Published online May 16, 2022. doi: 10.4253/wjge.v14.i5.320
Peer-review started: December 4, 2021
First decision: January 8, 2022
Revised: February 11, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 16, 2022
Processing time: 162 Days and 23.8 Hours
The diagnosis of residual tumors using endoscopic ultrasound (EUS) after neoadjuvant therapy for esophageal cancer is considered challenging. However, the reasons for this difficulty are not well understood.
To investigate the ultrasound imaging features of residual tumors and identify the limitations and potential of EUS.
This exploratory prospective observational study enrolled 23 esophageal squamous cell carcinoma patients receiving esophagectomy after neoadjuvant therapy [15 patients after neoadjuvant chemotherapy (NAC) and 8 patients after chemoradiotherapy (CRT)] at the Department of Surgery, Chiba University Hospital, between May 2020 and October 2021. We diagnosed the T stage for specimens using ultrasound just after surgery and compared ultrasound images with the cut surface of the fixed specimens of the same level of residual tumor. The ratio of esophageal muscle layer defect measured by ultrasound was compared with clinicopathological factors. Furthermore, the rate of reduction for the muscle layer defect was evaluated using EUS images obtained before and after neoadjuvant therapy.
The accuracy of T stage rate was 61% (n = 14/23), which worsened after CRT (38%, n = 3/8) than after NAC (73%, n = 11/15) because of overstaging. Moreover, pT0 could not be diagnosed in all cases. The detection rate of residual tumor for specimens using ultrasound retrospectively was 75% (n = 15/20). There was no correlation between after-NAC (79%, n = 11/14) and after-CRT (67%, n = 4/6) detection rate. The detection of superficial and submucosal types was poor. The pathologic tumor size and pathological response were correlated. Tumor borders were irregular and echogenicity was mixed type after CRT. There was a correlation between the pT stage (pT0/1 vs pT2/3) and the length of muscle layer circumference (P = 0.025), the length of muscle layer defect (P < 0.001), and the ratio of muscle layer defect (P < 0.001). There was also a correlation between the pT stage and the rate of muscle layer defect reduction measured by EUS (P = 0.001).
Compared to pathological images, some tumors are undetectable by ultrasound. Focusing on the esophageal muscle layer might help diagnose the depth of the residual tumor.
Core Tip: This exploratory prospective observational study evaluated the effectiveness of endoscopic ultrasound (EUS) in diagnosing residual tumors after neoadjuvant therapy for esophageal squamous cell carcinoma. It is well known that the diagnosis using EUS after neoadjuvant therapy is inaccurate. The results of ultrasound for surgical specimens are not satisfactory as well. Our study found that the inability to distinguish scar tissue from the tumor made detection and diagnosis impossible in some residual tumors. Esophageal muscle layer defect as an indirect finding correlated with the depth of the residual tumor. These insights could help improve the diagnosis of residual tumors.
- Citation: Yonemoto S, Uesato M, Nakano A, Murakami K, Toyozumi T, Maruyama T, Suito H, Tamachi T, Kato M, Kainuma S, Matsusaka K, Matsubara H. Why is endosonography insufficient for residual diagnosis after neoadjuvant therapy for esophageal cancer? Solutions using muscle layer evaluation. World J Gastrointest Endosc 2022; 14(5): 320-334
- URL: https://www.wjgnet.com/1948-5190/full/v14/i5/320.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i5.320
Esophageal cancer is the seventh most common cancer worldwide in terms of incidence and the sixth most common in terms of mortality. Especially in Asia, esophageal squamous cell carcinoma (ESCC) accounts for more than 90% of all esophageal cancers[1]. There is strong evidence supporting the superiority of neoadjuvant chemoradiotherapy (CRT) and neoadjuvant chemotherapy (NAC) plus surgery over surgery alone for locally advanced esophageal cancer[2]. In ESCC patients, pathological complete response (pCR) was 62% after CRT and 2%-7% after NAC[3-5]. While patients with pCR may have avoided unnecessary esophagectomy, the residual tumor must be accurately identified to justify not performing a surgical resection.
In contrast, residual tumors after CRT and NAC are often present only at a depth of the esophageal wall, without any exposure to the superficial mucosa[6,7]. Although Endoscopic ultrasound (EUS) has a well-established role in the initial staging of esophageal cancer[8], the diagnosis of esophageal cancer after neoadjuvant therapy has been controversial. EUS sensitivity for residual tumors at the primary site after neoadjuvant CRT is as high as 0.96; however, the specificity is as low as 0.08, and thus it does not seem to be sufficiently accurate to detect residual tumor[9]. In addition, the accuracy of staging after NAC is not sufficient[10]. Several studies have correlated EUS measurements with tumor regression grade and survival. However, it is unclear whether the echogenic lesions detected using EUS are indeed residual tumors and how they appear on ultrasound. The purpose of this study was to characterize the ultrasound images of residual tumors, explore the limitations of EUS, and assess its potential in residual diagnosis.
This exploratory prospective observational study was conducted in two steps. The first step (study 1) aimed to investigate the limitations and characteristics of residual tumor diagnosis using ultrasound. Based on study 1, the second step (study 2) aimed to implement EUS to detect remanent tumors deep in the muscle layer. Study 1 enrolled 23 ESCC patients undergoing esophagectomy after neoadjuvant therapy, including NAC or CRT in the Department of Surgery, Chiba University Hospital, between May 2020 and October 2021. All patients were histologically proven to have ESCC based on biopsy specimens. The clinical stage was determined by endoscopy, barium esophagography, chest and abdominal computed tomography (CT) scans, and 18F-fluorodeoxyglucose positron emission tomography, based on the 11th Edition of the Japanese Classification of Esophageal Cancer[11]. Study 2 enrolled 20 out of the initial 23 participants in the first study who underwent EUS for staging and were diagnosed with cT2 or deeper. Our Institutional Review Board (IRB No. 3550) approved this study. We obtained written informed consent from patients for all examinations and treatments.
As recommended by the Japanese Clinical Oncology Group (JCOG) 9907 Study, we performed preoperative chemotherapy postoperatively for patients with clinically UICC stage II/III resectable ESCC in our department's criteria[5]. NAC was composed of two cycles of 5-fluorouracil (800 mg/m2 infusion for five consecutive days) and cisplatin (80 mg/m2 on day 1). Some patients received three cycles of docetaxel (70 mg/m2 on day 1), cisplatin (70 mg/m2 on day 1) and 5-fluorouracil (750 mg/m2 infusion for five consecutive days) based on the JCOG 1109 study[12]. After NAC, all patients were evaluated by CT, PET, and endoscopy, and underwent radical esophagectomy with three-field lymphadenectomy, including cervical, mediastinal, and abdominal lymph node dissection. CRT was composed of 2 Gy/fraction at a total dose of 40 Gy with a long-T radiation field from the cricoid cartilage to the upper abdomen, including the gross tumor volume. Concurrent chemotherapy was performed with 5-fluorouracil (500 mg/m2 infusion on day 0-4) and cisplatin (15 mg/m2 on day 1-5). After receiving a 40 Gy dose, all patients were evaluated by CT, PET, and endoscopy. An additional 20 Gy dose was delivered to patients with potentially resectable tumors, making the total irradiation dose 60 Gy (definitive CRT), and concurrent chemotherapy with the same regimen was also provided. After CRT, patients with resectable tumors underwent radical esophagectomy with three-field lymphadenectomy four weeks after CRT. The criteria for the pathological response of primary tumor were categorized as ineffective (Grade 0); viable cancer cells accounted for 1/3 or more of tumor tissue (Grade 1); viable cancer cells accounted for less than 1/3 of tumor tissue (Grade 2); no viable cancer cells (Grade 3).
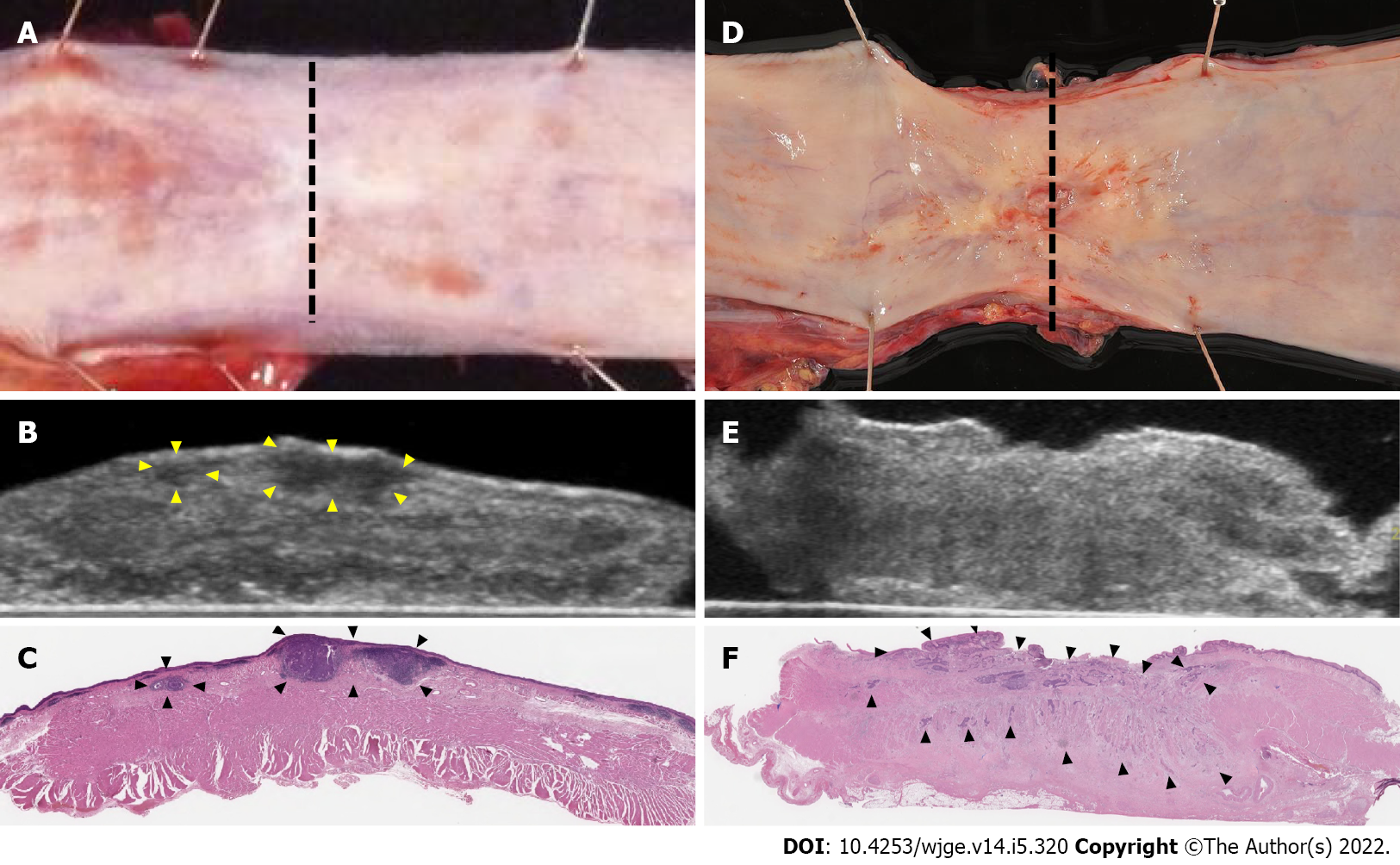
In study 1, the surgical specimens of all patients were collected from the operation room, and an ultrasound was performed immediately. The unfixed specimens immersed in saline solution were scanned vertically and horizontally using 15 MHz electronic linear ultrasound. The imaging procedure was recorded on video. We used LOGIQ S8 (GE Healthcare Japan Corporation, Tokyo, Japan) ultrasound platform in all studies. The ultrasound for specimens showed the mucosal layer, submucosal layer, inner muscle layer, intermuscular connective tissue layer, and outer muscle layer, as shown in EUS. We diagnosed the presence and depth of the tumor on the day of surgery before pathology results were known. We assessed the accuracy of diagnosing residual tumor depth using ultrasound. The prefix “u” indicates ultrasound diagnosis. Furthermore, to clarify the characteristic features of residual tumor, we compared ultrasound images with the cut surface of the fixed specimens at the same level of tumor site in the esophageal wall.
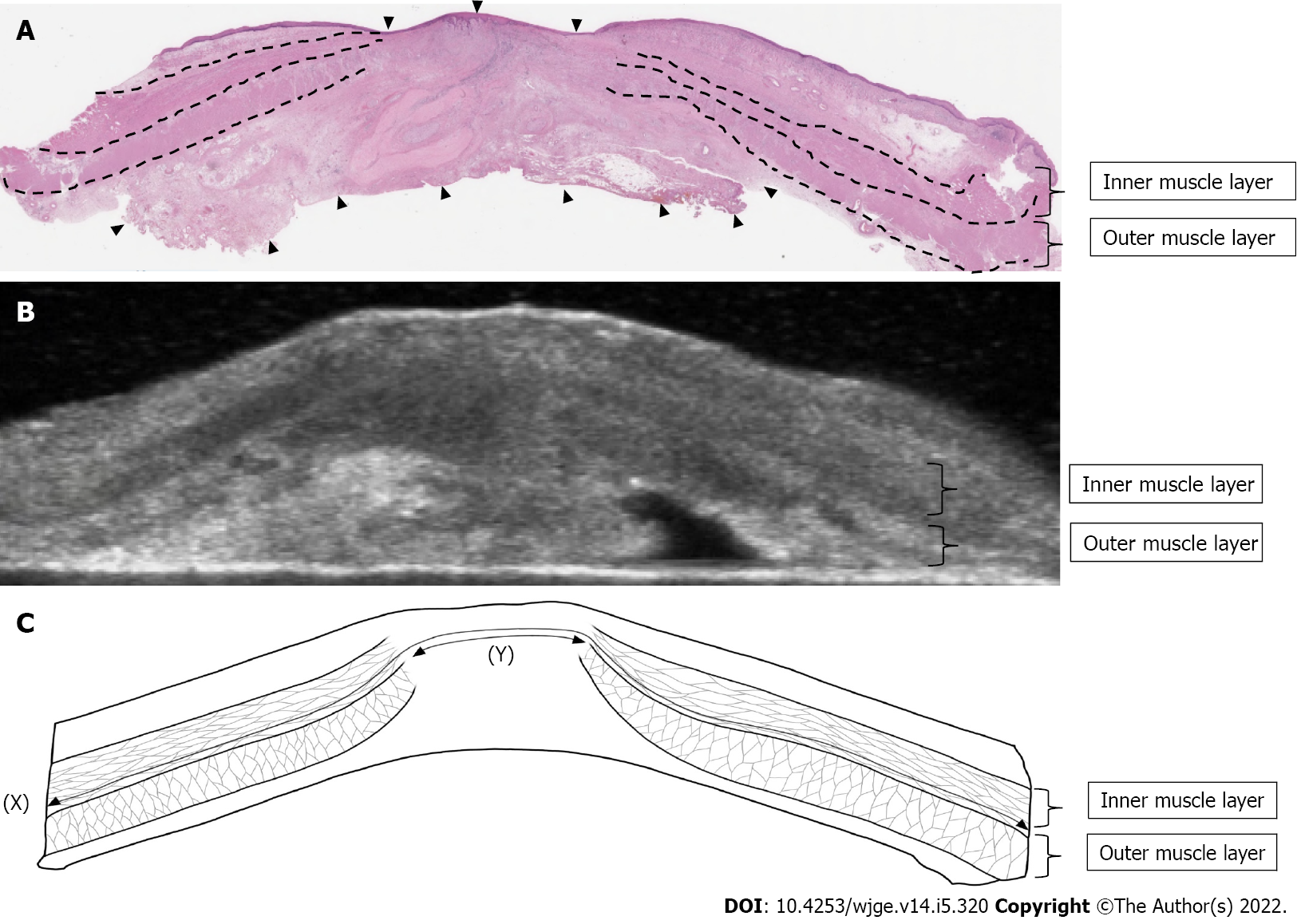
In study 1, in addition to the direct finding of the tumor, we focused on the esophageal muscle layer as an indirect finding, which is the most visible on ultrasound. We set up a cross-sectional image vertical to the esophagus at the center of the tumor. We measured the length of muscle layer circumference and the length of muscle layer defect. We calculated the ratio of muscle layer defect and compared each pathological factor.
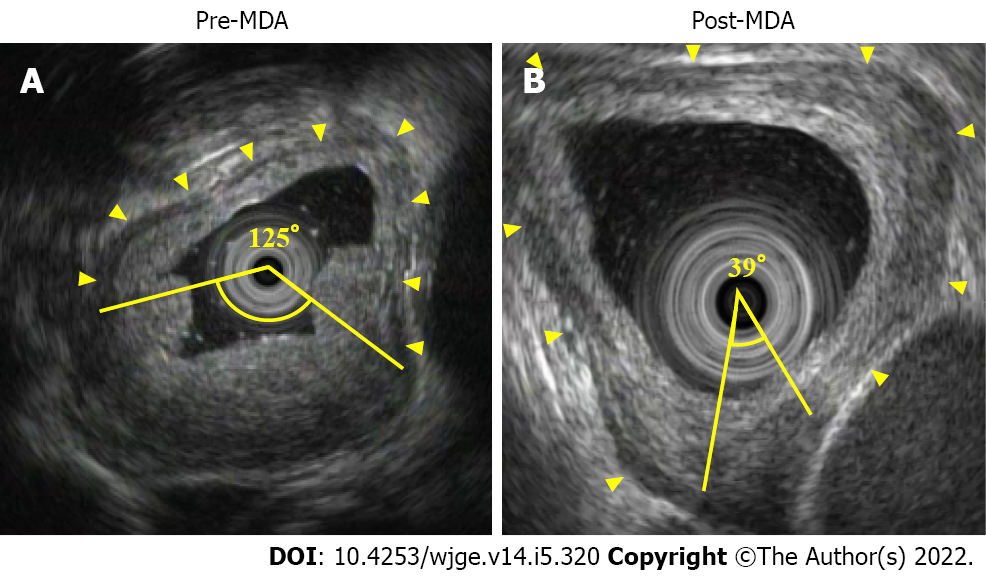
Study 2 aimed to evaluate the muscle layer defect using EUS. However, the EUS and ultrasound findings for specimens were different since the specimens were fully stretched. Keeping the esophageal wall stretched in vivo and measuring the circumference of the muscle layer by EUS would be challenging. Therefore we substituted the ratio of muscle layer defect with the total circumference of the muscle layer by the angle and named it as muscle layer defect angle (MDA). MDA was defined as the angle between the center of the lumen and the two points where EUS could not help visualize the inner muscle and intermuscular connective tissue layer. Using MDA, we measured the percentage of improvement in muscle layer defect caused by neoadjuvant therapy using the still images as well as video images of EUS. EUS was performed before and after neoadjuvant therapy by three or more skilled endoscopists. We calculated the MDA reduction rate using Pre-MDA and Post-MDA. MDA reduction rate was expressed using the following equation:
MDA reduction rate (%) = {[PreMDA(°) - PostMDA(°)] / PreMDA(°)} × 100
We compared each MDA factor with the pathological T stage. The echo images were analyzed using ImageJ software (National Institutes of Health, available at http://rsb.info.nih.gov/ij) specialized for morphological evaluation.
This study compared the results of prospectively collected data after confirming pathology. All statistical analyses were conducted with the JMP® Pro software program, version 13.2 (SAS Institute Inc., Cary, NC, United States). Continuous variables were expressed as median (min–max) or mean (± SD). Fisher’s exact test was used to compare and analyze categorical variables. Continuous variables were analyzed using Wilcoxon’s signed-rank sum test. P values of < 0.05 were considered statistically significant. Receiver operating characteristics (ROC) analysis was performed to assess the highest diagnostic values to determine the optimal cut-off points.
From May 2020 to October 2021, 61 patients underwent esophagectomy for esophageal cancer, and 37 patients underwent neoadjuvant therapy in our department. Of these, we excluded 5 patients with adenocarcinoma, 2 patients with neuroendocrine carcinoma, and 7 patients whose surgical specimens could not be analyzed using ultrasound. The clinical characteristics and pathological examination are summarized in Table 1. Fifteen patients received NAC, of which 13 patients received cisplatin plus 5-fluorouracil (CF), and 2 patients received docetaxel plus cisplatin plus 5-fluorouracil (DCF). Eight patients received CRT, of which 6 patients received 38-40 Gy irradiation, and 2 patients received additional irradiation to the total of 60 Gy as their tumors were considered unresectable by the end of 40 Gy irradiation. These two patients underwent salvage surgery after the additional irradiation. Three patients achieved pathological pCR (pathological grade 3); of these, 2 patients received CRT, and 1 patient received NAC only.
| All population (n = 23) | NAC (n = 15) | CRT (n = 8) | |
| Age (yr) | |||
| Median (range) | 72 (43-81) | 72 (43-78) | 72 (49-81) |
| Sex | |||
| Male | 19 | 12 | 7 |
| Female | 4 | 3 | 1 |
| Tumor location | |||
| Ut | 2 | 2 | 0 |
| Mt | 15 | 8 | 7 |
| Lt | 4 | 4 | 0 |
| Ae | 2 | 1 | 1 |
| Clinical T stage | |||
| cT1b | 1 | 1 | 0 |
| cT2 | 3 | 3 | 0 |
| cT3 | 11 | 11 | 0 |
| cT4a | 1 | 0 | 1 |
| cT4b | 7 | 0 | 7 |
| Chemotherapy regimen | |||
| CF | 21 | 13 | 8 |
| DCF | 2 | 2 | |
| Total irradiation dose | |||
| 38-40Gy | 6 | 6 | |
| 60Gy | 2 | 2 | |
| Time of surgery after therapy (d) | |||
| Median (range) | 37 (31-61) | 36 (31-61) | 40 (35-57) |
| Pathological T stage | |||
| pT0 | 3 | 1 | 2 |
| pT1a | 3 | 1 | 2 |
| pT1b | 6 | 6 | 0 |
| pT2 | 3 | 1 | 2 |
| pT3 | 8 | 6 | 2 |
| Pathological response | |||
| Grade1 | 13 | 11 | 2 |
| Grade2 | 7 | 3 | 4 |
| Grade3 | 3 | 1 | 2 |
We diagnosed uT stage by ultrasound for specimens just after surgery (Table 2). There was poor agreement between uT and pT stages. The overall accuracy uT stage rate was 61% (n = 14/23). The respective accuracy uT stage rate was 0% (n = 0/3) for pT0, 0% (n = 0/3) for pT1a, 67% (n = 4/6) for pT1b, 67% (n = 2/3) for pT2, and 100% (n = 8/8) for pT3. All pT0 and pT1a patients could not be diagnosed. Regarding comparison with NAC and CRT, the overall accuracy of uT stage rates were 73% (n = 11/15) and 38% (n = 3/8), respectively. The overall accuracy of overstaging uT stage rates was 13% (n = 2/15) and 62% (n = 5/8), respectively.
| Ultrasound T stages | pT0 | pT1a | pT1b | pT2 | pT3 | Total |
| Pathological T stages after NAC and CRT | ||||||
| uT0 | 0 | 1 | 1 | 0 | 0 | 2 |
| uT1a | 0 | 0 | 0 | 0 | 0 | 0 |
| uT1b | 0 | 0 | 4 | 0 | 0 | 4 |
| uT2 | 1 | 1 | 1 | 2 | 0 | 5 |
| uT3 | 2 | 1 | 0 | 1 | 8 | 12 |
| Total | 3 | 3 | 6 | 3 | 8 | 23 |
| Accuracy (%) | 0 | 0 | 67 | 67 | 100 | 61 |
| Overstaging (%) | 100 | 67 | 17 | 33 | 0 | 30 |
| Understaging (%) | 33 | 16 | 0 | 0 | 9 | |
| Pathological T stages after NAC | ||||||
| uT0 | 0 | 1 | 1 | 0 | 0 | 2 |
| uT1a | 0 | 0 | 0 | 0 | 0 | 0 |
| uT1b | 0 | 0 | 4 | 0 | 0 | 4 |
| uT2 | 1 | 0 | 1 | 1 | 0 | 3 |
| uT3 | 0 | 0 | 0 | 0 | 6 | 6 |
| Total | 1 | 1 | 6 | 1 | 6 | 15 |
| Accuracy (%) | 0 | 0 | 67 | 100 | 100 | 73 |
| Overstaging (%) | 100 | 0 | 17 | 0 | 0 | 13 |
| Understaging (%) | 100 | 17 | 0 | 0 | 13 | |
| Pathological T stages after CRT | ||||||
| uT0 | 0 | 0 | 0 | 0 | 0 | 0 |
| uT1a | 0 | 0 | 0 | 0 | 0 | 0 |
| uT1b | 0 | 0 | 0 | 0 | 0 | 0 |
| uT2 | 0 | 1 | 0 | 1 | 0 | 2 |
| uT3 | 2 | 1 | 0 | 1 | 2 | 6 |
| Total | 2 | 2 | 0 | 2 | 2 | 8 |
| Accuracy (%) | 0 | 0 | 0 | 50 | 100 | 38 |
| Overstaging (%) | 100 | 100 | 0 | 50 | 0 | 62 |
| Understaging (%) | 0 | 0 | 0 | 0 | 0 | |
Among 20 patients, excluding 3 patients who achieved complete response, we compared ultrasound images with the cut surface of the fixed specimens of the same level of residual tumor site in the esophageal wall to examine whether the residual tumor itself could be detected (Table 3). The overall detection rate for residual tumors was 75% (n = 15/20), with no correlation between after NAC (79%, n = 11/14) and after CRT (67%, n = 4/6). The macroscopic types after neoadjuvant therapy were classified into two groups; 11 patients had ulcerative and protruding tumor types, while 9 patients had superficial and submucosal tumors. The superficial and submucosal types were poorly detected (P = 0.008). In addition, pathologic tumor size and the pathological response showed a significant correlation (P = 0.008, 0.127). Echoic characteristics of the residual tumor are shown in Table 4.
| Detection of residual tumor | |||
| Possible | Impossible | P | |
| All, n (%) | 15 (75) | 5 (25) | |
| Preoperative treatment, n (%) | |||
| NAC | 11 (79) | 3 (21) | |
| CRT | 4 (67) | 2 (33) | 0.613 |
| Macroscopic type after neoadjuvant therapy, n (%) | |||
| Ulcerative and protruding type | 11 (100) | 0 (0) | |
| Superficial and SMT type | 4 (44) | 5 (56) | 0.008 |
| Pathologic tumor size (mm) | |||
| Median (range) | 42 (5-65) | 4 (2-34) | 0.008 |
| Pathological T stage, n (%) | |||
| pT1a/1b | 5 (56) | 4 (44) | |
| pT2/3 | 10 (91) | 1 (9) | 0.127 |
| Pathological response, n (%) | |||
| Grade1 | 12 (92) | 1 (8) | |
| Grade2 | 3 (43) | 4 (57) | 0.031 |
| All population (n = 15) | NAC (n = 11) | CRT (n = 4) | P | |
| Border | ||||
| Regular | 10 | 10 | 0 | |
| Irregular | 5 | 1 | 4 | 0.004 |
| Echogenicity | ||||
| Hypoechoic | 5 | 5 | 0 | |
| Hypo and isoechoic (mixed) | 10 | 6 | 4 | 0.231 |
The tumor borders were relatively regular, and echogenicity was hypoechoic after NAC. In contrast, tumor borders were irregular, and echogenicity was hypo and iso (mixed) echoic type in all patients after CRT (Figure 1).
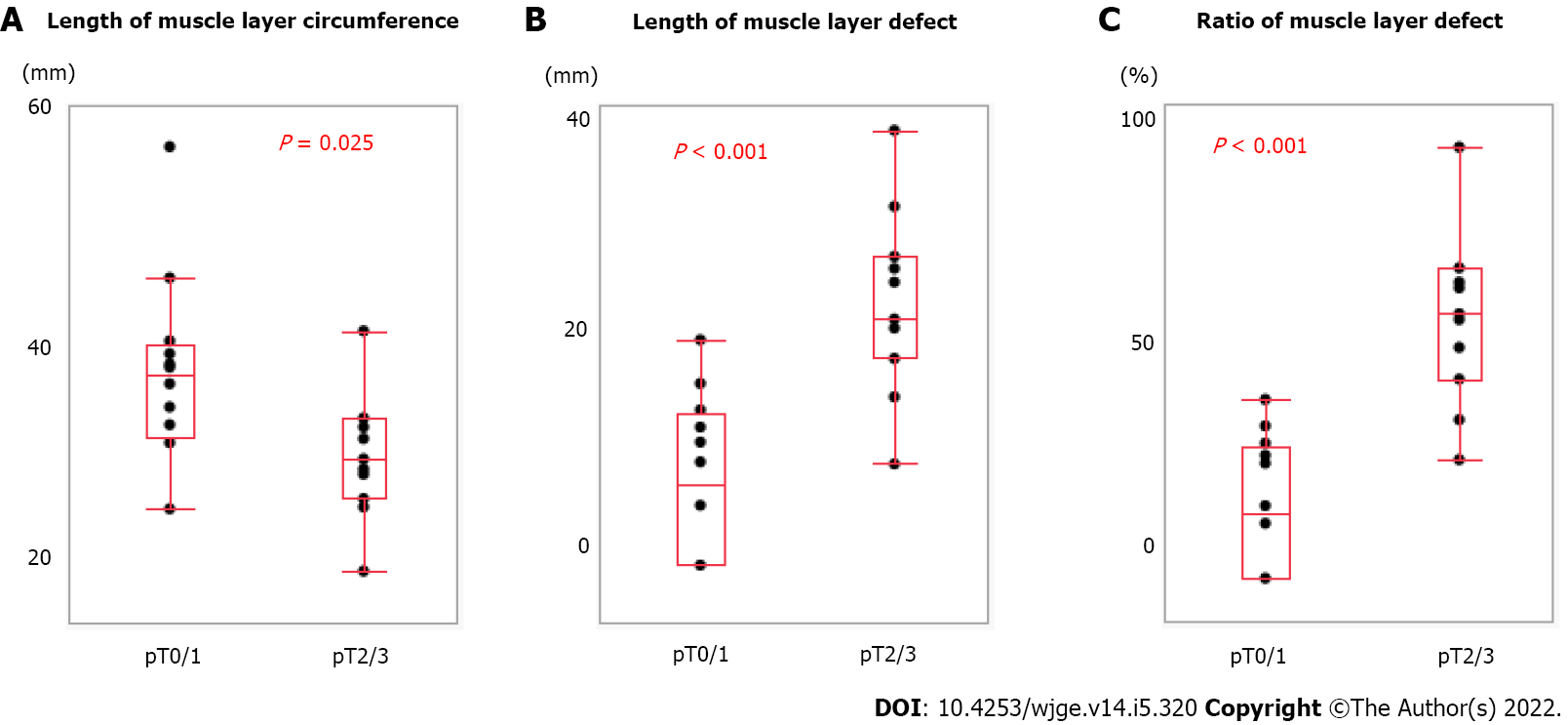
We measured the muscle layer using ultrasound images (Figure 2). Ultrasound showed a clearly defined disruption of the muscle layer. We compared muscle layer factors with pathological characteristics (Figure 3). There was a significant correlation between pT stage (pT0/1, n = 12 vs pT2/3, n = 11) and length of muscle layer circumference (36.2 ± 5.9 mm vs 44.3 ± 8.9 mm, P = 0.025), length of muscle layer defect (22.5 ± 8.0 mm vs 7.1 ± 7.2 mm, P < 0.001), and the ratio of muscle layer defect (63.0 ± 22.8% vs 16.1 ± 16.0%, P < 0.001).
There was no correlation between pathological response (Grade 1/2, n = 20 vs Grade 3, n = 3) and length of muscle layer circumference (40.0 ± 9.0 mm vs 42.6 ± 4.9 mm, P = 0.438), length of muscle layer defect (14.5 ± 11.5 mm vs 14.6 ± 4.5 mm, P = 1.00), and the ratio of muscle layer defect (39.2 ± 32.9% vs 33.8 ± 6.8%, P = 0.927).
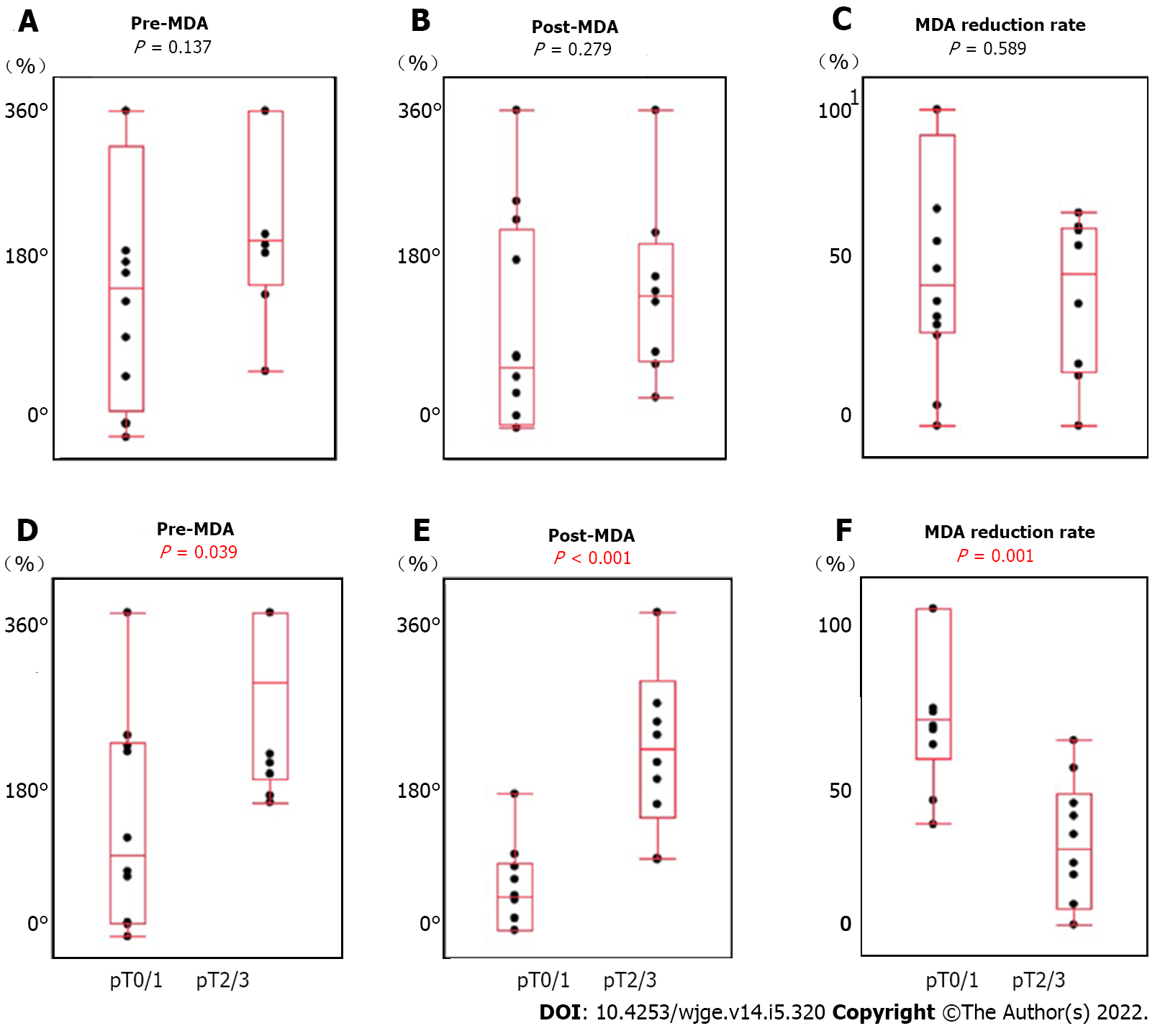
In study 2, we measured MDA using EUS images (Figure 4). To confirm the reduction of muscle layer defect after adjuvant therapy compared to before, we excluded 3 patients (EUS before therapy did not show muscle layer invasion in 2 patients, and 1 patient did not undergo EUS before therapy). The clinical characteristics and pathological examination results are summarized in Table 5. There was no significant difference between pT0/1 and pT2/3 in terms of clinical characteristics. MDA factors were compared with pathological T stage (Figure 5). There was no correlation between preoperative treatment (NAC, n = 12 vs CRT, n = 8), pre-MDA (50.0 ± 35.3° vs 70.0 ± 27.9°, P = 0.137), post-MDA (30.5 ± 33.6° vs 43.2 ± 28.4°, P = 0.279), and MDA reduction rate (51.4 ± 34.9% vs 40.4 ± 25.7%, P = 0.589). There was a significant correlation between pT stage (pT0/1, n = 10 vs pT2/3, n = 10), pre-MDA (142.5 ± 110.6° vs 274.0 ± 91.7°, P = 0.039), post-MDA (45.9 ± 49.3° vs 210.0 ± 98.7°, P < 0.001), and MDA reduction rate (68.9 ± 24.4% vs 25.1 ± 20.3%, P = 0.001).
| pT0/1 (n = 10) | pT2/3 (n = 10) | P | |
| Age (yr) | |||
| Median (range) | 73 (52-79) | 72 (43-81) | 0.94 |
| Sex | |||
| Male/Female | 9/1 | 7/3 | 0.582 |
| Tumor location | |||
| Ut, Mt, Lt/Ae | 10/0 | 8/2 | 0.473 |
| Clinical T stage | |||
| cT2, 3/cT4a, b | 6/4 | 6/4 | 1 |
| Preoperative treatment | |||
| NAC/CRT | 6/4 | 6/4 | 1 |
| Chemo regimen | |||
| CF/DCF | 9/1 | 9/1 | 1 |
| Total irradiation dose | |||
| 38-40Gy/60Gy | 2/2 | 4/0 | 0.429 |
| Time of EUS after therapy (d) | |||
| Median (range) | 37 (21-49) | 29 (14-50) | 0.172 |
| Time of surgery after therapy (d) | |||
| Median (range) | 41 (34-57) | 37 (31-61) | 0.471 |
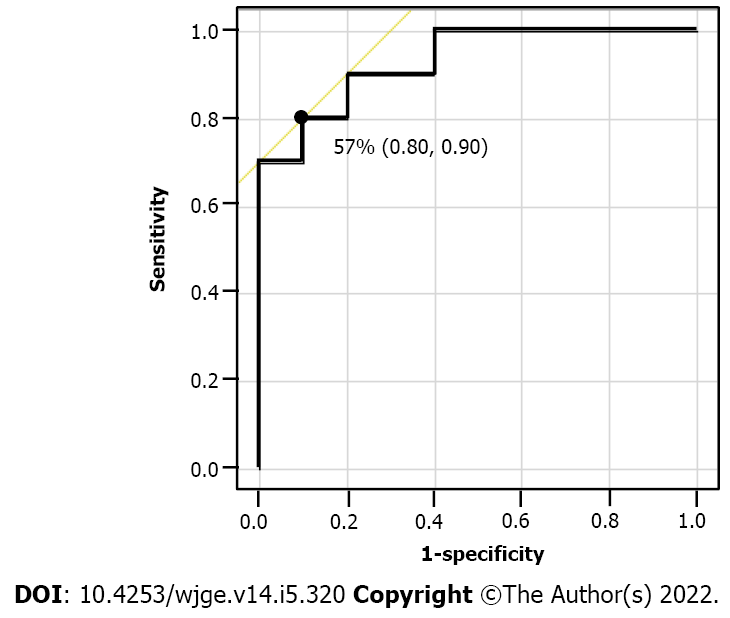
We conducted ROC analysis to determine the optimal MDA reduction rate cut-off points that could yield the maximum difference between the two groups (Figure 6). From this ROC curve analysis, 57.0% was determined as the best cut-off rate to detect the patients in the pT0/1 group with the highest accuracy. Based on the optimal cut-off values of the MDA reduction rate, that could distinguish the pT0/1 group with a sensitivity of 0.80, specificity of 0.90, and accuracy of 0.93.
We conducted two studies; study 1 was performed to investigate the limitations and characteristics of residual tumor diagnosis using ultrasound and study 2 aimed to implement EUS to detect remanent tumors deep in the muscle layer. The first study revealed the limitations and potential of ultrasound for residual tumors. After cross-referencing ultrasound images with the correct pathological diagnosis, some residual tumors were found to be undetectable on ultrasound. In contrast, the ratio of the esophageal muscle layer defect, which was not focused upon so far, was considered helpful in diagnosing the depth of the residual tumor. In the second study, muscle layer defect was measured using EUS. The results showed that the rate of muscle layer defect reduction in neoadjuvant therapy correlated with the pathological depth of the tumor. Our findings can help improve EUS diagnosis and provide more treatment options for ESCC patients after neoadjuvant therapy.
We considered comparing pathological and ultrasound images. However, using only EUS was considered unreliable for the following reasons. First, it was difficult to compare the measured level of tumor site in the esophagus with the level of the fixed specimens. Second, EUS was good for evaluating targeted areas but not for scanning large areas. In contrast, ultrasound for surgical specimens allowed us to compare pathological and ultrasound images with the same level of ultrasound images and scans of the entire lesion. This could help clarify whether the modality of echo itself contributes to the residual diagnosis after neoadjuvant therapy.
According to several meta-analyses examining the accuracy of detecting residual tumors for esophageal cancer after CRT, the consensus was that EUS had high sensitivity but low specificity[10,13]. Even after NAC, the concordance rate between EUS and pathological T-stage was reportedly as low as 29%, and the depth was overstaged in more than half of the cases (51%)[14]. It is well known that tumor invasion might be overestimated due to inflammation within and surrounding the tumor[15]. Our study showed 61% accuracy and 30% overstaging of uT, which was better than previous studies. Even though the ultrasound on surgical specimens was performed in a stable environment, these results are not sufficiently accurate. A previous study analyzing the accuracy of EUS in patients with esophageal cancer after NAC or CRT showed that accuracy of uT was significantly worse after CRT (16%) than after NAC (43%)[16]. In line with this previous study, our results showed that the accuracy of uT worsened after CRT (38%) than after NAC (73%). Our study showed that CRT downstaged tumors more effectively than NAC. As a result, there were more tumors with pT0 and pT1a, which were difficult to detect using ultrasound. All pT0 and pT1a patients could not be diagnosed because the scar tissue associated with tumor disappearance was misidentified as a residual tumor, causing overstaging. Diagnosing T3 was easy because the esophageal muscle layer was destroyed or replaced by fibrosis. However, distinguishing between a residual tumor and a fibrosis tissue seemed impossible.
We also examined the retrospective detection rate for residual tumor and the echoic characteristics of the residual tumor by comparing ultrasound images with the cut surface of the fixed specimens of the same level of the esophageal wall. Our results showed no difference in the detection rate after CRT and after NAC; however, the after CRT specimens appeared to have an irregular border and mixed echogenicity. According to a study that classified the echogenicity of gastrointestinal tumors, most esophageal cancers expressed echo levels between the muscularis propria and the deep mucosa[17]. However, our study showed that the residual tumors lost heterogeneity and higher echogenicity after CRT compared to deep mucosa. This result indicated that the preoperative treatment increased the brightness of echogenicity. In a previous pathological study, chemotherapy was found to generally decrease tumor cellularity and cause fragmentation of cell nuclei. Additionally, in squamous cell carcinoma, chemotherapy is known to increase keratinization with the formation of keratin pearls, acellular keratin with islands of nonviable tumor cells, histiocytic giant cells, and lymphocytes surrounding tumor cells in squamous cell carcinoma[18]. Our pathological findings after neoadjuvant therapy, particularly after CRT, showed that the density of collagen fibers increased as the cancer cells disappeared. Consequently, the ratio of cancer cells to stromal components also changed, which might have led to a difference in echo level, such as mixed echogenicity. The increase in the echogenicity of tumors is reportedly related to the positive response to NAC in breast tumors[19]. Although such phenomena correlating echogenicity and treatment effect are not reported for esophageal cancers, and our study could not prove the relationship, some changes in echogenicity of ESCC could be attributed to treatment.
When predicting patient prognosis after CRT or NAC, it is reasonable to measure the reduction in tumor volume using EUS. However, the conventional measurement method involving direct identification and measurement of the tumor is not accurate. Several studies have assessed the predictive value of tumor thickness and area using EUS to determine patient prognosis and tumor regression in patients with esophageal cancer undergoing NAC or CRT[20-23]. Although these studies focused on lesions identified on EUS, our results showed that EUS could not detect the residual tumor. Tumors were either scattered on the esophageal wall, had unclear borders, or were scar tissue that appeared like a tumor.
For this reason, we considered it inappropriate to include EUS-confirmed echo lesions as residual tumors. In our clinical experience, we have observed that the esophageal muscle layer can be clearly visualized using EUS in patients with a good response to neoadjuvant therapy. Therefore, we focused on the esophageal muscle layer as indirect findings instead of the tumor. In the first study, ultrasound findings for specimens in the group with pT0 and pT1 showed that the muscle layer circumference was longer, the length of muscle layer defect was shorter, and the rate of muscle layer defect was lower than in the group with pT2 and pT3. Tissue heterogeneity was noted if residual cancer cells remained in the muscle layer or deeper; in such cases, we could not explore the muscle layer using ultrasound findings. In addition, it was improbable that the muscle layer destroyed by tumor invasion could be regenerated, at least during the observation period. We considered that the reduction in the muscle layer defect in the specimens with stages pT0 and pT1 was because of scar contraction caused by the disappearance of the tumor due to neoadjuvant therapy. In the second study, findings of EUS performed before and after neoadjuvant therapy in the group with pT0 and pT1 showed that pre-MDA was smaller, post-MDA was smaller, and MDA reduction rate was larger in the groups with pT2 and pT3 staging. The improvement of the muscle layer defect was considered useful in EUS depth diagnosis.
If EUS helps diagnose pCR or superficial residual tumors and deep remanent tumors in patients after neoadjuvant therapy by focusing on the muscle layer, the clinical treatment options can be expanded significantly. In recent years, endoscopic salvage resection has been preferred over esophagectomy for patients with superficial localized residual tumors after CRT[24,25]. In addition, it was reported that overall, 29% of patients with esophageal cancer achieved pCR after neoadjuvant CRT[26], and 62% of patients with ESCC achieved pCR according to the JCOG9906 study in Japan[3]. A study reported that 2%-7% of patients with ESCC achieved pCR after NAC; however, they included only a small number of cases[4,5]. Because of such response rates, recent studies have focused on assessing the efficacy of active surveillance to help avoid highly invasive esophagectomy[27]. In addition to the usual endoscopic diagnosis, which mainly involves biopsy, subsequent MDA reduction rate may allow the selection of endoscopic salvage resection instead of esophagectomy.
Our study had some limitations. First, it was a single-center study with a small sample size. The usefulness of EUS must be evaluated in the future by conducting larger prospective studies. Second, it was difficult to seamlessly match the sites measured before and after preoperative treatment with EUS. We attempted to match the measurement sites by recording the scope length from the mouth and comparing it to the surrounding vessels and structures. Third, the value of post-MDA could be different depending on the time since preoperative treatment. We assessed MDA 4 to 6 wk after the last preoperative treatment. However, to determine the effectiveness of neoadjuvant therapy and for active surveillance, it is necessary to examine the differences in MDA according to the time since treatment.
This study showed that ultrasound could not detect some residual tumors after neoadjuvant therapy. Meanwhile, focusing on the esophageal muscle layer as indirect findings rather than the residual tumor as direct findings could help diagnose the depth of the tumor. Applying these results in clinical practice may help clinicians provide more treatment options for patients with ESCC after neoadjuvant therapy.
The diagnosis of endoscopic ultrasound (EUS) for esophageal cancer after neoadjuvant therapy is controversial. In addition, it is unclear whether the echogenic lesions detected using EUS are indeed residual tumors and how they appear on ultrasound.
There are few studies that contrast echographic and pathologic images of esophageal cancer after neoadjuvant therapy. In our clinical experience, we have observed that the esophageal muscle layer can be clearly visualized using EUS in patients with a good response to neoadjuvant therapy.
To investigate the ultrasound imaging features of residual tumors and identify the limitations and potential of EUS.
Twenty-three patients receiving esophagectomy after neoadjuvant therapy [15 patients after neoadjuvant chemotherapy (NAC) and 8 patients after chemoradiotherapy (CRT)] were studied. We diagnosed the T stage and compared ultrasound images with pathological findings using ultrasound for surgical specimens. Furthermore, the rate of reduction for the muscle layer defect was evaluated using EUS images obtained before and after neoadjuvant therapy.
The accuracy of T stage rate was 61%, which worsened after CRT (38%) than after NAC (73%). Moreover, pT0 could not be diagnosed in all cases. The detection rate of residual tumor for specimens using ultrasound retrospectively was 75%. Tumor borders were irregular and echogenicity was mixed type after CRT. There was a correlation between the pT stage and the rate of muscle layer defect reduction measured by EUS.
Some tumors are undetectable on ultrasound when compared to pathological images. However, focusing on the esophageal muscle layer may improve the accuracy of T stage diagnosis of residual tumors.
If EUS helps diagnose T stage of residual tumors in patients after neoadjuvant therapy by focusing on the muscle layer, the clinical treatment options can be expanded significantly.
We are deeply grateful to Ishimura R, Department of Photo Center, Chiba University Hospital, Japan, who provided great illustrations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Altonbary AY, Egypt; Chen T, China; Yang X, China S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55833] [Article Influence: 7976.1] [Reference Citation Analysis (132)] |
| 2. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 3. | Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, Takiuchi H, Komatsu Y, Miyata Y, Fukuda H; Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (JCOG). Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, Siersema PD, Dinjens WN, van Lanschot JJ, Tilanus HW, van der Gaast A. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1052] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 6. | Chao YK, Tsai CY, Chang HK, Tseng CK, Liu YH, Yeh CJ. A Pathological Study of Residual Cancer in the Esophageal Wall Following Neoadjuvant Chemoradiotherapy: Focus on Esophageal Squamous Cell Carcinoma Patients with False Negative Preoperative Endoscopic Biopsies. Ann Surg Oncol. 2015;22:3647-3652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, Morii E, Mori M, Doki Y. The Pattern of Residual Tumor After Neoadjuvant Chemotherapy for Locally Advanced Esophageal Cancer and Its Clinical Significance. Ann Surg. 2020;271:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 243] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 9. | Eyck BM, Onstenk BD, Noordman BJ, Nieboer D, Spaander MCW, Valkema R, Lagarde SM, Wijnhoven BPL, van Lanschot JJB. Accuracy of Detecting Residual Disease After Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg. 2020;271:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Sun F, Chen T, Han J, Ye P, Hu J. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: a meta-analysis and systematic review. Dis Esophagus. 2015;28:757-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. 2017.. |
| 12. | Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, Fukuda H, Kitagawa Y; Japan Esophageal Oncology Group/Japan Clinical Oncology Group. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | van Rossum PSN, Goense L, Meziani J, Reitsma JB, Siersema PD, Vleggaar FP, van Vulpen M, Meijer GJ, Ruurda JP, van Hillegersberg R. Endoscopic biopsy and EUS for the detection of pathologic complete response after neoadjuvant chemoradiotherapy in esophageal cancer: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:866-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Heinzow HS, Seifert H, Tsepetonidis S, Wolters H, Kucharzik T, Domschke W, Domagk D, Meister T. Endoscopic ultrasound in staging esophageal cancer after neoadjuvant chemotherapy--results of a multicenter cohort analysis. J Gastrointest Surg. 2013;17:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Misra S, Choi M, Livingstone AS, Franceschi D. The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg Endosc. 2012;26:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Bohle W, Kasper M, Zoller WG. Different accuracy of endosonographic tumor staging after neoadjuvant chemotherapy and chemoradiotherapy in esophageal cancer. Surg Endosc. 2016;30:2922-2928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Okanobu H, Hata J, Haruma K, Mitsuoka Y, Kunihiro K, Manabe N, Tanaka S, Chayama K. A classification system of echogenicity for gastrointestinal neoplasms. Digestion. 2005;72:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Sethi D, Sen R, Parshad S, Khetarpal S, Garg M, Sen J. Histopathologic changes following neoadjuvant chemotherapy in various malignancies. Int J Appl Basic Med Res. 2012;2:111-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Dobruch-Sobczak K, Piotrzkowska-Wróblewska H, Klimonda Z, Karwat P, Roszkowska-Purska K, Clauser P, Baltzer PAT, Litniewski J. Multiparametric ultrasound examination for response assessment in breast cancer patients undergoing neoadjuvant therapy. Sci Rep. 2021;11:2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Jost C, Binek J, Schuller JC, Bauerfeind P, Metzger U, Werth B, Knuchel J, Frossard JL, Bertschinger P, Brauchli P, Meyenberger C, Ruhstaller T. Endosonographic radial tumor thickness after neoadjuvant chemoradiation therapy to predict response and survival in patients with locally advanced esophageal cancer: a prospective multicenter phase ll study by the Swiss Group for Clinical Cancer Research (SAKK 75/02). Gastrointest Endosc. 2010;71:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Ribeiro A, Franceschi D, Parra J, Livingstone A, Lima M, Hamilton-Nelson K, Ardalan B. Endoscopic ultrasound restaging after neoadjuvant chemotherapy in esophageal cancer. Am J Gastroenterol. 2006;101:1216-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | van der Bogt RD, Noordman BJ, Krishnadath KK, Roumans CAM, Schoon EJ, Oostenbrug LE, Siersema PD, Vleggaar FP, van Lanschot JJB, Spaander MCW. Endoscopic ultrasound measurements for detection of residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. Endoscopy. 2019;51:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Bohle W, Kasper M, Zoller WG. Prognostic relevance of serial endoscopic ultrasound after chemoradiation in esophageal cancer. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Yano T, Muto M, Hattori S, Minashi K, Onozawa M, Nihei K, Ishikura S, Ohtsu A, Yoshida S. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Al-Kaabi A, Schoon EJ, Deprez PH, Seewald S, Groth S, Giovannini M, Braden B, Berr F, Lemmers A, Hoare J, Bhandari P, van der Post RS, Verhoeven RHA, Siersema PD. Salvage endoscopic resection after definitive chemoradiotherapy for esophageal cancer: a Western experience. Gastrointest Endosc. 2021;93:888-898.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4080] [Article Influence: 313.8] [Reference Citation Analysis (0)] |
| 27. | Noordman BJ, Wijnhoven BPL, Lagarde SM, Boonstra JJ, Coene PPLO, Dekker JWT, Doukas M, van der Gaast A, Heisterkamp J, Kouwenhoven EA, Nieuwenhuijzen GAP, Pierie JEN, Rosman C, van Sandick JW, van der Sangen MJC, Sosef MN, Spaander MCW, Valkema R, van der Zaag ES, Steyerberg EW, van Lanschot JJB; SANO-study group. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer. 2018;18:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |