Published online Feb 16, 2022. doi: 10.4253/wjge.v14.i2.96
Peer-review started: August 15, 2021
First decision: September 12, 2021
Revised: September 18, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 16, 2022
Processing time: 178 Days and 22.3 Hours
Olympus Corporation has developed texture and color enhancement imaging (TXI) as a novel image-enhancing endoscopic technique.
To investigate the effectiveness of TXI in identifying colorectal adenomas using magnifying observation.
Colorectal adenomas were observed by magnified endoscopy using white light imaging (WLI), TXI, narrow band imaging (NBI), and chromoendoscopy (CE). This study adopted mode 1 of TXI. Adenomas were confirmed by histological examination. TXI visibility was compared with the visibility of WLI, NBI, and CE for tumor margin, and vessel and surface patterns of the Japan NBI expert team (JNET) classification. Three expert endoscopists and three non-expert endo
Sixty-one consecutive adenomas were evaluated. The visibility score for tumor margin of TXI (3.47 ± 0.79) was significantly higher than that of WLI (2.86 ± 1.02, P < 0.001), but lower than that of NBI (3.76 ± 0.52, P < 0.001), regardless of the endoscopist’s expertise. TXI (3.05 ± 0.79) had a higher visibility score for the vessel pattern of JNET classification than WLI (2.17 ± 0.90, P < 0.001) and CE (2.47 ± 0.87, P < 0.001), but lower visibility score than NBI (3.79 ± 0.47, P < 0.001), regardless of the experience of endoscopists. For the visibility score for the surface pattern of JNET classification, TXI (2.89 ± 0.85) was superior to WLI (1.95 ± 0.79, P < 0.01) and CE (2.75 ± 0.90, P = 0.002), but inferior to NBI (3.67 ± 0.55, P < 0.001).
TXI provided higher visibility than WLI, lower than NBI, and comparable to or higher than CE in the magnified observation of colorectal adenomas.
Core Tip: Texture and color enhancement imaging (TXI) has been developed as a novel image-enhancing endoscopy. Colorectal adenomas were observed by magnified endoscopy using white light imaging (WLI), TXI, narrow band imaging (NBI), and chromoendoscopy (CE). TXI visibility was compared with the visibility of WLI, NBI, and CE for tumor margin, and vessel and surface patterns of the Japan NBI Expert Team (JNET) classification. TXI provided higher visibility than WLI and lower than NBI for tumor margin. TXI showed higher visibility than WLI and CE, and lower than NBI for the vessel and surface patterns of the JNET classification.
- Citation: Toyoshima O, Nishizawa T, Yoshida S, Yamada T, Odawara N, Matsuno T, Obata M, Kurokawa K, Uekura C, Fujishiro M. Texture and color enhancement imaging in magnifying endoscopic evaluation of colorectal adenomas. World J Gastrointest Endosc 2022; 14(2): 96-105
- URL: https://www.wjgnet.com/1948-5190/full/v14/i2/96.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i2.96
Colorectal adenomas are precursors to colorectal cancer and their removal prevents occurrence of cancer in this region. Endoscopists with higher adenoma detection rates have lower colorectal cancer incidence and mortality in their patients than those with lower adenoma detection rates[1,2]. Currently, adenomas are a common finding. Hilsden et al[3] reported the following benchmarks of adenoma detection rates: minimally acceptable, 25%; standard of care, 30%; and aspirational, 39%. It is recommended that the endoscopists overcome the “minimally acceptable” threshold[3,4]. Therefore, accurate diagnosis of colorectal adenomas is crucial in clinical practice[5-7].
Recent advances in endoscopic technology have improved the accuracy of endoscopy using image-enhanced endoscopy (IEE) for lesions that are difficult to observe using conventional white light imaging (WLI). Since narrow band imaging (NBI) was developed as an IEE modality, evidence on the usefulness of IEE has been accumulated and IEE is commonly used in daily practice. NBI selects blue and green wavelengths using optical filters with the elimination of red light, thus emphasizing mucosal surface structures and blood vessels[8]. NBI has been reported to be effective in detecting[9] and characterizing lesions[10-12]. Following NBI, blue light imaging (BLI) and linked color imaging (LCI) have become available as new IEE modalities. BLI and LCI irradiate mucosa with a short wavelength, narrow-band light, which includes light amplification by stimulated emission of radiation or light emitting diode, without an optical filter. Furthermore, the acquired color information is reallocated to different colors that are similar to the mucosal color, resulting in improved performance in depicting blood vessels. In addition, image processing that enhances color separation for red color permits clear visualization of red blood vessels and white pits in LCI[13]. The efficacy of BLI and LCI has also been extensively reported[14]. Texture and color enhancement imaging (TXI), which is a novel method to enhance images, was developed in the new endoscopy system EVIS X1 (Olympus Corporation, Tokyo, Japan) in 2020.
TXI is designed to enhance three image factors, including texture, brightness, and color, in WLI to clearly define subtle tissue differences by applying the retinex theory[15,16]. Retinex is based on the theory of “color constancy” and “brightness constancy”, in which the human eye can perceive color and brightness regardless of the illumination light. TXI consists of the following six processes. First, the input image is split into two layers, base and detail. Next, the brightness in the dark regions of the base layer is adjusted. Tone-mapping is applied to the corrected base layer in step three. Fourth, texture enhancement is applied to the detail layer to enhance the subtle contrast. In step five, the base layer after tone-mapping and the detail layer after texture enhancement are recombined. A TXI image produced in the fifth step is immediately displayed in TXI mode 2. In the final step, color enhancement is applied to the output of TXI mode 1 to more clearly define the slight color contrast. The color enhancement algorithm of TXI was designed to expand the color difference between red and white hues in the image[16].
The Japan NBI Expert Team (JNET) classification is a standard for diagnosing the histology of a neoplasm by observing the surface structure (vessel pattern and surface pattern) of the neoplasm using magnified NBI. The JNET classification is widely used in clinical practice for the diagnosis of adenoma. It has proven to be useful for the diagnosis of superficial colorectal neoplasms in a clinical setting by both expert and non-expert endoscopists[12]. A meta-analysis suggested that the diagnostic efficacy of the JNET classification may be equivalent to that of the Pit pattern classification[17]. Furthermore, the algorithm for the treatment of colorectal polyps using the JNET classification was reported to be valid[18]. Meanwhile, evidence supports that chromoendoscopy (CE) increases colorectal polyp detection and contributes to accurate polyp diagnosis[6,19-22].
Currently, the only clinical studies on TXI that have already been published are those by Ishikawa et al[23] and Abe et al[24], wherein TXI was used for imaging the stomach. Some clinical trials on the efficacy of TXI in colorectal polyp observation are ongoing; however, no published reports on colonoscopy are available in PubMed or the Cochrane Library. Therefore, the aim of this study was to investigate the effectiveness of TXI for colorectal adenomas. The visibility of TXI was compared with the visibility of WLI, NBI, and CE for the tumor margin and JNET classification pattern using magnifying observation.
Patients who underwent colonoscopy at Toyoshima Endoscopy Clinic (Tokyo, Japan), which is a representative clinic in Japan, from April to May 2021, were enrolled. Patients with removed adenomas were eligible for the study. When patients had multiple adenomas, they were treated individually. Adenomas were diagnosed histopathologically. Indications for colonoscopy included screening, examination of symptoms, investigation for a positive fecal immunochemical test, and polyp surveillance. Patients with inflammatory bowel disease were excluded.
This study was conducted in accordance with the ethical guidelines for medical studies in Japan. Written informed consent was obtained from the patients at the time of colonoscopy to use their data for research purposes. The study design was described in a protocol prepared by Toyoshima Endoscopy Clinic and was approved by the Certificated Review Board, Yoyogi Mental Clinic on July 16, 2021 (approval No. RKK227). We published this study’s protocol on our institute’s website (http://www.ichou.com) so that patients could opt out of the study if they did not wish to participate. All clinical investigations were conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
EVIS X1 video system center (CV-1500), 4 K resolution ultra-high-definition liquid crystal display monitor (OEV321UH), and colonoscope CF-HQ290Z (Olympus Corporation, Tokyo, Japan) were used in this study setting. TXI has two methods, namely modes 1 and 2, and the enhancement of brightness and texture is similar between them. Because the enhancement of the color contrast of mode 1 is superior to that of mode 2[16], this study adopted mode 1. For the enhanced structure level, A8 was selected for WLI, NBI, and CE. The type A mode is ideal for observation of larger mucosal tissues with high contrast, whereas the type B mode is suitable for observation of vascular tissues. There are eight levels among the type A mode, of which A8 is the most emphasized, and A1 is the least emphasized mode. A 0.05% indigo carmine was used for the CE. The T-File System (STS-Medic Inc., Tokyo, Japan) was used to file the endoscopic images and document the endoscopic findings.
One expert endoscopist performed colonoscopy and magnified observation using the WLI, TXI, NBI, and CE modalities. Lesions were first washed carefully with water to remove the mucus and dye from the mucosal surface; then, images were obtained through WL, TXI, and NBI. The lesions were subsequently stained for CE. The endoscopist took an image within 15 s for each modality.
We investigated the visibility of the tumor margin, and the vessel and surface patterns according to the JNET classification. The vessel pattern shows the pattern of superficial microvessels, which appear red in WLI, TXI, and CE, and brown in NBI. The surface pattern indicates the pattern of superficial crypts, which appear whitish in all modalities. JNET type 2A corresponds to the histopathological classification of low-grade intramucosal neoplasia, including adenoma. The vessel pattern of type 2A is of a regular caliber and distribution (meshed and/or spiral pattern). The surface pattern of type 2A is defined as regular (tubular, branched, and/or papillary)[10-12].
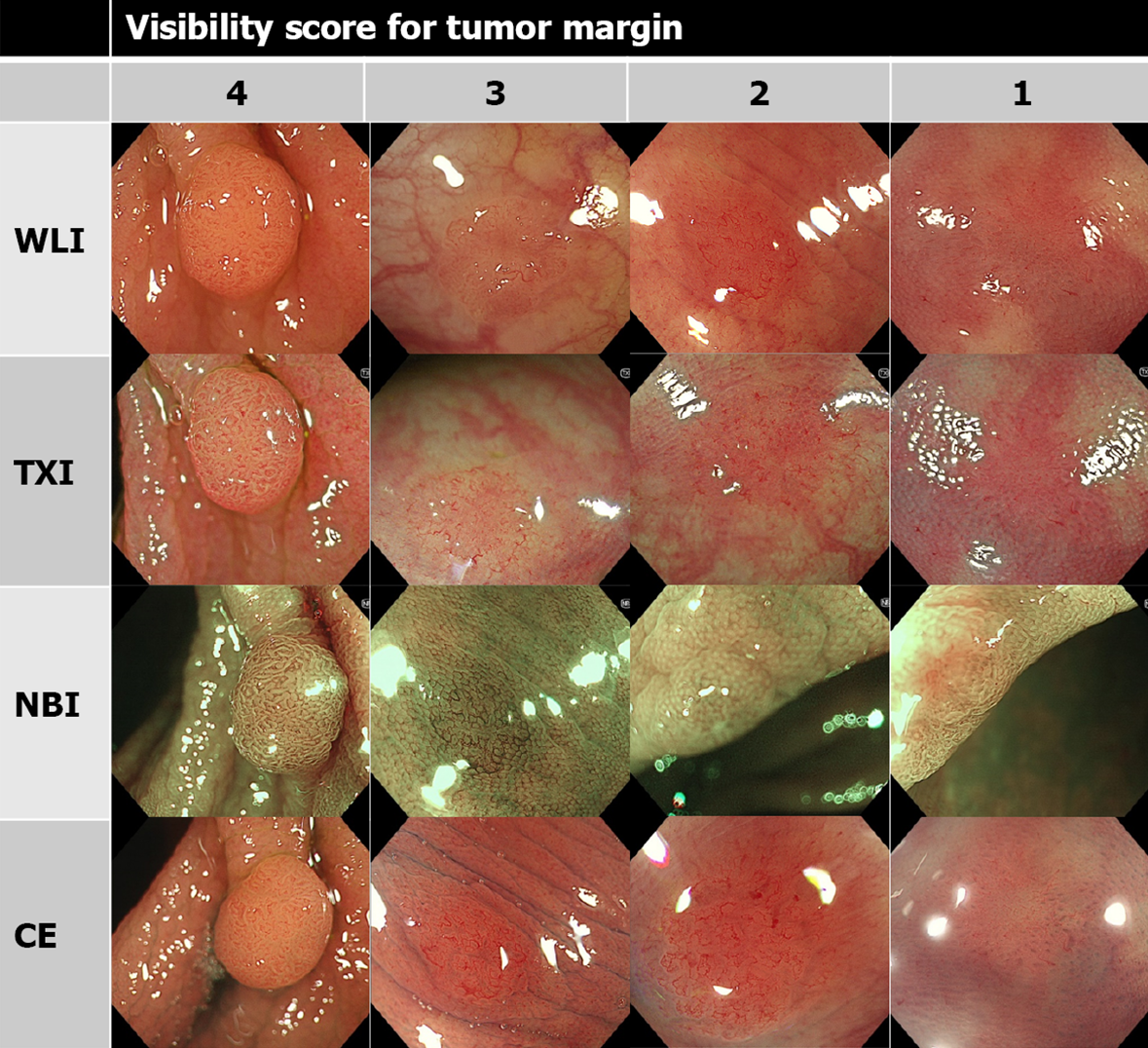
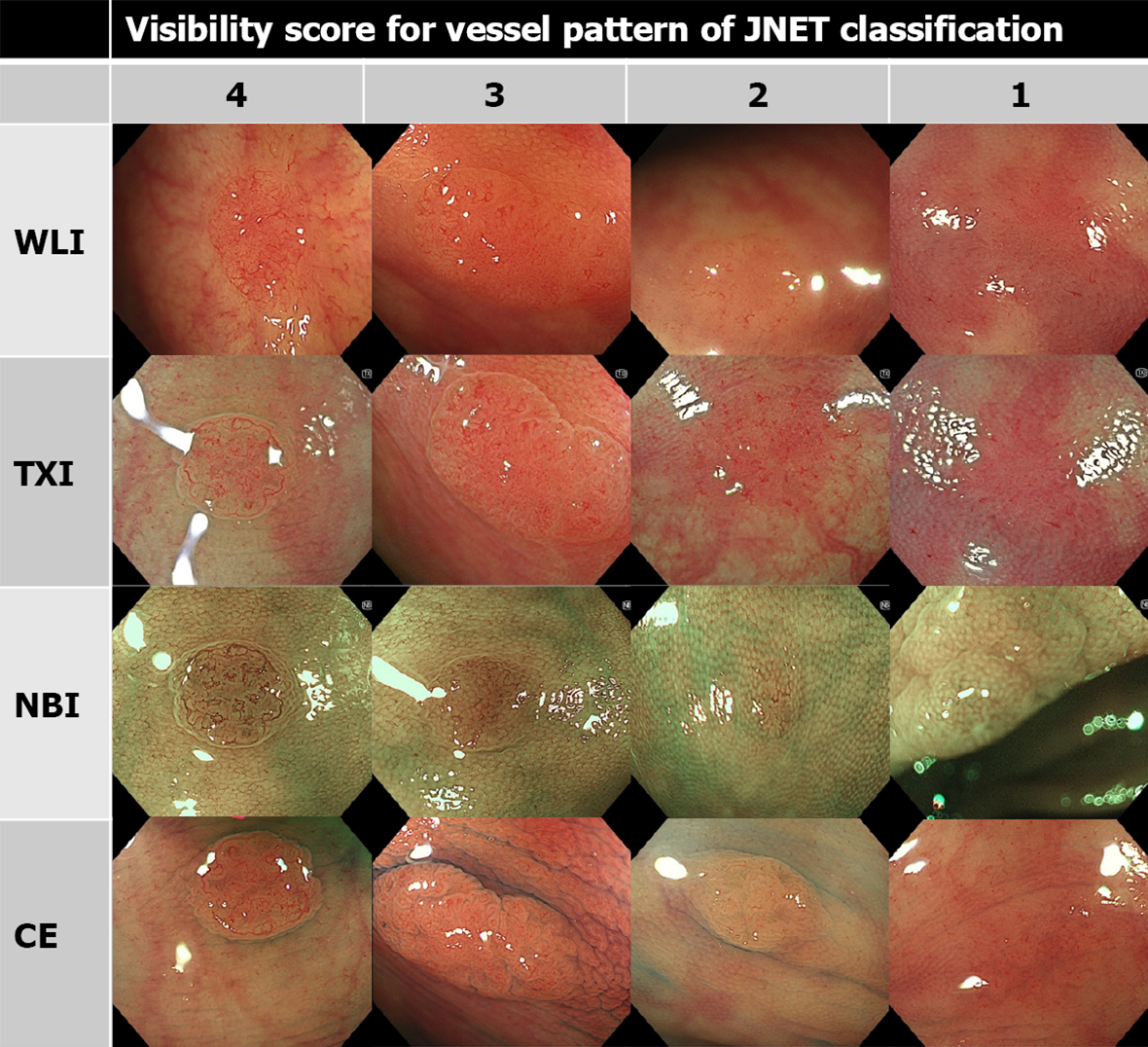
As in previous reports, the visibility score was defined as follows: score 4, excellent (easily detectable); score 3, good (detectable with careful observation); score 2, fair (hardly detectable without careful examination); score 1, poor (not detectable without repeated careful examination)[12,14]. Representative images of each score are shown in Figures 1, 2, and 3.
Three expert endoscopists and three non-expert endoscopists evaluated the visibility score. The images studied were observed without zooming. The endoscopist assessed all images at the same size and magnification. A physician with more than 5000 experiences in colonoscopy was defined as an expert endoscopist and one with less than 5000 experiences was considered a non-expert[12].
The main outcomes of this study were the mean visibility scores for tumor margin, vessel pattern of JNET classification, and surface pattern of JNET classification based on WLI, TXI, NBI, and CE observations. We collected data on age and sex of the patients, the location of adenomas, size of adenomas, morphology of adenomas based on the Paris endoscopic classification of neoplastic lesions[25], histological subtype (i.e., tubular or villous) of adenomas, and atypia of adenomas as clinicopathological characteristics.
The visibility scores of TXI and other modalities were compared using the Wilcoxon signed-rank test. Statistical significance was defined as a P value less than 0.05. All statistical data were analyzed using the statistical software Ekuseru-Toukei 2015 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
The clinicopathological characteristics of the 37 consecutive patients with 61 adenomas evaluated in this study are shown in Table 1. The mean age was 59.1 years, and men accounted for 51.4%. Of the adenomas with an average size of 4.2 mm, 78.7% were located on the right side, 86.9% had a flat morphology, and all were tubular subtype with low-grade dysplasia.
| Patients, n | 37 |
| Age, mean (range, SD), yr | 59.1 (41-79, 9.0) |
| Sex, male/female, n | 19/18 |
| Adenomas, n | 61 |
| Location,cecum/ascending/transverse/descending/sigmoid/rectum, n | 5/8/35/3/10/0 |
| Size, mean (range, SD), mm | 4.2 (1-12, 2.3) |
| Morphology1, Ip/Is/IIa/IIb, n | 2/6/48/5 |
| Histological subtype, tubular/villous, n | 61/0 |
| Dysplasia, low-grade/high-grade, n | 61/0 |
The visibility score for the tumor margin of TXI was higher than that of WLI, but lower than that of NBI. Similar tendencies were obtained regardless of the endoscopist’s expertise (Table 2).
| WLI | TXI | NBI | CE | WLI vs TXI, P value | TXI vs NBI, P value | TXI vs CE, P value | |
| Tumor margin | |||||||
| All, mean (SD) | 2.86 (1.02) | 3.47 (0.79) | 3.76 (0.52) | 3.52 (0.84) | < 0.001 | < 0.001 | 0.21 |
| Expert, mean (SD) | 2.85 (0.96) | 3.57 (0.66) | 3.81 (0.43) | 3.64 (0.70) | < 0.001 | < 0.001 | 0.14 |
| Nonexpert, mean (SD) | 2.86 (1.08) | 3.37 (0.90) | 3.72 (0.59) | 3.39 (0.94) | < 0.001 | < 0.001 | 0.73 |
| Vessel pattern | |||||||
| All, mean (SD) | 2.17 (0.90) | 3.05 (0.79) | 3.79 (0.47) | 2.47 (0.87) | < 0.001 | < 0.001 | < 0.001 |
| Expert, mean (SD) | 2.31 (0.87) | 3.24 (0.67) | 3.80 (0.41) | 2.57 (0.85) | < 0.001 | < 0.001 | < 0.001 |
| Nonexpert, mean (SD) | 2.03 (0.90) | 2.86 (0.85) | 3.78 (0.52) | 2.37 (0.88) | < 0.001 | < 0.001 | < 0.001 |
| Surface pattern | |||||||
| All, mean (SD) | 1.95 (0.79) | 2.89 (0.85) | 3.67 (0.55) | 2.75 (0.90) | < 0.001 | < 0.001 | 0.002 |
| Expert, mean (SD) | 1.92 (0.74) | 2.96 (0.78) | 3.70 (0.47) | 2.67 (0.81) | < 0.001 | < 0.001 | < 0.001 |
| Nonexpert, mean (SD) | 1.97 (0.83) | 2.83 (0.92) | 3.64 (0.61) | 2.83 (0.97) | < 0.001 | < 0.001 | 0.94 |
TXI had a higher visibility score for vessel pattern of JNET classification than WLI and CE, but lower visibility score than NBI. Similar tendencies were observed regardless of the endoscopist’s experience (Table 2).
The visibility score of TXI for surface pattern of JNET classification was higher than those of WLI or CE, but lower than that of NBI. However, no difference was observed in the visibility scores between TXI and CE for non-expert endoscopists (Table 2).
This study showed that TXI provided higher visibility than WLI, but lower visibility than NBI for margin and surface structure (i.e., JNET patterns) of adenoma. Moreover, TXI had superior visibility for the surface structure of adenoma to CE. TXI is designed to enhance the three image components (i.e., texture, brightness, and color) of WLI because it clearly defines subtle tissue differences and minimizes gross changes that negatively impact familiarity.
Although TXI was inferior to NBI in a detailed observation of the lesions, many endoscopists prefer to maintain consistency regarding the brightness and color in the original WLI because WLI is used as the standard practice for observation of the entire mucosa. As shown in this study, TXI may improve the balance of image features vital to an endoscopist searching for abnormalities, with texture enhancement, color enhancement, and selectively increased brightness.
Olympus Corporation first developed the NBI in 2007. Fujifilm Corporation developed a similar BLI product. NBI uses ambient light with wavelengths of 415 nm and 540 nm, whereas BLI uses wavelengths of 410 and 450 nm. The images of NBI and BLI are similar. The diagnostic performances of NBI and BLI were also similar for colorectal and esophageal lesions[26]. Fujifilm Corporation developed the LCI. A randomized controlled trial showed that LCI was significantly superior to standard WLI colonoscopy for polyp detection[13]. Currently, LCI-based observations are becoming mainstream. However, Olympus did not have a mode corresponding to that of LCI until recently. Recently, Olympus released TXI as a mode similar to that of LCI.
Although LCI and TXI have similar images, there are several differences in their principles. LCI uses the same illumination as BLI-bright, the images are converted to resemble those of WLI, and color is enhanced such that red is changed to vivid red and white to clear white. On the other hand, TXI uses white light, brightness is adjusted, and texture and color are enhanced. In this study, TXI showed improved tumor margin visibility than WLI. Similar to LCI, TXI may contribute to the improvement in adenoma detection rate; however, future studies are warranted.
In this study, the magnified TXI was inferior to the magnified NBI. Several reports have shown that magnified LCI with CE is superior to magnified BLI. Sakamoto et al[27] reported that magnified LCI with crystal violet staining provided more diagnostic information than magnified BLI and WLI. Kitagawa et al[28] reported that magnified LCI with indigo carmine was superior to magnified BLI. Magnified TXI with CE needs to be further investigated in future studies.
The strength of this study is that it is the first report on the efficacy of TXI in colonoscopy. Second, this study targeted colorectal adenomas, which are common in daily practice; however, evaluation of visibility of malignant tumors is required. Artificial intelligence (AI) has made remarkable progress in the field of endoscopy[29], and we have shown the possible usefulness of TXI for AI endoscopy in the future.
The present study has some limitations. This was a single-center, retrospective study. However, since our institution specializes in endoscopy, the endoscopic environment is well managed. Multicenter randomized control trials are required in the future. Since this study is only for magnified observation, it is desirable to study non-magnified observations as well. TXI has two modes: mode 1 and mode 2. Mode 2 includes brightness adjustment and texture enhancement, and mode 1 adds color enhancement to mode 2. Mode 2 is more natural than mode 1. Since TXI mode 1 was shown to be superior to TXI mode 2 in visibility for gastric neoplasms[23], only mode 1 was investigated in this study. However, comparative studies of visibility between modes 1 and 2 in colonoscopy should be conducted in the future. Additionally, since this study only used CF-HQ290Z, evaluation in various other scopes is necessary. Finally, colorectal adenomas that we investigated were as small as 4.2 mm, and most of them were morphologically flat (86.9%) and located in the proximal colon (78.7%), compared with the adenomas in previous Japanese studies[12]. Our previous study showed that an expert endoscopist with a high adenoma detection rate frequently detected diminutive and flat adenomas in the proximal colon[22]. In the present study, one expert endoscopist conducted all colonoscopies; hence, the adenomas investigated cannot be generalized. In the future, studies with a larger number of cases evaluated by non-expert endoscopists are warranted.
TXI provided higher visibility than WLI, lower than NBI, and comparable to or higher than CE in the magnified observation of colorectal adenomas. Further accumulation of evidence on the performance of TXI is required in the future.
Olympus Corporation has developed texture and color enhancement imaging (TXI) as a novel image-enhancing endoscopic technique.
There are no reports on the use of TXI in the colon.
To investigated the effectiveness of TXI in identifying colorectal adenomas using magnifying observation.
Colorectal adenomas were observed by magnified endoscopy using white light imaging (WLI), TXI, narrow band imaging (NBI), and chromoendoscopy (CE). TXI visibility was compared with the visibility of WLI, NBI, and CE for tumor margin, and vessel and surface patterns of the Japan NBI Expert Team (JNET) classification. The visibility scores were classified as 1, 2, 3, and 4.
Sixty-one consecutive adenomas were evaluated. The visibility score for tumor margin of TXI was significantly higher than that of WLI, but lower than that of NBI. TXI had a higher visibility score for the vessel pattern of JNET classification than WLI and CE, but lower visibility score than NBI. For the visibility score for the surface pattern of JNET classification, TXI was superior to WLI and CE, but inferior to NBI.
TXI provided higher visibility than WLI, lower than NBI, and comparable to or higher than CE in the magnified observation of colorectal adenomas.
TXI may contribute to the improvement in adenoma detection rate. Further accumulation of evidence on the performance of TXI is required in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shaikh DH S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1151] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 2. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1551] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 3. | Hilsden RJ, Rose SM, Dube C, Rostom A, Bridges R, McGregor SE, Brenner DR, Heitman SJ. Defining and Applying Locally Relevant Benchmarks for the Adenoma Detection Rate. Am J Gastroenterol. 2019;114:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Arano T, Isomura Y, Kanazawa T, Ando H, Tsuji Y, Koike K. Simple feedback of colonoscopy performance improved the number of adenomas per colonoscopy and serrated polyp detection rate. Endosc Int Open. 2021;9:E1032-E1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Toyoshima O, Hata K, Yoshida S, Arita M. New-generation chromoendoscopy may increase confidence in the DISCARD2 study. Gut. 2017;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Karsenti D, Tharsis G, Burtin P, Venezia F, Tordjman G, Gillet A, Samama J, Nahon-Uzan K, Cattan P, Cavicchi M. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Boeriu A, Boeriu C, Drasovean S, Pascarenco O, Mocan S, Stoian M, Dobru D. Narrow-band imaging with magnifying endoscopy for the evaluation of gastrointestinal lesions. World J Gastrointest Endosc. 2015;7:110-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, Inoue H, Ishikawa H, Ochiai A, Shimoda T, Watanabe H, Tajiri H, Saito D. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 522] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 10. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 11. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Kobayashi S, Yamada M, Takamaru H, Sakamoto T, Matsuda T, Sekine S, Igarashi Y, Saito Y. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J. 2019;7:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Min M, Deng P, Zhang W, Sun X, Liu Y, Nong B. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc. 2017;86:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Suzuki T, Hara T, Kitagawa Y, Takashiro H, Nankinzan R, Sugita O, Yamaguchi T. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc. 2017;86:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Meylan L, Süsstrunk S. High dynamic range image rendering with a Retinex-based adaptive filter. IEEE Trans Image Process. 2006;15:2820-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Sato T. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J Healthc Eng. 2021;2021:5518948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Chen HY, Zhou XL, Pan WS, Zhou XX, Pan HH. Diagnostic efficacy of the Japan Narrow-band-imaging Expert Team and Pit pattern classifications for colorectal lesions: A meta-analysis. World J Gastroenterol. 2020;26:6279-6294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 18. | Kato M, Abe K, Kubosawa Y, Sunata Y, Hirai Y, Hirata T, Takada Y, Wada M, Takatori Y, Banno S, Kinoshita S, Mori H, Takabayashi K, Kikuchi M, Shiraishi J, Uraoka T. Validation of treatment algorithm based on the Japan narrow-band imaging expert team classification for sub-centimeter colorectal polyps. Endosc Int Open. 2018;6:E934-E940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Rahmi G, Lecomte T, Malka D, Maniere T, Le Rhun M, Guimbaud R, Lapalus MG, Le Sidaner A, Moussata D, Caron O, Barbieux JP, Gaudric M, Coron E, Barange K, Ponchon T, Sautereau D, Samaha E, Saurin JC, Chaussade S, Laurent-Puig P, Chatellier G, Cellier C. Impact of chromoscopy on adenoma detection in patients with Lynch syndrome: a prospective, multicenter, blinded, tandem colonoscopy study. Am J Gastroenterol. 2015;110:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Buchner AM. The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia. Gastroenterol Hepatol (N Y). 2017;13:336-347. [PubMed] |
| 21. | Hurt C, Ramaraj R, Farr A, Morgan M, Williams N, Philips CJ, Williams GT, Gardner G, Porter C, Sampson J, Hillier S, Heard H, Dolwani S; CONSCOP Clinical Research Consortium. Feasibility and economic assessment of chromocolonoscopy for detection of proximal serrated neoplasia within a population-based colorectal cancer screening programme (CONSCOP): an open-label, randomised controlled non-inferiority trial. Lancet Gastroenterol Hepatol. 2019;4:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Toyoshima O, Nishizawa T, Yoshida S, Sekiba K, Kataoka Y, Hata K, Watanabe H, Tsuji Y, Koike K. Expert endoscopists with high adenoma detection rates frequently detect diminutive adenomas in proximal colon. Endosc Int Open. 2020;8:E775-E782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 23. | Ishikawa T, Matsumura T, Okimoto K, Nagashima A, Shiratori W, Kaneko T, Oura H, Tokunaga M, Akizue N, Ohta Y, Saito K, Arai M, Kato J, Kato N. Efficacy of Texture and Color Enhancement Imaging in visualizing gastric mucosal atrophy and gastric neoplasms. Sci Rep. 2021;11:6910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Abe S, Yamazaki T, Hisada IT, Makiguchi ME, Yoshinaga S, Sato T, Nonaka S, Suzuki H, Oda I, Saito Y. Visibility of early gastric cancer in texture and color enhancement imaging. DEN Open. 2022;2:e46. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1318] [Article Influence: 59.9] [Reference Citation Analysis (4)] |
| 26. | Ueda T, Dohi O, Naito Y, Yoshida T, Azuma Y, Ishida T, Matsumura S, Kitae H, Takayama S, Mizuno N, Nakano T, Iwai N, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Konishi H, Nishimura A, Kishimoto M, Itoh Y. Diagnostic performance of magnifying blue laser imaging vs magnifying narrow-band imaging for identifying the depth of invasion of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Sakamoto T, Inoki K, Takamaru H, Sekiguchi M, Yamada M, Nakajima T, Matsuda T, Saito Y. Efficacy of linked colour imaging in magnifying chromoendoscopy with crystal violet staining: a pilot study. Int J Colorectal Dis. 2019;34:1341-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Kitagawa Y, Hara T, Ikebe D, Nankinzan R, Takashiro H, Kobayashi R, Nakamura K, Yamaguchi T, Suzuki T. Magnified endoscopic observation of small depressed gastric lesions using linked color imaging with indigo carmine dye. Endoscopy. 2018;50:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Sinagra E, Badalamenti M, Maida M, Spadaccini M, Maselli R, Rossi F, Conoscenti G, Raimondo D, Pallio S, Repici A, Anderloni A. Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped. World J Gastroenterol. 2020;26:5911-5918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |