Published online May 16, 2022. doi: 10.4253/wjge.v14.i5.267
Peer-review started: December 31, 2021
First decision: January 23, 2022
Revised: February 14, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 16, 2022
Processing time: 136 Days and 9.6 Hours
Gastroenteropancreatic neuroendocrine neoplasms are a heterogenous group of rare neoplasms that are increasingly being discovered, often incidentally, throughout the gastrointestinal tract with varying degrees of activity and malignant potential. Confusing nomenclature has added to the complexity of managing these lesions. The term carcinoid tumor and embryonic classification have been replaced with gastroenteropancreatic neuroendocrine neoplasm, which includes gastrointestinal neuroendocrine and pancreatic neuroendocrine neoplasms. A comprehensive multidisciplinary approach is important for clinicians to diagnose, stage and manage these lesions. While histological diagnosis is the gold standard, recent advancements in endoscopy, conventional imaging, functional imaging, and serum biomarkers complement histology for tailoring specific treatment options. In light of developing technology, our review sets out to characterize diagnostic and therapeutic advancements for managing gastroenteropancreatic neuroendocrine tumors, including innovations in radiolabeled peptide imaging, circulating biomarkers, and endoscopic treatment approaches adapted to different locations throughout the gastrointestinal system.
Core Tip: Diagnostic technology for neuroendocrine tumors continues to advance. Radiomics promises to enhance morphologic imaging. Gallium-68 DOTA-peptide positron emission tomography/computed tomography has replaced Octreoscan as the preferred functional imaging modality. Newer radiolabeled peptides may further improve detection. A novel liquid biopsy biomarker (NETest) has proven more accurate than chromogranin A in monitoring treatment response and predicting disease activity. Therapy has also progressed with treatment adapted based on the predicted behavior of the tumor. Advanced endoscopic resection techniques have revolutionized treatment. Preliminary evidence suggests endoscopic ultrasound guided radiofrequency ablation may prove useful in treating pancreatic lesions. Multimodality therapy continues to evolve for metastatic pancreatic tumors.
- Citation: Canakis A, Lee LS. Current updates and future directions in diagnosis and management of gastroenteropancreatic neuroendocrine neoplasms. World J Gastrointest Endosc 2022; 14(5): 267-290
- URL: https://www.wjgnet.com/1948-5190/full/v14/i5/267.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i5.267
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are a heterogenous group of rare neoplasms with a wide clinicopathologic spectrum of disease activity[1]. These neoplasms arise from the secretory cells of the neuroendocrine system and can occur anywhere along the gastrointestinal tract[2]. Nearly 95% occur sporadically, though genetic testing should be considered for patients less than 40 years old, family history of NENs, features concerning for multiple endocrine neoplasia type 1, von Hippel-Lindau disease, tuberous sclerosis or neurofibromatosis type 1[3]. Traditional terminology including carcinoid tumor and APUDoma were replaced by neuroendocrine neoplasm in 2010 by the World Health Organization (WHO), which also discouraged using the terms benign and malignant. NENs are grouped as well-differentiated neuroendocrine tumors (NET) or poorly differentiated neuroendocrine carcinomas (NEC)[4]. NECs are highly aggressive with significantly worse prognosis. Nearly 80%-90% of GEP-NENs are NETs, which are slow growing and graded from G1 (low), G2 (intermediate), to G3 (high)[2].
With the advent of high-resolution cross-sectional imaging, GEP-NENs are increasingly being discovered-notably without any significant change in rates of metastasis[5]. In a large population-based study of 64971 patients, the age-adjusted incidence rate of NETs increased from 1.09 per 100000 in 1973 to 6.98 per 100000 in 2012, with the greatest increase occurring in localized NETs and G1 NETs[6]. These observations suggest that many of these lesions are incidental and/or asymptomatic at the time of discovery. Incidence of gastric and rectal NETs has increased the greatest unlike small bowel NETs, which likely correlates with greater use of endoscopic procedures. Similar trends have been noted in Europe and Asia[7].
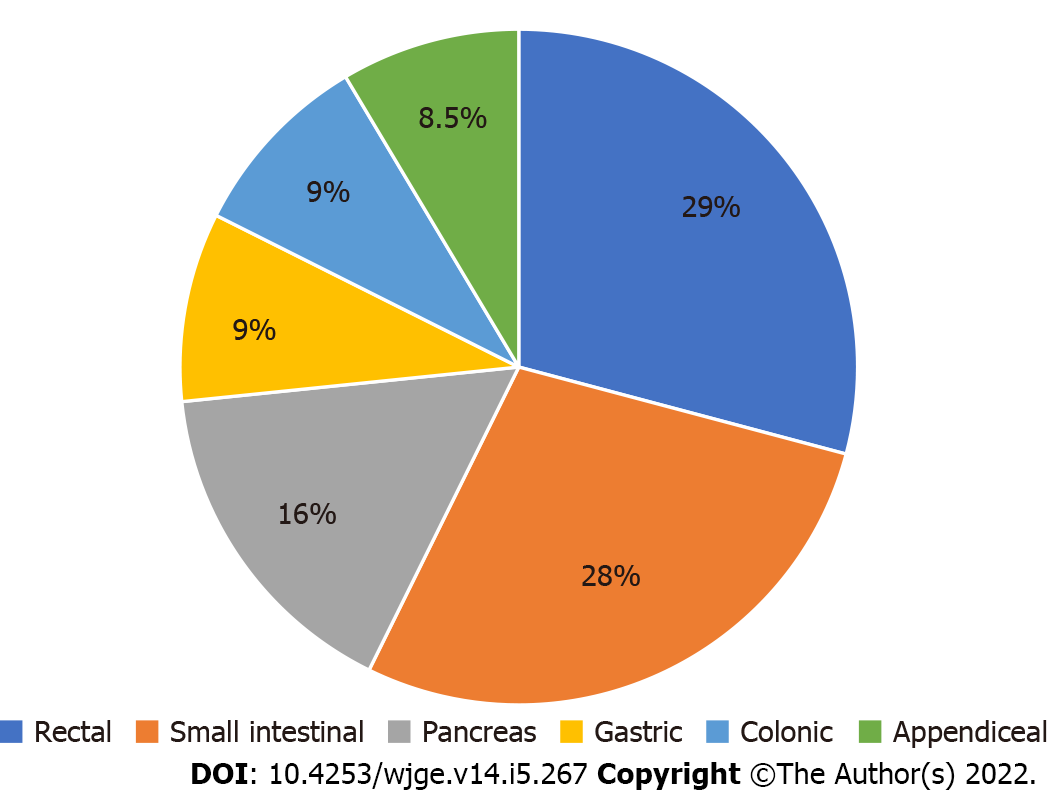
GEP-NENs are divided into gastrointestinal and pancreatic NENs with the most common being rectal (29%) and small intestinal (28%) (Figures 1 and 2)[8,9]. These tumors exhibit a wide range of behavior with varying degrees of disease activity including growth rate, grade, differentiation and metastatic potential[10]. Generally speaking, small intestinal NENs have high malignant potential while gastric, duodenal, appendiceal, and rectal NETs are less likely to metastasize[11]. A recent cohort of 43751 patients in the United States noted that the majority of GEP-NENs were localized (51.7%) and grade 1 (71.7%)[9]. This study also found that the most lesions (73%) occurred in whites, followed by black (16.2%) and Asian (7.3%) populations with no difference in three or five year survival based on race.
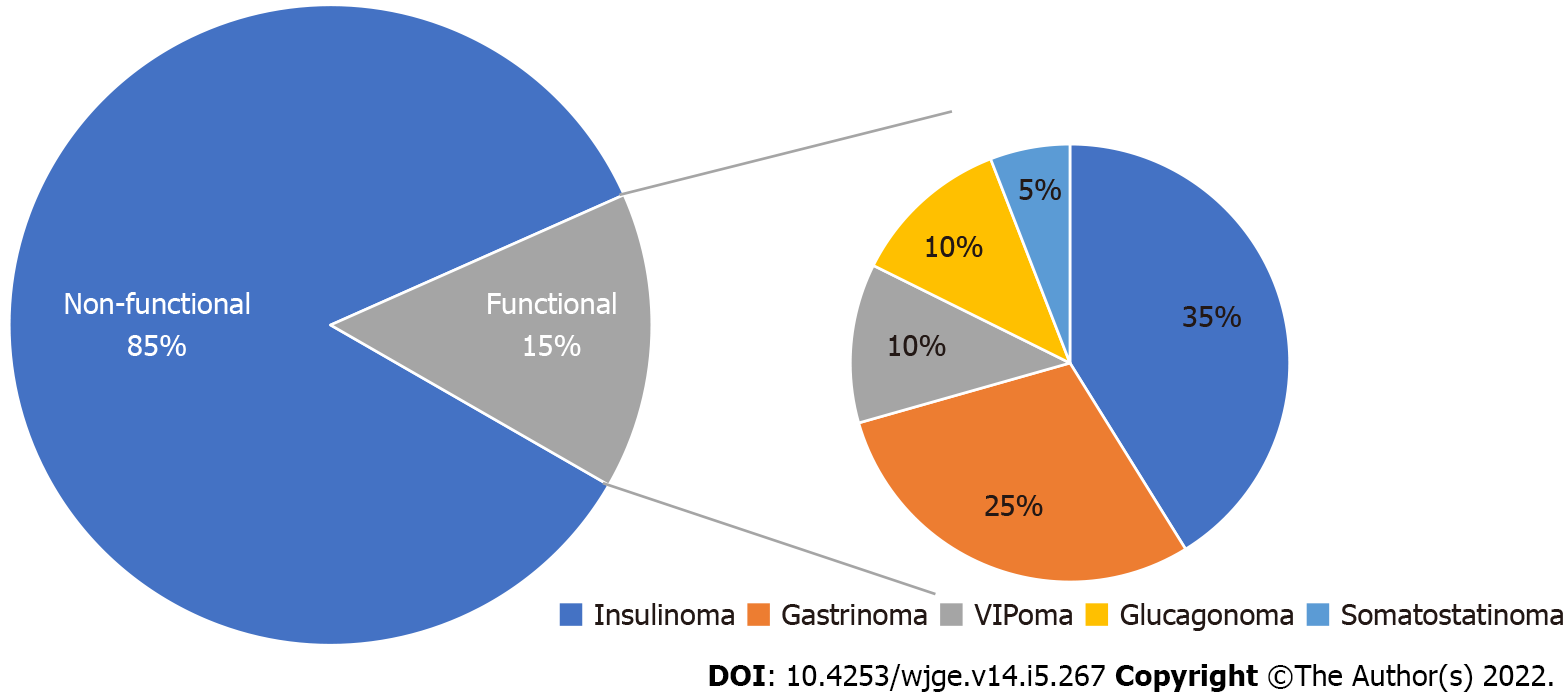
The majority of GEP-NENs are non-functional while functional NENs secrete hormones and substances that lead to clinical symptoms. Functioning gastrointestinal NENs are not classified separately from nonfunctioning gastrointestinal NENs and manifest with carcinoid syndrome while functioning pancreatic NENs are classified distinctly according to the hormone secreted by the tumor. Nonhormonal products are also produced by both non-functional and functional NENs, which include chromogranin A, pancreastatin and pancreatic polypeptide, and may offer aid in diagnosis and follow-up.
Diagnosis relies on morphological imaging, functional imaging, endoscopic procedures, biomarkers, and pathology. All patients should undergo computed tomography (CT) and/or magnetic resonance imaging (MRI). Functional imaging serves as an adjunct to conventional imaging in advanced NETs and is helpful for identifying primary tumors and staging. Endoscopic procedure with biopsy diagnoses gastric, duodenal and colorectal NENs while endoscopic ultrasound (EUS) aids in identification of pancreatic NENs. The mainstay of biomarkers is chromogranin A although newer markers have been identified, which may expand the role of biomarkers in post-treatment surveillance and detection of recurrence.
Tumor staging and grading are essential to assess prognosis and disease activity as reflected in the 2019 WHO classification based on tumor differentiation and grading (mitotic rate or Ki-67 index) (Table 1)[2,4]. The degree of differentiation is based on the extent the tumor cells resemble their endocrine cell counterparts[11]. Grading is based on the proliferative rate from either mitotic counts or Ki-67 labeling index with higher values associated with more aggressive behavior, independent of stage[2]. Mitotic counts rely on the number of mitotic figures in 10 consecutive high-power fields while Ki-67 Labeling index is the percent of positive tumor cells. Small biopsy samples and heterogeneity within the tumor all pose challenges to accurate assessment of tumor grade of the entire lesion. Whether there is incremental benefit from larger core samples obtained during EUS-fine needle biopsy and whether artificial intelligence technology will help partially automate calculating Ki-67 index require further study[12]. Radiomics may supplant or supplement histologic diagnosis by assessing the whole lesion and will be discussed further below.
| Terminology, grade | Differentiation | Mitotic count (HPF2) | Ki-67 index (%) |
| NET, G1 | Well-differentiated | < 2/10 | < 3 |
| NET, G2 | Well-differentiated | 2-20/10 | 3-20 |
| NET, G3 | Well-differentiated | > 20/10 | > 20 |
| NEC, G3 (small or large cell type) | Poorly differentiated | > 20/10 | > 20 |
NETs typically are highly vascular, hyperenhancing in the early arterial phase with washout during the delay portal venous phase of CT (Figure 3). Differentiating liver metastases from hepatocellular carcinomas may be aided by exploiting the fact that hepatocellular carcinomas have higher attenuation levels with contrast and higher iodine uptake with a threshold value of 0.22 for normalized iodine uptake having 100% sensitivity and 90% specificity[13]. Attenuation assessment of lymph nodes on CT may also help identify malignant nodes with a cutoff value of 7.5 Hounsfield units distinguishing 96% of positron emission tomography (PET) positive and 89% of PET negative lymph nodes[14]. Limitations of CT include lower sensitivity with a recent study suggesting only 76% of CT scans identified the primary tumor in patients with metastatic GEP-NETs, and difficulties with identifying small (< 1 cm) lesions especially in the small bowel where only 21% of small intestine NETs were identified in one study[15-17]. CT enteroclysis has been used for localization of small bowel tumors[18] with luminal distension using neutral contrast aiding in defining small mucosal features with a positive predictive value of 95%[18].
MRI with contrast enhancement is superior in detecting lesions in the liver and pancreas[15]. With higher tissue resolution, MRI is also better for evaluating bone and liver metastases[19,20]. NENs typically have low T1 and high T2 signal on imaging (Figure 4). Adding diffusion weighted MRI to standard MRI imaging increased metastatic findings in 71% of patients, which changed patient management in 19% of patients[21]. A comparative study showed that while contrast enhanced MRI is superior, adding diffusion weighted to non-contrast MRI imaging may suffice for everyday practice[22].
Radiomics appears to augment the ability of MRI to differentiate pancreatic NET from adenocarcinoma and solid pseudopapillary neoplasms[23,24].
Grading pancreatic NETs by CT or MRI is challenging and relies on assessing tumor margins, pattern of venous phase contrast washout, and enhancement pattern[10,18]. Irregular margins on CT have 71% sensitivity and 82% specificity for predicting grade 2/3 tumors while a model incorporating margins and fusion signature had 0.90 AUC for differentiating grade 1 from grade 2/3 tumors. Tumor texture analysis of CT and MRI images suggests entropy may be most useful in differentiating the different grades with 91% sensitivity and 85% specificity for distinguishing grade 1/2 NET from grade 3 NEC on CT and 83% sensitivity and 61% specificity for separating G2/3 from G1 tumors on MRI[25,26]. Whole tumor apparent diffusion coefficient histogram analysis may help predict the aggressiveness of pancreatic NET with kurtosis being the most useful marker[26]. While exciting, further studies are needed to understand the capabilities and role of radiomics in diagnosing, grading, and potentially prognosticating and guiding treatment.
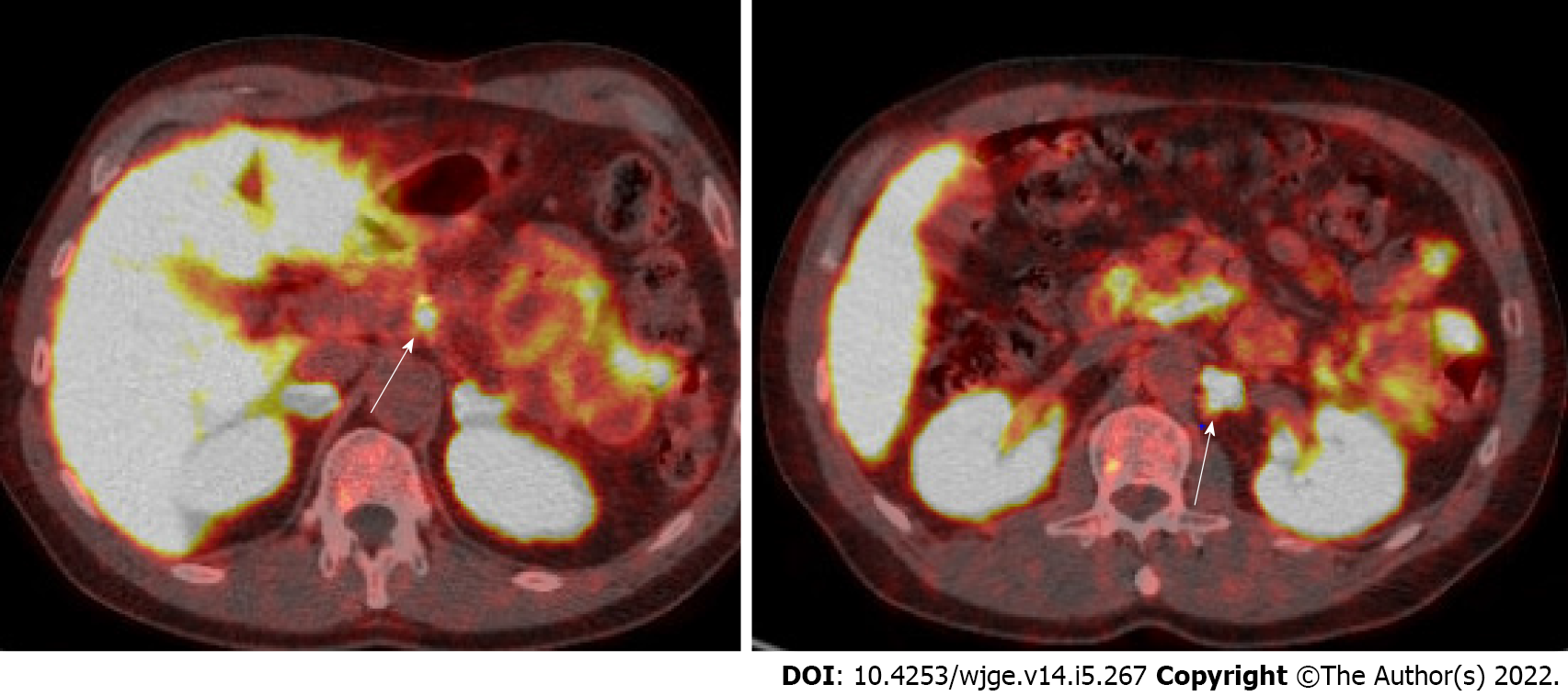
Somatostatin receptor imaging provides whole body imaging for NETs based on the wide expression of somatostatin receptors in most well-differentiated NETs. Nearly 70%-90% of gastrointestinal NETs and 50%-70% of pancreatic NETs express somatostatin receptors[27]. Quantification of somatostatin receptor expression can diagnose, stage, and assess response to therapy with somatostatin analogues (SSAs) or peptide receptor radionuclide therapy (PRRT)[15]. Gallium (Ga)-68 DOTA-peptides with PET/CT have replaced traditional Octreoscan [octreotide single-photon emission computed tomography (SPECT)/CT or 111-Inpentetreotide with SPECT] as the preferred modality due to its higher accuracy and shortened procedure time, which reduces radiation exposure (Figure 5)[28]. The sensitivity and specificity to detect NET is 92% and 95%, respectively[29]. Of note, there are different labeled peptides that can be used (DOTA-TOC, DOTA-NOC, and DOTA-TATE), but they are regarded as equally efficient[30]. One meta-analysis of 1561 patients found that using 68-DOTATATE changed management in one third of patients who previously had an Octreoscan[31]. Another study of 101 patients with well/moderately differentiated NETs showed that 68-DOTATATE imaging altered management in 36 patients, which included avoiding the need for biopsy (n = 4), initiating systemic therapy (n = 14), and altering operative plans in half of patients referred to surgery (n = 14)[32]. When available, this modality is preferred due to its high sensitivity and ability to influence management strategies in more than 70% of cases[33,34].
However, it should be noted that the accuracy of Ga-DOTA-peptides PET-CT imaging declines as NET tumor grading increases due to decrease in somatostatin receptor expression[15]. As NETs lose somatostatin receptors, their cells increase glucose utilization[35]. In this context, 18F-fluorodeoxyglucose (18F-FDG) PET/CT may be the preferred method for identifying high grade lesions. In a large study with 104 biopsy proven NETs where both Ga-DOTATATE and 18F-FDG PET/CT were performed, 18F-FDG PET/CT was most useful in changing management of G3 tumors while not helpful for G1 tumors[36]. Therefore, these authors suggested only limited use of 18F-FDG PET/CT for tumors with Ki-67 ≤ 12%. Ga-DOTATATE and 18F-FDG PET/CT may be complementary imaging modalities. Other studies have suggested this as well with FDG PET-CT being 100% sensitive for identifying poorly differentiated G3 tumors while Ga-DOTATATE had 83% sensitivity for well-differentiated G2/3 tumors[37]. A retrospective study of pathology-proven NENs demonstrated increased sensitivity (94%) for diagnosing NENs when both tracers were used compared to either alone (Ga-DOTATATE 63.8% and 18F-FDG 74.7%)[37]. Ki-67 index also negatively correlated with Ga-DOTATATE while positively correlated with 18F-FDG. Another group developed a NETPET grade from 0 to 5: P0 is negative for both 18F-FDG and 68Ga-DOTA-peptide scans, P1 is 68Ga-DOTA scan positive and 18F-FDG negative, P2-4 are positive for both with varying intensity of uptake, P5 is 18F-FDG positive and 68Ga-DOTA scan negative. This grading system correlated with tumor grade and survival with P5 having lowest median overall survival (11 mo)[38]. NETPET may allow selection of patients for PRRT which relies on the presence of somatostatin receptors to uptake therapeutic radionuclide into the NET cells. Patients with significant 18F-FDG positivity and 68Ga-DOTA negative disease may not respond well to PRRT alone and likely would benefit greater from systemic chemotherapy.
64Cu-DOTA is a new tracer with longer half-life and potentially superior spatial resolution compared to 68Ga[39]. The longer half-life (12.7 h vs 1.1 h) would potentially allow 64Cu-DOTA to be used more routinely and readily compared with 68Ga-DOTA. In 59 patients who underwent both 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT, more patients had more lesions detected using 64Cu-DOTA than with 68Ga-DOTA (13 vs 3, respectively, P = 0.013)[40]. A phase III US study confirmed the safety and high accuracy of 64Cu-DOTA PET/CT[41].
18F (fluoro-dihydroxyphenylalanine)-DOPA is another radiopharmaceutical that has high sensitivity of 97% and specificity of 90% for small intestinal NETs, and alters management in 50% of small intestinal NETs[42]. In a comparative prospective study, 18F-DOPA outperformed combined CT and somatostatin-receptor scintigraphy imaging in localizing low grade small intestinal NETs[43]. However, other studies have suggested 68Ga-DOTA is superior to 18F-DOPA for detecting well-differentiated NETs, including small intestinal NETs. 18F-DOPA is not readily available in Western countries, but may be complementary in the evaluation of small intestinal NETs[39,44].
Insulinomas are notoriously difficult to detect using morphological and somatostatin receptor imaging. Because they over-express glucagon-like peptide-1-receptors (GLP-1R), these offer targets for PET-based imaging[39]. 68Ga-DOTA-exendin-4 is a PET agent targeting GLP-1R. In a prospective randomized crossover study of 52 patients with suspected insulinoma, patients underwent 68Ga-DOTA-exendin-4 PET/CT, SPECT/CT and MRI with 68Ga-DOTA-exendin-4 imaging being more accurate than MRI for detecting insulinomas (93.9% and 67.6%, respectively)[45].
There is little literature on the value of PET-MRI, however, one small study demonstrated comparable image quality between 68Ga-DOTA-TOC PET/CT with PET/MRI while another suggested more lesions were identified with PET/MRI[46,47]. Advantages of PET/MRI include use in patients with renal insufficiency and better detection of liver lesions.
Functional NETs secrete hormones that lead to various clinical symptoms and syndromes. These hormone levels should only be checked in patients with clinical symptoms and syndromes suggestive of a functional NET. Hormone levels may be followed in patients with functional pancreatic NETs to monitor response to treatment and recurrence[48].
Carcinoid syndrome may occur with metastatic NETs, typically from the small intestine. Twenty four hours measurement of urinary 5-hydroxyindoleacetic acid (5-HIAA), an end product of serotonin metabolism, has a specificity and sensitivity of over 90%[49]. Patients should avoid tryptophan-rich foods and certain medications for several days before urine collection. Urinary 5-HIAA may also help predict patients at risk for carcinoid heart disease and carcinoid crisis during surgery as well as those who may respond to SSAs and PRRT[50]. If urine collection is difficult, plasma testing may be more convenient. Compared to urinary measurements, plasma 5-HIAA has a sensitivity and specificity of 89% and 97%, respectively, in diagnosing carcinoid patients[51]. Its widespread use is limited by institutional preferences and lack of validation in clinical studies.
Nonhormonal secretory products are also produced by both functional and nonfunctional NETs and can serve as biomarkers. Chromogranin A, a nonhormonal serum glycoprotein, is the main biochemical marker. However, its use has been deemphasized with the National Comprehensive Cancer Network and North American Neuroendocrine Tumor Society (NANET) not recommending its routine use due to limitations in accuracy, lack of standardization across laboratories (differing assays and isoforms), and unclear added value beyond imaging findings[48,52,53]. It should be measured fasting and at least 2 wk after discontinuation of proton pump inhibitors[54]. Sensitivity is lower in localized disease[55], and chromogranin A levels may drop with use of SSAs due to decreased production of hormones from cells rather than reduction in tumor burden[56].
Consequently, other biomarkers have been investigated. Genetic mutations in DAXX and ATRX expression (which interact with centromeric and telomeric regions) have recently been associated with well-differentiated NENs and poor survival in pancreatic NETs[57]. DNA hypermethylation has been associated with worse prognosis in pancreatic NETs. There is also interest in a new biomarker that measures cell-free DNA which circulates in the plasma following apoptosis, necrosis or active secretion, whereby it may have the potential to differentiate metastatic vs localized pancreatic NETs[57,58].
A novel liquid biopsy biomarker (NETest) measures 51 different RNA transcripts relevant to NET using quantitative real-time polymerase chain reaction[59]. Scores range from 0%-100% with 0-20 normal, 4-80 intermediate and ≥ 80 high activity. NETest has recently been reported with favorable results compared to chromogranin A for monitoring treatment response following both surgery and PRRT[60,61]. In a cohort of 253 GEP-NENs, NETest out performed chromogranin A in terms of accuracy (99% vs 53%) and also proved reliable in correlating the grade, stage and progression of GEP-NENs[62]. Another prospective study confirmed high diagnostic accuracy (91%) of NETest, ability to differentiate metastatic from local disease, 91% concordance with CT/MRI/ Ga 68-DOTA peptide PET, correlation with curative vs palliative surgeries, and higher diagnostic accuracy compared with chromogranin A[63]. NETest predicted postoperative recurrence at postop day 30 with 94% accuracy while chromogranin A was not helpful[64]. No patients with R0 resection and normal NETest developed recurrence while all R1/R2 patients had elevated NETest. This would allow early identification of patients with residual disease postoperatively who need to be followed more intensely while those with R0 resection and normal NETest likely can have fewer follow-up imaging studies. These exciting results need further confirmation in larger studies, and the utility of using this blood test rather than imaging to adjust treatment in advanced disease requires study as well.
For gastrointestinal NETs, endoscopy with biopsy should be performed to obtain pathological diagnosis[20]. Endoscopic imaging is insufficient for definitive diagnosis as differential diagnosis includes other subepithelial lesions, such as gastrointestinal stromal tumor especially in the stomach and duodenum and cysts and Brunner’s gland hyperplasia also in the duodenum. When imaging modalities fail to localize a small bowel tumor, video-capsule endoscopy (VCE) and device-assisted enteroscopy (DBE) are often needed[10]. VCE has a diagnostic yield of 45% for detecting tumors in the small intestine[65]. A retrospective study conducted over a seven year period found that small bowel tumors were detected in 1.5% of patients undergoing VCE (with a mean number of 4.7 tests used prior to VCE)[66]. In a study of 390 patients with metastatic NETs, radiology failed to localize a primary tumor in 2.8% whereas VCE identified NETs in 8/10 patients, which were confirmed histologically. As such, VCE should be used in select patients to identify small intestine NETs. While more invasive, antegrade and retrograde DBE may serve as an adjunctive tool prior to surgery by providing a histologic diagnosis and allowing tattooing areas of interest for surgeons[65]. Its diagnostic yield for detecting small intestine NETs ranges from 33%-80%[67,68]. Multifocal small intestinal NETs occur in 20%-30% of patients. CT and MRI have low accuracy for detecting these, and while CT or MR enterography, VCE, and DBE improve detection, the gold standard remains digital palpation of the small bowel intraoperatively[69].
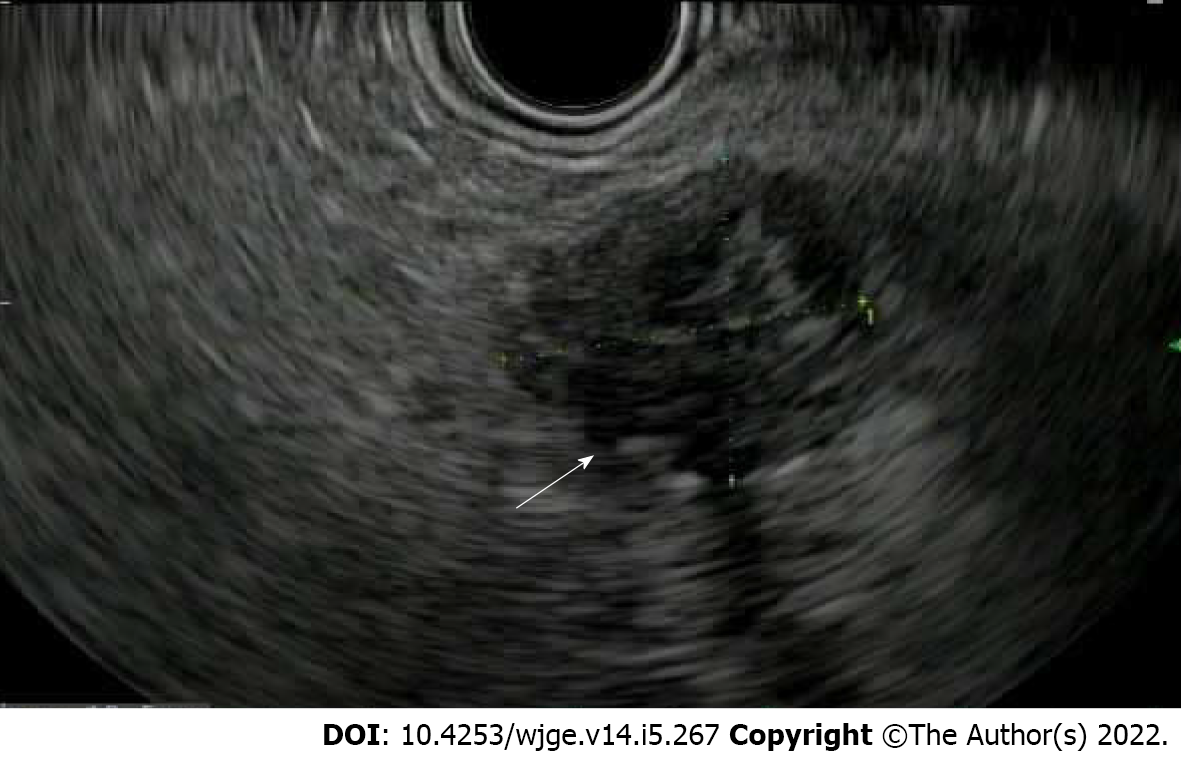
EUS is valuable for diagnosing pancreatic NETs and differentiating from pancreatic adenocarcinoma or metastatic disease with 87.2% sensitivity of 87.2% and 98% specificity (Figure 6)[70]. Mean detection rate of pancreatic NET for EUS is 90% while about 73% for both CT and MRI[71]. EUS identified pancreatic NET in 26% of cases where CT and other radiology studies including MRI and PET were negative[72]. EUS is particularly helpful for detecting small pancreatic NETs < 10 mm, 68% of which were missed by CT[73]. EUS also provides more accurate size estimate than CT (11.2% vs 46.5% inaccurate, respectively). Therefore, in patients with suspected pancreatic NET and negative imaging, EUS should be performed.
A limitation of EUS sampling is inaccurate assessment of grade and Ki67 index compared with surgical specimens. This discordance is accentuated in tumors > 2 cm because Ki-67 immunoreactivity can be focal and therefore, potentially missed by EUS sampling[74]. EUS-FNB may improve assessment of Ki-67 as well as diagnostic yield compared with EUS-FNA[75,76]. Diagnostic yield of EUS-FNA in cystic pancreatic NETs is lower at 73% compared with solid NETs although higher than mucinous cysts. Cystic pancreatic NETs may have thick wall with low carcinoembryonic antigen levels (< 5 ng/mL)[77].
Adjunctive EUS technologies include elastography and contrast harmonic EUS (CH-EUS). Elastography assesses the relative stiffness of tissue qualitatively and semi-quantitatively with strain elastography and more recently shear wave elastography. It may help differentiate pancreatic ductal adenocarcinoma from pancreatic NET, but was unable to distinguish NET from benign lesions in one study[78]. Another study suggested modest ability to diagnose malignant vs benign pancreatic NETs (67% sensitivity and 71% specificity)[79]. Further studies are needed with shear wave elastography, which may lead to improved results. CH-EUS uses intravenous microbubble-based contrast agents to assess microvasculature in lesions. With pancreatic NETs being hypervascular, they appear hyperenhancing on CH-EUS with sensitivity 79% and specificity 99%[80]. CH-EUS may be particularly helpful in assessing tumor grade as microvasculature density inversely correlates with grade. Therefore, higher grade tumors have more heterogeneous enhancement with 90% accuracy for predicting malignancy and > 95% negative predictive value for tumor aggressiveness[81]. Quantitative CH-EUS may allow accurate differentiation of G1/G2 pancreatic NET from G3 pancreatic NEC[82].
The next sections will highlight updates and controversial areas needing further research for the various GEP-NETs.
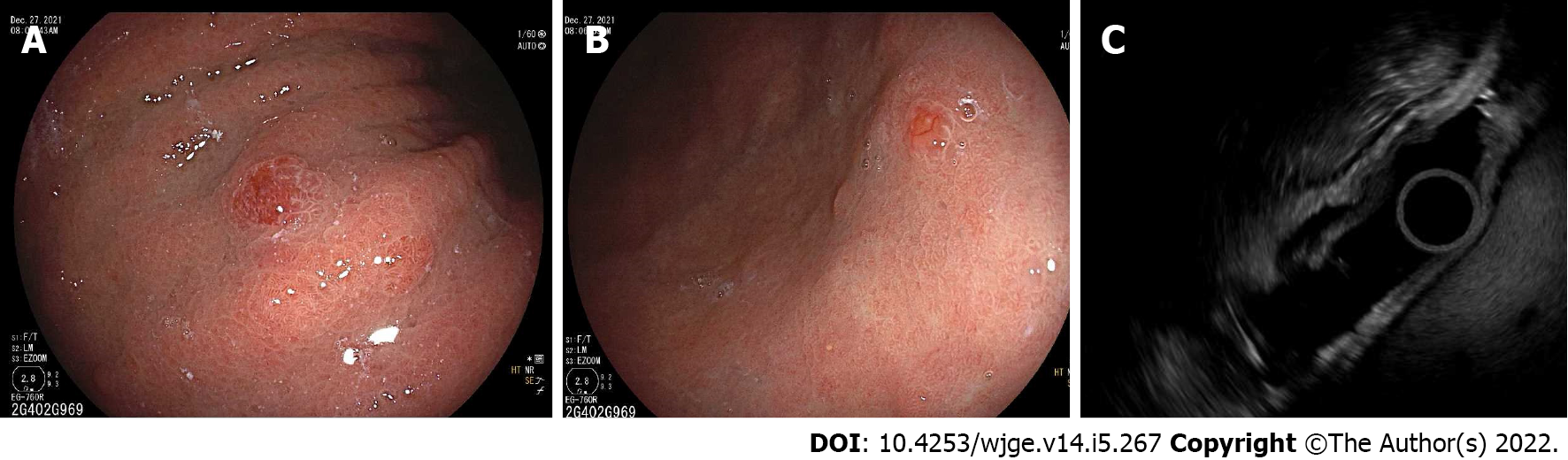
Gastric NETs are typically diagnosed incidentally during endoscopy, and it is important to understand the subtypes of gastric NETs and their corresponding treatment recommendations (Table 2). Metastases occur in less than 10% of type I gastric NETs ≤ 2 cm (Figure 7), but in nearly 20% greater than 2 cm[83,84]. A long-term study of small (< 1 cm) type I gastric NETs followed endoscopically over an average of 7 years found that none developed advanced disease or significant growth of the tumor[85]. For larger lesions, EUS should be performed to assess depth of invasion and presence of lymph node metastases before performing endoscopic or surgical resection. Regarding endoscopic resection, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) can be considered although ESD should be reserved for larger lesions with superficial submucosal invasion[86]. A retrospective study of 87 Lesions less than 1 cm resected by ESD or EMR found that while complete resection rates trended higher with ESD (94.9% vs 83.3%, P = 0.174), it was associated with increased procedural time (26.1 min vs 9.5 min) and a tendency towards higher complications (15% vs 6%, P = 0.28)[87]. For rare type 1 gastric NETs with invasive disease, regional metastases, or grade 3 Lesions, surgery may be considered[88]. Antrectomy is an option in patients with numerous tumors, which may be curative with decreased recurrence compared to endoscopic resection (11% vs 44%)[89]. The role of medical therapy with SSAs (lanreotide and octreotide) to suppress gastrin levels as a means to reduce tumor progression remains to be determined[86].
| Type 1 | Type 2 | Type 3 | Type 4 | |
| Proportion of gastric neuroendocrine tumors | 70%-80% | 5% | 15%-25% | Very rare |
| Associated conditions | Atrophic gastritis | Zollinger-Ellison and MEN-1 | Sporadic | Sporadic |
| Location | Gastric fundus and body | Gastric fundus and body | Antrum | Anywhere |
| Endoscopic findings | Multiple, small polyps | Multiple, small polyps | Solitary, larger | Solitary, larger |
| Gastrin level | Increased | Increased | Normal | Normal |
| pH | Increased | Decreased | Normal | Normal |
| Prognosis | Excellent | Good | Poor | Very poor |
| Metastasis | 10%-20% | 10%-30% | 30%-80% | 80%-100% |
| Evaluation | Gastric pH, gastrin, EUS 1-2 cm lesions | Gastric pH, gastrin, EUS 1-2 cm lesions, abdominal imaging | Gastric pH, gastrin, EUS, abdominal imaging | Gastric pH, gastrin, EUS, abdominal imaging |
| Treatment | Endoscopic resection for larger lesions and surveillance for lesions < 2 cm | Similar to type 1 | Surgery, endoscopic resection for superficial, well-differentiated lesions < 1 cm | Surgery for local disease, systemic chemotherapy for metastatic |
| Surveillance | EGD every year | EGD every 6-12 mo, abdominal imaging every year | EGD every 6-12 mo, abdominal imaging every 3 mo |
Because type III gastric NETs behave differently from type I and II and are very aggressive tumors, traditionally surgical resection was recommended (Table 3)[90,91]. However, for small < 1 cm well-differentiated lesions without EUS evidence of deep invasion or regional metastases, endoscopic resection may be feasible[92]. A Japanese multi-center study of 144 Lesions (90 G1 and 54 G2) with median size 8 mm compared surgical (81 patients) and endoscopic (63 patients) resection outcomes during long-term follow-up[93]. Patients undergoing endoscopic resection had smaller lesions confined to the mucosa or submucosa, and 24% of these patients needed subsequent surgical resection. Overall, 5-year survival was similar for both groups, and in the endoscopic resection alone cohort, only one patient developed recurrence with no mortality over median 32-mo follow-up. Another recent study comparing 45 patients undergoing surgical or endoscopic resection found that tumor size greater than 1 cm was associated with lymph node metastases[94]. In a cohort of 50 patients undergoing endoscopic resection (41 EMR and 9 ESD) with a median follow up of 46 mo, mean size was 10 mm with nonsignificant trend towards larger lesions resected with ESD (14.2 mm vs 9.3 mm) and greater lymphovascular invasion in ESD patients (22.2% vs 2.4%). However, there was no evidence of tumor recurrence in either group. Of note, all lesions were no deeper than the submucosa layer and well-differentiated[95]. Given the more aggressive biology of type III gastric NETs, ESD may be favored over EMR although further study is needed. The resection approach should be carefully tailored to a patient’s tumor size, depth of invasion, grade and presence of regional metastases[71].
| Duodenal | Ampullary | Jejuno-ileal | |
| Epidemiology | 2%-3% GEP-NETs | 0.3%-1% GEP-NETs | 1.2 cases/100000 incidence quadrupled over past 30 yr |
| Evaluation | > 2 cm: CT and EUS | CT, EUS | Chromogranin A, urine 5-HIAA, CT/MRI, gallium-DOTATATE PET CT, colonoscopy into terminal ileum |
| 5-yr survival | No metastases: 80%-95%; Regional metastases: 65%-75%; Zollinger-Ellison or MEN-1: > 90% | 59% | Local disease: 80%-100%; Regional disease: 70%-80%; Distant metastases: 35%-80% |
| Treatment | < 1 cm: Endoscopic resection; 1-2 cm: Endoscopic or surgical resection; > 2 cm: EMR or ESD, surgical resection for regional disease | < 2 cm superficial without metastases: Pancreaticoduodenectomy or consider endoscopic ampullectomy; > 2 cm: Pancreaticoduodenectomy | Surgery; Carcinoid syndrome: Long-acting SSA (octreotide LAR 20-30 mg IM) |
| Surveillance | EGD at least every 2 yr | EGD at 1-2 yr interval | NANETS: Curative surgery-CT every 3-6 mo then 6-12 mo for 7 yr; Advanced disease- CT every 6 mo; ENETS: Curative surgery: Chromogranin A, urine 5-HIAA, CT every 6-12 mo; Slow-growing treated without curative intent: every 3-6 mo |
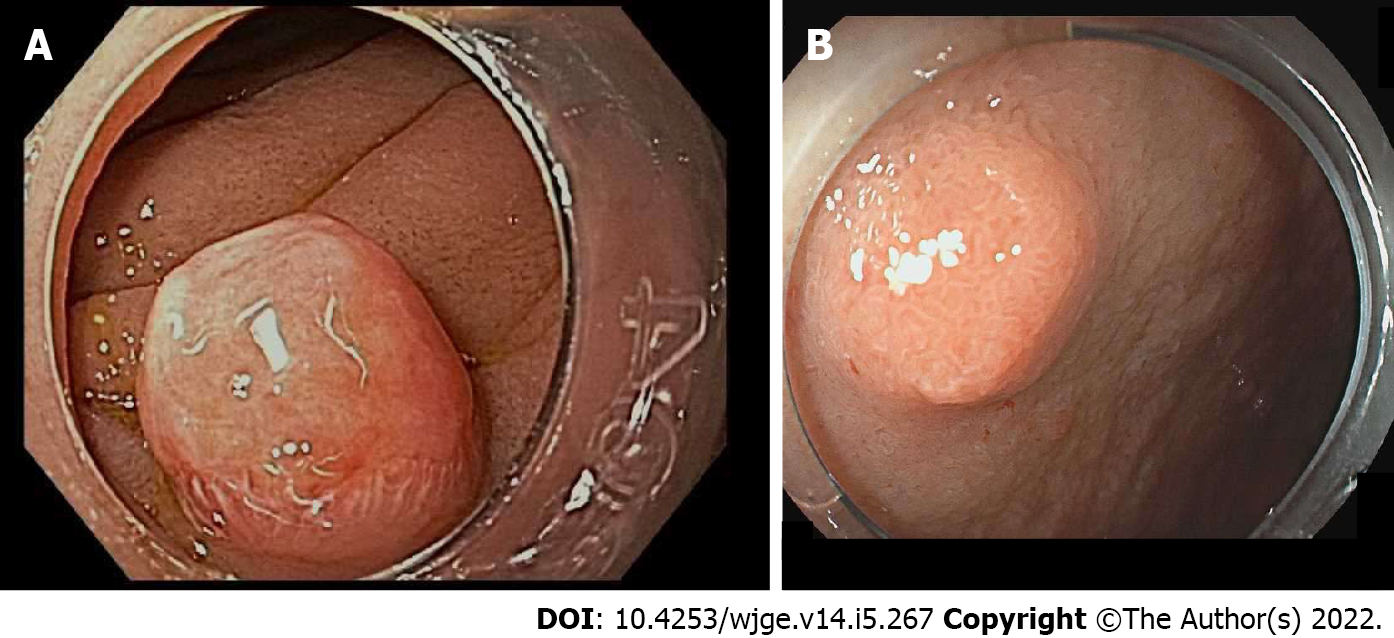
Table 3 summarizes evaluation and management of small intestinal (duodenal, ampullary, and jejuno-ileal) NETs[96,97]. Nearly 90% of duodenal NETs are non-functional, well-differentiated and incidentally discovered as small, polypoid lesions in the first and second portion of the duodenum (Figure 8)[88]. For small duodenal NETs undergoing EMR, the optimal EMR technique remains unclear (standard, underwater, ligation, ligation without resection) with the main complications being bleeding in up to 20% of patients and perforation. For lesions greater than 2 cm without evidence of metastatic disease, ESD should be reserved for larger lesions because perforation and bleeding appear higher than with EMR or ESD[20,86,98].
The optimal strategy for duodenal NETS between 1 and 2 cm remains unclear. A multicenter study of 60 patients found that lesions larger than 11 mm had significantly higher rates of lymphovascular invasion and incomplete endoscopic resection with none having complete pathologic resection compared with smaller lesions[99]. Therefore, the authors suggested surgical resection for lesions larger than 11 mm. However, a recent study suggested EMR is efficacious and safe for 1-2 cm lesions without regional or distant metastases with similar overall survival to surgical resection during median 56-mo follow-up[100]. As expected, patients undergoing EMR were older (72.6 years vs 59.2 years, respectively) with more node negative disease (89.5% vs 50%, respectively). The decision to pursue endoscopic or surgical resection should be considered based on local expertise and the individual case.
Ampullary NETs (Table 3) appear different in nature than non-ampullary duodenal NETs, and are often more advanced at presentation (G3 in 17% vs 2% for duodenal NETs) with higher incidence of lymph node metastasis (34% vs 10% for duodenal NETs)[101,102]. In a large pathology series of 203 duodenal NETs, most of the 27 NECs occurred in the ampullary region[103]. While pancreaticoduodenectomy is recommended regardless of size, its morbidity and mortality make endoscopic resection an attractive option. Small ampullary NETs less than 2 cm without muscularis propria invasion or lymph node metastases were completely resected endoscopically in one small study, and 71% had no recurrence during median 56 mo follow-up[104]. Further studies are needed to understand which patients may be managed with endoscopic ampullectomy.
The true incidence of jejuno-ileal NETs (Table 3) likely remains underappreciated as in autopsy studies, the incidence is much higher (1.2 cases per 100000) than in population studies (0.67 cases per 100000)[96,105]. This implies that many early jejuno-ileal NETs remain undiagnosed[106]. Early diagnosis remains challenging as most patients are asymptomatic or have nonspecific symptoms, and carcinoid syndrome occurs in only 20%-30% of patients with metastatic disease[106]. Unlike gastric, duodenal and colorectal NETs, incidental diagnosis of jejuno-ileal NETs is unlikely with 89% found in the ileum[105,107].
Segmental resection and wide lymphadenectomy is the definitive approach for jejuno-ileal NETs with localized and regional metastatic disease[108]. Intraoperative exploration with small bowel palpation is recommended as up to 70% of pre-operative imaging may understage tumors[109]. This is likely due to limitations of diagnostic imaging including VCE and DBE, which may miss small, multifocal lesions[52,110]. For patients with distant metastatic disease, surgical resection of the primary tumor may still be considered to alleviate symptoms resulting from the lesion (for example, obstructive symptoms or bleeding), to achieve potential cure if the distant metastases may be completely resected as well, and to improve outcome although data on this is mixed and further study is needed[106].
Traditionally appendiceal NETs were the most common appendiceal tumors although recent data suggests mucinous neoplasms may have surpassed them[111,112]. Most present incidentally and are asymptomatic as the majority are located in the distal one-third of the appendix rather than the base. Because risk of metastases correlates with tumor size, recommendations for evaluation and management vary depending on the size (Tables 4 and 5). However, a study of 418 patients noted that risk of nodal metastases was affected by age, depth of invasion, extent of surgery as well as tumor size with 0.89 area under the curve[113]. Another study analyzing 435 patients found that tumor size > 1.5 cm, G2 grade, lymphovascular infiltration, and mesoappendiceal invasion were associated with nodal metastasis[114]. Therefore, some guidelines suggest right hemicolectomy for 1-2 cm tumors with any of these high-risk features. However, in a study of 916 patients with 1-2 cm NETs, right hemicolectomy was not associated with increased survival despite being associated with larger and higher stage tumors (hazard ratio = 1.14, P = 0.72)[115]. The most appropriate surgical approach for appendiceal NETs especially between 1-2 cm remains unclear as well as the definitive triggers to send a patient for completion right hemicolectomy.
| Tumor size | Nodal metastases | Distant metastases |
| ≤ 1 cm | 0% | 0% |
| 1-2 cm | 7.5% | 4% |
| ≥ 2 cm | 33% | 12% |
| Appendiceal | Colonic | Rectal | |
| Epidemiology | 1.45% of appendectomies | < 10% NETs | 29% GEP-NETs |
| Presentation | Incidental or acute appendicitis; Carcinoid syndrome rare | Incidental (yellowish polypoid or donut-shaped); 46% advanced at diagnosis | Incidental (small, yellowish polypoid) |
| Evaluation | (1) Colonoscopy; (2) CT/MRI if > 2 cm, incomplete resection1, suspected metastases; (3) Gallium DOTATATE PET CT: Incomplete resection1, suspected metastases, carcinoid syndrome; and (4) Chromogranin A and urine 5-HIAA: liver metastases or carcinoid syndrome | CT, EUS, Gallium DOTATATE PET CT | Colonoscopy; EUS; > 2 cm, invasion beyond submucosa, lymph node disease: Gallium DOTATATE PET CT |
| 5-yr survival | < 2 cm without regional or distant disease: 100%; 2-3 cm with regional nodes or ≥ 3 cm: 78%; Distant metastases: 32% | Stage I: 90%; Stage II: 77%; Stage III: 53%; Stage IV: 14% | Localized: 98%-100%; Regional metastases: 54%-74%; Distant metastases: 15%-37% |
| Treatment | Right hemicolectomy with lymph node dissection: (1) > 2 cm; and (2) 1-2 cm with high-risk features2; Appendectomy: (1) < 1 cm, well-differentiated; and (2) 1-2 cm without high-risk features2 | Local disease: segmental colectomy and lymphadenectomy; Metastatic disease: chemotherapy | < 1 cm without invasion beyond submucosa: Endoscopic resection; 1-2 cm: Endoscopic resection or transanal resection; > 2 cm without metastatic disease: Radical surgical resection |
| Surveillance | (1) ≤ 2 cm without high-risk features2 and confined to appendix: No follow-up; and (2) Larger or node positive, and right hemicolectomy: CT/MRI 3-12 mo post-surgery; consider baseline gallium DOTATATE PET CTAfter first year, annual CT/MRI | < 1 cm: None; 1-2 cm: EUS or MRI at 6 and 12 mo; > 2 cm: CT/MRI at 3 and 12 mo, then every 12-24 mo |
With increased colon cancer screening, the incidence of colonic NETs has increased dramatically from 0.02 to 0.2 per 100000 people in the United States between 1973 to 2004[116]. The majority are high-grade, poorly differentiated lesions that typically occur in the right colon (70%), especially in the cecum[117,118]. Well-differentiated colonic NETs have significantly worse prognosis than well-differentiated NETs anywhere else in the GI tract. A recent study using the SEER database developed a novel nomogram to predict survival incorporating patient’s age ≥ 68 years, sex, tumor size, grade, chemotherapy, N stage and M stage. This outperformed the traditional TNM staging system in predicting overall survival[119].
With aggressive behavior and poor survival outcomes, colonic NETs require multidisciplinary care (Table 5). Tumors < 2 cm may be considered for endoscopic resection, however surgery is required for incomplete resection or high-grade pathology[116]. Very little data exists about the efficacy and safety of ESD with one study including only 6 non-rectal, colonic NETs. This study demonstrated that non-rectal NETs were significantly associated with risk of non-R0 resection and while complications were higher, this was not significant compared with ESD of rectal NETs[120]. On the other end of the spectrum in patients with metastatic disease, chemotherapy can also be utilized[117]. Survival improved with chemotherapy alone, surgery alone and even more with the combination of surgery and chemotherapy (5-year survival 37% for combination vs 32% surgery alone, P < 0.001)[121]. However, other studies noted that surgery did not provide significant survival benefit in localized and metastatic disease[122,123]. Further study is necessary to understand the optimal treatment combination as well as role of immunotherapy.
Similar to colonic NETs, rectal NETs have been increasingly diagnosed with improved screening colonoscopy rates, experiencing a 10-fold rise in incidence over the past 30 years[124,125]. They are more common in women in the United States although in Korea men are more likely to have rectal NETs. In the United States, Asian and African American patients have higher incidence than Caucasians[126]. The majority (70%-88%) of rectal NETs are small (< 1 cm) and localized at the time of diagnosis[124,127]. Lymph node metastasis occurs in about 2% and distant metastases in about 8% of rectal NETs at diagnosis. Tumor size, depth of invasion, grade and lymphovascular invasion all affect prognosis. Regarding tumor size, it appears to correlate with metastasis at the time of diagnosis (3%, 66%, and 73% metastases with tumor size ≤ 1 cm, 1-1.9 cm, and ≥ 2 cm, respectively)[128]. A study using the SEER database of 788 patients with T1 rectal NETs noted tumor size and submucosal invasion were predictive of metastasis, and no tumors ≤ 19 mm without submucosal invasion had metastases[129]. At diagnosis, 1.5% of patients had metastases with 1.1% in tumors ≤ 10 mm and 6.6% in NETs 11-19 mm.
Usually, rectal NETs are not recognized before polypectomy by the endoscopist and only later discovered when pathology returns. If the endoscopist is suspicious of a rectal NET during the procedure, biopsies can be obtained with photograph documentation and tattoo adjacent to the lesion. In terms of treatment, endoscopic resection should be performed for lesions smaller than 1 cm without invasion beyond the submucosa. Options include EMR, EMR band ligation, and ESD; however, given the greater procedure time and complications with ESD, EMR or EMR band ligation are preferred. A prospective study comparing EMR band ligation (n = 53) to ESD (n = 24) in lesions ≤ 10 mm demonstrated the superiority of EMR band ligation with higher complete resection rates (100% vs 54.2%, P = 0.00)[130]. In addition to 100% negative margins, EMR band ligation was associated with shorter procedure times (5.3 vs 17.9 min, P = 0.00). Similarly, a retrospective study of 82 tumors < 10 mm reported higher complete resection rates with EMR band ligation compared to ESD (95% and 75%, P = 0.025) with shorter procedure times[131]. A recent retrospective comparative study of underwater EMR (n = 36) to ESD (n = 79) found no difference in achieving R0 resection for lesions ≤ 10 mm[132]. Yet underwater EMR was associated with a significantly shorter procedure time (5.8 min vs 26.6 min, P = 0.0001) and no adverse events while there were two cases of delayed bleeding and minor perforation in the ESD group. Therefore, for small rectal NETs < 1 cm, EMR band ligation is the endoscopic method of choice while underwater EMR may be considered as well.
If incomplete resection occurs, then salvage therapy with ESD or transanal endoscopic microsurgery should be pursued to minimize recurrence[133,134]. Optimal management for rectal NETs 1-2 cm remains uncertain. NANETS recommends transanal excision although noted this could be considered after endoscopic resection if that resulted in positive margins. ESD may have a role and may be preferred to cap-assisted EMR as higher complete resection (100% vs 70%) and lower recurrence (0% vs 17%) was achieved with ESD[135]. However, ESD may not be the ideal approach in patients with lymphovascular invasion, grade 2, and/or positive margins as distant metastasis occurred in 2.5% following ESD of small (< 2 cm) rectal NETs[120]. With advanced metastatic disease, palliative surgery and systemic therapies should considered through a multidisciplinary approach considering availability of local resources.
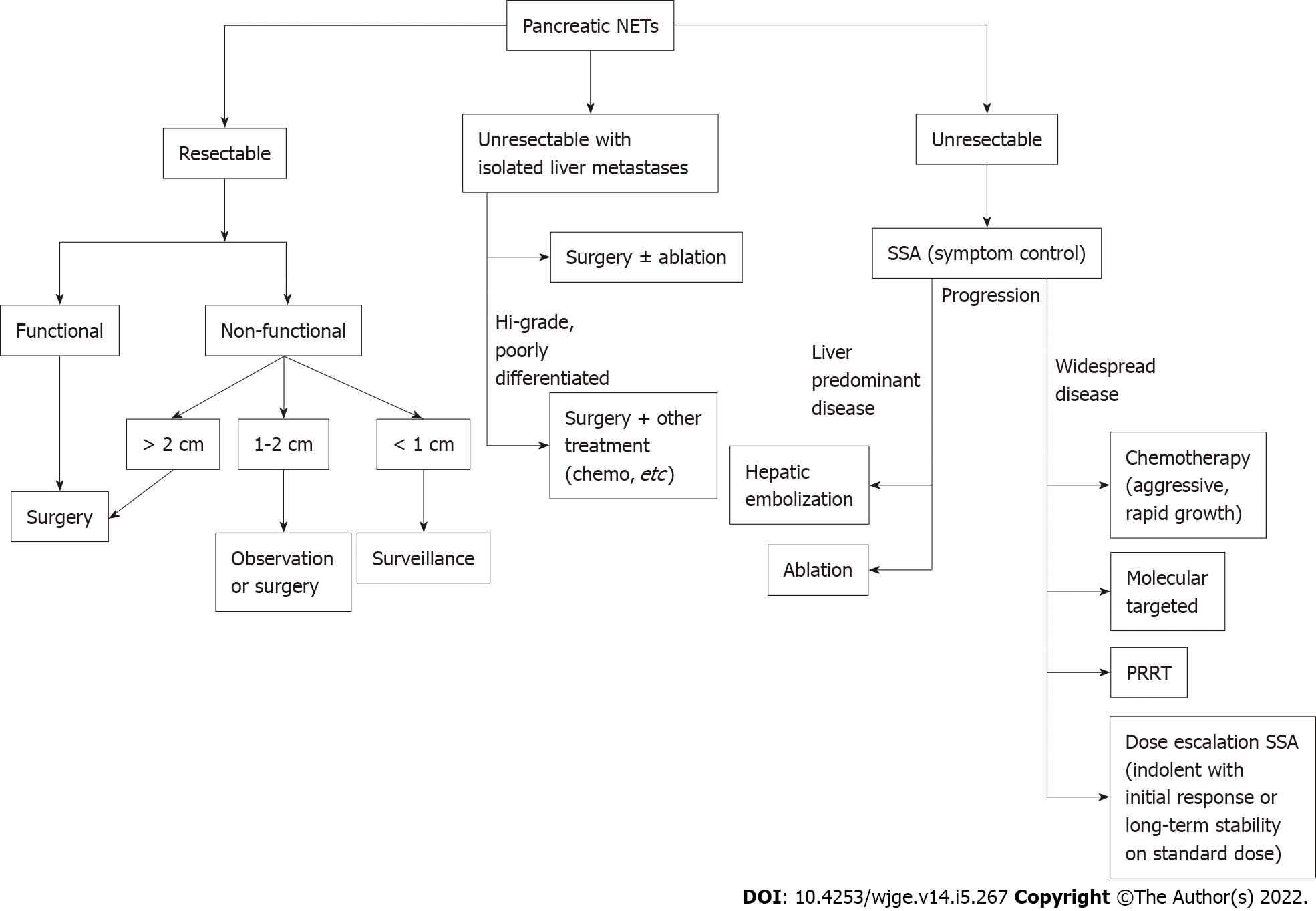
Pancreatic NETs make up 16% of GEP-NETs with annual incidence of 0.5 per 100000 people[6,9]. The majority are sporadic and malignant with metastatic disease present in 60% of patients at the time of diagnosis (Table 6)[96,136]. If there are no distant metastases or if the metastatic disease is resectable (for example, isolated hepatic metastases), surgery is the primary method of treatment for all functioning pancreatic NETS, irrespective of size (Figure 9). It is also recommended for localized (confined to the pancreas and regional lymph nodes) nonfunctioning pancreatic NETs greater than 2 cm. Lesions less than 1 cm can safely undergo surveillance in the absence of symptoms and pancreatic duct dilation[137]. In a cohort comparing nonoperative and operative management of nonfunctioning NETs less than 1 cm, there was no difference in mortality or disease progression over median 45-mo follow up with surgical patients experiencing relatively high 46% rate of complications postoperatively[138].
| Diagnostic evaluation | |
| All pancreatic NET | Multiphasic CT/MRI |
| If results impact management, gallium DOTATATE PET CTEUS with biopsy | |
| Insulinoma | 72 h fast test: Hypoglycemia with elevated insulin |
| Oral glucose tolerance test: May be necessary in minority with only postprandial hypoglycemia | |
| Gastrinoma | Fasting gastrin 10 times upper limit of normal + gastric pH < 2 |
| If gastrin less elevated + gastric pH < 2, measure BAO with secretin test | |
| BAO > 15 mEq/h or serum gastrin increase > 120 pg/mL | |
| Glucagonoma | Fasting serum glucagon > 500 pg/mL |
| Somatostatinoma | Fasting plasma somatostatin > 30 pg/mL |
| VIPoma | Large volume diarrhea + serum VIP > 75 pg/mL |
However, observation vs surgery for nonfunctioning pancreatic NETs measuring between 1-2 cm remains controversial. Several studies have supported observation, as smaller tumor size correlates with lower malignancy potential[138-141]. On the other hand, other studies have suggested surgery is superior[142-145]. One study that followed 39 resected lesions less than 2 cm for a median 34.2 mo found that 7.7% developed late metastasis or recurrence[143]. Two other comparative studies supported surgical resection for pancreatic NETs less than 2 cm, as five-year overall survival rates were greater than the observation group (82.2%-92.8% vs 34.3%-67.4%, respectively)[142,145]. Regardless of tumor size, if surgery is pursued, follow up with cross-sectional imaging is recommended annually for the first three years then every two years for a total of 10 years[146].
EUS-guided radiofrequency ablation (RFA) has recently been studied as a potentially safe and minimally invasive treatment option. Through the use of targeted electromagnetic energy and alternating high-frequency currents, EUS-RFA induces coagulative necrosis, fibrotic changes, and a delayed immune response to the pancreatic tissue of interest[147]. Only a few human studies have investigated treatment outcomes, but have demonstrated feasible and promising results[148,149]. In one study, 18 patients (including seven insulinomas and 11 non-functioning lesions) with a mean diameter of 1.4 cm demonstrated no signs of recurrence during mean follow-up of 8.7 mo[149]. Furthermore, all seven patients with insulinomas had normalization of glucose within 24 h of EUS-RFA. A prospective multicenter study of 14 pancreatic NETs (G1 lesions with median size 1.3 cm) found that 12 (85.7%) lesions completely resolved at 12 mo follow up[148]. The other two lesions were considered treatment failures with one increasing by 3 mm and the other remaining unchanged in size. A recent video case report used EUS-guided microwave ablation to safely and effectively treat a symptomatic inoperable pancreatic neck NET (35 × 32 mm) invading the splenic artery without any complications[150]. Further prospective and longer-term studies are needed to determine how this technology may improve patient outcomes and how it fits into the treatment algorithm.
For patients with isolated liver metastases, optimal management remains uncertain in the absence of randomized controlled studies and ranges from surgical resection of all visible metastatic disease to local therapy with ablation. Candidates for resection of liver metastases include those with isolated unilobar disease, preserved liver function and well-differentiated pathology[151]. However, even patients with bilobar disease could undergo multiple wedge resections and/or hepatectomy provided at least 20 percent of the total liver volume remains preserved. Five-year survival rates ranging from 85% to 90% have been reported with selected patients undergoing curative resection[152,153]. However, recurrence rates are as high as 54% despite negative margins, which implies that preoperative imaging misses small metastatic disease[154].
Whether the primary tumor should be resected as well in these patients remains debated although retrospective studies suggest improved survival with this approach[155].
Ablation is mainly effective for small (< 3 cm) lesions and includes RFA, cryoablation and microwave ablation with a more favorable morbidity profile than surgery or hepatic arterial embolization. The optimal use of this technique remains unclear although it is often used as an adjunct to surgical resection especially when complete resection of multifocal or bilateral disease is not feasible or in patients who have already undergone hepatic resection. Comparative studies remain limited with one nonrandomized study suggesting high overall 5-year survival (84%) following RFA compared to surgery (90%)[152]. If RFA is contraindicated (especially for lesions near the liver surface or adjacent to vital structure) or technically not possible, cryoablation can be used[156]. While cryoablation is relatively underutilized, a small case series demonstrated 77.8% complete response and 22.2% partial response in 9 patients undergoing ablation with a median follow of 7 mo[157]. Cryoablation may be considered in technically challenging tumor locations. Further studies are needed to delineate its role relative to other ablative techniques.
For unresectable liver disease in symptomatic patients, hepatic arterial embolization is suggested for palliation as an alternative to medical treatment alone. Techniques include injection of different substances [bland embolization (gel foam powder), chemoembolization (chemotherapy), radioembolization (radioactive isotopes)]. In liver predominate disease, chemoembolization is associated with a tumor response rate over 50%, which appears comparable to the other techniques[158]. A randomized trial is underway to compare liver progression-free survival and complications of these three techniques.
For unresectable widespread disease, treatment options include systematic therapy with SSAs to treat symptoms and control disease, chemotherapy, molecular targeted therapy, PRRT, and immunotherapy. SSAs suppress hormone release in pancreatic NETs by binding somatostatin receptors, which prevents the release of hormonal peptides, and is thus most helpful for VIPomas, glucagonomas, and somatostatinomas and less helpful for insulinomas and gastrinomas. When used to control disease by exploiting the ability of SSAs to decrease proliferation in nonfunctioning NETs, SSAs are administered to patients with high tumor burden[159]. The CLARINET study, a randomized, double blind placebo trial, provided support for lanreotide in preventing disease progression in advanced well to moderately differentiated nonfunctioning pancreatic NETs (prolonged progression-free survival 65% vs 33% at 24 mo)[160]. Short-acting octreotide may be used and if effective, changed to long-acting depot with monthly injections.
Chemotherapy is particularly helpful in aggressive disease with rapidly growing metastases[10]. Compared to temozolomide, the use of combination chemotherapy with capecitabine and temozolomide (CAPTEM) demonstrated high response, progression free survival, and manageable toxicity in patients with well-differentiated intermediate to high grade pancreatic NETs[161,162]. Given its favorable toxicity profile as an oral regimen, CAPTEM is typically favored over streptozocin-containing regimens. Expression of methylguanine DNA methyltransferase (MGMT) may predict response to alkylating chemotherapeutics as studies suggested that patients without MGMT had better response[163]. However, prospective studies are necessary.
Molecular targeted therapy has a role in patients with disease progression on SSAs by inhibiting the mammalian target of rapamycin or tyrosine kinase with everolimus and sunitinib, respectively[164]. Compared to placebo, everolimus was able to prolong progression free survival (11 mo vs 4.6 mo) in a cohort of 410 patients with advanced, progressive low and intermediate grade pancreatic NETs[165]. Sunitinib has also demonstrated safe and reliable results in progressive, well-differentiated pancreatic NETs where progression free survival was double placebo (11.4 mo vs 5.5 mo)[161]. with a response rate of 24.5%[166]. Other promising agents include tyrosine kinase inhibitors sorafenib, pazopanib, vascular endothelial growth factor receptor inhibitor cabozantinib and lenvatinib, which all require further prospective study.
PRRT uses radiolabeled SSAs (90Yttrium or 177Lutetium) to bind somatostatin receptors as a means to emit localized radiation in advanced pancreatic NETs[164]. Therefore, it is an option in patients who have progressed through SSAs. A phase III trial compared 177Lu-Dotatate (116 patients) to long acting octreotide (113 patients) and found longer progression free survival (65.2% vs 10.8%) and higher response rates (18% vs 3%) with 177Lu-Dotatate[167]. A larger study of 610 patients (which included bronchial NETs) also reported a favorable survival and response rate, especially in the pancreatic NET group[168]. Despite encouraging results, concern remains over potential long-term toxicity including acute leukemia (0.7%) and myelodysplastic syndrome (1.5%)[168]. As such, risk and benefits of treatment should be carefully discussed with patients before embarking on PRRT. Further studies are needed to understand the role and safety of PRRT as well as whether combination therapy with SSAs is more efficacious.
Although immunotherapy has revolutionized oncology, its utility in treating pancreatic NETs remains unclear. Early trials evaluating anti-programmed cell death 1 antibodies including spartalizumab and pembrolizumab have not been encouraging with minimal response in pancreatic NETs. Further studies are certainly needed.
GEP-NENs represent a complex and diverse physiologic and pathologic spectrum of neoplasms with varying disease activity that benefit from multidisciplinary care. With advancements in functional imaging, serum biomarkers, and endoscopic techniques for diagnosis including EUS as well as therapy with EMR, ESD and EUS-RFA, identification and management of these protean lesions continue to improve and allow for tailored treatment plans based on prognostic information and location throughout the gastrointestinal tract.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India; Liu L, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Díez M, Teulé A, Salazar R. Gastroenteropancreatic neuroendocrine tumors: diagnosis and treatment. Ann Gastroenterol. 2013;26:29-36. [PubMed] |
| 2. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 698] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 3. | Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, Lawlor RT, Johns AL, Miller DK, Mafficini A, Rusev B, Scardoni M, Antonello D, Barbi S, Sikora KO, Cingarlini S, Vicentini C, McKay S, Quinn MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, McLean S, Nourse C, Nourbakhsh E, Wilson PJ, Anderson MJ, Fink JL, Newell F, Waddell N, Holmes O, Kazakoff SH, Leonard C, Wood S, Xu Q, Nagaraj SH, Amato E, Dalai I, Bersani S, Cataldo I, Dei Tos AP, Capelli P, Davì MV, Landoni L, Malpaga A, Miotto M, Whitehall VL, Leggett BA, Harris JL, Harris J, Jones MD, Humphris J, Chantrill LA, Chin V, Nagrial AM, Pajic M, Scarlett CJ, Pinho A, Rooman I, Toon C, Wu J, Pinese M, Cowley M, Barbour A, Mawson A, Humphrey ES, Colvin EK, Chou A, Lovell JA, Jamieson NB, Duthie F, Gingras MC, Fisher WE, Dagg RA, Lau LM, Lee M, Pickett HA, Reddel RR, Samra JS, Kench JG, Merrett ND, Epari K, Nguyen NQ, Zeps N, Falconi M, Simbolo M, Butturini G, Van Buren G, Partelli S, Fassan M; Australian Pancreatic Cancer Genome Initiative, Khanna KK, Gill AJ, Wheeler DA, Gibbs RA, Musgrove EA, Bassi C, Tortora G, Pederzoli P, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 5. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 6. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2491] [Article Influence: 311.4] [Reference Citation Analysis (4)] |
| 7. | Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21:R153-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Fernandez CJ, Agarwal M, Pottakkat B, Haroon NN, George AS, Pappachan JM. Gastroenteropancreatic neuroendocrine neoplasms: A clinical snapshot. World J Gastrointest Surg. 2021;13:231-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 9. | Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, Luo F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open. 2021;4:e2124750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 10. | Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M. Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis. 2021;53:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 405] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 12. | Govind D, Jen KY, Matsukuma K, Gao G, Olson KA, Gui D, Wilding GE, Border SP, Sarder P. Improving the accuracy of gastrointestinal neuroendocrine tumor grading with deep learning. Sci Rep. 2020;10:11064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Kaltenbach B, Wichmann JL, Pfeifer S, Albrecht MH, Booz C, Lenga L, Hammerstingl R, D'Angelo T, Vogl TJ, Martin SS. Iodine quantification to distinguish hepatic neuroendocrine tumor metastasis from hepatocellular carcinoma at dual-source dual-energy liver CT. Eur J Radiol. 2018;105:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 14. | Giesel FL, Schneider F, Kratochwil C, Rath D, Moltz J, Holland-Letz T, Kauczor HU, Schwartz LH, Haberkorn U, Flechsig P. Correlation Between SUVmax and CT Radiomic Analysis Using Lymph Node Density in PET/CT-Based Lymph Node Staging. J Nucl Med. 2017;58:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Hofland J, Kaltsas G, de Herder WW. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 16. | Keck KJ, Maxwell JE, Menda Y, Bellizzi A, Dillon J, O'Dorisio TM, Howe JR. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. 2017;161:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Pasquer A, Walter T, Hervieu V, Forestier J, Scoazec JY, Lombard-Bohas C, Poncet G. Surgical Management of Small Bowel Neuroendocrine Tumors: Specific Requirements and Their Impact on Staging and Prognosis. Ann Surg Oncol. 2015;22 Suppl 3:S742-S749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Półtorak-Szymczak G, Budlewski T, Furmanek MI, Wierzba W, Sklinda K, Walecki J, Mruk B. Radiological Imaging of Gastro-Entero-Pancreatic Neuroendocrine Tumors. The Review of Current Literature Emphasizing the Diagnostic Value of Chosen Imaging Methods. Front Oncol. 2021;11:670233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Dromain C, de Baere T, Lumbroso J, Caillet H, Laplanche A, Boige V, Ducreux M, Duvillard P, Elias D, Schlumberger M, Sigal R, Baudin E. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | de Mestier L, Lepage C, Baudin E, Coriat R, Courbon F, Couvelard A, Do Cao C, Frampas E, Gaujoux S, Gincul R, Goudet P, Lombard-Bohas C, Poncet G, Smith D, Ruszniewski P, Lecomte T, Bouché O, Walter T, Cadiot G; Thésaurus National de Cancérologie Digestive (TNCD). Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2020;52:473-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 21. | Moryoussef F, de Mestier L, Belkebir M, Deguelte-Lardière S, Brixi H, Kianmanesh R, Hoeffel C, Cadiot G. Impact of Liver and Whole-Body Diffusion-Weighted MRI for Neuroendocrine Tumors on Patient Management: A Pilot Study. Neuroendocrinology. 2017;104:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Flechsig P, Zechmann CM, Schreiweis J, Kratochwil C, Rath D, Schwartz LH, Schlemmer HP, Kauczor HU, Haberkorn U, Giesel FL. Qualitative and quantitative image analysis of CT and MR imaging in patients with neuroendocrine liver metastases in comparison to (68)Ga-DOTATOC PET. Eur J Radiol. 2015;84:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Li X, Zhu H, Qian X, Chen N, Lin X. MRI Texture Analysis for Differentiating Nonfunctional Pancreatic Neuroendocrine Neoplasms From Solid Pseudopapillary Neoplasms of the Pancreas. Acad Radiol. 2020;27:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Shindo T, Fukukura Y, Umanodan T, Takumi K, Hakamada H, Nakajo M, Umanodan A, Ideue J, Kamimura K, Yoshiura T. Histogram Analysis of Apparent Diffusion Coefficient in Differentiating Pancreatic Adenocarcinoma and Neuroendocrine Tumor. Medicine (Baltimore). 2016;95:e2574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Guo C, Zhuge X, Wang Z, Wang Q, Sun K, Feng Z, Chen X. Textural analysis on contrast-enhanced CT in pancreatic neuroendocrine neoplasms: association with WHO grade. Abdom Radiol (NY). 2019;44:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | De Robertis R, Maris B, Cardobi N, Tinazzi Martini P, Gobbo S, Capelli P, Ortolani S, Cingarlini S, Paiella S, Landoni L, Butturini G, Regi P, Scarpa A, Tortora G, D'Onofrio M. Can histogram analysis of MR images predict aggressiveness in pancreatic neuroendocrine tumors? Eur Radiol. 2018;28:2582-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Fernandes MR, Ghezzi CLA, Grezzana-Filho TJ, Feier FH, Leipnitz I, Chedid AD, Cerski CTS, Chedid MF, Kruel CRP. Giant hepatic extra-gastrointestinal stromal tumor treated with cytoreductive surgery and adjuvant systemic therapy: A case report and review of literature. World J Gastrointest Surg. 2021;13:315-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Van Binnebeek S, Vanbilloen B, Baete K, Terwinghe C, Koole M, Mottaghy FM, Clement PM, Mortelmans L, Bogaerts K, Haustermans K, Nackaerts K, Van Cutsem E, Verslype C, Verbruggen A, Deroose CM. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: A lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol. 2016;26:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40:1770-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Oberg K, Krenning E, Sundin A, Bodei L, Kidd M, Tesselaar M, Ambrosini V, Baum RP, Kulke M, Pavel M, Cwikla J, Drozdov I, Falconi M, Fazio N, Frilling A, Jensen R, Koopmans K, Korse T, Kwekkeboom D, Maecke H, Paganelli G, Salazar R, Severi S, Strosberg J, Prasad V, Scarpa A, Grossman A, Walenkamp A, Cives M, Virgolini I, Kjaer A, Modlin IM. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect. 2016;5:174-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Barrio M, Czernin J, Fanti S, Ambrosini V, Binse I, Du L, Eiber M, Herrmann K, Fendler WP. The Impact of Somatostatin Receptor-Directed PET/CT on the Management of Patients with Neuroendocrine Tumor: A Systematic Review and Meta-Analysis. J Nucl Med. 2017;58:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 32. | Crown A, Rocha FG, Raghu P, Lin B, Funk G, Alseidi A, Hubka M, Rosales J, Lee M, Kennecke H. Impact of initial imaging with gallium-68 dotatate PET/CT on diagnosis and management of patients with neuroendocrine tumors. J Surg Oncol. 2020;121:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 689] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 34. | Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med. 2010;51:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 35. | Krenning EP, Valkema R, Kwekkeboom DJ, de Herder WW, van Eijck CH, de Jong M, Pauwels S, Reubi JC. Molecular imaging as in vivo molecular pathology for gastroenteropancreatic neuroendocrine tumors: implications for follow-up after therapy. J Nucl Med. 2005;46 Suppl 1:76S-82S. [PubMed] |
| 36. | Panagiotidis E, Alshammari A, Michopoulou S, Skoura E, Naik K, Maragkoudakis E, Mohmaduvesh M, Al-Harbi M, Belda M, Caplin ME, Toumpanakis C, Bomanji J. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J Nucl Med. 2017;58:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 37. | Zhang P, Yu J, Li J, Shen L, Li N, Zhu H, Zhai S, Zhang Y, Yang Z, Lu M. Clinical and Prognostic Value of PET/CT Imaging with Combination of 68Ga-DOTATATE and 18F-FDG in Gastroenteropancreatic Neuroendocrine Neoplasms. Contrast Media Mol Imaging. 2018;2018:2340389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A, Barnes T, Diakos C, Khasraw M, Samra J, Eslick E, Roach PJ, Engel A, Clarke SJ, Bailey DL. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics. 2017;7:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 39. | Papadakis GZ, Karantanas AH, Marias K, Millo C. Current status and future prospects of PET-imaging applications in patients with gastro-entero-pancreatic neuroendocrine tumors (GEP-NETs). Eur J Radiol. 2021;143:109932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Johnbeck CB, Knigge U, Loft A, Berthelsen AK, Mortensen J, Oturai P, Langer SW, Elema DR, Kjaer A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J Nucl Med. 2017;58:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 41. | Delpassand ES, Ranganathan D, Wagh N, Shafie A, Gaber A, Abbasi A, Kjaer A, Tworowska I, Núñez R. 64Cu-DOTATATE PET/CT for Imaging Patients with Known or Suspected Somatostatin Receptor-Positive Neuroendocrine Tumors: Results of the First U.S. Prospective, Reader-Masked Clinical Trial. J Nucl Med. 2020;61:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Montravers F, Kerrou K, Nataf V, Huchet V, Lotz JP, Ruszniewski P, Rougier P, Duron F, Bouchard P, Grangé JD, Houry S, Talbot JN. Impact of fluorodihydroxyphenylalanine-18F positron emission tomography on management of adult patients with documented or occult digestive endocrine tumors. J Clin Endocrinol Metab. 2009;94:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Koopmans KP, de Vries EG, Kema IP, Elsinga PH, Neels OC, Sluiter WJ, van der Horst-Schrivers AN, Jager PL. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 2006;7:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Deleval N, Pesque L, Dieudonné A, Viry F, Hentic O, Lebtahi R, Ruszniewski P, de Mestier L. Prognostic impact of bone metastases detected by 18F-DOPA PET in patients with metastatic midgut neuroendocrine tumors. Eur Radiol. 2021;31:4166-4174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Antwi K, Fani M, Heye T, Nicolas G, Rottenburger C, Kaul F, Merkle E, Zech CJ, Boll D, Vogt DR, Gloor B, Christ E, Wild D. Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging. 2018;45:2318-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 46. | Gaertner FC, Beer AJ, Souvatzoglou M, Eiber M, Fürst S, Ziegler SI, Brohl F, Schwaiger M, Scheidhauer K. Evaluation of feasibility and image quality of 68Ga-DOTATOC positron emission tomography/magnetic resonance in comparison with positron emission tomography/computed tomography in patients with neuroendocrine tumors. Invest Radiol. 2013;48:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Beiderwellen KJ, Poeppel TD, Hartung-Knemeyer V, Buchbender C, Kuehl H, Bockisch A, Lauenstein TC. Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: initial results. Invest Radiol. 2013;48:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O'Dorisio TM, Halperin DM, Fishbein L, Eads J, Hope TA, Singh S, Salem R, Metz DC, Naraev BG, Reidy-Lagunes DL, Howe JR, Pommier RF, Menda Y, Chan JA. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:863-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 49. | O'Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O'Connor J, Pape UF, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biochemical markers. Neuroendocrinology. 2009;90:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Buchanan-Hughes A, Pashley A, Feuilly M, Marteau F, Pritchard DM, Singh S. Carcinoid Heart Disease: Prognostic Value of 5-Hydroxyindoleacetic Acid Levels and Impact on Survival: A Systematic Literature Review. Neuroendocrinology. 2021;111:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Carling RS, Degg TJ, Allen KR, Bax ND, Barth JH. Evaluation of whole blood serotonin and plasma and urine 5-hydroxyindole acetic acid in diagnosis of carcinoid disease. Ann Clin Biochem. 2002;39:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, Kunz PL, O'Dorisio TM, Salem R, Segelov E, Howe JR, Pommier RF, Brendtro K, Bashir MA, Singh S, Soulen MC, Tang L, Zacks JS, Yao JC, Bergsland EK. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas. 2017;46:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 53. | Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 54. | Mosli HH, Dennis A, Kocha W, Asher LJ, Van Uum SH. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. J Clin Endocrinol Metab. 2012;97:E1731-E1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Wang YH, Yang QC, Lin Y, Xue L, Chen MH, Chen J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine (Baltimore). 2014;93:e247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Oberg K. Management of neuroendocrine tumours. Ann Oncol Off J Eur Soc Med Oncol. 2004;15 Suppl 4:iv293-298. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Ciobanu OA, Martin S, Fica S. Perspectives on the diagnostic, predictive and prognostic markers of neuroendocrine neoplasms (Review). Exp Ther Med. 2021;22:1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Boons G, Vandamme T, Peeters M, Beyens M, Driessen A, Janssens K, Zwaenepoel K, Roeyen G, Van Camp G, Op de Beeck K. Cell-Free DNA From Metastatic Pancreatic Neuroendocrine Tumor Patients Contains Tumor-Specific Mutations and Copy Number Variations. Front Oncol. 2018;8:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Modlin IM, Drozdov I, Kidd M. Gut neuroendocrine tumor blood qPCR fingerprint assay: characteristics and reproducibility. Clin Chem Lab Med. 2014;52:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, Malczewska A, Baum RP, Kwekkeboom DJ, Paganelli G, Krenning EP, Modlin IM. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imaging. 2020;47:895-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 61. | Partelli S, Andreasi V, Muffatti F, Schiavo Lena M, Falconi M. Circulating Neuroendocrine Gene Transcripts (NETest): A Postoperative Strategy for Early Identification of the Efficacy of Radical Surgery for Pancreatic Neuroendocrine Tumors. Ann Surg Oncol. 2020;27:3928-3936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Malczewska A, Witkowska M, Wójcik-Giertuga M, Kuśnierz K, Bocian A, Walter A, Rydel M, Robek A, Pierzchała S, Malczewska M, Leś-Zielińska I, Czyżewski D, Ziora D, Pilch-Kowalczyk J, Zajęcki W, Kos-Kudła B. Prospective Evaluation of the NETest as a Liquid Biopsy for Gastroenteropancreatic and Bronchopulmonary Neuroendocrine Tumors: An ENETS Center of Excellence Experience. Neuroendocrinology. 2021;111:304-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Modlin IM, Kidd M, Falconi M, Filosso PL, Frilling A, Malczewska A, Toumpanakis C, Valk G, Pacak K, Bodei L, Öberg KE. A multigenomic liquid biopsy biomarker for neuroendocrine tumor disease outperforms CgA and has surgical and clinical utility. Ann Oncol. 2021;32:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 64. | Modlin IM, Kidd M, Frilling A, Falconi M, Filosso PL, Malczewska A, Kitz A. Molecular Genomic Assessment Using a Blood-based mRNA Signature (NETest) is Cost-effective and Predicts Neuroendocrine Tumor Recurrence With 94% Accuracy. Ann Surg. 2021;274:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Rossi RE, Conte D, Elli L, Branchi F, Massironi S. Endoscopic techniques to detect small-bowel neuroendocrine tumors: A literature review. United European Gastroenterol J. 2017;5:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Sidhu R, McAlindon ME. The use of capsule endoscopy for the investigation of small bowel tumors: experience from a United Kingdom single center. Dig Dis Sci. 2011;56:2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Bellutti M, Fry LC, Schmitt J, Seemann M, Klose S, Malfertheiner P, Mönkemüller K. Detection of neuroendocrine tumors of the small bowel by double balloon enteroscopy. Dig Dis Sci. 2009;54:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Scherübl H, Faiss S, Tschöpe R, Zeitz M. Double-balloon enteroscopy for the detection of midgut carcinoids. Gastrointest Endosc. 2005;62:994; author reply 994-994; author reply 995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, Nieveen van Dijkum EJM, Pape UF, Pascher A, Ramage J, Reed N, Ruszniewski P, Scoazec JY, Toumpanakis C, Kianmanesh R, Falconi M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2017;105:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 70. | Puli SR, Kalva N, Bechtold ML, Pamulaparthy SR, Cashman MD, Estes NC, Pearl RH, Volmar FH, Dillon S, Shekleton MF, Forcione D. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: a systematic review and meta analysis. World J Gastroenterol. 2013;19:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Sundin A, Vullierme MP, Kaltsas G, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009;90:167-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 72. | James PD, Tsolakis AV, Zhang M, Belletrutti PJ, Mohamed R, Roberts DJ, Heitman SJ. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: a meta-analysis. Gastrointest Endosc. 2015;81:848-56.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Manta R, Nardi E, Pagano N, Ricci C, Sica M, Castellani D, Bertani H, Piccoli M, Mullineris B, Tringali A, Marini F, Germani U, Villanacci V, Casadei R, Mutignani M, Conigliaro R, Bassotti G, Zullo A. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J Gastrointestin Liver Dis. 2016;25:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Zilli A, Arcidiacono PG, Conte D, Massironi S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: lights and shadows. Dig Liver Dis. 2018;50:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 75. | Crinò SF, Ammendola S, Meneghetti A, Bernardoni L, Conti Bellocchi MC, Gabbrielli A, Landoni L, Paiella S, Pin F, Parisi A, Mastrosimini MG, Amodio A, Frulloni L, Facciorusso A, Larghi A, Manfrin E. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology. 2021;21:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 76. | Leeds JS, Nayar MK, Bekkali NLH, Wilson CH, Johnson SJ, Haugk B, Darne A, Oppong KW. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc Int Open. 2019;7:E1281-E1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Yoon WJ, Daglilar ES, Pitman MB, Brugge WR. Cystic pancreatic neuroendocrine tumors: endoscopic ultrasound and fine-needle aspiration characteristics. Endoscopy. 2013;45:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Carrara S, Di Leo M, Grizzi F, Correale L, Rahal D, Anderloni A, Auriemma F, Fugazza A, Preatoni P, Maselli R, Hassan C, Finati E, Mangiavillano B, Repici A. EUS elastography (strain ratio) and fractal-based quantitative analysis for the diagnosis of solid pancreatic lesions. Gastrointest Endosc. 2018;87:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Havre RF, Ødegaard S, Gilja OH, Nesje LB. Characterization of solid focal pancreatic lesions using endoscopic ultrasonography with real-time elastography. Scand J Gastroenterol. 2014;49:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 81. | Palazzo M, Napoléon B, Gincul R, Pioche M, Pujol B, Lefort C, Fumex F, Hautefeuille V, Fabre M, Cros J, Felce M, Couvelard A, Sauvanet A, Lévy P, Ruszniewski P, Palazzo L. Contrast harmonic EUS for the prediction of pancreatic neuroendocrine tumor aggressiveness (with videos). Gastrointest Endosc. 2018;87:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 82. | Takada S, Kato H, Saragai Y, Muro S, Uchida D, Tomoda T, Matsumoto K, Horiguchi S, Tanaka N, Okada H. Contrast-enhanced harmonic endoscopic ultrasound using time-intensity curve analysis predicts pathological grade of pancreatic neuroendocrine neoplasm. J Med Ultrason (2001). 2019;46:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Saund MS, Al Natour RH, Sharma AM, Huang Q, Boosalis VA, Gold JS. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol. 2011;18:2826-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 444] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 85. | Sato Y, Imamura H, Kaizaki Y, Koizumi W, Ishido K, Kurahara K, Suzuki H, Fujisaki J, Hirakawa K, Hosokawa O, Ito M, Kaminishi M, Furuta T, Chiba T, Haruma K. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc. 2014;26:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 87. | Kim HH, Kim GH, Kim JH, Choi MG, Song GA, Kim SE. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol Res Pract. 2014;2014:253860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 88. | Delle Fave G, Kwekkeboom DJ, Van Cutsem E, Rindi G, Kos-Kudla B, Knigge U, Sasano H, Tomassetti P, Salazar R, Ruszniewski P; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 89. | Jenny HE, Ogando PA, Fujitani K, Warner RR, Divino CM. Laparoscopic antrectomy: a safe and definitive treatment in managing type 1 gastric carcinoids. Am J Surg. 2016;211:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Köseoğlu H, Duzenli T, Sezikli M. Gastric neuroendocrine neoplasms: A review. World J Clin Cases. 2021;9:7973-7985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Carvão J, Dinis-Ribeiro M, Pimentel-Nunes P, Libânio D. Neuroendocrine Tumors of the Gastrointestinal Tract: A Focused Review and Practical Approach for Gastroenterologists. GE Port J Gastroenterol. 2021;28:336-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 93. | Hirasawa T, Yamamoto N, Sano T. Is endoscopic resection appropriate for type 3 gastric neuroendocrine tumors? Dig Endosc. 2021;33:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Exarchou K, Kamieniarz L, Tsoli M, Victor A, Oleinikov K, Khan MS, Srirajaskanthan R, Mandair D, Grozinsky-Glasberg S, Kaltsas G, Howes N, Pritchard DM, Toumpanakis C. Is local excision sufficient in selected grade 1 or 2 type III gastric neuroendocrine neoplasms? Endocrine. 2021;74:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD, Moon JS. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 96. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 97. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1852] [Article Influence: 84.2] [Reference Citation Analysis (1)] |
| 98. | Chin JL, O'Toole D. Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors. Clin Endosc. 2017;50:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 99. | Lee SW, Sung JK, Cho YS, Bang KB, Kang SH, Kim KB, Kim SH, Moon HS, Song KH, Kim SM, Chung IK, Lee DS, Jeong HY, Youn SJ. Comparisons of therapeutic outcomes in patients with nonampullary duodenal neuroendocrine tumors (NADNETs): A multicenter retrospective study. Medicine (Baltimore). 2019;98:e16154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 100. | Tran CG, Sherman SK, Suraju MO, Nayyar A, Gerke H, Abiad RGE, Chandrasekharan C, Ear PH, O'Dorisio TM, Dillon JS, Bellizzi AM, Howe JR. Management of Duodenal Neuroendocrine Tumors: Surgical versus Endoscopic Mucosal Resection. Ann Surg Oncol. 2022;29:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Ruff SM, Standring O, Wu G, Levy A, Anantha S, Newman E, Karpeh MS Jr, Nealon W, Deutsch GB, Weiss MJ, DePeralta DK. Ampullary Neuroendocrine Tumors: Insight into a Rare Histology. Ann Surg Oncol. 2021;28:8318-8328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014;18:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 103. | Vanoli A, La Rosa S, Klersy C, Grillo F, Albarello L, Inzani F, Maragliano R, Manca R, Luinetti O, Milione M, Doglioni C, Rindi G, Capella C, Solcia E. Four Neuroendocrine Tumor Types and Neuroendocrine Carcinoma of the Duodenum: Analysis of 203 Cases. Neuroendocrinology. 2017;104:112-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 104. | Gincul R, Ponchon T, Napoleon B, Scoazec JY, Guillaud O, Saurin JC, Ciocirlan M, Lepilliez V, Pioche M, Lefort C, Adham M, Pialat J, Chayvialle JA, Walter T. Endoscopic treatment of sporadic small duodenal and ampullary neuroendocrine tumors. Endoscopy. 2016;48:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 105. | Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, Law CH, Liu EH, Kim MK, Menda Y, Morse BG, Bergsland EK, Strosberg JR, Nakakura EK, Pommier RF. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46:715-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 106. | Niederle B, Pape UF, Costa F, Gross D, Kelestimur F, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, O'Toole D, Krenning E, Reed N, Kianmanesh R; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology. 2016;103:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 107. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 108. | Moris D, Ntanasis-Stathopoulos I, Tsilimigras DI, Vagios S, Karamitros A, Karaolanis G, Griniatsos J, Papalampros A, Papaconstantinou I, Glantzounis GK, Spartalis E, Blazer DG 3rd, Felekouras E. Update on Surgical Management of Small Bowel Neuroendocrine Tumors. Anticancer Res. 2018;38:1267-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Clift AK, Faiz O, Al-Nahhas A, Bockisch A, Liedke MO, Schloericke E, Wasan H, Martin J, Ziprin P, Moorthy K, Frilling A. Role of Staging in Patients with Small Intestinal Neuroendocrine Tumours. J Gastrointest Surg. 2016;20:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 110. | Clift AK, Kidd M, Bodei L, Toumpanakis C, Baum RP, Oberg K, Modlin IM, Frilling A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology. 2020;110:444-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 111. | Moris D, Tsilimigras DI, Vagios S, Ntanasis-Stathopoulos I, Karachaliou GS, Papalampros A, Alexandrou A, Blazer DG 3RD, Felekouras E. Neuroendocrine Neoplasms of the Appendix: A Review of the Literature. Anticancer Res. 2018;38:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 112. | Clancy TE, Chan JA. Well-differentiated neuroendocrine tumors of the appendix-UpToDate [cited 2022 Feb 1]. Available from: https://www.uptodate.com/contents/well-differentiated-neuroendocrine-tumors-of-the-appendix?search=appendiceal%20NETS%20&source=search_result&selectedTitle=1~19&usage_type=default&display_rank=1. |
| 113. | Mosquera C, Fitzgerald TL, Vora H, Grzybowski M. Novel nomogram combining depth of invasion and size can accurately predict the risk for regional nodal metastases for appendiceal neuroendocrine tumors (A-NET). J Surg Oncol. 2017;116:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Brighi N, La Rosa S, Rossi G, Grillo F, Pusceddu S, Rinzivillo M, Spada F, Tafuto S, Massironi S, Faggiano A, Antonuzzo L, Santini D, Sessa F, Maragliano R, Gelsomino F, Albertelli M, Vernieri C, Panzuto F, Fazio N, De Divitiis C, Lamberti G, Colao A, Fave GD, Campana D. Morphological Factors Related to Nodal Metastases in Neuroendocrine Tumors of the Appendix: A Multicentric Retrospective Study. Ann Surg. 2020;271:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 115. | Nussbaum DP, Speicher PJ, Gulack BC, Keenan JE, Ganapathi AM, Englum BR, Tyler DS, Blazer DG 3rd. Management of 1- to 2-cm Carcinoid Tumors of the Appendix: Using the National Cancer Data Base to Address Controversies in General Surgery. J Am Coll Surg. 2015;220:894-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Byrne RM, Pommier RF. Small Bowel and Colorectal Carcinoids. Clin Colon Rectal Surg. 2018;31:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (11)] |
| 118. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB 3rd, Pommier RF; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 119. | Xu R, Zhou B, Hu P, Xue B, Gu D, Li X, Tang Q. Development and validation of prognostic nomograms for patients with colon neuroendocrine neoplasms. World J Surg Oncol. 2021;19:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 120. | Chen T, Yao LQ, Xu MD, Zhang YQ, Chen WF, Shi Q, Cai SL, Chen YY, Xie YH, Ji Y, Chen SY, Zhou PH, Zhong YS. Efficacy and Safety of Endoscopic Submucosal Dissection for Colorectal Carcinoids. Clin Gastroenterol Hepatol. 2016;14:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 121. | Fields AC, Lu P, Vierra BM, Hu F, Irani J, Bleday R, Goldberg JE, Nash GM, Melnitchouk N. Survival in Patients with High-Grade Colorectal Neuroendocrine Carcinomas: The Role of Surgery and Chemotherapy. Ann Surg Oncol. 2019;26:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 122. | Balasubramanyam S, O'Donnell BP, Musher BL, Jhaveri PM, Ludwig MS. Evaluating Treatment Patterns for Small Cell Carcinoma of the Colon Using the National Cancer Database (NCDB). J Gastrointest Cancer. 2019;50:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21:2956-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 124. | Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: the investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther. 2016;44:332-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 125. | Taghavi S, Jayarajan SN, Powers BD, Davey A, Willis AI. Examining rectal carcinoids in the era of screening colonoscopy: a surveillance, epidemiology, and end results analysis. Dis Colon Rectum. 2013;56:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 126. | Amorim LC, Ferreira AR, Perez RO, Peixoto RD. Localized Well-Differentiated Rectal Neuroendocrine Tumors - Where Are We in 2021? Clin Colorectal Cancer. 2022;21:e22-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Weinstock B, Ward SC, Harpaz N, Warner RR, Itzkowitz S, Kim MK. Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology. 2013;98:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Gleeson FC, Levy MJ, Dozois EJ, Larson DW, Wong Kee Song LM, Boardman LA. Endoscopically identified well-differentiated rectal carcinoid tumors: impact of tumor size on the natural history and outcomes. Gastrointest Endosc. 2014;80:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 129. | Ngamruengphong S, Kamal A, Akshintala V, Hajiyeva G, Hanada Y, Chen YI, Sanaei O, Fluxa D, Haito Chavez Y, Kumbhari V, Singh VK, Lennon AM, Canto MI, Khashab MA. Prevalence of metastasis and survival of 788 patients with T1 rectal carcinoid tumors. Gastrointest Endosc. 2019;89:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Bang BW, Park JS, Kim HK, Shin YW, Kwon KS, Kim JM. Endoscopic Resection for Small Rectal Neuroendocrine Tumors: Comparison of Endoscopic Submucosal Resection with Band Ligation and Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2016;2016:6198927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 131. | Lim HK, Lee SJ, Baek DH, Park DY, Lee BE, Park EY, Park JW, Kim GH, Song GA. Resectability of Rectal Neuroendocrine Tumors Using Endoscopic Mucosal Resection with a Ligation Band Device and Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2019;2019:8425157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 132. | Park SS, Han KS, Kim B, Chang Kim B, Hong CW, Sohn DK, Chang HJ. Comparison of underwater endoscopic mucosal resection and endoscopic submucosal dissection of rectal neuroendocrine tumors (with videos). Gastrointest Endosc. 2020;91:1164-1171.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 134. | Yangong H, Shi C, Shahbaz M, Zhengchuan N, Wang J, Liang B, Ruliang F, Gao H, Bo Q, Niu J. Diagnosis and treatment experience of rectal carcinoid (a report of 312 cases). Int J Surg. 2014;12:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Wang X, Xiang L, Li A, Han Z, Li Y, Wang Y, Guo Y, Zuang K, Yan Q, Zhong J, Xiong J, Yang H, Liu S. Endoscopic submucosal dissection for the treatment of rectal carcinoid tumors 7-16 mm in diameter. Int J Colorectal Dis. 2015;30:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Scott AT, Howe JR. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg Clin North Am. 2019;99:793-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 137. | Assi HA, Mukherjee S, Kunz PL, Machiorlatti M, Vesely S, Pareek V, Hatoum H. Surgery Versus Surveillance for Well-Differentiated, Nonfunctional Pancreatic Neuroendocrine Tumors: An 11-Year Analysis of the National Cancer Database. Oncologist. 2020;25:e276-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 138. | Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, Levy MJ, Huebner M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 139. | Massironi S, Rossi RE, Zilli A, Casazza G, Ciafardini C, Conte D. A wait-and-watch approach to small pancreatic neuroendocrine tumors: prognosis and survival. Oncotarget. 2016;7:18978-18983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Sallinen V, Le Large TY, Galeev S, Kovalenko Z, Tieftrunk E, Araujo R, Ceyhan GO, Gaujoux S. Surveillance strategy for small asymptomatic non-functional pancreatic neuroendocrine tumors - a systematic review and meta-analysis. HPB (Oxford). 2017;19:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 141. | Barenboim A, Lahat G, Nachmany I, Nakache R, Goykhman Y, Geva R, Osher E, Scapa E, Wolf I, Orbach L, Brazowski E, Isakov O, Klausner JM, Lubezky N. Resection Versus Observation of Small Asymptomatic Nonfunctioning Pancreatic Neuroendocrine Tumors. J Gastrointest Surg. 2020;24:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 142. | Sun Y, Wang Y, Li R, Kang G, Zhang M, Chen X, Jin M, Liu Y, He Y, Zhu X, Kang Q, Zhou F, Yu Q. Surgical resection of primary tumor is associated with prolonged survival in low-grade pancreatic neuroendocrine tumors. Clin Res Hepatol Gastroenterol. 2021;45:101432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146:534-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 144. | Finkelstein P, Sharma R, Picado O, Gadde R, Stuart H, Ripat C, Livingstone AS, Sleeman D, Merchant N, Yakoub D. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J Gastrointest Surg. 2017;21:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 145. | Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19:117-23; discussion 123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 146. | Singh S, Moody L, Chan DL, Metz DC, Strosberg J, Asmis T, Bailey DL, Bergsland E, Brendtro K, Carroll R, Cleary S, Kim M, Kong G, Law C, Lawrence B, McEwan A, McGregor C, Michael M, Pasieka J, Pavlakis N, Pommier R, Soulen M, Wyld D, Segelov E; Commonwealth Neuroendocrine Tumour Collaboration (CommNETS) Follow-up Working Group. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018;4:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 147. | Canakis A, Law R, Baron T. An updated review on ablative treatment of pancreatic cystic lesions. Gastrointest Endosc. 2020;91:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 148. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 149. | Oleinikov K, Dancour A, Epshtein J, Benson A, Mazeh H, Tal I, Matalon S, Benbassat CA, Livovsky DM, Goldin E, Gross DJ, Jacob H, Grozinsky-Glasberg S. Endoscopic Ultrasound-Guided Radiofrequency Ablation: A New Therapeutic Approach for Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2019;104:2637-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 150. | Robles-Medranda C, Arevalo-Mora M, Oleas R, Alcivar-Vasquez J, Del Valle R. Novel EUS-guided microwave ablation of an unresectable pancreatic neuroendocrine tumor. VideoGIE. 2022;7:74-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 151. | Akirov A, Larouche V, Alshehri S, Asa SL, Ezzat S. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 152. | Fairweather M, Swanson R, Wang J, Brais LK, Dutton T, Kulke MH, Clancy TE. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann Surg Oncol. 2017;24:2319-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 153. | Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, Davidson B, Siegler M, Caplin M, Solcia E, Schilsky R; Working Group on Neuroendocrine Liver Metastases. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 368] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 154. | Bagante F, Spolverato G, Merath K, Postlewait LM, Poultsides GA, Mullen MG, Bauer TW, Fields RC, Lamelas J, Marques HP, Aldrighetti L, Tran T, Maithel SK, Pawlik TM. Neuroendocrine liver metastasis: The chance to be cured after liver surgery. J Surg Oncol. 2017;115:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 155. | Nguyen SQ, Angel LP, Divino CM, Schluender S, Warner RR. Surgery in malignant pancreatic neuroendocrine tumors. J Surg Oncol. 2007;96:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Rossi RE, Massironi S, Conte D, Peracchi M. Therapy for metastatic pancreatic neuroendocrine tumors. Ann Transl Med. 2014;2:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 157. | Kalra N, Gupta P, Jugpal T, Naik SS, Gorsi U, Chaluvashetty SB, Bhujade H, Duseja A, Singh V, Dhiman RK, Sandhu MS. Percutaneous Cryoablation of Liver Tumors: Initial Experience from a Tertiary Care Center in India. J Clin Exp Hepatol. 2021;11:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 158. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 159. | Li D, Rock A, Kessler J, Ballena R, Hyder S, Mo C, Chang S, Singh G. Understanding the Management and Treatment of Well-Differentiated Pancreatic Neuroendocrine Tumors: A Clinician's Guide to a Complex Illness. JCO Oncol Pract. 2020;16:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 160. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 161. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1829] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 162. | Sahu A, Jefford M, Lai-Kwon J, Thai A, Hicks RJ, Michael M. CAPTEM in Metastatic Well-Differentiated Intermediate to High Grade Neuroendocrine Tumors: A Single Centre Experience. J Oncol. 2019;2019:9032753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 163. | Walter T, van Brakel B, Vercherat C, Hervieu V, Forestier J, Chayvialle JA, Molin Y, Lombard-Bohas C, Joly MO, Scoazec JY. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: prognostic relevance and association with response to alkylating agents. Br J Cancer. 2015;112:523-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 164. | Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (5)] |
| 165. | Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016;34:3906-3913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 166. | Raymond E, Kulke MH, Qin S, Yu X, Schenker M, Cubillo A, Lou W, Tomasek J, Thiis-Evensen E, Xu JM, Croitoru AE, Khasraw M, Sedlackova E, Borbath I, Ruff P, Oberstein PE, Ito T, Jia L, Hammel P, Shen L, Shrikhande SV, Shen Y, Sufliarsky J, Khan GN, Morizane C, Galdy S, Khosravan R, Fernandez KC, Rosbrook B, Fazio N. Efficacy and Safety of Sunitinib in Patients with Well-Differentiated Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2018;107:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 167. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2238] [Article Influence: 279.8] [Reference Citation Analysis (0)] |
| 168. | Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017;23:4617-4624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 169. | Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 170. | Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, Krenning E, Reed N, O'Toole D; Vienna Consensus Conference participants. ENETS Consensus Guidelines for Neuroendocrine Neoplasms of the Appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology. 2016;103:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 171. | Chagpar R, Chiang YJ, Xing Y, Cormier JN, Feig BW, Rashid A, Chang GJ, You YN. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 172. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 173. | National Comprehensive Cancer Network. Treatment by Cancer Type. [cited 2021 Dec 2]. Available from: https://www.nccn.org/guidelines/category_1. |
| 174. | Frilling A, Akerström G, Falconi M, Pavel M, Ramos J, Kidd M, Modlin IM. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19:R163-R185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |