Published online Apr 16, 2022. doi: 10.4253/wjge.v14.i4.226
Peer-review started: July 14, 2021
First decision: September 5, 2021
Revised: September 13, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: April 16, 2022
Processing time: 267 Days and 10.8 Hours
Sessile serrated adenomas (SSAs) are important premalignant lesions that are difficult to detect during colonoscopy due to poor definition, concealment by mucous caps, and flat appearance. High definition (HD) colonoscopy may uniquely aid in the detection of these inconspicuous lesions compared to standard definition (SD) colonoscopes. In the absence of existing clinical guidelines to obligate the use of HD colonoscopy for colorectal cancer screening in average-risk patients, demonstrating the benefit of HD colonoscopy on SSA detection rate (SSADR) may help strengthen the evidence to recommend its use in all settings.
To evaluate the benefit of HD colonoscopy compared to SD colonoscopy on SSADR in average-risk patients undergoing screening colonoscopy.
Data from screening colonoscopies for patients aged 50-76 years two years before and two years after the transition from SD colonoscopy to HD colonoscopy at our large, academic teaching center were collected. Patients with symptoms of colorectal disease, positive occult blood test, history of colon polyps, cancer, polyposis syndrome, inflammatory bowel disease or family history of colon cancer or polyps were excluded. Patients whose endoscopists did not perform colonoscopies both before and after scope definition change were also excluded. Differences in individual endoscopist SSADR, average SSADR, and overall SSADR with SD colonoscopy vs HD colonoscopy were also evaluated for significance.
A total of 3657 colonoscopies met eligibility criteria with 2012 colonoscopies from the SD colonoscopy period and 1645 colonoscopies from the HD colonoscopy period from a pool of 11 endoscopists. Statistically significant improvements of 2.30% in mean SSADR and 2.53% in overall SSADR were noted with HD colonoscopy (P = 0.00028 and P = 0.00849, respectively). On the individual level, three endoscopists experienced statistically significant benefit with HD colonoscopy (+5.74%, P = 0.0056; +4.50%, P = 0.0278; +4.84%, P = 0.03486).
Our study suggests that HD colonoscopy statistically significantly improves sessile serrated adenoma detection rate in the screening of average risk patients during screening colonoscopy. By improving the detection and removal of these lesions, adoption of HD colonoscopy may reduce the significant premalignant burden of sessile serrated adenomas.
Core Tip: Sessile serrated adenomas (SSA) have become increasingly recognized as important premalignant lesions that are difficult to detect during colonoscopy due to similarity in appearance to surrounding colonic mucosa. We performed a retrospective study to evaluate the impact of high definition (HD) colonoscopy compared to standard definition colonoscopy on SSA detection rate (SSADR) during screening colonoscopy. Our study found a statistically significant benefit to SSADR with HD colonoscopy that also met benchmark detection rates. To our knowledge, this study is the first to show the utility of HD colonoscopy for SSADR in average-risk patients, thereby demonstrating it as an important tool for routine colorectal cancer screening. In the absence of a strong clinical guideline to obligate the use of HD colonoscopy, the benefit demonstrated to SSADR by HD colonoscopy in our study may help strengthen the evidence to recommend its use in all settings.
- Citation: Sehgal A, Aggarwal S, Mandaliya R, Loughney T, Mattar MC. Improving sessile serrated adenoma detection rates with high definition colonoscopy: A retrospective study. World J Gastrointest Endosc 2022; 14(4): 226-234
- URL: https://www.wjgnet.com/1948-5190/full/v14/i4/226.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i4.226
Serrated adenomatous lesions have been increasingly recognized for their potential for transformation into malignancy more rapidly than conventional adenomas, contributing to approximately 15%-30% of all colorectal cancers (CRC). Serrated adenomas are typically classified into three types: sessile serrated polyps/adenomas (SSA), hyperplastic polyps (HP), and traditional serrated adenomas (TSA). Among these subtypes, SSAs are important due to their malignant potential and difficulty in detection during colonoscopy given poor circumscription, concealment by mucous caps, and flat appearance[1,2]. An analysis of two databases of screening colonoscopies in 2012 approximated that the prevalence of proximal serrated polyps (SSA, HP, and TSA) may be as high as 18%-20%[3]. Given the prevalence of SSAs, their difficulty in detection and their significant malignant potential, there is a critical need to improve the detection of this subtype of serrated lesions during screening colonoscopy[1].
Few endoscopic interventions have been found to meaningfully improve SSA detection rate (SSADR). Slower withdrawal time has shown efficacy according to a Dutch study that reported an OR of 1.12 (95%CI: 1.10-1.16) for proximal serrated polyp (SSA, HP, and TSA) detection with longer withdrawal times[4]. This is supported by data from the New Hampshire colonoscopy registry that demonstrated an increasing rate of serrated lesion detection (SSA and HP) per minute between 6-9 min of withdrawal time[4,5]. Similarly, chromoendoscopy with indigocarmine dye as surface contrast agent has also been suggested to enhance the detection of sessile lesions (SSA and HP) compared to conventional colonoscopy (1.19 vs 0.49 per patient, P < 0.001)[6]. Finally, use of the mucolytic agent acetic acid compared to normal saline during colonoscopy has been shown to significantly improve SSA detection in the right colon (13.5% vs 0.5%, P < 0.001)[7]. Interventions that have shown negligible improvement in SSADR include: narrowed spectrum endoscopy, antispasmodics, and wide angle and enhanced mucosal views. High definition (HD) colonoscopy, on the other hand, has been cited as possibly beneficial in the detection of serrated polyps by the British Society of Gastroenterology, although data is lacking on its efficacy[1].
Though HD colonoscopy has been touted for its perceived benefits in the detection of adenomas due to heightened image resolution and magnification, there is still a lack of sufficient high quality data to obligate its use. The most recent position by the European Society of Gastrointestinal Endoscopy (ESGE) on the adoption of HD colonoscopy for overall adenoma detection in average risk patients is weak, citing inconsistent trial results, which may deter centers that currently use SD colonoscopy from adopting HD colonoscopy[8,9]. Given the lack of data on the adoption rate of HD colonoscopy outside of tertiary care centers, proving the benefit of HD colonoscopy on the detection of premalignant SSAs, specifically, may help strengthen the evidence behind its use in all settings.
Given the limited high-quality data supporting the use of HD colonoscopy in screening average-risk populations, it is understandable that there is also minimal data specifically on the impact of HD colonoscopy and SSADR. A recent study by Roelandt et al[10] that compared effects of endoscopy system, colonoscope definition, and virtual chromoendoscopy performed a subgroup SSADR analysis found significant benefit with 582 HD colonoscopies compared to 505 SD colonoscopies (8.2% vs 3.8%, respectively). However, a significant limitation of this study, was its inclusion of diagnostic (32.1%) as well as surveillance colonoscopies (29.3%), likely performed to increase sample size but potentially misrepresenting the improvement in SSADR that can be attributed to HD colonoscopy[10,11]. Another study by East et al[12] of 72 standard colonoscopies and 58 HD colonoscopies that investigated improvements in hyperplastic polyp detection (defined to include SSA and HP) with optimized withdrawal technique found a nonsignificant improvement with HD colonoscopy. It should be noted, however, that given the small study size, the benefit to SSADR may not be detectable especially given that SSAs make up a relatively lower proportion of all polyps detected on colonoscopy[12].
Based on the limited high powered, high quality studies available on detection of SSAs in HD colonoscopy, there is room in the literature for additional study on this subject. As such, we performed a retrospective study to evaluate the impact of HD colonoscopy compared to SD colonoscopy on SSADR exclusively during screening colonoscopy. Our secondary analysis compared overall adenoma detection rates with HD colonoscopy vs SD colonoscopy at our center.
All colonoscopies performed at our tertiary medical center in the two years before and after the transition from SD colonoscopy to HD colonoscopy on June 2nd, 2018 were identified. All other procedural elements were uniform during the 4-year study period. All pathology specimens were reviewed solely by the pathology department at our institution. For the primary SSADR analysis, each colonoscopy report and associated pathology report during the defined study period were collected, from which patient demographics, colonoscopy date, colonoscopy indication, colonoscopy findings (polyp/lesion presence and type), and endoscopist data were compiled. For the secondary analysis involving adenoma detection rate (ADR), preexisting ADR data from our center with the same inclusion criteria during the same time period was used.
All patients aged 50-76 years who underwent a screening colonoscopy between June 1, 2016 – June 2, 2020 were included. Patients with any symptoms of colorectal disease, positive occult blood test, history of colon polyps, cancer, polyposis syndrome, inflammatory bowel disease or family history of colon cancer or polyps were excluded. Patients whose endoscopists did not perform colonoscopies both before and after scope definition change were also excluded.
All statistical analyses were performed with Microsoft Excel and JMP PRO 15 software. Two-sided P-values < 0.05 were considered significant. Biostatistical analysis was performed by the authors.
The average age and the sex distribution of the SD colonoscopy group (June 1, 2016 – June 1, 2018) and the HD colonoscopy group (June 2, 2018 – June 2, 2020) were compared for demographic data. These comparisons were only performed with data from the SSADR analysis.
The primary outcome measure was SSA detection rate (SSADR), defined as the proportion of eligible colonoscopies in which at least one SSA was identified, for both the SD and HD colonoscopy periods. Individual differences in endoscopist SSADRs with SD colonoscopy and HD colonoscopy were evaluated by Z-test. Mean SSADR and overall SSADR were also reported. Mean SSADRs were calculated as the average of the individual endoscopist SSADRs. The difference in mean SSADRs with SD and HD colonoscopy was evaluated with the paired t-test. Overall SSADRs were calculated as the sum of all SSA-positive colonoscopies over the total number of eligible colonoscopies. The difference in overall SSADR with SD and HD colonoscopy was evaluated with the Z-test.
A secondary outcome measure was ADR, defined as the proportion of eligible colonoscopies in which at least one adenoma of any type was identified. Individual differences in endoscopist ADRs with SD and HD colonoscopy were evaluated with the Z-test. Mean ADR and overall ADR were also reported. Mean ADRs were calculated as the average of the individual endoscopist ADRs. The difference in mean ADRs with SD and HD colonoscopy was evaluated with the paired t-test. Overall ADRs were calculated as the sum of all SSA-positive colonoscopies over the total number of eligible colonoscopies. The difference in overall ADR with SD and HD colonoscopy was evaluated with the Z-test.
Following review of the data, 3657 cases met eligibility criteria with 2012 colonoscopies in the SD group and 1645 colonoscopies in the HD group for the SSADR analysis. Eleven endoscopists performed colonoscopies both before and after implementation of HD colonoscopy on June 2, 2018.
Demographic analysis of the SD and HD groups (Table 1) show the average age in both groups was 59 years and that males comprised approximately 45% of both groups. There was no significant difference in average age or sex distribution between the SD and HD groups.
| Variable | Standard definition, n = 2012 | High definition, n = 1645 | P value |
| Age (yr), mean (range) | 59.3 (50-76) | 59.2 (50-76) | 0.985 |
| Gender, male (%) | 896 (44.5%) | 757 (46.0%) | 0.36812 |
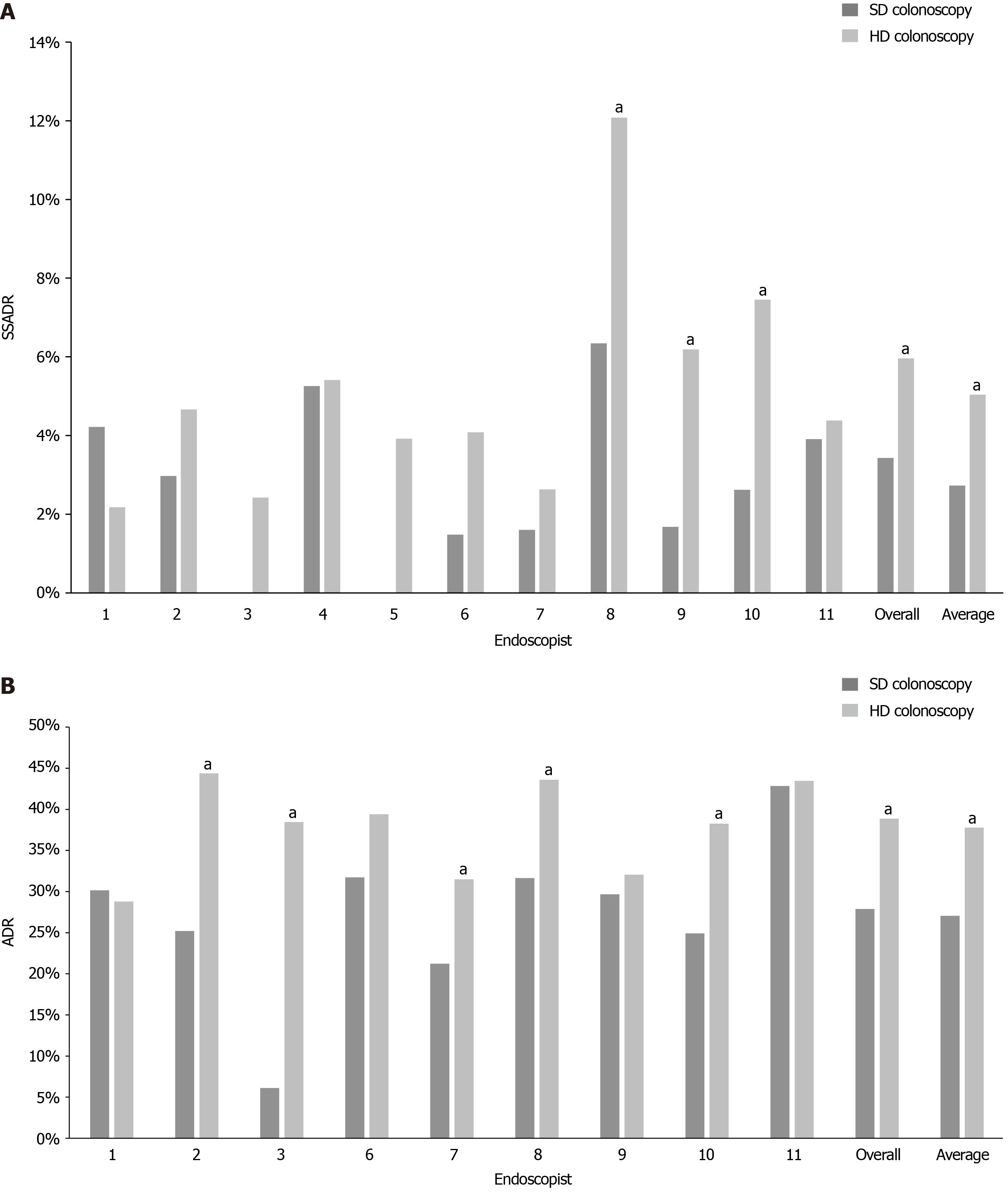
The mean SSADRs with SD colonoscopy and HD colonoscopy were 2.73% and 5.04%, respectively, yielding a statistically significant improvement of 2.30% (P = 0.00028). Comparison of the overall SSADRs also showed a statistically significant improvement from 3.43% with SD colonoscopy to 5.96% with HD colonoscopy (Δ 2.53%, P = 0.00849). Most of the endoscopists also demonstrated individual increases in SSADR with HD colonoscopy. On the individual level, three endoscopists experienced statistically significant benefit with HD colonoscopy (+5.74%, P = 0.0056, +4.50%, P = 0.0278, +4.84%, P = 0.03486). One endoscopist had a reduction in SSADR, but this difference was statistically nonsignificant (-2.03%, P = 0.24604) (Table 2 and Figure 1A).
| Endoscopist | Standard definition | High definition | Δ | P value (α < 0.05) | ||
| Eligible colonoscopies | SSADR | Eligible colonoscopies | SSADR | |||
| 1 | 166 | 4.22% | 229 | 2.18% | -2.03% | 0.24604 |
| 2 | 303 | 2.97% | 279 | 4.66% | 1.69% | 0.28462 |
| 3 | 82 | 0.00% | 124 | 2.42% | 2.42% | 0.1556 |
| 4 | 171 | 5.26% | 37 | 5.41% | 0.14% | 0.9681 |
| 5 | 63 | 0.00% | 51 | 3.92% | 3.92% | 0.11184 |
| 6 | 135 | 1.48% | 98 | 4.08% | 2.60% | 0.21498 |
| 7 | 125 | 1.60% | 76 | 2.63% | 1.03% | 0.61006 |
| 8 | 410 | 6.34% | 356 | 12.08% | 5.74% | 0.0056 |
| 9 | 238 | 1.68% | 97 | 6.19% | 4.50% | 0.0278 |
| 10 | 191 | 2.62% | 161 | 7.45% | 4.84% | 0.03486 |
| 11 | 128 | 3.91% | 137 | 4.38% | 0.47% | 0.8493 |
| Overall | 2012 | 3.43% | 1645 | 5.96% | 2.53% | 0.00028 |
| Average | 182.91 | 2.73% | 149.54 | 5.04% | 2.30% | 0.00849 |
Preexisting ADR data was only available for nine of the eleven endoscopists. The mean ADRs with SD colonoscopy and HD colonoscopy were 27.06% and 37.77%, respectively, yielding a significant improvement of 10.72% (P = 0.01522). Comparison of the overall ADRs also showed a significant improvement with HD colonoscopy (Δ 10.98%, P < 0.00001). Most of the endoscopists demonstrated individual increases in ADR with HD colonoscopy. Five of these endoscopists saw significant benefit. One endoscopist had a minimal reduction in ADR, but this difference was nonsignificant (Table 3 and Figure 1B).
| Endoscopist | Standard definition | High definition | Δ | P value (α < 0.05) | ||
| Eligible colonoscopies | ADR | Eligible colonoscopies | ADR | |||
| 1 | 262 | 30.15% | 250 | 28.80% | -1.35% | 0.72786 |
| 2 | 492 | 25.20% | 311 | 44.37% | 19.17% | < 0.00001 |
| 3 | 49 | 6.12% | 104 | 38.46% | 32.34% | < 0.00001 |
| 6 | 145 | 31.72% | 104 | 39.42% | 7.70% | 0.20766 |
| 7 | 245 | 21.22% | 127 | 31.50% | 10.27% | 0.02926 |
| 8 | 493 | 31.64% | 360 | 43.61% | 11.97% | 0.00034 |
| 9 | 283 | 29.68% | 78 | 32.05% | 2.37% | 0.68916 |
| 10 | 289 | 24.91% | 162 | 38.27% | 13.36% | 0.00288 |
| 11 | 91 | 42.86% | 138 | 43.48% | 0.62% | 0.92828 |
| Overall | 2349 | 27.88% | 1634 | 38.86% | 10.98% | < 0.00001 |
| Average | 261 | 27.06% | 181.6 | 37.77% | 10.72% | 0.01522 |
Identifying techniques that improve the detection of SSAs will help reduce interval colon cancer in screening colonoscopy[1,3]. In the absence of high-quality evidence to obligate the use of HD colonoscopy for the average-risk population, we performed a retrospective study to evaluate the benefit of HD colonoscopy compared to SD colonoscopy on SSADR during screening colonoscopy[8]. In addition to the significant improvements to both average and overall SSADRs, benefit from HD colonoscopy was further underscored by the average SSADR surpassing the serrated lesion benchmark detection rate of 7% (inclusive of HPs)[1,11]. To our knowledge, this study is the first to illustrate the utility of HD colonoscopy for SSADR in average risk patients, solidifying its role as a tool in high quality CRC screening.
Notably, our study demonstrated significant benefit to all adenoma/polyp detection rates, not simply SSADR. It should be acknowledged, however, that it is possible that our ADR outcomes were improved slightly by the independent improvement of endoscopists during the four-year study period or by HD colonoscopy itself. Interestingly, our data is also consistent with an existing study by Waldmann et al[13] that reported significant increases in ADR with HD colonoscopy in endoscopists with historically lower ADR , as each of the four endoscopists in our study with an ADR < 30% experienced statistically significant increases in ADR with HD colonoscopy. In contrast, four of the five endoscopists with an ADR ≥ 30% with SD colonoscopy did not experience such improvement with HD colonoscopy in our study, further supporting the selective benefit of HD colonoscopy for endoscopists with lower ADRs.
A major strength to our study is the exclusion of surveillance and diagnostic procedures to focus solely on screening colonoscopies. This is in contrast to the existing study by Roelandt et al[10] on HD colonoscopy and SSADR that included both diagnostic and surveillance colonoscopies in its analysis. Our criteria allow for our results to be more generalizable to average risk patients and more applicable to benchmark detection rates set for the screening population[11]. Another advantage was that our study was sufficiently powered compared to any other available literature similarly studying SSADR with HD colonoscopy to date[10,12].
In acknowledging the strengths to our data, it is also important to consider why this improvement to SSADR has not clearly been reflected in the overall ADRs in existing study on HD colonoscopy, as demonstrated by the weak recommendation by the ESGE on the utility of HD colonoscopy[8]. It is possible that higher quality endoscopes have more utility in the detection of subtle SSA lesions than in the detection of adenomatous polyps that have been historically easier to identify, perhaps limiting the overall benefit of HD colonoscopy on detection of the conventional adenomas. Thus, as SSAs make up a relatively small component of overall ADR compared to conventional adenomas, the significant improvement to SSADR may be undetectable when assessing the improvement to all adenoma detection with HD colonoscopy. In this way, our results help to highlight a significant benefit of HD colonoscopy that may have been overlooked in prior studies of HD colonoscopy focused on overall ADR. This allows for stronger recommendations for the use of HD colonoscopy given that improved SSA detection is an unmet need in screening colonoscopy.
We acknowledge some limitations to our study. A main limitation is the retrospective design of the study. In addition, while the longitudinal nature of the study permitted a relatively large number of colonoscopies to be included in our analysis, the four-year period allowed for changing skill level of endoscopists over time. Another limitation is that our study did not control for withdrawal time. In studies past, this has been one factor that has been demonstrated to significantly improve SSADR with maximum benefit at 9 min of withdrawal time[4,5]. Nevertheless, the withdrawal times of our endoscopists may have been optimized on average as the mean withdrawal time of academic gastroenterologists has been reported to be 9.1 min[5,14]. Another consideration arises from a lack of control for bowel preparation quality in our study. Although two prior studies that have evaluated the impact of bowel preparation on SSA detection found a nonsignificant impact of bowel preparation on SSADR, a 2016 prospective study reported significant decrease in SSADR with bowel preparation quality that is below high quality in a population of veterans with high adenoma prevalence, suggesting that our study’s lack of exclusion of colonoscopies with suboptimal bowel preparation may have falsely lowered our SSADR results[4,15,16]. We also acknowledge discrepancies of eligible colonoscopy totals for the SSADR data collected directly for this study and ADR data collected from a preexisting study at our center, likely due to differences in the manual review of eligible colonoscopies during respective data compilations. COVID-19 also significantly impacted elective procedures in 2020, reducing the number of colonoscopies in the HD colonoscopy group.
In conclusion, our study suggests that high definition colonoscopy significantly improves sessile serrated adenoma detection in the screening of average risk patients. By improving the detection and removal of these lesions, adoption of high definition colonoscopy may reduce the significant premalignant burden of sessile serrated adenomas.
Sessile serrated adenomas (SSA) have become increasingly recognized as important premalignant lesions that are difficult to detect during colonoscopy due to similarity in appearance to surrounding colonic mucosa. Hypothesizing that higher resolution colonoscopy may improve SSA detection rates (SSADR), we performed a retrospective study to evaluate the impact of high definition (HD) colonoscopy compared to standard definition (SD) colonoscopy on SSADR during screening colonoscopy. To our knowledge, this study is the first to study the utility of HD colonoscopy for SSADR in average-risk patients. In the absence of a strong clinical guideline to obligate the use of HD colonoscopy, the benefit demonstrated to SSADR by HD colonoscopy in our study may help strengthen the evidence to recommend its use in all settings.
To our knowledge, there has been no study on the efficacy of HD colonoscopy vs SD colonoscopy on SSADR in average risk patients undergoing screening colonoscopy only. Furtheremore, the most recent position by the European Society of Gastrointestinal Endoscopy on the adoption of HD colonoscopy for overall adenoma detection in average risk patients is weak, citing inconsistent trial results, which may deter centers that currently use SD colonoscopy from adopting HD colonoscopy. Given the lack of data on the adoption rate of HD colonoscopy outside of tertiary care centers, proving the benefit of HD colonoscopy on the detection of premalignant SSAs, specifically, may help strengthen the evidence behind its use in all settings.
We performed a retrospective study to evaluate the impact of HD colonoscopy compared to SD colonoscopy on SSADR exclusively during screening colonoscopy. Our secondary analysis compared overall adenoma detection rates (ADR) with HD colonoscopy vs SD colonoscopy at our center. By demonstrating that high definition colonoscopy significantly improves sessile serrated adenoma detection in the screening of average risk patients, the adoption of high definition colonoscopy may be universally recommended to reduce the significant premalignant burden of sessile serrated adenomas.
All colonoscopies performed at our tertiary medical center in the two years before and after the transition from SD colonoscopy to HD colonoscopy on June 2nd, 2018 were identified. For the primary SSADR analysis, each colonoscopy report and associated pathology report during the defined study period were collected, from which patient demographics, colonoscopy date, colonoscopy indication, colonoscopy findings (polyp/Lesion presence and type), and endoscopist data were compiled. For the secondary analysis involving ADR, preexisting ADR data from our center with the same inclusion criteria during the same time period was used. The average age and the sex distribution of the SD colonoscopy group (June 1, 2016 – June 1, 2018) and the HD colonoscopy group (June 2, 2018 – June 2, 2020) were compared for demographic data, using only data from the SSADR analysis. The primary outcome measure were differences in individual endoscopist, overall, and mean SSA detection rate (SSADR) (defined as the proportion of eligible colonoscopies in which at least one SSA was identified) for the SD and HD colonoscopy periods. The secondary outcome measure was differences in individual endoscopist, overall, and mean overall adenoma detection rate (defined as the proportion of eligible colonoscopies in which at least one adenoma of any type was identified) for the SD and HD colonoscopy periods.
There was no significant difference in average age or sex distribution between the SD and HD groups. The mean SSADRs with SD colonoscopy and HD colonoscopy were 2.73% and 5.04%, respectively, yielding a statistically significant improvement of 2.30% (P = 0.00028). Comparison of the overall SSADRs also showed a statistically significant improvement from 3.43% with SD colonoscopy to 5.96% with HD colonoscopy (Δ 2.53%, P = 0.00849). On the individual level, three endoscopists experienced statistically significant benefit with HD colonoscopy (+5.74%, P = 0.0056, +4.50%, P = 0.0278, +4.84%, P = 0.03486). Preexisting ADR data was only available for nine of the eleven endoscopists. The mean ADRs with SD colonoscopy and HD colonoscopy were 27.06% and 37.77%, respectively, yielding a significant improvement of 10.72% (P = 0.01522). Comparison of the overall ADRs also showed a significant improvement with HD colonoscopy (Δ 10.98%, P < 0.00001). Most of the endoscopists demonstrated individual increases in ADR with HD colonoscopy. Five of these endoscopists saw significant benefit.
To our knowledge, this study is the first to show the utility of HD colonoscopy for SSADR in average-risk patients, thereby demonstrating it as an important tool to improve the detection and removal of these premalignant lesions during routine colorectal cancer screening. Furthermore, in the absence of a strong clinical guideline to obligate the use of HD colonoscopy, the benefit demonstrated to SSADR by HD colonoscopy in our study may help strengthen the evidence to recommend its use in all settings.
Future research endeavors should include randomized control trials to assess the efficacy of HD vs SD colonoscopy in average-risk patients undergoing screening colonoscopy only.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin Y S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN, Leedham SJ, Phull PS, Rutter MD, Shepherd NA, Tomlinson I, Rees CJ. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949-966.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 3. | Kahi CJ, Li X, Eckert GJ, Rex DK. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc. 2012;75:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Tytgat KM, Dees J, Mathus-Vliegen EM, Kuipers EJ, Fockens P, van Leerdam ME, Dekker E. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Butterly L, Robinson CM, Anderson JC, Weiss JE, Goodrich M, Onega TL, Amos CI, Beach ML. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol. 2014;109:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Pohl J, Schneider A, Vogell H, Mayer G, Kaiser G, Ell C. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut. 2011;60:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Tribonias G, Theodoropoulou A, Stylianou K, Giotis I, Mpitouli A, Moschovis D, Komeda Y, Manola ME, Paspatis G, Tzouvala M. Irrigating Acetic Acid Solution During Colonoscopy for the Detection of Sessile Serrated Neoplasia: A Randomized Controlled Trial. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, Pellisé M, Antonelli G, Bustamante Balen M, Coron E, Cortas G, Iacucci M, Yuichi M, Longcroft-Wheaton G, Mouzyka S, Pilonis N, Puig I, van Hooft JE, Dekker E. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:1155-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (1)] |
| 9. | Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, Pellisé M, Antonelli G, Bustamante Balen M, Coron E, Cortas G, Iacucci M, Yuichi M, Longcroft-Wheaton G, Mouzyka S, Pilonis N, Puig I, van Hooft JE, Dekker E. Correction: Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:C6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Roelandt P, Demedts I, Willekens H, Bessissow T, Braeye L, Coremans G, Cuyle PJ, Ferrante M, Gevers AM, Hiele M, Osselaer M, Tack J, Tejpar S, Ulenaers M, Van Assche G, Van Cutsem E, Van Gool S, Vannoote J, Vermeire S, Bisschops R. Impact of endoscopy system, high definition, and virtual chromoendoscopy in daily routine colonoscopy: a randomized trial. Endoscopy. 2019;51:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Anderson JC, Butterly LF, Weiss JE, Robinson CM. Providing data for serrated polyp detection rate benchmarks: an analysis of the New Hampshire Colonoscopy Registry. Gastrointest Endosc. 2017;85:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | East JE, Stavrindis M, Thomas-Gibson S, Guenther T, Tekkis PP, Saunders BP. A comparative study of standard vs. high definition colonoscopy for adenoma and hyperplastic polyp detection with optimized withdrawal technique. Aliment Pharmacol Ther. 2008;28:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Waldmann E, Britto-Arias M, Gessl I, Heinze G, Salzl P, Sallinger D, Trauner M, Weiss W, Ferlitsch A, Ferlitsch M. Endoscopists with low adenoma detection rates benefit from high-definition endoscopy. Surg Endosc. 2015;29:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Mandaliya R, Baig K, Barnhill M, Murugesan V, Som A, Mohammed U, Jhaveri K, Vangimalla SS, Raymond A, Tran J, Hasan L, Lewis JH, Cho W. Significant Variation in the Detection Rates of Proximal Serrated Polyps Among Academic Gastroenterologists, Community Gastroenterologists, and Colorectal Surgeons in a Single Tertiary Care Center. Dig Dis Sci. 2019;64:2614-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Anderson JC, Butterly LF, Robinson CM, Goodrich M, Weiss JE. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest Endosc. 2014;80:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Clark BT, Laine L. High-quality Bowel Preparation Is Required for Detection of Sessile Serrated Polyps. Clin Gastroenterol Hepatol. 2016;14:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |