Published online Oct 16, 2022. doi: 10.4253/wjge.v14.i10.648
Peer-review started: July 17, 2022
First decision: September 5, 2022
Revised: September 5, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: October 16, 2022
Processing time: 86 Days and 13.6 Hours
Infection with Histoplasma capsulatum (H. capsulatum) can lead to disseminated disease involving the gastrointestinal tract presenting as diffuse abdominal pain and diarrhea which may mimic inflammatory bowel disease (IBD).
We report a case of 12-year-old boy with presumptive diagnosis of Crohn disease (CD) that presented with several months of abdominal pain, weight loss and bloody diarrhea. Colonoscopy showed patchy moderate inflammation characterized by erythema and numerous pseudopolyps involving the terminal ileum, cecum, and ascending colon. Histologic sections from the colon biopsy revealed diffuse cellular infiltrate within the lamina propria with scattered histiocytic aggregates, and occasional non-necrotizing granulomas. Grocott-Gomori’s Me
Gastrointestinal involvement with H. capsulatum with no accompanying respiratory symptoms is exceedingly rare and recognition is often delayed due to the overlapping clinical manifestations of IBD. This case illustrates the importance of excluding infectious etiologies in patients with “biopsy-proven” CD prior to initiating immunosuppressive therapies. Communication between clinicians and pathologists is crucial as blood cultures and antigen testing are key studies that should be performed in all suspected cases of histoplasmosis to avoid misdiagnosis and inappropriate treatment.
Core Tip: Impaired cell-mediated immunity is known to increase the risk for disseminated histoplasmosis and has been described in the setting of Crohn disease (CD) treated with immunosuppressant agents. Endoscopically, the appearance of histoplasmosis varies and includes features of inflammatory mucosal changes. Increasing awareness of this condition is critical to avoid misdiagnosis and inappropriate treatment, particularly in the setting of underlying CD. While no specific recommendations are available, immunosuppressive therapy may be safely initiated in some cases when there appears to be effective response to antifungal therapy and the patient can be monitored closely.
- Citation: Miller CQ, Saeed OAM, Collins K. Gastrointestinal histoplasmosis complicating pediatric Crohn disease: A case report and review of literature. World J Gastrointest Endosc 2022; 14(10): 648-656
- URL: https://www.wjgnet.com/1948-5190/full/v14/i10/648.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i10.648
Histoplasmosis is an infection caused by inhalation of spores from the fungus Histoplasma capsulatum (H. capsulatum), found in soil enriched with bird and bat droppings and is endemic to the central and eastern states, prevalent in the Ohio and Mississippi River Valleys[1,2]. Clinical manifestations are typically self-limiting in immunocompetent children, whereas immunocompromised children are likely to present with more severe or disseminated disease and may be indistinguishable from malignancy or tuberculosis[3,4]. Single-organ histoplasmosis is rare, primarily affecting the lungs, occasionally lymph nodes, liver, bone marrow, skin and mucosal membranes[5-8]. While the literature contains many reports of disseminated histoplasmosis reminiscent of Crohn disease (CD) radiographically and endoscopically in immunocompromised patients, there are relatively few reports of symptomatic gastrointestinal histoplasmosis occurring in immunocompetent patients. The most commonly involved sites are the terminal ileum and the colon[9]. We report a case of an immunocompetent pediatric patient presenting with possible disseminated histoplasmosis after presumed initial diagnosis of CD. Early detection is critical to avoid treatment with immunosuppressive therapy and potential complications.
The patient is a 12-year-old boy who presented with several months of abdominal pain, weight loss, and bloody diarrhea.
The patient experienced abdominal pain, weight loss, and bloody diarrhea and was referred for upper and lower GI endoscopy with biopsy.
His medical history was remarkable for several mild and self-limiting respiratory illnesses with non-productive cough. The most recent episode occurred fourteen months prior to his current presentation.
No notable personal or family medical history.
Unremarkable physical examination.
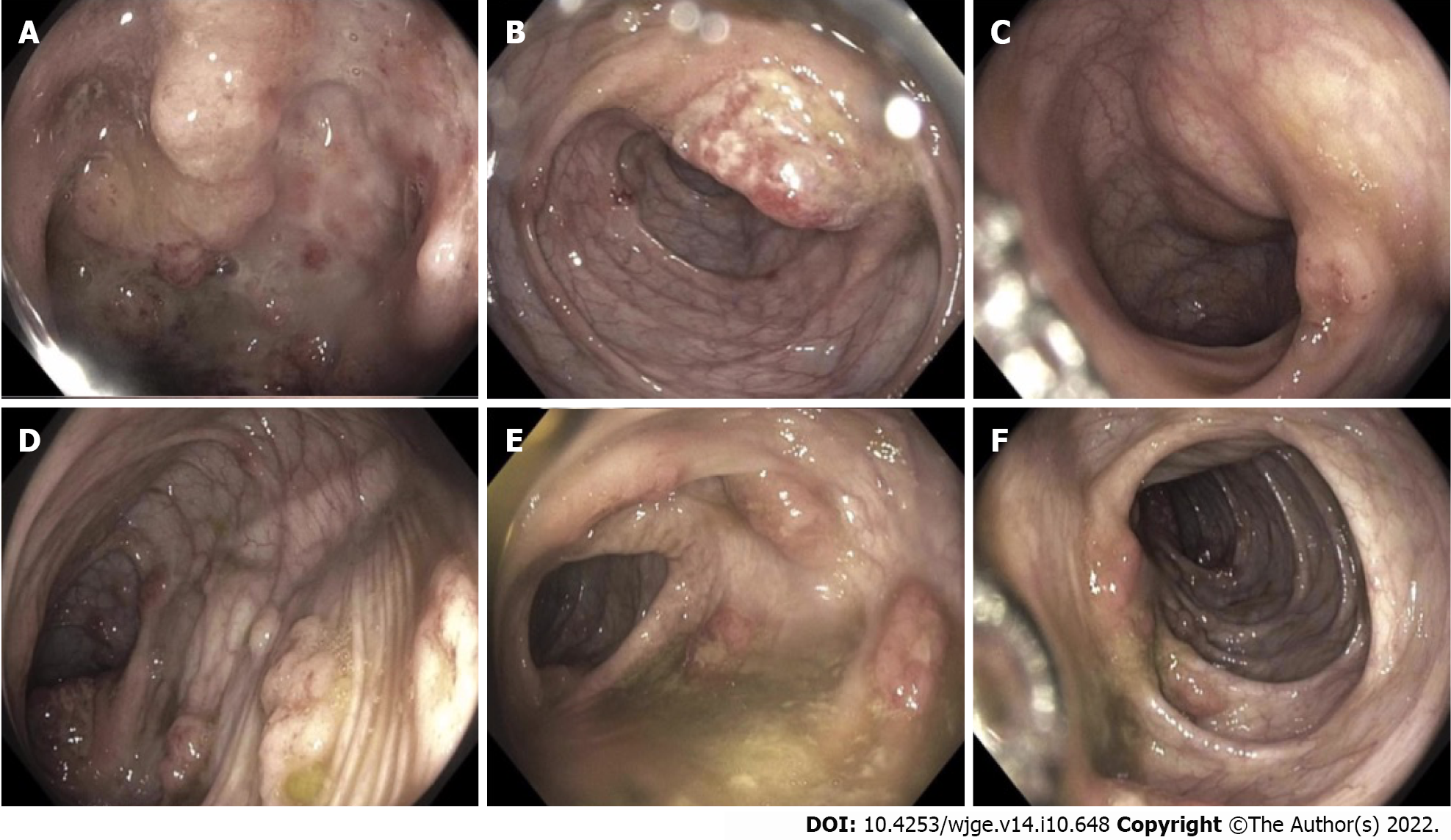
Esophagogastroduodenoscopy was performed and revealed focally ulcerated gastric mucosa and several inflammatory polyps arising within the second and third portions of the duodenum. Colonoscopy revealed patchy moderate inflammation characterized by erythema and numerous pseudopolyps involving the terminal ileum, cecum, and ascending colon (Figure 1). An erythematous region containing shallow ulcers was identified at the hepatic flexure. Multiple biopsies were taken from throughout the colon. A presumptive diagnosis of CD was made, methylprednisolone (40 mg/kg/d, IV) was administered and the patient was then discharged on oral prednisone (40 mg, QD) and oral mesalamine (1000 mg, TID).
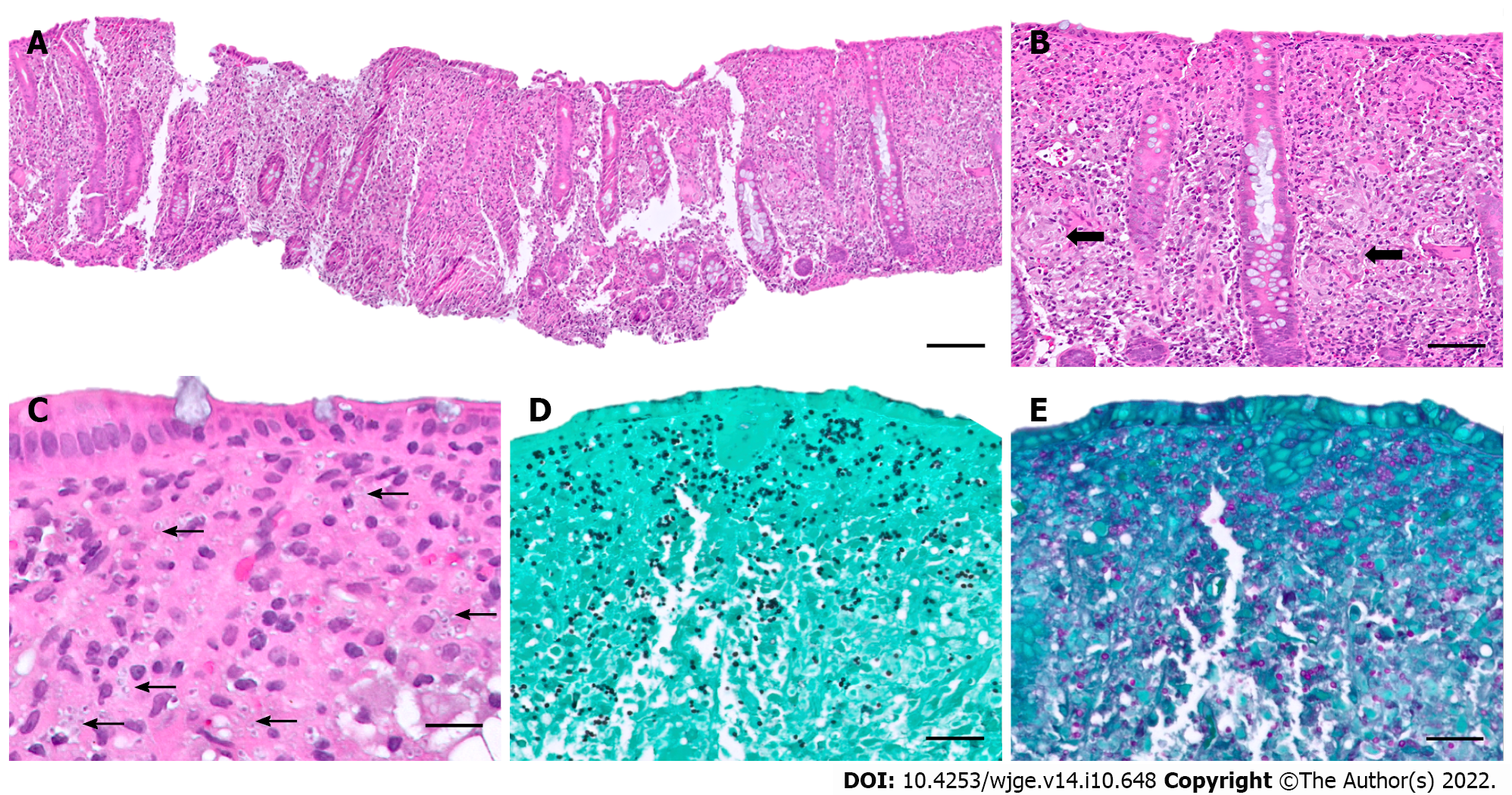
Histologic examination of an H&E-stained colonic biopsy revealed a diffuse cellular infiltrate within the lamina propria with scattered histiocytic aggregates and occasional non-necrotizing granulomas (Figure 2A-C). Grocott-Gomori’s methenamine silver (GMS) and Periodic acid-Schiff stains confirmed the presence of numerous yeast forms morphologically suggestive of H. capsulatum (Figure 2D and E), further confirmed with positive urine Histoplasma antigen (6.58 ng/mL, positive range 0.2-20 ng/mL) and serum immunoglobulin G (IgG) antibodies to Histoplasma (35.9 EU, positive ≥ 10.0 EU).
Given the unusual nature of the histoplasmosis infection, an immunological workup was initiated and revealed profound hypogammaglobulinemia: Serum IgG 94 mg/dL (range 638-1453), IgM 9 mg/dL (range 56-242), and IgA 40 mg/dL (range 45-285) as well as CD8 lymphopenia (253/mm3, range 331-1445). Genetic testing was ordered for inborn error of immunity using Invitae Primary Immunodeficiency Panel and one pathogenic variant was identified in CD40LG c.43del (pThr15Leufs*7), associated with X-linked hyper-IgM syndrome (XHIGM) and two likely pathogenic variants in TNFRSF13B c.310T>C (p.Cys104RG) (homozygous), associated with recessive common variable immunodeficiency (CVID).
Computed tomography (CT) of the chest, abdomen, and pelvis demonstrated a calcified left lower lobe lung nodule with associated hilar lymphadenopathy, diffuse colitis with wall thickening of the distal small bowel through the cecum, abdominal lymphadenopathy, and abnormal-appearing adrenal glands, likely related to disseminated histoplasmosis infection.
Combined with the patient’s medical history, the final diagnosis was isolated gastrointestinal histoplasmosis complicating newly diagnosed, presumed CD.
An induction regimen of liposomal amphotericin was administered (3 mg/kg/d, IV) followed by 1 year of oral itraconazole (200 mg, BID) and treatment with oral mesalamine (1000 mg, TID) to maintain endoscopic remission with plans for endoscopy and colonoscopy in the future after trailing off medication at 6 mo.
Ongoing follow-up is planned for diagnostic evaluation of CD and the treatment plan includes maintaining clinical improvement and Histoplasma antigen clearance. Decisions on whether to initiate treatment for CD are pending as duration of antifungal therapy and safety of immunosuppressive therapy are to be determined. To date, our patient has completed 5 mo of a 12-mo course of antifungal therapy and is maintained on mesalamine until follow-up endoscopy and colonoscopy. The patient’s symptoms have largely resolved and remain stable after 5 mo of follow-up.
Gastrointestinal involvement commonly occurs as part of disseminated histoplasmosis; however isolated colonic involvement with lack of respiratory symptoms is rare[10]. Histoplasmosis can occur at any age. Nonspecific clinical manifestations of gastrointestinal involvement such as abdominal pain, fever, weight loss, and diarrhea are variably present and may only be mild[6,10,11]. Immunocompromised patients are at increased risk of developing disseminated disease and may experience complications such as bleeding or intestinal obstruction more readily than immunocompetent individuals. A high index of suspicion is required for diagnosing histoplasmosis and the gold standard for diagnosis includes isolation of the fungus in blood culture and antigen testing in suspected cases, as utilizing both serum and urine consistently provides the highest sensitivity for detection. Testing for anti-Histoplasma antibodies further increases the sensitivity for diagnosis[12].
The terminal ileum is most commonly involved, presumably because of the lymphoid-rich tissue in this area, but can be found throughout the gastrointestinal tract[9]. The pathologic findings of gastrointestinal histoplasmosis include mucosal ulceration, polypoid lesions, and obstructing masses[6,11,13]. Histologically, tissue shows diffuse expansion of lamina propria and submucosa by macrophages containing intracellular yeast forms[6,10]. As in our case, due to similarities in presentation, pattern of involvement and associated granulomatous inflammation, gastrointestinal histoplasmosis can mimic CD[6,14-17].
To our knowledge, only 7 cases of isolated gastrointestinal histoplasmosis occurring in the pediatric age group (younger than 18 years of age) have been previously reported, mostly from individual case reports (Table 1)[18-22] and one small case series[23]. Ages ranged from 4 to 16 years with a median age of 13 years. Of the previously described cases, the male/female ratio was 5:2. Our patient presented at a slightly younger age than the median (12 years vs 13 years). The most common presenting symptoms included abdominal pain and weight loss, with diarrhea, anorexia, and fever appearing occasionally. Pulmonary symptoms at presentation or during the disease course were not reported in any case. Five patients were presumed immunocompetent[20-22], while two patients were known to have immunocompromising conditions (hyper-IgE syndrome) prior to their presentation[18,19]. One patient with hyper-IgE syndrome was effectively treated seven months prior for cough and fever of unknown origin[19]. As in our case, five patients were given a presumptive diagnosis of CD based on clinical presentation and endoscopic findings[20-23]. A broad range of diagnostic laboratory tests were performed including immunological tests for antigen and/or antibody detection. Microscopic examination revealed the presence of yeast forms (by routine hematoxylin and eosin staining and/or special staining methods) in all cases.
| Ref. | No. of cases | Age/Sex | Clinical presentation | Initial concern | Immune status | Laboratory investigations |
| Soper et al[23], 1970 | 2 | 15/M | Periumbilical pain with radiation to back; prior exposure to Coccidioides and Histoplasma | Presumed CD | Immunocompetent | Histoplasma antibody titers 1:1024 |
| 13/M | Abd pain, bilious vomiting, weight loss, fever; prior exposure to Histoplasma | Presumed CD | Immunocompetent | Not performed | ||
| Alberti-Flor and Granda[18], 1986 | 1 | 16/M | Abd pain, diarrhea, weakness, fever; history of Job syndrome | Presumed CD | Hyper-IgE syndrome | Complement fixation 1:64; yeast antigen 1:8; preciptin (H/M bands), GMS+ yeast forms (resection specimen) |
| Steiner et al[19], 2009 | 1 | 14/F | Fatigue, abd pain, fever, weight loss | Presumed CD | Hyper-IgE syndrome | Urine Histoplasma antigen (8.34 ng/mL), Histoplasma complement fixation titers 1:32 (mycelial phase) 1:64 (yeast phase), preciptin (H/M bands), Yeast forms (terminal ileum, ileocecal valve) |
| Agarwal et al[20], 2015 | 1 | 7/F | Intermittent fever and chills, weight loss | Presumed CD | Immunocompetent | Yeast forms (peripheral blood), GMS/PAS+ yeast forms (bone marrow) |
| Kweyamba et al[21], 2016 | 1 | 4/M | Intermittent vague abd pain, anorexia, occasional vomiting and nausea; obstructing mesenteric chylous cyst | Intestinal obstruction | Immunocompetent | PAS+ yeast forms (cyst lining) |
| Acharyya et al[22], 2021 | 1 | 8/M | Colicky abd pain, weight loss, constipation, subsequent ileal stricture | Presumed intestinal tuberculsosis, unresponsive to antitubercular medication × 9 mo | Immunocompetent | GMS+ yeast forms (ileum, mesenteric nodes) |
| Current case, 2022 | 1 | 12/M | Abdominal pain × several months, weight loss, bloody diarrhea | Presumed CD | Immunocompetent | GMS+ yeast forms (colon) |
In our present case, the patient presented with gastrointestinal symptoms alone and endoscopic findings suggestive for CD and was started on corticosteroids and subsequently mesalamine. An interesting feature of our case is that while the gastrointestinal tract was the only site of symptomatic disease, it is unlikely to be the primary focus of infection. It is more likely that after inhalation of the fungus, dissemination by the bloodstream occurred before an immune response was mounted with some unidentifiable factor favoring persistence in the gastrointestinal tract exclusively. After additional workup, the patient was identified as more susceptible to histoplasmosis because of the dysregulation of cell-mediated immunity associated with his XHIGM and CVID, as suggested by his immunological testing results. Distinction of these entities is vital as the optimal treatment for one disease could lead to exacerbation of the other. A list of infectious diseases that should be excluded in patients diagnosed as inflammatory bowel disease (IBD) is provided in Table 2.
| Infectious etiology | Gastrointestinal site | Routine stain | Ancillary stain(s) |
| Bacterial | |||
| E. coli, O157-H7[24] | Colon | H&E stain | Gram stain |
| Shigella spp.[25] | Colon | ||
| Salmonella spp.[26] | Colon, terminal ileum | ||
| Campylobacter spp.[27] | Colon, terminal ileum | ||
| Yersinia enterocolitica[28] | Colon, terminal ileum | ||
| Clostridiodes difficle[29] | Colon | ||
| Nesisseria gonorrhoeae[30] | Colorectal | ||
| Treponema pallidum[31] | Colorectal | ||
| Chlamydia trachomatis[32] | Colorectal | ||
| Aeromonas spp.[33] | Colon | ||
| Mycobacterial tuberculosis[34] | Gastrointestinal tract, mostly terminal ileum | Gram stain | |
| Acid-fast stain (Ziehl-Neelsen or Kinyoun) | |||
| Fungal | |||
| Cryptococcus spp.[35] | Terminal ileum | H&E stain | GMS stain |
| Histoplasma capsulatum[36] | Terminal ileum | PAS stain | |
| Coccidioides spp.[37] | Colon | ||
| Paracoccidioides spp.[38] | Colorectal | ||
| Viral | |||
| Cytomegalovirus[39] | Jejunoileal | H&E stain | CMV immunostain |
| Herpes simplex virus[40] | Colorectal | HSV I/II immunostain | |
| Parasite | |||
| Entamoeba histolytica[41] | Colon | H&E stain | Giemsa stain |
| Enterobius vermicularis[42] | Colorectal | Serology | |
| Taenia saginata[43] | Ileum | Stool examination | |
| Strongyloides stercoralis[44] | Colon | ||
| Anisakis spp.[45] | Ileum | ||
| Hookworm (Ancylostoma duodenale, Necator americanus)[46] | Jejunoileal | ||
Gastrointestinal involvement with H. capsulatum in the absence of pulmonary manifestations is exceedingly rare and may lead to delay in recognition due to overlapping symptoms with IBD. This case highlights the importance of excluding infectious etiologies in patients with “biopsy-proven” CD prior to initiating immunosuppressive therapies, especially in the setting of recent travel or exposure in an endemic area. Communication between clinicians and pathologists is crucial as tests for Histoplasma antigen in urine or serum should be performed once histoplasmosis is suspected.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bao CH, China; Sitkin S, Russia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Goodwin RA Jr, Des Prez RM. State of the art: histoplasmosis. Am Rev Respir Dis. 1978;117:929-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 2. | Goodwin RA, Loyd JE, Des Prez RM. Histoplasmosis in normal hosts. Medicine (Baltimore). 1981;60:231-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 170] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Liu B, Qu L, Zhu J, Yang Z, Yan S. Histoplasmosis mimicking metastatic spinal tumour. J Int Med Res. 2017;45:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Kabangila R, Semvua K, Rambau P, Jackson K, Mshana SE, Jaka H, Peck RN. Pulmonary histoplasmosis presenting as chronic productive cough, fever, and massive unilateral consolidation in a 15-year-old immune-competent boy: a case report. J Med Case Rep. 2011;5:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 767] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 6. | Goodwin RA Jr, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore). 1980;59:1-33. [PubMed] |
| 7. | Reddy P, Gorelick DF, Brasher CA, Larsh H. Progressive disseminated histoplasmosis as seen in adults. Am J Med. 1970;48:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Smith JW, Utz JP. Progressive disseminated histoplasmosis. A prospective study of 26 patients. Ann Intern Med. 1972;76:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 100] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Lamps LW, Molina CP, West AB, Haggitt RC, Scott MA. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am J Clin Pathol. 2000;113:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kahi CJ, Wheat LJ, Allen SD, Sarosi GA. Gastrointestinal histoplasmosis. Am J Gastroenterol. 2005;100:220-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Psarros G, Kauffman CA. Colonic histoplasmosis: a difficult diagnostic problem. Gastroenterol Hepatol (N Y). 2007;3:461-463. [PubMed] |
| 12. | Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, Hammoud K, Alapat D, Babady NE, Parker M, Fuller D, Noor A, Davis TE, Rodgers M, Connolly PA, El Haddad B, Wheat LJ. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 13. | Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore). 2007;86:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Xi L, Qin X, Song Y, Han J, Li Z, Zhang J. Gut Microbial Alterations in Diarrheal Baer's Pochards (Aythya baeri). Front Vet Sci. 2021;8:756486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Turner D, Griffiths AM. Esophageal, gastric, and duodenal manifestations of IBD and the role of upper endoscopy in IBD diagnosis. Curr Gastroenterol Rep. 2007;9:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Kuriyama M, Kato J, Morimoto N, Fujimoto T, Okada H, Yamamoto K. Specific gastroduodenoscopic findings in Crohn's disease: Comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig Liver Dis. 2008;40:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Mekhjian HS, Switz DM, Melnyk CS, Rankin GB, Brooks RK. Clinical features and natural history of Crohn's disease. Gastroenterology. 1979;77:898-906. [PubMed] |
| 18. | Alberti-Flor JJ, Granda A. Ileocecal histoplasmosis mimicking Crohn's disease in a patient with Job's syndrome. Digestion. 1986;33:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Steiner SJ, Kleiman MB, Corkins MR, Christenson JC, Wheat LJ. Ileocecal histoplasmosis simulating Crohn disease in a patient with hyperimmunoglobulin E syndrome. Pediatr Infect Dis J. 2009;28:744-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Agarwal P, Capoor MR, Singh M, Gupta A, Chhakchhuak A, Debatta P. An Unusual Presentation of Disseminated Histoplasmosis: Case Report and Review of Pediatric Immunocompetent Patients from India. Mycopathologia. 2015;180:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Kweyamba V, Apiyo M, Olika B, Kituuka O. A Case of a 4-Year-Old Boy with a Mesenteric Chylous Cyst Infected with Histoplasma capsulatum. Case Rep Surg. 2016;2016:4296059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Acharyya BC, Acharyya S, Chakrabarty H. Isolated gastrointestinal histoplasmosis: A rare diagnosis of pediatric chronic abdominal pain. Indian J Pathol Microbiol. 2021;64:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Soper RT, Silber DL, Holcomb GW Jr. Gastrointestinal histoplasmosis in children. J Pediatr Surg. 1970;5:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Ilnyckyj A, Greenberg H, Bernstein CN. Escherichia coli O157:H7 infection mimicking Crohn's disease. Gastroenterology. 1997;112:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Lin WC, Chang CW, Chen MJ, Chu CH, Shih SC, Hsu TC, Wang HY. Challenges in the diagnosis of ulcerative colitis with concomitant bacterial infections and chronic infectious colitis. PLoS One. 2017;12:e0189377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Friesen C, Hill I, Woods C. Salmonella gastroenteritis mimicking onset of inflammatory bowel disease in children. J Pediatr Gastroenterol Nutr. 2008;46:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Cohen GS, Prasad M. Campylobacter Pouchitis Mimicking the Appearance of Crohn's Disease. Case Rep Gastrointest Med. 2016;2016:5254914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Naddei R, Martinelli M, Strisciuglio C, DʼArmiento M, Vollaro A, Staiano A, Miele E. Yersinia Enterocolitica Ileitis Mimicking Pediatric Crohn's Disease. Inflamm Bowel Dis. 2017;23:E15-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1432-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Arnold CA, Roth R, Arsenescu R, Harzman A, Lam-Himlin DM, Limketkai BN, Montgomery EA, Voltaggio L. Sexually transmitted infectious colitis vs inflammatory bowel disease: distinguishing features from a case-controlled study. Am J Clin Pathol. 2015;144:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Yilmaz M, Memisoglu R, Aydin S, Tabak O, Mete B, Memisoglu N, Tabak F. Anorectal syphilis mimicking Crohn's disease. J Infect Chemother. 2011;17:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Gallegos M, Bradly D, Jakate S, Keshavarzian A. Lymphogranuloma venereum proctosigmoiditis is a mimicker of inflammatory bowel disease. World J Gastroenterol. 2012;18:3317-3321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 33. | van der Gaag EJ, Roelofsen E, Tummers RF. [Aeromonas caviae infection mimicking inflammatory bowel disease in a child]. Ned Tijdschr Geneeskd. 2005;149:712-714. [PubMed] |
| 34. | Rafael MA, Martins Figueiredo L, Oliveira AM, Nuno Costa M, Theias Manso R, Martins A. Gastrointestinal Tuberculosis Mimicking Crohn's Disease. GE Port J Gastroenterol. 2020;27:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Chavapradit N, Angkasekwinai N. Disseminated cryptococcosis in Crohn's disease: a case report. BMC Infect Dis. 2018;18:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Ahmed A, Homsi N, Kapila R. Crohn's disease or histoplasmosis? Med Mycol Case Rep. 2020;30:8-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Mitter SS, Derhovanessian A, Hillman JD, Uslan DZ. Disseminated coccidioidomycosis in a patient managed with adalimumab for Crohn's disease. Nat Rev Gastroenterol Hepatol. 2010;7:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Lomazi EA, de Negreiros LMV, Magalhães PVVS, Togni RCS, de Paiva NM, Ribeiro AF, Leal RF. Intestinal paracoccidioidomycosis resembling Crohn's disease in a teenager: a case report. J Med Case Rep. 2018;12:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Khan FN, Prasad V, Klein MD. Cytomegalovirus enteritis mimicking Crohn's disease in a lupus nephritis patient: a case report. World J Gastroenterol. 2009;15:4327-4330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Sandgren KE, Price NB, Bishop WP, McCarthy PJ. Herpes Simplex Proctitis Mimicking Inflammatory Bowel Disease in a Teenaged Male. Case Rep Pediatr. 2017;2017:3547230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Cheng CW, Feng CM, Chua CS. Cecal amebiasis mimicking inflammatory bowel disease. J Int Med Res. 2020;48:300060520922379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Johansson J, Ignatova S, Ekstedt M. Pinworm infestation mimicking crohns' disease. Case Rep Gastrointest Med. 2013;2013:706197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Nussinson E, Yair-Sabag S, Shibli F. Detection of Taenia saginata infection mimicking Crohn's disease using video capsule endoscopy. Clin Case Rep. 2018;6:741-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 44. | Yoon J, Kaur M. Strongyloides stercoralis infection mimicking Crohn’s disease [Abstract]. Am J Gastroenterol. 2013;108:S429-S430. |
| 45. | Montalto M, Miele L, Marcheggiano A, Santoro L, Curigliano V, Vastola M, Gasbarrini G. Anisakis infestation: a case of acute abdomen mimicking Crohn's disease and eosinophilic gastroenteritis. Dig Liver Dis. 2005;37:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Aktay AN. Hookworm infection mimicking Crohn’s disease: diagnosed with wireless capsule endoscopy. Ann Pediatr Child Health. 2015;3:1083. |
| 47. | Shojaei E, Walsh JC, Sangle N, Yan B, Silverman MS, Hosseini-Moghaddam SM. Gastrointestinal Histoplasmosis Mimicking Crohn's Disease. Open Forum Infect Dis. 2021;8:ofab249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |