Published online Dec 16, 2022. doi: 10.4253/wjge.v14.i12.789
Peer-review started: September 20, 2022
First decision: October 13, 2022
Revised: October 20, 2022
Accepted: November 19, 2022
Article in press: November 19, 2022
Published online: December 16, 2022
Processing time: 84 Days and 4.5 Hours
Schistosomiasis is a chronic parasitic infection endemic in many countries. Col
We present the case of a 21-year-old male who suffered from chronic diarrhea and abdominal pain. Physical examination found no abnormalities, blood tests were normal, and stool examination was negative. A colonoscopy revealed a nodular terminal ileal mucosa, two cecal polypoid lesions with no particular surface pat
Colonic schistosomiasis should be considered as a differential diagnosis, es
Core Tip: Colonic schistosomiasis is a rare disease, often mistaken for other pathologies, such as inflammatory bowel disease, because the clinical and endoscopic manifestations are non-specific and can be misleading. Histopathological examination is key to diagnosis when the stool examination shows no ova. We present a case of colonic schistosomiasis in a 21-year-old male presenting with chronic diarrhea and abdominal pain. The stool examination was negative and colposcopy showed multiple polyps. Histopathological examination confirmed the diagnosis of colonic schistosomiasis. Antiparasitic treatment was effective.
- Citation: Koulali H, Zazour A, Khannoussi W, Kharrasse G, Ismaili Z. Colonic schistosomiasis: A case report. World J Gastrointest Endosc 2022; 14(12): 789-794
- URL: https://www.wjgnet.com/1948-5190/full/v14/i12/789.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i12.789
Schistosomiasis is a serious chronic parasitic infection caused by trematodes, primarily Schistosoma mansoni and Schistosoma japonicum. Humans are accidental hosts; infection occurs after ingesting larva-infested water. According to the World Health Organization, 236.6 million people needed preventative treatment in 2019 and the global death rate ranged between 24000 and 200000. Schistosoma commonly infects the urinary tract, and intestinal infection is rare. Its clinical manifestations are non-specific, ranging from asymptomatic to intestinal occlusion secondary to larva deposits, diarrhea, abdominal pain, malnutrition, and chronic anemia. Colonoscopy can reveal lesions, among which mucosal edema, ulcerations, and polypoid lesions are frequently observed[1].
Herein, we present a case of a 21-year-old male with colonic schistosomiasis.
A 21-year-old male, originally from Madagascar but living in Morocco for the past 5 years, presented with chronic diarrhea up to 3-4 times a day, diffuse abdominal pain prominent to the right iliac fossa and intermittent subocclusive symptoms for 3 years with no recent aggravation.
The patient suffered from his complaints for 3 years prior to presentation, and they occurred in a flare-up/remission pattern.
The physical examination found no abnormalities. The patient had a normal body mass index. No abdominal tenderness nor mass was noted.
Blood tests gave normal findings, showing negativity for C-reactive protein levels. Stool examination for parasite ova and bacterial culture were negative.
A thoracic abdominopelvic computed tomography scan revealed no abnormalities.
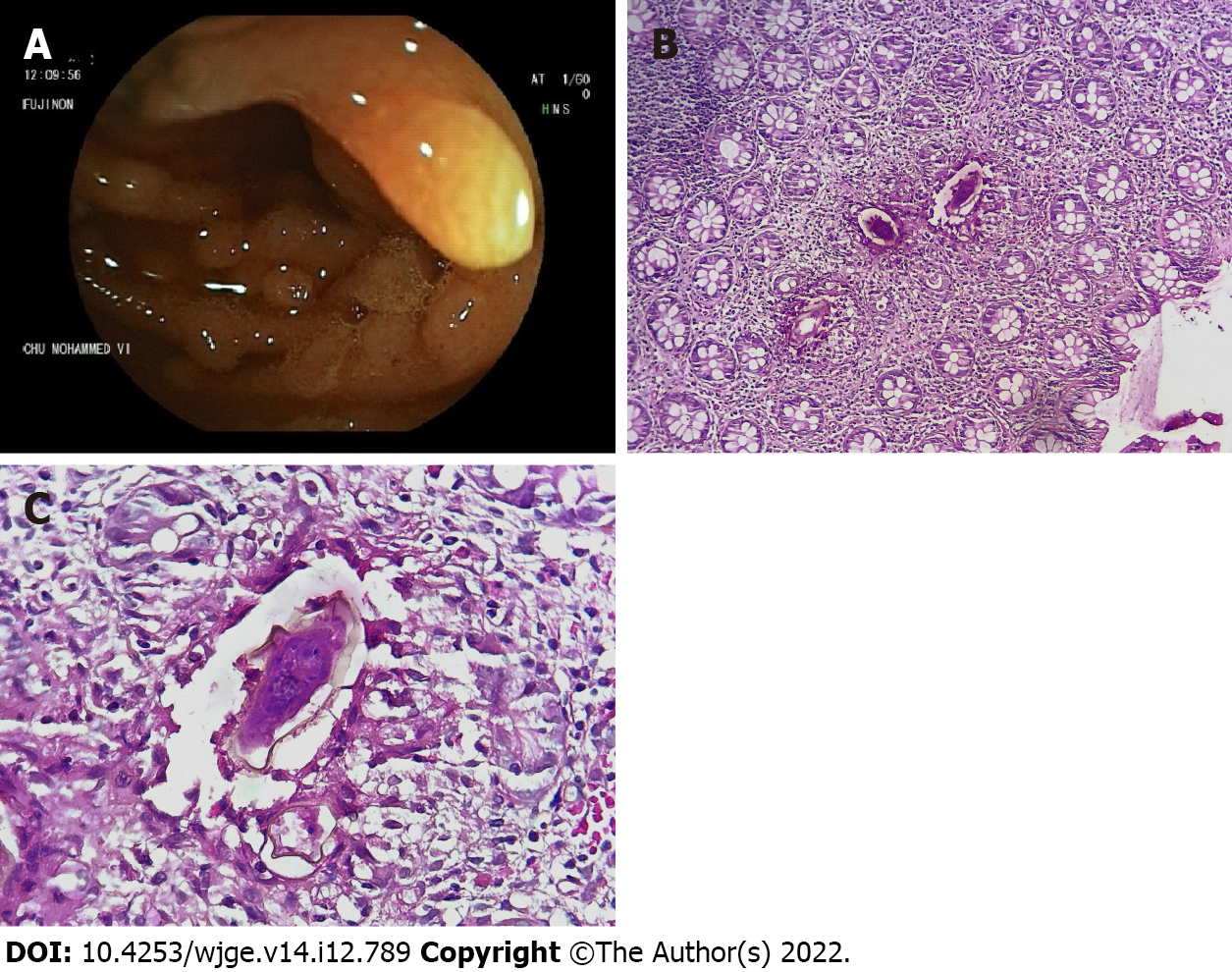
Colonoscopy revealed a nodular terminal ileal mucosa, two cecal polypoid lesions with no particular surface pattern, and millimetric erosions in the rectum (Figure 1A). Biopsies were taken with jumbo forceps. Histopathological examination showed the presence of Schistosoma eggs with thick peripheral capsules and viable embryos inside (Figure 1B). The egg capsules were surrounded by numerous eosinophils (Figure 1C).
Colonic schistosomiasis.
The patient received praziquantel (60 mg/kg in two doses over a 1-d period).
The treatment resolved the diarrhea and alleviated the abdominal pain.
Schistosomiasis, also known as Bilharzia, is a parasitic infectious disease caused by schistosomes. Its geographical distribution is widespread, with endemic foci in some regions of the world (Africa, South America and Asia). S. mansoni and S. japonicum are typically involved in digestive schistosomiasis. In Africa, colonic polyposis is generally associated with S. mansoni infection[2]. Patients are infected after direct contact with water contaminated with snails carrying the parasite. The urinary system is preferentially affected, while intestinal involvement is rare.
Symptoms can be non-specific, and the evolution of the infection can last for long periods (as reported in our case). Diarrhea is the main symptom, as 3%-55% of a population study presented with diarrhea, with 11%-50% of cases presenting with bloody diarrhea[1]. In a study of 216 patients with intestinal schistomiasis, by Mohamed et al[2], abdominal pain and diarrhea were the most frequent symptoms, accounting for 39 % and 27% of cases respectively. In another study by Rocha et al[3], diarrhea was also the most common symptom, observed in 56% of cases. Abdominal pain, constipation, weight loss and fatigue are commonly observed, while obstructive symptoms, such as intestinal stenosis, are rare.
Differential diagnosis with inflammatory bowel disease and malignancy can be challenging. Hypereosinophelia is a nonspecific finding of schistomiasis correlating to the stage, intensity, and duration of infection. Stool examination may reveal ova, which is essential in determining larva species[1,2]. However, detecting ova in the stool can be difficult, as the numbers decrease as the infection evolves. Quantitative sampling according to the Kato-Katz technique coupled with concentration technique improves the sensitivity of egg detection; the diagnosis sensitivity could also be improved by associating Kato-Katz sampling examination with serological testing (e.g., IgG anti-Schistosoma mansoni-enzyme-linked immunosorbent assay technique)[4]. Serological diagnosis by detection of serum antibody titer is also available, especially in endemic areas, but it cannot differentiate between active or chronic infection; meanwhile, a negative serological test can rule out infection in endemic areas but cannot be used in post-treatment follow-up due to prolonged positivity post-therapy[5]. Detection of free circulating DNA by polymerase chain reaction can be used for early diagnosis of prepatent schistosomiasis infection[6], with good sensitivity and specificity for urine samples (94.4% and 99.9% respectively)[7]. Serologic tests for the detection of one of the two gut-associated parasite proteins ¾ circulating anodic antigen and circulating cathodic antigen ¾ can also be used for diagnosis[8].
When digestive colonization occurs, superficial submucosal deposits of Schistosoma eggs lead to the formation of polypoid lesions corresponding to inflammatory granulation tissue and hypertrophy of the adjacent muscular layer. Colonoscopy can show polypoid lesions, edema, ulcers, and granular patterns[9-13]. In the study mentioned above by Mohamed et al[2], polyps were found in only 8 cases (3 were rectal and 5 were colonic), and histopathological examination showed schistosomal ova in all 8 of the polyps. Cao et al[10] observed that nodular lesions and polyps are more frequent in the left colon, while mucosal edema, erythema, granular pattern, and ulcers are often seen in the right colon. In this study, 4 patients were misdiagnosed as ulcerative colitis, 1 as Crohn’s disease, and 7 as ischemic colitis. While intestinal lesions associated with S. mansoni are usually observed in the ileum and the colon, duodenal involvement has been reported as well. Based upon visualization of schistosomal ova, biopsies and histopathological examination are the golden diagnostic standard of colonic schistomiasis. The ova are mainly deposited in the lamina propria and/or submucosa[11], with an observable inflammatory reaction in the tissue surrounding them[10,12]. Other characteristic features are excessive mucus and diffuse or focal infiltration of eosinophilic granulocytes, which may be highly suggestive of colonic schistosomiasis[14], as seen in our patient. In addition, intestinal ultrasound and computed tomography may reveal wall thickening, but they show no abnormalities in most cases. Abdominal X-rays and barium enemas can show images of polyps and structures but are not typically utilized due to their lack of specificity.
Intestinal schistosomiasis is amenable to medical treatment, including praziquantel, with a safe and effective outcome and cure rates ranging between 60% and 90%[15]. It has been shown that antigen tests become negative as early as 5-10 d after successful therapy[16]. A study from Africa that aimed to evaluate the efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma concluded the efficacy of crushed praziquantel administered to preschool-aged children at a dose of 40 mg/kg against S. mansoni and Schistosoma haematobium[17]. Mutapi et al[18] had also concluded from their study that praziquantel is safe and efficacious in children aged 1-10 years.
Praziquantel is substantially excreted by the kidney, and elderly patients with decreased renal function may be at greater risk of toxic reactions. In a study conducted by Putri et al[19], the group aged 45 to 69 experienced a high proportion of side effects.
A second praziquantel regimen can be prescribed in case of persistence of the infection; oxamniquine alone or combinated with praziquantel and trioxolane can also be used as second-line therapy.
Following treatment, stool analysis or colon biopsy could be considered for assessment of treatment success but should be performed at least 6 wk post-treatment[20]. No data are available in the literature regarding colonic polyps’ endoscopic follow-up and monitoring.
Cases of colon cancer associated with S. japonicum have been reported. However, the carcinogenic pathways are unclear, and the association is not well established[2,10,21]. A Chinese study including 454 colorectal carcinoma specimens showed that more than half (n = 289) were associated with S japonicum infection[22]. Furthermore, a study by Kaw et al[23] including 1277 colonic carcinoma patients showed that schistosomiasis was often accompanied by rectal cancer.
Schistosomiasis prevention is key to its elimination; public health awareness campaigns, water sanitation, hygiene programs, and chemotherapy programs are necessary. Preventive chemotherapy in preschool-aged children is deemed appropriate for those aged ≥ 2 years in endemic communities, according to the World Health Organization. While an antischistosomal vaccine will be ideal for long-term protection, clinical trials for its development are still in progress.
Colonic schistosomiasis is a rare disease that should be considered a differential diagnosis in endemic regions. Endoscopic appearance is non-specific. Histopathological and stool examinations have a significant role in diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Morocco
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Z, China; Wu C, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Akere A, Oluwasola AO, Fakoya TO, Lawan A. SCHISTOSOMIASIS PRESENTING AS COLONIC POLYPOID MASSES IN A NIGERIAN PATIENT. Ann Ib Postgrad Med. 2017;15:61-64. [PubMed] |
| 2. | Mohamed AR, al Karawi M, Yasawy MI. Schistosomal colonic disease. Gut. 1990;31:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Rocha MO, Pedroso ER, Lambertucci JR, Greco DB, Rocha RL, Rezende DF, Neves J. Gastro-intestinal manifestations of the initial phase of schistosomiasis mansoni. Ann Trop Med Parasitol. 1995;89:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Carneiro TR, Pinheiro MC, de Oliveira SM, Hanemann AL, Queiroz JA, Bezerra FS. Increased detection of schistosomiasis with Kato-Katz and SWAP-IgG-ELISA in a Northeastern Brazil low-intensity transmission area. Rev Soc Bras Med Trop. 2012;45:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Leder K, Weller PF. Eosinophilia and helminthic infections. Baillieres Best Pract Res Clin Haematol. 2000;13:301-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Hussein HM, El-Tonsy MM, Tawfik RA, Ahmed SA. Experimental study for early diagnosis of prepatent schistosomiasis mansoni by detection of free circulating DNA in serum. Parasitol Res. 2012;111:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Gundersen SG, Ravn J, Haagensen I. Early detection of circulating anodic antigen (CAA) in a case of acute schistosomiasis mansoni with Katayama fever. Scand J Infect Dis. 1992;24:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Sandoval N, Siles-Lucas M, Pérez-Arellano JL, Carranza C, Puente S, López-Abán J, Muro A. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology. 2006;133:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Ebigbo A, Kahn M, Zellmer S, Messmann H. Advanced endoscopic imaging of colonic schistosomiasis. Endoscopy. 2021;53:E251-E252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Cao J, Liu WJ, Xu XY, Zou XP. Endoscopic findings and clinicopathologic characteristics of colonic schistosomiasis: a report of 46 cases. World J Gastroenterol. 2010;16:723-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Godyn JJ, Siderits R, Hazra A. Schistosoma mansoni in colon and liver. Arch Pathol Lab Med. 2005;129:544-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Nebel OT, el-Masry NA, Castell DO, Farid Z, Fornes MF, Sparks HA. Schistosomal colonic polyposis: endoscopic and histologic characteristics. Gastrointest Endosc. 1974;20:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Qin X, Liu CY, Xiong YL, Bai T, Zhang L, Hou XH, Song J. The clinical features of chronic intestinal schistosomiasis-related intestinal lesions. BMC Gastroenterol. 2021;21:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Radhakrishnan S, Al Nakib B, Shaikh H, Menon NK. The value of colonoscopy in schistosomal, tuberculous, and amebic colitis. Two-year experience. Dis Colon Rectum. 1986;29:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Elbaz T, Esmat G. Hepatic and intestinal schistosomiasis: review. J Adv Res. 2013;4:445-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Van 't Wout AB, De Jonge N, Tiu WU, Garcia EE, Mitchell GF, Deelder AM. Schistosome circulating anodic antigen in serum of individuals infected with Schistosoma japonicum from the Philippines before and after chemotherapy with praziquantel. Trans R Soc Trop Med Hyg. 1992;86:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Coulibaly JT, N'gbesso YK, Knopp S, Keiser J, N'Goran EK, Utzinger J. Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis. 2012;6:e1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Mutapi F, Rujeni N, Bourke C, Mitchell K, Appleby L, Nausch N, Midzi N, Mduluza T. Schistosoma haematobium treatment in 1-5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis. 2011;5:e1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Putri ASD, Vera Diana T, Daris R, Afriana F, Hidayat SH. Does the presence of praziquantel-related adverse events affect the health community's perception toward the mass chemopreventive program in the highest prevalence area of Schistosomiasis in Indonesia? Gac Sanit. 2021;35 Suppl 2:S487-S490. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Issa I, Osman M, Aftimos G. Schistosomiasis manifesting as a colon polyp: a case report. J Med Case Rep. 2014;8:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Li WC, Pan ZG, Sun YH. Sigmoid colonic carcinoma associated with deposited ova of Schistosoma japonicum: a case report. World J Gastroenterol. 2006;12:6077-6079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Ming-Chai C, Chi-Yuan C, Pei-Yu C, Jen-Chun H. Evolution of colorectal cancer in schistsosomiasis: transitional mucosal changes adjacent to large intestinal carcinoma in colectomy specimens. Cancer. 1980;46:1661-1675. [PubMed] [DOI] [Full Text] |
| 23. | Kaw LL Jr, Punzalan CK, Crisostomo AC, Bowyer MW, Wherry DC. Surgical pathology of colorectal cancer in Filipinos: implications for clinical practice. J Am Coll Surg. 2002;195:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |