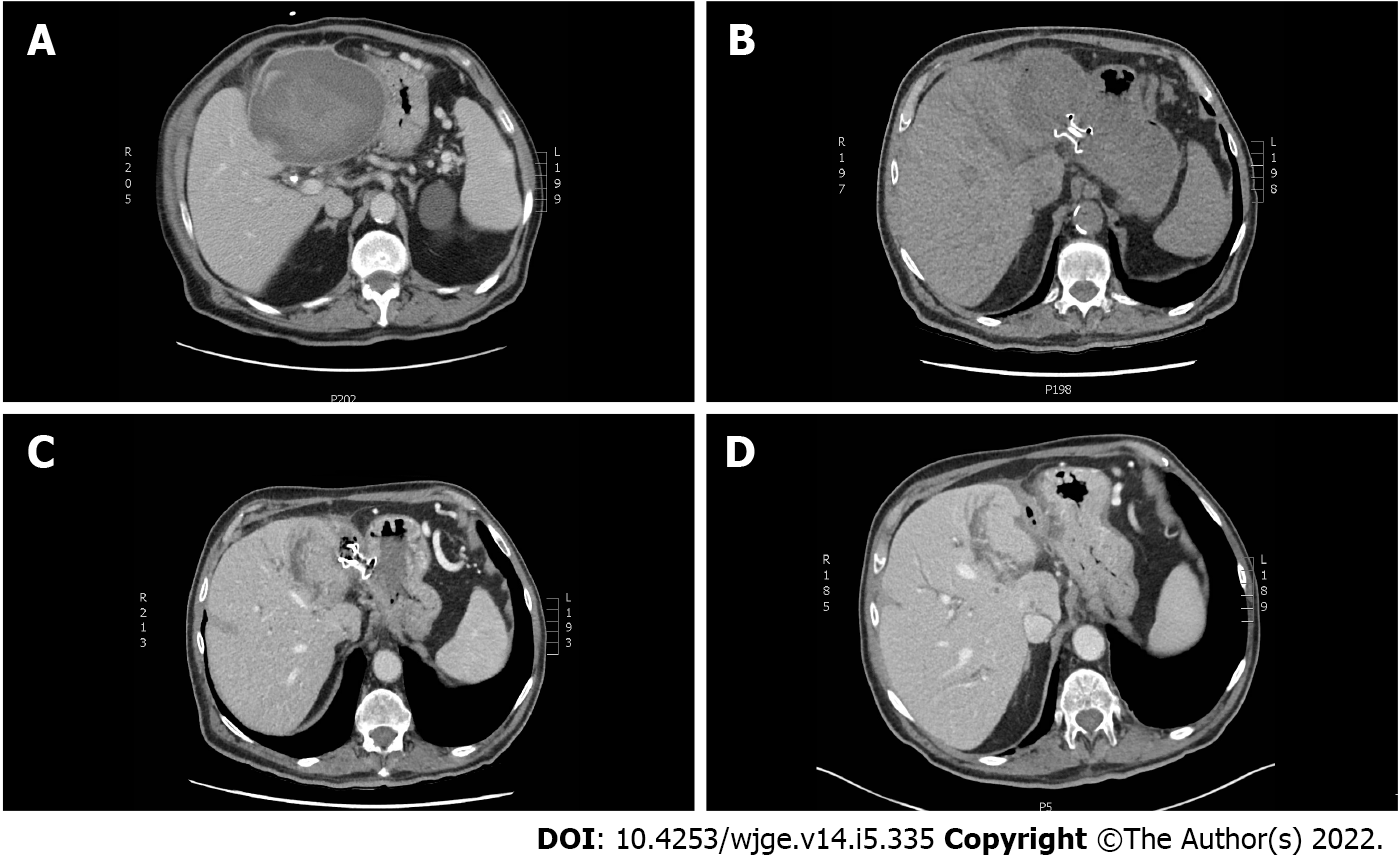
A control computed tomography (CT) scan revealed a 12.5 cm × 10.5 cm × 12.5 cm hypodense lesion compatible with a SHH in the left lobe (segment 3) (Figure 1). The patient was sent back in radiology and there was no active bleeding or pseudoaneurysm during the arteriography. Over the next days the patient developed a fever. A percutaneous 10 Fr catheter was inserted in the hematoma to attempt drainage and was repositioned once. Only a modest amount of bloody fluid was collected (150 mL). After a month of conservative treatment and a failed attempt to wean the patient from antibiotics, a control CT scan showed an expansion of the SHH with air bubbles within. Percutaneous drainage was again performed in radiology using a multiperforated 10 Fr stent and drained 100 cc of bloody liquid. Control CT showed a slow regression of the SHH and a thick wall around it.
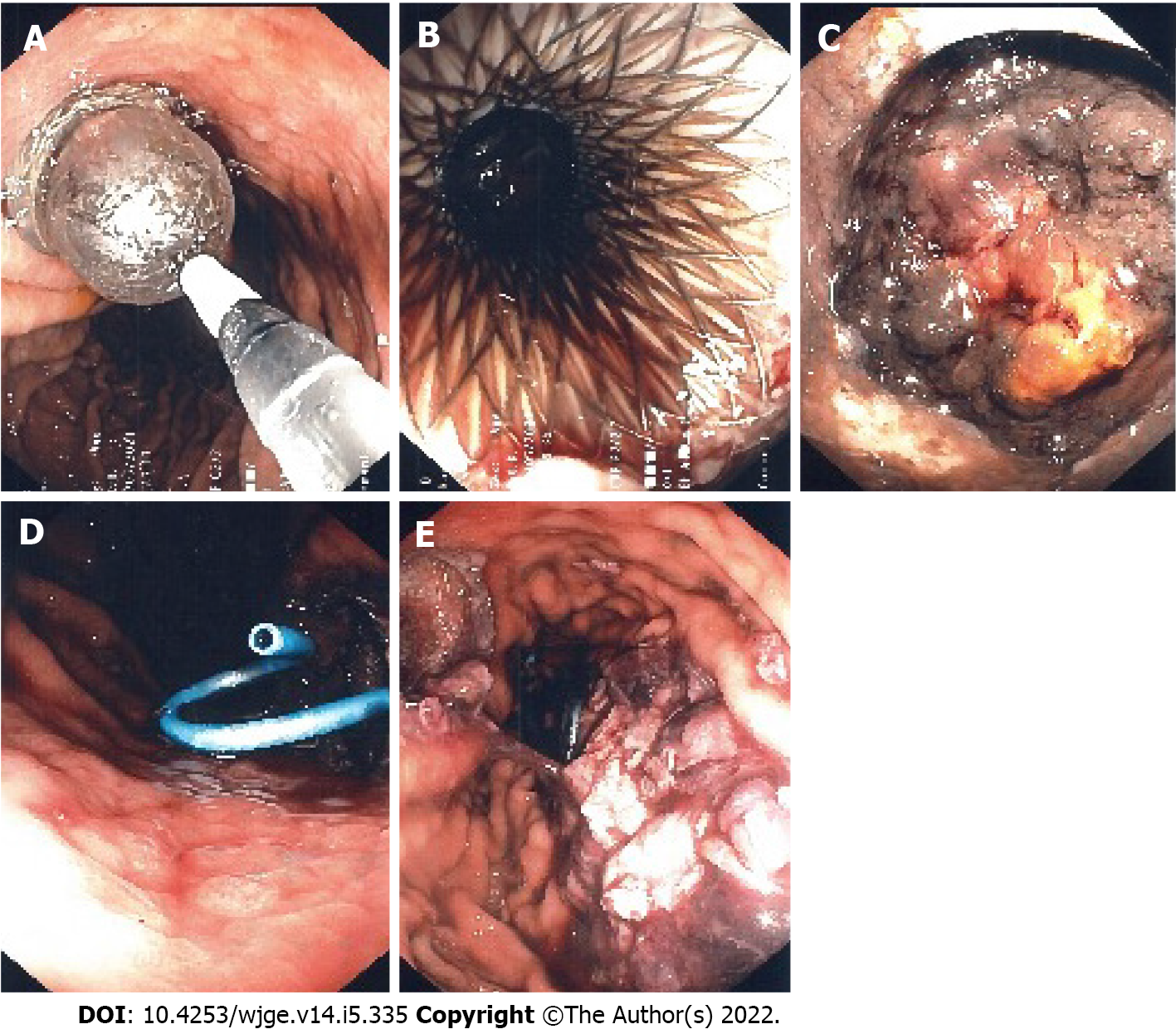
Seeing the slow rate of resorption of the infected SHH, a consultation in hepatobiliary surgery was obtained but the patient was deemed too sick to withstand surgery. After consent from the patient, we decided to perform a EUS drainage of the infected SHH with a 10 mm × 15 mm LAMS (Hot-Axios, Boston scientific) by a transgastric approach under conscious sedation. The collection appeared heterogenous, surrounded by a thick wall and very close to the stomach smaller curvature. Considering the location of the SHH, the puncture was easy, and deployment of the LAMS was done using the standard Seldinger technique. Pus and blood were drained from the hematoma into the stomach immediately after deployment. After the procedure, the patient recovered well, with no adverse event. The two percutaneous drains were removed. The following day, the first of four debridement sessions under conscious sedation were performed with a standard gastroscope through the LAMS (Figure 2). Dilatation of the LAMS at 18 mm was needed at the first debridement. Each debridement session lasted between 30-35 min. Informed consent was obtained before each session. At the end of each debridement, a double-sided pigtail 7 Fr drain was installed inside the LAMS stent to help drain the SHH and maintain position and patency.
After the fourth debridement, the endoscopic appearance of the SHH cavity was clean with whitish walls and a CT scan revealed a massive regression of the SHH (2.2 cm × 3.1 cm); showing that the EUS procedure was a success. The LAMS was then removed endoscopically and the fistula between the stomach and the SHH closed immediately. The patient recovered well (Figure 3).
SHH is an “accumulation of blood between the Glisson’s capsule and the liver parenchyma; rupture into the peritoneum has a 75% mortality rate”[10] which makes it life threatening[11]. In this case, the SHH was present for more than 3 mo, giving it time to organize itself and coagulate making it refractory to percutaneous drainage. Moreover, the SHH was infected, and the patient was under antibiotics for 6 wk without any success. Finally, the patient couldn’t withstand surgery, so we had no choice but to try EUS drainage as a therapeutic procedure.
Important factors helped us choose this approach: The patient didn’t have any coagulopathy; the encapsulated look and thick walls of the SHH; the anatomy of this region and the proximity of the SHH, in segment 3 of the liver, with the small curvature of the stomach; the absence of pseudoaneurysm or active bleeding on the arteriogram and our experience in the debridement of WOPN. Altogether, it made us confident that EUS drainage and debridement under conscious sedation was the right approach. This way we were able to use a known and proven technique to a novel situation (i.e., SHH). The procedure was a success, since after drainage and debridement, there was a significant reduction in the volume of the SHH (Figure 1).
This makes it the first EUS drainage and debridement of a SHH to our knowledge in the medical literature. We warn that this technique may be used only in cases where the collection is near the gastric or duodenal wall and when there is an experienced endoscopist who has competence in therapeutic EUS. The use of a naso-cystic tube to improve irrigation and shorten the resolution of SHH is debatable. Those tube are used also for common bile duct infection but are not well tolerated by patients. We decided to keep the LAMS in place for 2 mo to maintain the fistula wide open and make the access to the SHH easier. We removed it after the fourth debridement when the SHH was resolved. It is usually advised to remove those stents after 4-6 wk to avoid potential bleeding due to mucosal erosion[17].
There are many risks associated with the procedure. Aside from the general risks related to endoscopic anesthesia (respiratory failure, aspiration), the specific risk are bile leak, bleeding, infection, perforation, peritonitis and death. To assess and minimize the bleeding risk, doppler was used before the first endoscopic access to avoid any vascular structure in the gastric wall. The SHH was scanned with multiphasic acquisitions to rule out the presence of a pseudoaneurysm. If significant bleeding was to happen, we would have referred to angiography and arterial embolization. For peritonitis, the decision to send the patient to the operating room to proceed with conservative management would have been based on the severity and extent on imaging studies.
Furthermore, since the access to the SHH was in the smaller curvature, there was a potential risk of reflux of digestive flora into the SHH. This is a potential risk of all trans-gastric drainage techniques for which the consequences are unknown to our knowledge. Some have stated that it could be beneficial in the way that stomach acidity can provide a kind of chemical debridement [some even stop proton pump inhibitors (PPIs) between sessions of pancreatic necrosis debridement][18]; others fear potential supra-infection from the digestive flora and food relux from the digestive lumen[19]. In our case, the patient remained on large spectrum IV antibiotics from the first to the last endoscopic intervention to prevent supra-infection. PPIs were maintained.
We did not study the cost effectiveness of this approach compared to surgery. This is certainly an interesting question. Surgery remains for us the gold standard for refractory SHH; we proceeded this way because the risk of surgery was too high in our case. In the future, we think that EUS should be considered along the other modalities (surgery and radiological drainage) for the treatment of all kinds of peri-digestive infections (pseudocyst, pancreatic necrosis, liver and perihepatic abscesses, acute cholecystitis). The choice of the best modality should be based on available scientific data, specific risks for the patient, local expertise, and availability of the technology.
There are many potential advantages to the use of EUS: It is less invasive than surgery, there is no need for a transcutaneous tube or collecting bag, it can be a permanent drainage (ex: For gallbladders and pseudocyst) and larger stents allow for potential endoscopic debridement if needed. However, the lack of availability and expertise and the cost of material and technology make using EUS as a therapeutical option challenging.
To our knowledge, this is the first case of successful endoscopic debridement of a SHH using a LAMS which appear to be feasible and safe in this specific case. Thus, EUS drainage of an infected SHH seems like an alternative therapeutic approach to consider, but clinical indications remain to be defined. More experience from other centers around the world will be needed before applying this treatment in a widespread fashion.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cen LS, China; Ghannam WM, Egypt; Peltec A, Moldova; Sugimoto M, Japan; Zharikov YO, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Morita S, Kamimura K, Suda T, Oda C, Hoshi T, Kanefuji T, Yagi K, Terai S. Endoscopic ultrasound-guided transmural drainage for subphrenic abscess: report of two cases and a literature review. BMC Gastroenterol. 2018;18:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Campos S, Poley JW, van Driel L, Bruno MJ. The role of EUS in diagnosis and treatment of liver disorders. Endosc Int Open. 2019;7:E1262-E1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Braden B, Gupta V, Dietrich CF. Therapeutic EUS: New tools, new devices, new applications. Endosc Ultrasound. 2019;8:370-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Chin YK, Asokkumar R. Endoscopic ultrasound-guided drainage of difficult-to-access liver abscesses. SAGE Open Med. 2020;8:2050312120921273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Singhal S, Changela K, Lane D, Anand S, Duddempudi S. Endoscopic ultrasound-guided hepatic and perihepatic abscess drainage: an evolving technique. Therap Adv Gastroenterol. 2014;7:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Fei BY, Li CH. Subcapsular hepatic haematoma after endoscopic retrograde cholangiopancreatography: an unusual case. World J Gastroenterol. 2013;19:1502-1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Carvajal JJ, Betancur Salazar K, Mosquera-Klinger G. Transgastric drainage of a liver abscess through endoscopic ultrasound in a patient with multiple organ failure. Rev Gastroenterol Mex (Engl Ed). 2021;86:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Quencer KB, Tadros AS, Marashi KB, Cizman Z, Reiner E, O'Hara R, Oklu R. Bleeding after Percutaneous Transhepatic Biliary Drainage: Incidence, Causes and Treatments. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Small AJ, Irani S. Endoscopic ultrasound gallbladder drainage: Patient selection, preparation, and performance. Techniques Gastrointest Endosc. 2017;19:230234. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Ndzengue A, Hammoudeh F, Brutus P, Ajah O, Purcell R, Leadon J, Rafal RB, Balmir S, Enriquez DA, Posner GL, Jaffe EA, Chandra P. An obscure case of hepatic subcapsular hematoma. Case Rep Gastroenterol. 2011;5:223-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Zappa MA, Aiolfi A, Antonini I, Musolino CD, Porta A. Subcapsular hepatic haematoma of the right lobe following endoscopic retrograde cholangiopancreatography: Case report and literature review. World J Gastroenterol. 2016;22:4411-4415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | García Tamez A, López Cossio JA, Hernández Hernández G, González Huezo MS, Rosales Solís AA, Corona Esquivel E. Subcapsular hepatic hematoma: An unusual, but potentially life-threating post-ERCP complication. Case report and literature review. Endoscopia. 2016;28:75-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Corazza LR, D’Ambrosio L, D’Ascoli B, Dilorenzo MF. Subcapsular Hepatic Hematoma. Is it still an unusual Complication Post ERC? Gastroenterol Hepatol Open Access. 2017;6:149-152. [DOI] [Full Text] |
| 14. | Brown V, Martin J, Magee D. A rare case of subcapsular liver haematoma following laparoscopic cholecystectomy. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Jha AK, Goenka MK, Kumar R, Suchismita A. Endotherapy for pancreatic necrosis: An update. JGH Open. 2019;3:80-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Jagielski M, Smoczyński M, Adrych K. The role of endoscopic ultrasonography in transmural drainage/debridement of walled-off pancreatic necrosis. Prz Gastroenterol. 2018;13:160-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Ahmad W, Fehmi SA, Savides TJ, Anand G, Chang MA, Kwong WT. Protocol of early lumen apposing metal stent removal for pseudocysts and walled off necrosis avoids bleeding complications. Scand J Gastroenterol. 2020;55:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Thompson CC, Kumar N, Slattery J, Clancy TE, Ryan MB, Ryou M, Swanson RS, Banks PA, Conwell DL. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology. 2016;16:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Kim JJ, Hiotis SP, Sur MD. Gastric Reflux Into the Gallbladder After EUS-guided Stenting-Letter to the Editor Regarding "EUS-guided Versus Percutaneous Gallbladder Drainage: Isn't It Time to Convert? J Clin Gastroenterol. 2019;53:392-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |