Published online Dec 16, 2022. doi: 10.4253/wjge.v14.i12.777
Peer-review started: September 18, 2022
First decision: October 19, 2022
Revised: October 26, 2022
Accepted: November 9, 2022
Article in press: November 9, 2022
Published online: December 16, 2022
Processing time: 86 Days and 22.6 Hours
Anaesthetic care during upper gastrointestinal (GI) endoscopy has the unique challenge of maintaining ventilation and oxygenation via a shared upper airway. Supplemental oxygen is recommended by international society guidelines, how
To assess the incidence of hypoxaemia (SpO2 < 90%) in patients undergoing upper GI endoscopy receiving supplemental oxygen using an oxygenating mouthguard at 20 L/min flow compared to standard nasal cannula (SNC) at 2 L/min flow.
A single centre, prospective, randomised clinical trial at two sites of an Australian tertiary hospital between October 2020 and September 2021 was conducted. Patients undergoing elective upper gastrointestinal endoscopy under deep sedation were randomised to receive supplemental oxygen via high-flow via oxygenating mouthguard (HFMG) at 20 L/min flow or SNC at 2 L/min flow. The primary outcome was the incidence of hypoxaemia of any duration measured by pulse oximetry. Intraprocedural-related, procedural-related, and sedation-related adverse events and patient-reported outcomes were also recorded.
Three hundred patients were randomised. Eight patients were excluded after randomisation. 292 patients were included in the intention-to-treat analysis. The incidence of hypoxaemia was significantly reduced in those allocated HFMG. Six patients (4.4%) allocated to HFMG experienced an episode of hypoxaemia, compared to thirty-four (22.1%) patients allocated to SNC (P value < 0.001). No significant difference was observed in the rates of adverse events or patient-reported outcome measures.
The use of HFMG offers a novel approach to reducing the incidence of hypoxaemia during short upper gastrointestinal endoscopic procedures in low-risk patients undergoing deep sedation.
Core Tip: This randomised controlled trial compared the incidence of hypoxaemia in those receiving supplemental oxygen at 20 L/min via an oxygenating mouthguard to those receiving supplemental oxygen at 2 L/min via standard nasal cannula during upper gastrointestinal endoscopy performed under deep sedation. A statistically significant difference in the incidence of hypoxaemia was demonstrated. No significant difference was observed in rates of adverse events or patient-reported outcome measures. We conclude that the use of supplemental oxygen at 20 L/min via an oxygenating mouthguard offers a novel approach to reducing the incidence of hypoxaemia in patients undergoing upper gastrointestinal endoscopy under deep sedation.
- Citation: Be KH, Zorron Cheng Tao Pu L, Pearce B, Lee M, Fletcher L, Cogan R, Peyton P, Vaughan R, Efthymiou M, Chandran S. High-flow oxygen via oxygenating mouthguard in short upper gastrointestinal endoscopy: A randomised controlled trial. World J Gastrointest Endosc 2022; 14(12): 777-788
- URL: https://www.wjgnet.com/1948-5190/full/v14/i12/777.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i12.777
Upper gastrointestinal (GI) endoscopic procedures are commonly performed under monitored anesthesia to facilitate endoscopic examination. Anaesthetic care during upper GI endoscopy has the unique challenges of balancing adequate patient sedation while maintaining sufficient ventilation and oxygenation via a shared upper airway[1]. In addition, anaesthetic agents routinely used during sedation for GI endoscopies, such as propofol, in combination with benzodiazepines and opioids can cause respiratory depression, predisposing patients to upper airway obstruction, hypoventilation, and hypoxaemia[2]. Therefore, supplementary oxygen during upper GI endoscopy under deep sedation is considered the standard practice to reduce the incidence and severity of hypoxaemia[3].
Although supplemental oxygen is a recommendation of various national and international societies, it is unclear what the optimal routes or rates of supplemental oxygen delivery are[4,5]. The incidence of hypoxaemia during upper GI endoscopy with deep sedation is common, and reported to occur in up to 33% of procedures depending on the route and rate of supplemental oxygen used[6,7]. Although transient and mild episodes of hypoxaemia are likely inconsequential, prolonged or severe hypoxaemia is associated with tachycardia and myocardial ischemia[8,9]. Various oxygen delivery devices have been investigated to improve oxygenation during upper GI endoscopy. These include standard nasal cannula (SNC), high-flow nasal cannula (HFNC), modified bite blocks, modified face masks and other more invasive nasopharyngeal (such as Wei Nasal Jet tube) and oropharyngeal devices (such as a gastro-laryngeal tube)[10-12]. The principles underlying these airway devices include the delivery of higher fractionated oxygen (FiO2) with or without positive pressure ventilation[1].
Oxygen supplementation via SNC is the most common approach to oxygen delivery during upper gastrointestinal endoscopy[11]. However, its use is limited to flow rates of 6 L/min, as higher flow rates cause drying of the nasal passages and nasal mucosa irritation. The advent of HFNC has circumvented these limitations of SNC by passing supplementary oxygen through a humidifier. Flows of up 60 L/min can be achieved, which has added advantages of generating a positive end-expiratory pressure, and reducing physiological dead space, whilst delivering higher FiO2[7]. The routine use of HFNP is limited by its high costs and the required training and education to set up. Other airway devices described above are limited by the commercial availability, costs and expertise required for insertion[11].
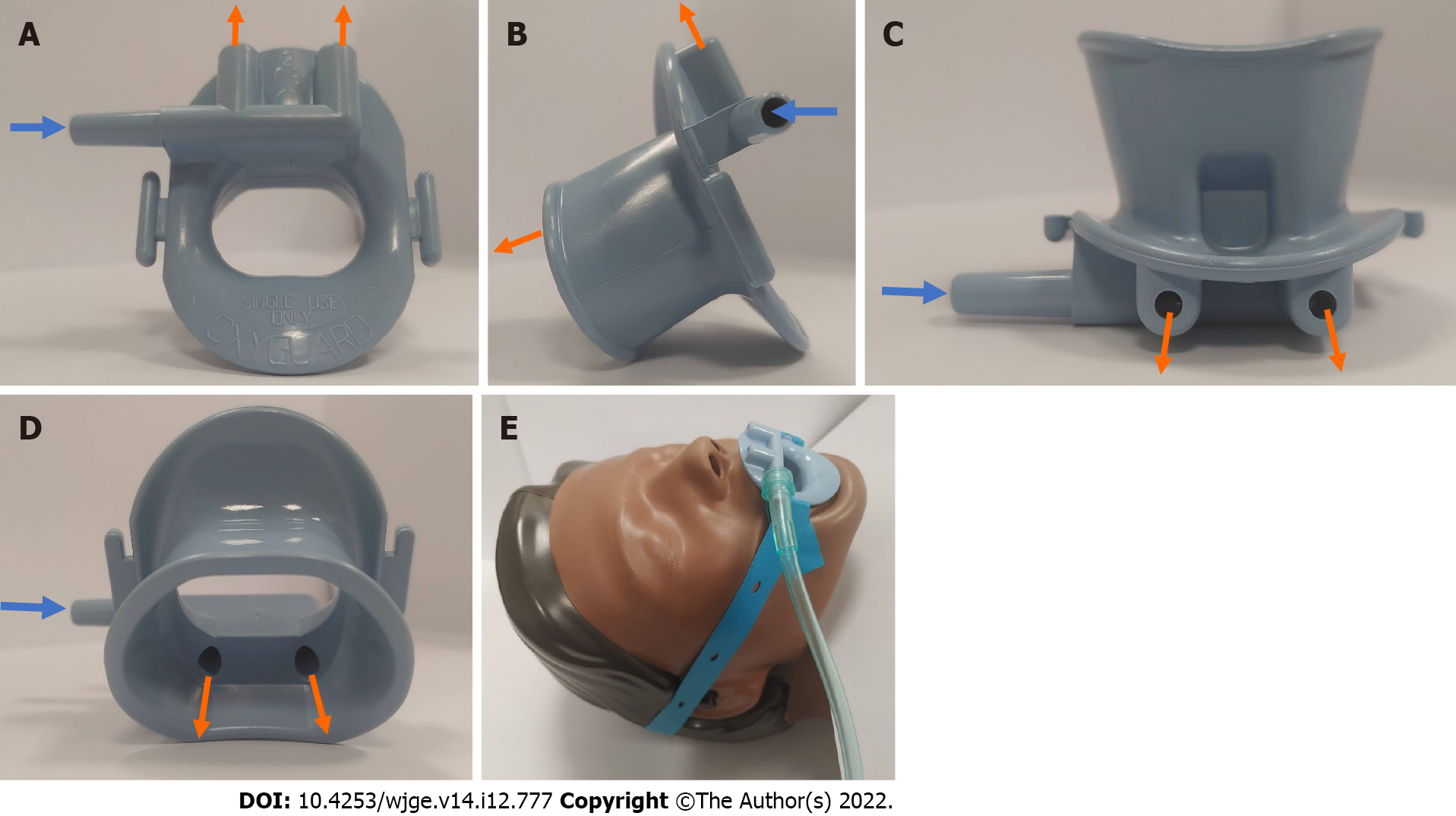
At our centre, an oxygenating mouthguard (OxyguardTM; North Yorkshire, England) is routinely used for all upper GI endoscopy procedures to minimise dental injury and damage to the endoscope, whilst maintaining the mouth in an open position during the procedure. This mouthguard can be used to deliver supplementary oxygen by directing the flow of oxygen via a dedicated oxygen port into the oral and nasal cavities simultaneously (Figure 1A-D). It is held in place with a rubber strap wrapped around a patient’s head (Figure 1E). This product is commercially available throughout Australia, Europe, and South Africa at the time of writing. Though the benefit of using 3L/min supplementary oxygen via this mouthguard in alleviating hypoxaemia during gastroscopy has been demonstrated, compared to a standard plastic mouthguard using room air, there are no publications to date on the use of high flows of supplemental oxygen[13]. Anecdotally, our team found that higher flows of supplemental oxygen can be safely delivered via this mouthguard during upper GI endoscopic procedures. An impetus to further investigate the clinical efficacy of delivering higher flows of oxygen via this mouthguard was the recent publication by Lin et al[7] The use of HFNC at 60 L/min, when compared to a supplemental oxygen flow rate of 2 L/min in a low-risk population for sedation-related adverse events undergoing a short gastroscopy performed under propofol sedation, demonstrated a significant reduction in the incidence of hypoxia (defined as oxygen saturation (SpO2) < 90% and ≥ 75% for < 60 s) and severe hypoxia (defined as SpO2 < 75% for any duration, or SpO2 < 90% and ≥ 75% for ≥ 60 s) from 8.4% to 0% (P value < 0.001) and from 0.6% to 0% (P value = 0.03), respectively[7].
In this article, we report a randomised controlled trial on the novel use of high-flow supplemental oxygen via an oxygenating mouthguard in low-risk patients of sedation-related adverse events under propofol sedation.
This is a single-centre, prospective, randomised clinical trial conducted at two sites of an Australian tertiary health service, between October 2020 and September 2021. Local ethics committee approval (ND 63130/2020) and registration at ANZCTR.org.au (ACTRN12620000930987) were attained before patient recruitment.
All patients referred for an endoscopy at our centre were considered during the study period. Inpatients scheduled a non-emergent upper GI endoscopy (gastroscopy, endoscopic retrograde cholangiopancreatography (ERCP), upper enteroscopy or upper endoscopic ultrasound (EUS), alone or in combination with another upper GI endoscopy) were offered the patient information and consent form (PICF) at least 12 h before their scheduled procedure. Non-emergent endoscopy was defined as a patient with vital signs within normal limits without evidence of upper GI bleeding or an active infection. Outpatients scheduled for upper GI endoscopies were sent the PICF via post or email. Patients scheduled for a combined lower GI tract endoscopy (such as colonoscopy, lower enteroscopy or lower endoscopic ultrasound) or scheduled for endoscopist administered sedation lists were excluded.
Patients scheduled for upper GI endoscopy were assessed for the following inclusion and exclusion criteria by an investigator at the time of their procedure. Inclusion criteria: (1) Age >18 years; (2) Ability to provide informed consent; and (3) An anticipated endoscopic procedure time of fewer than 20 min, as assessed by the accredited gastroenterologist or surgeon responsible for the case. Exclusion criteria: (1) America Society of Anesthesiologist[14] class greater than III; (2) Mallampati score[15] of greater than 3; (3) Body mass index > 35 kg/m2; (4) Supplementary oxygen dependence; (5) Pregnancy; (6) Deemed high-risk of a sedated-related adverse event by the duty anaesthetist; and (7) Anticipated requirement or plan for general anaesthesia involving airway instrumentation including a laryngeal mask or tracheal intubation.
Enrolled participants were randomly assigned to one of two groups: high-flow via oxygenating mouthguard (HFMG) at 20 L/min or SNC (Softi Smoothflow®; Victoria, Australia) at 2 L/min flow. Of note, the design of this SNC allows oxygen delivery through one nasal prong and sampling of expired carbon dioxide from the other prong simultaneously.
Supplemental oxygen at 20 L/min was supplied from a high-flow oxygen rotameter and delivered via a dedicated oxygen port as depicted in Figure 1A-E. Patients allocated to the SNC received oxygen at a fixed rate of 2 L/min. Initial flow rates were maintained throughout the endoscopic examination unless a hypoxemic event occurred. At the discretion of the anesthetist, the rate or route of oxygen delivery could be changed.
Proceduralists and anaesthesiologists were instructed to provide usual care except for the assigned initial oxygen delivery method and rate. Standard monitoring, including heart rate, blood pressure and SpO2 were measured and recorded. The use of capnography was at the discretion of the duty anaesthetist. All physiological measurements were recorded using the GE Datex-Ohmeda Aisys Anaesthesia Machine (General Electric, Boston, United States).
Gastroscopy, EUS and enteroscopy were performed in the left lateral position, unless performed together with an ERCP which were performed in the semi-prone position under intravenous sedation with propofol with or without benzodiazepine and/or opioids.
Data on participants’ symptoms post-procedure were collected using a Likert scale questionnaire (Supplementary Appendix III) before the patient’s discharge from the endoscopy unit. Incomplete patient-reported symptom forms were excluded.
The primary outcome was the occurrence of hypoxaemia, defined as SpO2 < 90%, of any duration measured by pulse oximetry during the procedure[7,16,17].
Secondary outcomes included the lowest SpO2 measured by pulse oximetry during the procedure, the incidence of hypoxaemia defined as mild (SpO2 90%-94%), moderate (SpO2 89%-76%) and severe (SpO2 ≤ 75%) of durations less than 1 minute, between 1 and 5 minutes and more than 5 min, procedure-related adverse events, sedation-related events, and patient-reported symptoms.
A clinically significant episode of hypoxaemia was defined as a need to change the flow or method of oxygen delivery that the patient was randomised to in response to an episode of hypoxaemia.
In addition, a posthoc analysis of the incidence of hypoxaemia defined as SpO2 < 85% was performed[18].
Intraprocedural-related adverse events included a need to pause or stop the procedure due to an episode of oxygen desaturation or as directed by the duty anaesthetist. Procedure-related complications including bleeding requiring intervention, perforation, and post-procedure complications including pain, bleeding or sepsis necessitating a hospital admission or delayed discharge from the endoscopy unit were also recorded. Sedation-related adverse events included hypotension, bradycardia, tachycardia, seizure, cardiac arrest, nausea or vomiting, recovery agitation and delayed recovery whilst in the procedure room were noted.
Patient-reported symptoms after the procedure included overall comfort, abdominal pain, abdominal bloating, nose, mouth or throat dryness or pain, and headache.
Endoscopy procedure time was routinely collected and defined as the time the endoscope entered and exited the oral orifice. When more than one upper GI endoscopy was performed, the endoscopy procedure time was defined as the time of the first endoscope entering the oral orifice and the last endoscope exiting. Anaesthetic time was defined as the duration of time during which intravenous propofol was administered.
Allocation was pre-defined through an online research randomiser (https://www.randomizer.org). The allocation was placed into 300 sealed opaque envelopes by an independent person who was not a member of the research team. The envelopes were labelled from 1 to 300 and were consecutively opened. The envelopes were evenly split between the two sites and continued to be evenly distributed until the last patient was recruited.
The clinical care team (e.g., anaesthetists, endoscopists, nurses) was advised of the patient’s randomisation. Patients were not blinded to their allocation due to the obvious difference in the oxygen delivery devices.
Two-tailed 0.05 alpha error and power of 80% were used for the sample size calculation. A 10% loss after randomisation was also accounted for. We aimed to enrol 300 patients, based on an anticipated difference of 8.4% previously observed when comparing HFNC at 40-60 L/min and 2 L/min in upper GI endoscopy[7]. The incidence rates used were 9.4% and 1.0% in the control and interventional group, respectively.
SPSS was used for statistical analyses. Collected data were summarised as mean ± standard deviation (SD) or median (25th and 75th percentile) for continuous data, and as frequency and percentages for categorical data. For continuous data, the characteristics, and outcomes for the two groups were compared using Student's t-test or Wilcoxon-Mann-Whitney test based on the normality assumption. Categorical data were compared with Chi-square or Fisher's exact test as appropriate. A P value of < 0.05 was considered significant. Statistical analyses were performed with SPSS Version 28.0.1.1.
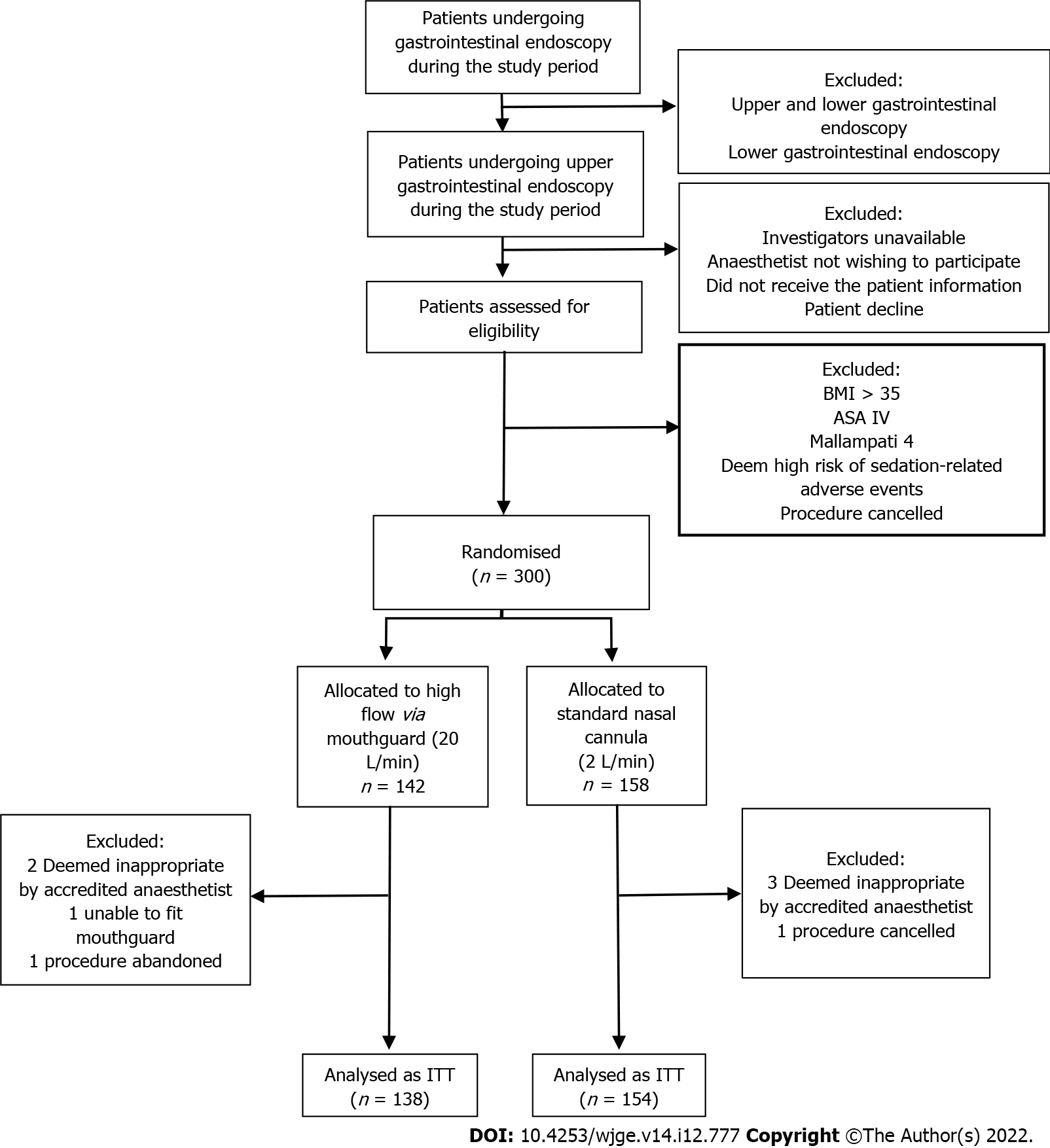
From October 2020 to September 2021, 300 patients were enrolled and randomised; 8 patients were excluded after randomisation. Five patients were excluded as the accredited anaesthesiologist deemed the patient not appropriate for the study (e.g., change in the anaesthetic plan after review by the accredited anaesthetist for intubation under general anaesthesia), one patient’s procedure was cancelled by the proceduralist as anti-coagulation was not ceased as planned, one patient’s procedure was abandoned due to the presence of food in the oesophagus and another patient was unable to wear the oxygenating mouthguard as their mouth opening was insufficient.
A total of 292 patients were included in our intention-to-treat analysis. Figure 2 flow chart describes the patient allocation.
In addition, ten patients did not receive their allocated rate and/or route of supplementary oxygen. Three of these patients allocated to HFMG did not receive 20 L/min as per protocol. Instead, two patients received 10 L/min, and one patient received 15 L/min via the mouthguard. Furthermore, seven patients were incorrectly allocated to the wrong group. Four patients allocated to HFMG received 2 L/min via SNC, and three patients allocated to SNC received 20 L/min via mouthguard. A per-protocol analysis was performed to determine the impact of these discrepancies on the primary outcome. The three patients receiving 10 L/min and 15 L/min via mouthguard were excluded from the per-protocol analysis. The per-protocol analysis for the primary outcome is described below in the results.
The baseline characteristics of the two groups are described in Table 1.
| Characteristics | SNC (n = 154) | HFMG (n = 138) | |
| Age (median, IQR) | 64, 56 to 72 | 59, 48.5 to 69.5 | |
| Male | 71, 46.1% | 67, 48.6% | |
| Weight, kg (mean, SD) | 76.4, 13.6 | 76.1, 14.8 | |
| BMI, kg/m2 (mean, SD) | 26.6, 4.1 | 26.4, 3.9 | |
| ASA classification, I/II/III | 14/67/73, 9.1%/43.5%/47.4% | 16/58/64, 11.6%/42.0%/46.4% | |
| Mallampati class, I/II/III | 54/70/30, 35.1%/45.4%/19.5% | 48/70/20, 34.8%/50.7%/14.5% | |
| Baseline oximetry, SpO2 (median, IQR) | 97%, 95% to 99% | 98%, 97% to 99% | |
| Past medical history | |||
| Current smoking history | 14, 9.1% | 14, 10.1% | |
| Obstructive sleep apnoea | 8, 5.2% | 6, 4.3% | |
| Hypertension | 69, 44.8% | 46, 33.3% | |
| Ischemic heart disease | 19, 12.3% | 9, 6.5% | |
| Diabetes mellitus | 34, 22.1% | 33, 23.9% | |
| Dyslipidemia | 36, 23.4% | 26, 18.8% | |
| Chronic obstructive pulmonary disease | 8, 5.2% | 11, 8% | |
| Asthma | 9, 5.8% | 11, 8% | |
| Cirrhosis | 25, 16.2% | 34, 24.6% | |
| Orthotopic liver transplantation | 19, 12.3% | 25, 18.1% | |
Details of the anaesthetic care and endoscopy procedure are summarised in Tables 2 and 3, respectively. Of note, the weighted dose of propofol per hour of the two groups and the number of anaesthetic agents used were similar. In addition, the duration of sedation and upper GI endoscopies performed were comparable between the two groups. Most procedures (86.3%) were 20 minutes or shorter. A sub-group analysis of longer procedures for the primary outcome was performed and is described below. More than half (52.7%) of the upper GI endoscopies were diagnostic. The most common procedures were gastroscopies (69.2%) and ERCPs (22.6%).
| Anaesthetic care | SNC (n = 154) | HFMG (n = 138) | P value | |
| Duration of sedation, min (median, IQR) | 12, 6.9 to 17.1 | 12, 6.5 to 17.5 | 0.421 | |
| Propofol dose, mg/kg/hr (median, IQR) | 13.3, 8.5 to 18.1 | 14.1, 7.8 to 20.5 | 0.189 | |
| Opioids | 89, 57.8% | 73, 52.9% | 0.631 | |
| Fentanyl | 52, 33.8% | 40, 29.0% | ||
| Alfentanil | 37, 24.0% | 33, 23.9% | ||
| Midazolam | 26, 16.9% | 23, 16.7% | 0.961 | |
| Endoscopy parameters | SNC, (n = 154) | HFMG, (n = 138) | P value | |
| Duration of procedure, min (median, IQR) | 10, 5.5 to 14.5 | 10, 4.5 to 15.5 | 0.684 | |
| Types of procedure | 0.175 | |||
| Diagnostic Procedure | 87, 56.5% | 67, 48.6% | ||
| Therapeutic Procedure | 67, 43.5% | 71, 51.4% | ||
| Types of upper GI endoscopy | 0.27 | |||
| Gastroscopy | 106, 68.8% | 96, 69.6% | ||
| Duodenoscope | 1, 0.6% | 1, 0.7% | ||
| ERCP | 32, 20.8% | 34, 24.6% | ||
| EUS | 12, 7.8% | 3, 2.2% | ||
| Gastroscopy + EUS | 3, 1.9% | 4, 2.9% | ||
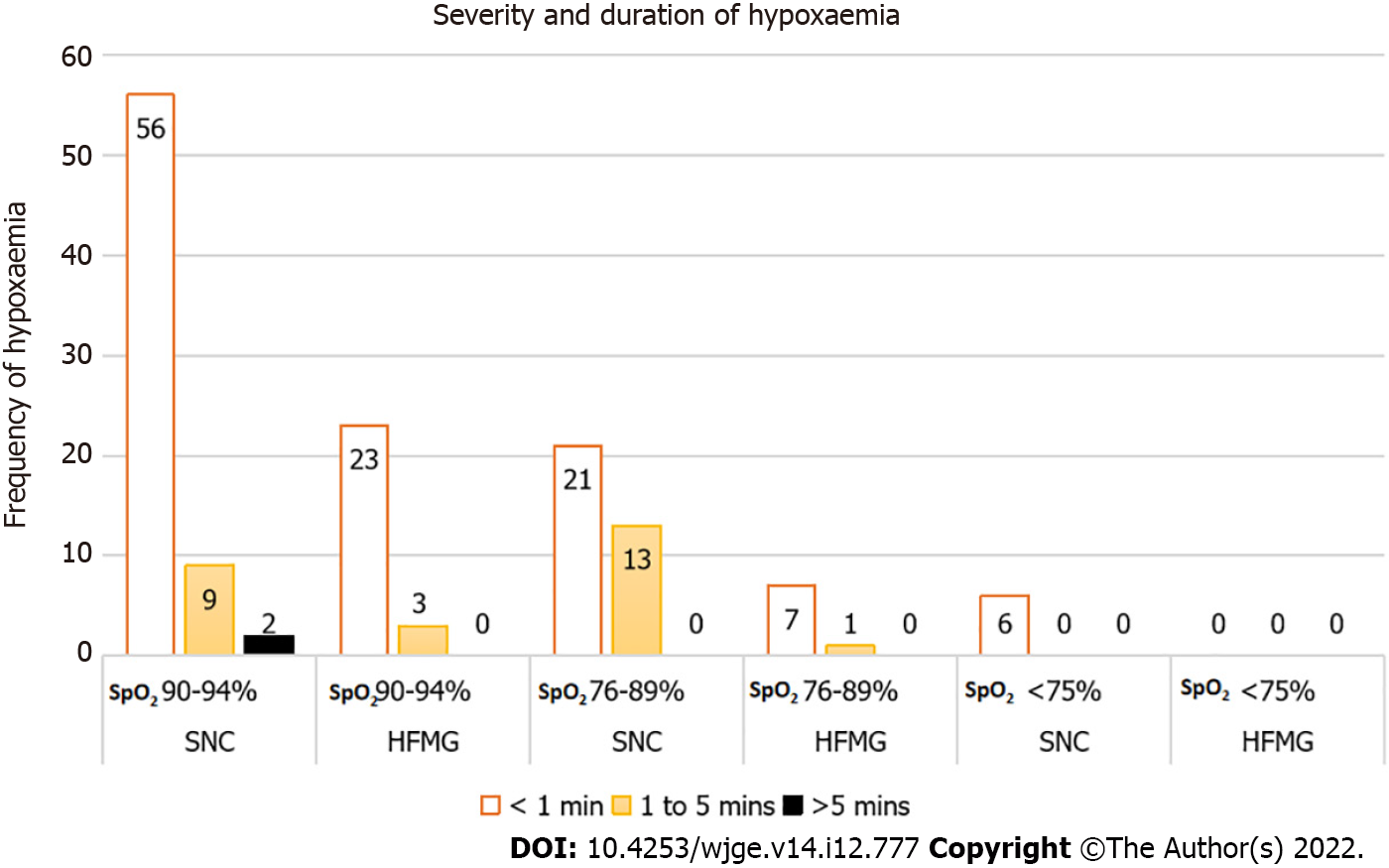
We found a statistically significant difference in the primary outcome of hypoxaemia (SpO2 < 90%) of any duration. Six patients (4.4%) allocated to HFMG experienced at least an episode of hypoxaemia compared to 34 (22.1%) patients allocated to SNC (Table 4). In addition, a statistically significant difference in all secondary outcomes was also observed between the two groups. No episode of severe hypoxaemia (SpO2 ≤ 75%) was observed in the HFMG group (Figure 3).
| End point | SNC (n = 154) | HFMG (n = 138) | P value | |
| Primary endpoint | ||||
| SpO2 < 90% of any duration | 34, 22.1% | 6, 4.4% | < 0.001 | |
| Secondary endpoint | ||||
| Lowest SpO2 (median, IQR) | 95%, 91% to 99% | 98%, 96.5% to 99.5% | < 0.001 | |
| Any episode of hypoxaemia | 74, 48.1% | 26, 18.8% | < 0.001 | |
| SpO2 90%-94% of any duration | 40, 26.0% | 20, 14.5% | 0.015 | |
| SpO2 76%-89% of any duration | 28, 18.2% | 6, 4.3% | < 0.001 | |
| SpO2 ≤ 75% of any duration | 6, 3.9% | 0, 0% | 0.019 | |
| Clinically significant episode of hypoxaemia1 | 32, 20.8% | 1, 0.7% | < 0.001 | |
| SpO2 < 85% of any duration | 19, 12.3% | 3, 2.2% | 0.001 | |
A per-protocol analysis performed for the primary outcome of hypoxaemia still demonstrated a statistically significant difference (P value < 0.001). A subgroup analysis of longer procedures for the primary outcome was performed. However, the number of patients and event rates were too few to provide a meaningful interpretation. Two patients (8.7%) allocated to HFMG, and four patients (23.5%) allocated to SNC experienced an episode of hypoxaemia in procedures longer than 20 min. The majority (68.3%) of procedures longer than 20 minutes were therapeutic, with ERCPs (48.8%) the most common procedure.
A clinically significant episode of hypoxaemia requiring a need to change the flow or route of oxygen delivery was observed in one patient (0.7%) in the HFMG and 32 patients (20.8%) in the SNC group based on an intention-to-treat analysis. This patient allocated to HFMG incorrectly received SNC and required a higher flow of supplemental oxygen to complete their procedure. Only three patients in the SNC group required a change in the method of oxygen delivery. Two of these patients received a short period of bag-valve-mask ventilation, and a third patient received supplemental oxygen via a facemask for a brief period, before completing their upper GI endoscopies on higher flows of supplemental oxygen either via SNC or HFNC. No patients required intubation in the study. With regards to airway manoeuvres, a greater proportion of patients in the SNC group (42.9%) required a chin lift and/or jaw thrust manoeuvres compared to those in the HFMG group (17.4%) (P value < 0.001).
A total of 7 intraprocedural-related adverse events occurred, the endoscope was either withdrawn and re-inserted or the procedure paused in response to an episode of hypoxaemia or as directed by the duty anaesthetist. Only one of these patients was allocated to HFMG. No procedure-related or post-procedure complications were observed in the study. Sedation-related adverse events were infrequent and observed in ten patients (3.4%). These include hypotension, bradycardia, tachycardia, nausea and vomiting. One patient with hypotension in the HFMG group required two doses of 0.5mg dose of metaraminol. In the SNC group, one patient had bradycardia requiring a dose of atropine for bradycardia and two others received rescue antiemetics.
No statistically significant difference in patient-reported symptoms was demonstrated. Patient-reported symptoms forms were completed by 74.3% of patients and no statistically significant difference in response rate was found between the two groups (Table 5).
| Patient-reported outcomes – Likert scale | SNC (n = 154) | HFMG (n = 138) | P value |
| (1 = Very uncomfortable or unbearable, 5 = Very comfortable or not at all) | |||
| Response rate | 115, 74.7% | 102, 73.9% | 0.882 |
| Comfort level ≤ 2 | 4, 3.5% | 5, 4.9% | 0.6 |
| Abdominal pain ≤ 2 | 3, 2.6% | 0, 0.0% | 0.1 |
| Bloating ≤ 2 | 1, 0.9% | 1, 1.0% | 0.932 |
| Mouth dryness ≤ 2 | 2, 1.7% | 1, 1.0% | 0.633 |
| Mouth pain ≤ 2 | 2, 1.7% | 1, 1.0% | 0.633 |
| Headache ≤ 2 | 1, 0.9% | 1, 1.0% | 0.932 |
In this single centre, randomised controlled trial, HFMG at 20 L/min of supplemental oxygen significantly reduced the incidence of hypoxaemia, defined as SpO2 < 90% of any duration, when compared to SNC at 2 L/min of supplemental oxygen in patients undergoing elective upper GI endoscopy under deep sedation. Further, clinically significant hypoxaemia events were significantly reduced in patients assigned to HFMG compared to SNC. No statistically significant difference in patient-rated outcomes was observed between the two groups. To the best of our knowledge, this is the first study comparing the use of supplemental oxygen at 20 L/min via a commercially available mouthguard to 2 L/min via a standard nasal cannula.
Though further studies are required to elucidate the mechanisms by which HFMG reduces the incidence of hypoxaemia in patients undergoing upper GI endoscopy, we postulate that oxygen delivery into the oral cavity has additional benefits. During upper GI endoscopy, an open-mouth respiratory system, the oropharyngeal cavity serves as a large oxygen reservoir.[19] As such, we hypothesize that higher flows delivered into both the nasal and oral cavities result in higher FiO2 delivery, greater physiological dead space washout, and positive end-expiratory pressure similar to that seen in HFNC[1].
Most importantly, we acknowledge the criticisms of choosing an oxygen flow rate of 2 L/min[11]. At the conception of the study, this decision was to allow inferences between HFMG and HFNC based on a recent publication by Lin et al[7]. In our study, of those allocated to HFMG, five patients (3.6%) experienced hypoxaemia and only one patient (0.7%) experienced an episode of severe hypoxaemia, as defined by Lin et al[7], respectively. Compared to HFNC, HFMG offers a relatively inexpensive and simpler method of delivering higher flows of supplemental oxygen. A single-use disposable mouthguard (OxyguardTM) with a rubber strap is approximately 2.33 USD. However, we acknowledge that further comparative studies are required to determine the cost-effectiveness of HFMG in upper GI endoscopy compared to HFNC and other airway devices.
Furthermore, this study has limitations. Firstly, we recognise that this is a single-centre study, and therefore further multicentre trials are required to validate our findings. Secondly, it is unclear whether a lower flow of supplemental oxygen would achieve the same observed benefits, and thus additional studies using different flows through this mouthguard would be warranted. Thirdly, procedures anticipated to be longer than 20 minutes, emergent or combined with a lower GI procedure were excluded. Further studies in these clinical scenarios are required. Finally, an adequate mouth opening is required to accommodate the 60Fr mouthguard. One patient allocated to HFMG did not have sufficient mouth opening which was only evident after randomisation. Although a smaller version of the OxyguardTM is commercially available, this is not available at our centre. Studies using the miniature version of the mouthguard (OxyguardTM mini; North Yorkshire, England) would be required to determine its clinical efficacy.
Concerning the use of pulse oximetry as our primary outcome measure, we appreciate its limitations relative to capnography[20]. Pulse oximetry is routinely used in all patients, and offers an objective and practical outcome measure. A strength of our study is the use of clinically significant hypoxemic events, as this encapsulates the anaesthetist’s clinical assessment and interpretation of an episode of hypoxaemia and thus is a more clinically relevant outcome.
The use of high-flow supplemental oxygen via a mouthguard offers a simple and novel approach to reducing the incidence of hypoxaemia during short upper GI endoscopy in low-risk patients undergoing propofol sedation.
Anaesthetic care during upper gastrointestinal (GI) endoscopy has the unique challenges of balancing adequate patient sedation while maintaining sufficient ventilation and oxygenation via a shared upper airway. Supplementary oxygen during upper GI endoscopy under deep sedation is considered the standard practice to reduce the incidence and severity of hypoxaemia. However, despite this being a recommendation of international society guidelines, the optimal route or rate of oxygen delivery is not known.
Various oxygen delivery devices have been investigated to improve oxygenation during upper GI endoscopy, however, these are limited by commercial availability, costs and in some cases, the expertise required for insertion. Anecdotally at our centre, higher flows of supplemental oxygen can safely be delivered via an oxygenating mouthguard. This oxygenating mouthguard is routinely used during upper GI endoscopic procedures in our practice and as such offers a practical solution to reducing the incidence and severity of hypoxaemia in patients undergoing upper GI endoscopic procedures under deep sedation.
To assess the incidence of hypoxaemia (SpO2 < 90%) in patients undergoing upper GI endoscopy receiving supplemental oxygen using an oxygenating mouthguard at 20 L/min flow compared to standard nasal cannula (SNC) at 2 L/min flow as a proof-of-concept study.
A single centre, prospective, randomised clinical trial at two sites of an Australian tertiary hospital between October 2020 and September 2021 was conducted. Patients undergoing elective upper gastrointestinal endoscopy under deep sedation were randomised to receive supplemental oxygen via high-flow via oxygenating mouthguard (HFMG) at 20 L/min flow or SNC at 2 L/min flow. The primary outcome was the incidence of hypoxaemia of any duration measured by pulse oximetry. Intraprocedural-related, procedural-related, and sedation-related adverse events and patient-reported outcomes were also recorded.
Three hundred patients were randomised. Eight patients were excluded after randomisation. 292 patients were included in the intention-to-treat analysis. The incidence of hypoxemia was significantly reduced in those allocated HFMG. Six patients (4.4%) allocated to HFMG experienced an episode of hypoxaemia, compared to thirty-four (22.1%) patients allocated to SNC (P value < 0.001). No significant difference was observed in the rates of adverse events or patient-reported outcome measures.
The use of HFMG offers a novel approach to reducing the incidence of hypoxaemia during short upper gastrointestinal endoscopic procedures in low-risk patients undergoing deep sedation.
Additional studies using different flows through the oxygenating mouthguard would be warranted to elucidate the mechanisms by which HFMG reduces the incidence of hypoxaemia in patients undergoing upper GI endoscopy. Further comparative studies are required to determine the cost-effectiveness of HFMG in upper GI endoscopy compared to high-flow nasal cannula and other airway devices.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bohara TP; João MPO, Portugal S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Goudra B, Singh PM. Airway Management During Upper GI Endoscopic Procedures: State of the Art Review. Dig Dis Sci. 2017;62:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar Risk of Cardiopulmonary Adverse Events Between Propofol and Traditional Anesthesia for Gastrointestinal Endoscopy: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Bell GD, Bown S, Morden A, Coady T, Logan RF. Prevention of hypoxaemia during upper-gastrointestinal endoscopy by means of oxygen via nasal cannulae. Lancet. 1987;1:1022-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Australian and New Zealand College of Anaesthetists. Professional Document: Guidelines on sedation and/or analgesia for diagnostic and interventional medical or surgical procedures (PS9). Melbourne: Australian and New Zealand College of Anaesthetists, 2014. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Leslie K, Sgroi J. Sedation for gastrointestinal endoscopy in Australia: what is the same and what is different? Curr Opin Anaesthesiol. 2018;31:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Nay MA, Fromont L, Eugene A, Marcueyz JL, Mfam WS, Baert O, Remerand F, Ravry C, Auvet A, Boulain T. High-flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: a multicentre randomised controlled trial (ODEPHI trial). Br J Anaesth. 2021;127:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Lin Y, Zhang X, Li L, Wei M, Zhao B, Wang X, Pan Z, Tian J, Yu W, Su D. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointest Endosc. 2019;90:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | Vargo JJ 2nd. Sedation-related complications in gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 2015;25:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Jurell KR, O'Connor KW, Slack J, Fraiz J, Shaar CJ, Kent L, Callon R. Effect of supplemental oxygen on cardiopulmonary changes during gastrointestinal endoscopy. Gastrointest Endosc. 1994;40:665-670. [PubMed] |
| 10. | Qin Y, Li LZ, Zhang XQ, Wei Y, Wang YL, Wei HF, Wang XR, Yu WF, Su DS. Supraglottic jet oxygenation and ventilation enhances oxygenation during upper gastrointestinal endoscopy in patients sedated with propofol: a randomized multicentre clinical trial. Br J Anaesth. 2017;119:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Goudra B, Gouda G, Singh PM. Recent Developments in Devices Used for Gastrointestinal Endoscopy Sedation. Clin Endosc. 2021;54:182-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Maimone A, Jovine E, D'Imperio N, Zanello M. The Gastro-Laryngeal Tube for interventional endoscopic biliopancreatic procedures in anesthetized patients. Endoscopy. 2012;44:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Brandl S, Borody TJ, Andrews P, Morgan A, Hyland L, Devine M. Oxygenating mouthguard alleviates hypoxia during gastroscopy. Gastrointest Endosc. 1992;38:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Americian Society of Anesthesiologist. ASA Physical Status Classification System. 2022 . [DOI] [Full Text] |
| 15. | Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, Liu PL. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1547] [Cited by in RCA: 1351] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 16. | Riccio CA, Sarmiento S, Minhajuddin A, Nasir D, Fox AA. High-flow vs standard nasal cannula in morbidly obese patients during colonoscopy: A prospective, randomized clinical trial. J Clin Anesth. 2019;54:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 17. | Doulberis M, Sampsonas F, Papaefthymiou A, Karamouzos V, Lagadinou M, Karampitsakos T, Stratakos G, Kuntzen T, Tzouvelekis A. High-flow vs conventional nasal cannula oxygen supplementation therapy and risk of hypoxia in gastrointestinal endoscopies: a systematic review and meta-analysis. Expert Rev Respir Med. 2022;16:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 18. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1847] [Article Influence: 123.1] [Reference Citation Analysis (1)] |
| 19. | Yamamoto N, Miyashita T, Takaki S, Goto T. Effects of Breathing Pattern on Oxygen Delivery Via a Nasal or Pharyngeal Cannula. Respiratory Care 2015; 60(12): 1804-1809. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Chan ED, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med. 2013;107:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |