Published online Jan 16, 2022. doi: 10.4253/wjge.v14.i1.17
Peer-review started: January 8, 2021
First decision: October 17, 2021
Revised: October 31, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 16, 2022
Processing time: 370 Days and 4.3 Hours
Endoscopic cryotherapy is a technique utilized for the ablation of target tissue within the gastrointestinal tract. A cryotherapy system utilizes the endoscopic application of cryogen such as liquid nitrogen, carbon dioxide or liquid nitrous oxide. This leads to disruption of cell membranes, apoptosis, and thrombosis of local blood vessels within the target tissue. Several trials utilizing cryotherapy for Barrett’s esophagus (BE) with variable dysplasia, gastric antral vascular ectasia (GAVE), esophageal carcinoma, radiation proctitis, and metastatic esophageal carcinomas have shown safety and efficacy. More recently, liquid nitrogen cryotherapy (cryodilation) was shown to be safe and effective for the treatment of a benign esophageal stricture which was refractory to dilations, steroid injections, and stenting. Moreover, liquid nitrogen cryotherapy is associated with less post procedure pain as compared to radiofrequency ablation in BE with comparable ablation rates. In patients with GAVE, cryotherapy was found to be less tedious as compared to argon plasma coagulation. Adverse events from cryotherapy most commonly include chest pain, esophageal strictures, and bleeding. Gastric perforations did occur as well, but less often. In summary, endoscopic cryotherapy is a promising and growing field, which was first demonstrated in BE, but the use now spans for several other disease processes. Larger randomized controlled trials are needed before its role can be established for these different diseases.
Core Tip: Cryotherapy involves freeze-thaw cycles of tissue to eradicate problematic lesions such as Barrett’s esophagus with variable dysplasia, gastric antral vascular ectasia, radiation proctitis, esophageal carcinomas and metastatic esophageal carcinomas. Two of the most used cryotherapy systems involve liquid nitrogen and carbon dioxide. Cryoballoon focal ablation system is another system, but not widely available. Cryotherapy systems have shown efficacy for these conditions even in patients who were refractory to the current standards of care.
- Citation: Dhaliwal A, Saghir SM, Mashiana HS, Braseth A, Dhindsa BS, Ramai D, Taunk P, Gomez-Esquivel R, Dam A, Klapman J, Adler DG. Endoscopic cryotherapy: Indications, techniques, and outcomes involving the gastrointestinal tract. World J Gastrointest Endosc 2022; 14(1): 17-28
- URL: https://www.wjgnet.com/1948-5190/full/v14/i1/17.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i1.17
Endoscopic cryotherapy is a technique utilized for the ablation of target tissue within the gastrointestinal (GI) tract. A cryotherapy system utilizes the endoscopic app
Cryotherapy achieves tissue destruction via two mechanisms, which include both immediate and delayed effects, while simultaneously preserving the cryo-resistant structures. The initial effect of cryotherapy is the formation of ice crystals by freezing the intracellular and extracellular water in the tissues. The ice crystals lead to the disruption of the cell membranes and protein denaturation. This creates an osmotic gradient, which draws water from the intracellular compartment leading to the cell dehydration and destruction[1-3]. The degree of cell death is similar to other modalities which are heat based like radiofrequency ablation (RFA) or argon plasma coagulation (APC) but this method preserves the architecture of the underlying tissue and the extracellular matrix which reduces scarring[1]. Cellular death of peripheral tissues that does not occur from direct injury by cryoablation may eventually die via apoptosis, caused by activation of cytochrome C due to the mitochondrial injury[4,5].
The thawing process follows the initial freezing mechanism[6]. During this phase, there is fusion of intracellular ice crystals, with the maximum effect occurring at -20-degrees-C to –50-degrees-C, which further damages the cell membranes. In addition, there is an indirect injury to the vascular endothelium via the fusion of ice crystals resulting in tissue necrosis and ischemia, due to the platelet aggregation, thrombus formation and regional hyperemia[7-9]. The risk of perforation in cryotherapy is decreased as collagen and elastin fibers are cryo-resistant as compared to the epithelial cells[10,11].
Currently, the two types of endoscopic cryotherapy methods which are commercially available include liquid nitrogen cryotherapy and carbon dioxide (CO2) cryotherapy.
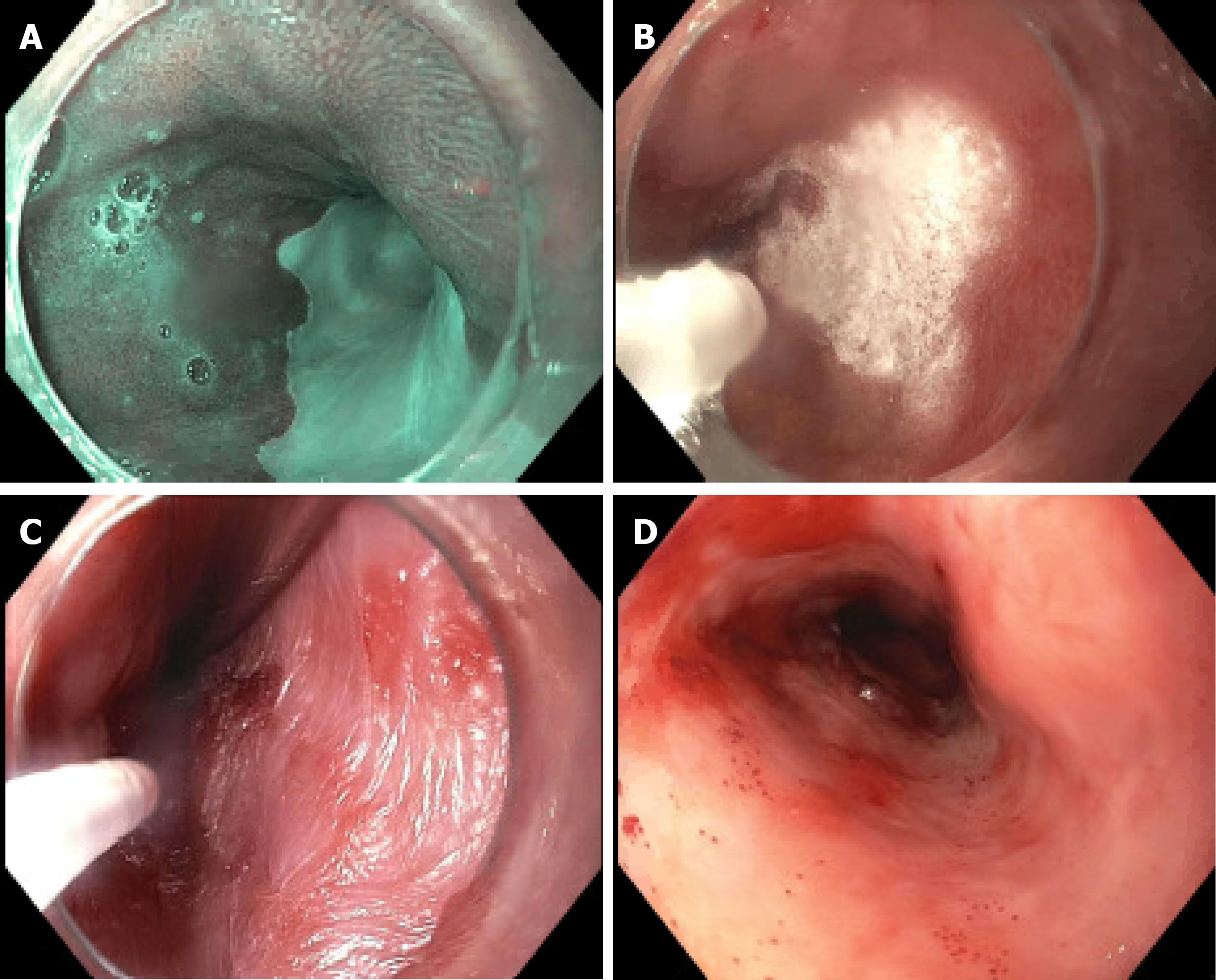
In this technique, a contact-free low-pressure spray of liquid nitrogen is delivered through a 7F catheter and reaches a temperature of -196-degrees-C, which freezes the GI mucosa (Figure 1). During this process, the catheter and the endoscope experience a rapid drop in temperature and become less compliant, which makes it difficult to operate the endoscope and/or move the catheter in the biopsy channel[2,12]. As nitrogen gas expands at room temperature, it leads to rapid cooling due to the Joule-Thompson effect (rapid expansion of a gas leading to a change in temperature of a gas). To warm the cryoprobe, the depressurized gas can be vented out and a heating circuit within in the catheter is necessary to maintain pliability of the device[2,13].
Prior to liquid nitrogen cryotherapy, a 20 F dual-channel decompression oral-gastric tube is placed to allow for both active and passive gas venting to reduce the risk of GI perforation[14]. This is utilized because after the liquid nitrogen spray freezes the tissue, the warmth transforms it into nitrogen gas, which expands at a rate of 6-8 L in a 20 s liquid nitrogen spray[15]. During the procedure, the abdomen is frequently examined by palpation, usually by an assistant, to ensure adequate decompression and to alert staff if distention is recognized[16].
In this technique, a compressed CO2 gas spray is applied through a catheter with a 0.005-inch diameter tip opening. The CO2 gas reaches a temperature of -78-degrees-C and is delivered at a rate of 6-8 L/min at a pressure of 450-750 psi[14]. A suction cap is placed on the distal end of the endoscope which is connected to the CO2 evacuation system, and this allows venting of the CO2 gas build up to avoid distention[14,17]. The CO2 gas is vented simultaneously as cryotherapy is being delivered. Unlike liquid nitrogen cryotherapy, a heating circuit is not necessary since the endoscope and the catheter delivering the CO2 gas are not at risk of freezing[12].
Differences between liquid nitrogen cryotherapy and CO2 based cryotherapy: Several differences exist between liquid nitrogen cryotherapy and CO2 cryotherapy systems aside from the type of gases and temperatures utilized. Both systems can cause abdominal distension as the cryogen changes to a gaseous state, however, this is less problematic with the CO2-based system because of a low-profile catheter which evacuates the excess CO2. Both systems have issues with fogging of the endoscope lens, which compromises visualization. The CO2 based system is comparatively cheaper and can be stored at room temperature as compared to the liquid nitrogen system, which requires storage in expensive containers to maintain a temperature between 195.8-210-degrees-C[12,18].
Duration and dosage of cryotherapy: Cryotherapy involves two stepwise processes: Freezing and thawing, often performed in cycles. The amount of tissue injury caused by cryotherapy depends on the rate and duration of cooling, the number of freeze-thaw cycles, and the distance from the target tissue to the origin of the spray. A critical limitation of cryotherapy is that dosimetry data for this technology is lacking, and is, for all intents and purposes, largely unknown. Initial dosing regimens on BE patients consisted of 3 cycles of 20 s each, which was changed to 4 cycles of 10 s each after over distention in a Marfans syndrome patient led to a gastric perforation. The clinical experience suggests that freeze times of 10-15 s may be efficacious for short term in ablation of BE[19].
In a study performed in a porcine animal model, the CO2 system demonstrated a dose-dependent effect on tissue damage based on seconds of CO2 spray. A 15 s spray caused minimal necrosis, a 30 s spray caused damage to the submucosa and a 120 s spray caused damage to the muscularis propria[20]. In another porcine study, liquid nitrogen was sprayed for 10-60 s and did not appear to show a dose-dependent effect on tissue[21]. This emphasizes how poorly the technology is understood.
Despite our poor understanding of dosimetry, the varying doses of cryotherapy used to date have shown efficacy with an acceptable safety profile in clinical settings. It is believed that longer freeze times maybe needed for the palliative treatment of esophageal cancer. There is limited data describing the clinical outcomes to compare the various freeze durations and number of freeze-thaw cycles[19].
BE, first described in 1950 by Dr. Norman Barrett, a British thoracic surgeon, refers to replacement of normal squamous epithelium of the esophagus by columnar epi
BE is traditionally classified based on endoscopic length of salmon colored mucosa, as long segment BE (LSBE > 3 cm) or short segment BE (SSBE < 3 cm). However, the diagnosis of BE needs histological correlation in addition to endoscopic appearance, which takes into account replacement of esophageal squamous epithelium by columnar epithelium along with presence of goblet cells, a marker of intestinal me
Endoscopic ablative techniques remain the treatment of choice for BE patients with dysplasia and/or early esophageal cancer without lymphatic spread[24]. The available endoscopic ablative techniques include RFA, photodynamic therapy and cryotherapy. RFA combined with endoscopic mucosal resection (EMR) has become the standard treatment for BE because of its demonstrated efficacy, cost effectiveness, and better side effect profile[25]. For limited surface areas, APC and bipolar probes are a less expensive alternative compared to cryotherapy. However, these procedures may have higher BE recurrence rates[13,26,27].
Liquid nitrogen cryotherapy in BE: A pilot study of liquid nitrogen cryotherapy published in 2005 reporting on only 11 patients with BE and variable dysplasia achieved complete endoscopic and histologic eradication in 82% of patients[28]. A subsequent multi-center study of 77 patients utilizing liquid nitrogen cryotherapy therapy for BE high grade dysplasia (HGD), BE dysplasia, and BE IM achieved complete eradication at rates of 94%, 88% and 53%, respectively. Additionally, complete remission of intramucosal cancer and carcinoma was seen in all 7 patients. The most common adverse event (AE) was chest pain at 17.6%. Three patients developed a stricture which was successfully managed endoscopically with dilation. Gastric distention from liquid nitrogen therapy led to a perforation in a patient with Marfan’s syndrome[29].
A recent study by Ramay et al[30] looked at the efficacy of liquid nitrogen cryotherapy on BE-HGD and intramucosal adenocarcinoma (IMC). This study included 50 patients who were analyzed over 3 years and 40 patients who were analyzed over 5 years. The initial rates of complete remission of HGD, dysplasia, and IM were 98%, 90%, and 60% and were found to be comparable at 3 and 5 years. Incidence rates of recurrent IM, dysplasia, and HGD/EAC on follow-up after initial complete eradication of IM were 12.2%, 4.0%, and 1.4% per person-year for the 5-year cohort.
Cryotherapy ablation compared against RFA for BE: A recently published non-inferiority trial comparing RFA with liquid nitrogen cryotherapy in 31 patients with HGD and early adenocarcinoma found similar results between the two groups. Complete remission of BE in patients undergoing RFA vs liquid nitrogen was 21% vs 12%, respectively. Pain scores were significantly lower in the liquid nitrogen cryotherapy group as compared to the RFA group. There was no major procedure related AEs. These results are preliminary as we are awaiting results of the complete trial[31]. Similar findings were demonstrated in a different study regarding lower post procedure pain scores in those undergoing liquid nitrogen cryotherapy as compared to RFA[32].
A retrospective study with 154 patients were treated for Barrett’s dysplasia, IM or HGD with either RFA or liquid nitrogen cryotherapy. Complete remission of HGD was comparable between both groups at 88%. Complete remission of IM was more successful in RFA vs cryotherapy (67% vs 41%) and statistically significant. Complete remission of dysplasia was also comparable between RFA vs cryotherapy (88% vs 79%)[33]. Similar results were also seen in a recent retrospective study by Fasullo et al[34] which included 100 patients in the RFA group and 62 patients in the liquid nitrogen cryotherapy group.
Cryotherapy has several potential advantages over RFA, which include fewer complications (pain, stricture), cost effectiveness and a no contact technique. Disadvantages of cryotherapy include the following: abdominal distention due to gas, difficulty in visualization during the endoscopic procedure due to freezing of tissue and barotrauma, poor dosimetry, and limited outcome data compared to RFA.
CO2 cryotherapy in BE: Data to establish the durability of CO2 cryotherapy as a treatment for BE is limited. In a single center study of 64 patients with BE reported complete remission of IMC, HGD and IM in 77%, 94% and 55% of patients, res
According to a small single center prospective case series of 10 patients, a negative experience led to an early termination of a study due to an insufficient effect of CO2 cryoablation in BE and early neoplasia. Most patients underwent EMR prior to cryotherapy. Complete remission of IM and dysplasia in 9 patients was reported to be 11% and 44% at the 6 mo follow up, respectively. Two noteworthy AEs included gastric perforation and esophageal laceration[36].
Cryoballoon focal ablation system using nitrous oxide for BE: A cryoballoon-based system is the most recent developed endoscopic cryotherapy system and ablates mucosa via direct contact of an inflated balloon tip catheter filled with nitrous oxide[14,37]. The balloon reaches temperatures close to -80-degrees-C[38]. The device has been slow to achieve widespread commercial release.
In a study published the same year by Sawas et al[39], 42 patients underwent cryoballoon focal ablation system (CbFAS) of which 37 had unsuccessful prior BE treatments indicating a more challenging cohort. Complete remission of dysplasia and IM were achieved in 54.8% and 9.5% of patients over a mean follow up period of 7.5 ± 5.7 mo.
A multicenter non-randomized comparative study of 46 patients utilizing CbFAS vs RFA showed comparable outcomes (88% vs 90%) for SSBE regression. There were 20 patients in the CbFAS group and 26 in the RFA group. Peak pain and duration of pain was reported to be significantly lower in the CbFAS group[40].
Canto et al[41] recently published a large multicenter trial on 120 patients of which 45% had previously received EMR for BE. The rates of complete remission of dysplasia and IM rates in 94 patients who have completed 12 mo of follow up are 97% and 91%, respectively. Fifteen patients developed strictures, which were treated with dilation. Three other patients developed serious AEs: 1 perforation after stricture dilation, 1 deep laceration after dilation, and 1 upper GI bleed. So far BE has not been seen on follow up biopsies post CbFAS. This is the largest trial to date representing the efficacy of CbFAS for BE.
Outcomes regarding this technique are variable and require confirmation by further studies. There are a few clinical trials being conducted for CbFAS effect on BE and we await their results.
CbFAS compared to liquid nitrogen cryotherapy for BE: Recently, a retrospective study compared cryoballoon therapy to liquid nitrogen cryospray. Forty-six patients were treated with CbFAS and 25 were treated with liquid nitrogen cryospray. They reported the complete eradication rates of dysplasia and IM to be comparable at 95.6% vs 96% and 84.75% vs 80% in the cryoballoon group vs liquid nitrogen cryospray group, respectively. Strictures were reported in 4 of the cryoballoon patients and 3 of the cryospray patients, which were treated with dilation. The authors reported cryoballoon to be more convenient since it uses cartridges prefilled with nitrous oxide as compared to handling a large nitrogen tank. In instances where patients had a large hiatal hernia, needed to be treated in a retroflexed position, or required a large surface area to be targeted, liquid nitrogen cryospray was used instead[37].
Gastric antral vascular ectasia (GAVE), also known as ‘watermelon stomach’, is an uncommon cause of GI bleeding but can often cause clinically significant chronic and severe bleeding. The prevalence of GAVE is estimated to be 0.3% in a large endoscopic series and 4% in highly selected cohorts for obscure GI bleeding. It is often misdiagnosed as antral gastritis and can be difficult to differentiate from portal hypertensive gastropathy[42,43]. Majority of patients with GAVE become transfusion dependent despite iron supplementation[43,44]. The best approach for the treatment has not yet been identified but the standard treatment in most countries is endoscopy based. APC has been a preferred treatment, however, can be very labor intensive due to the large surface area covered and multiple sessions required. Moreover, patients can develop recurrence overtime and may become transfusion dependent[45]. Cryotherapy is another intervention that has been utilized for GAVE, but the data is limited.
The etiology of GAVE is poorly understood however the histopathology demo
CO2 based cryotherapy for GAVE: In a single center pilot study by Cho et al[47], 12 patients with GAVE received 36 CO2 based cryotherapy treatments with complete response in 50% and partial response in 50%. Eight patients in this cohort had prior unsuccessful APC treatments of which 6 had complete response after CO2 based cryotherapy. There were no immediate cryotherapy related complications. Some late complications seen on follow up endoscopy included bleeding from a disrupted Schatzki’s ring and minor scarring/ulceration in the gastric antrum.
CbFAS with nitrous oxide for GAVE: In a pilot study of 7 patients, complete era
Cryotherapy for GAVE has seemingly promising results but has limited data and requires further investigation with larger trials. One major advantage of cryotherapy in comparison to APC is that it can treat larger surface areas in a shorter amount of time.
One of the most frequent complications after radiation therapy for pelvic malignancies is radiation proctitis[50,51]. The consensus has been that the incidence is related to the dose of radiation, exposure area, delivery method and the use of cytoprotective agents. The dose for most treatments is 45-50 Gy and up to 90 Gy. Complications are less for doses from 45-70 Gy, but doses above 70 Gy cause significant long-standing damage. Depending on the type of radiation therapy used, the incidence for proctitis varies from 1% to as high as 39%[50].
Radiation proctitis can be acute or chronic. Acute proctitis is an inflammatory process occurring within 3 mo of the initial therapy and is usually self-limiting after the radiation treatment has stopped. The treatment of acute proctitis is generally supportive with hydration, anti-diarrheal, steroids or 5-aminosalicylic acid enemas. Chronic proctitis on the other hand, can start during the acute phase of radiation but symptoms do not become obvious until the treatment is stopped around a median of 8-12 mo[52,53]. The treatment for chronic proctitis involves non-invasive methods such as anti-inflammatory agents, sucralfate, short-chain fatty acids, hyperbaric oxygen, antioxidants, or more invasive methods such as ablation and surgery. Invasive methods are reserved for refractory symptoms that have failed medical management. The ablation methods involve formalin, endoscopic coagulation with APC, yttrium-aluminum-garnet laser or potassium titanyl phosphate laser, cryotherapy, bipolar electrocoagulation, and hyperbaric oxygen[50,51,54,55]. Surgery carries the risk of morbidity and mortality[56]. APC has shown to be an effective and safe treatment for chronic proctitis with success rates of 80%-95% for bleeding cessation, but controlled trials are lacking[51,57,58]. Complications from these therapies may result in deep tissue injuries like ulcerations, perforation and fistulas, whereas cryotherapy has the potential to avoid these problems since the ablation of the mucosa is superficial[57].
CbFAS with nitrous oxide for radiation proctitis: In a small pilot study of 7 patients who underwent nitrous oxide cryotherapy, 100% resolution of lower GI bleeding was observed with no major AE. All patients had previous unsuccessful treatment with APC[48].
Liquid nitrogen cryotherapy for radiation proctitis: In a small prospective study of 10 patients who underwent liquid nitrogen cryoablation, the rectal telangiectasia density improved in 70% and the symptom severity scores improved in 80%. Cecal perforation due to gaseous overdistention occurred in 1 patient and was managed surgically. Rectal ulceration occurred in another patient, which improved from conservative management[57]. Similar results were seen in another small prospective study of 10 patients. There were no major complications[55].
Differences between APC and cryotherapy for radiation proctitis: Best results with APC have been achieved in mild to moderate radiation proctitis but its role has been limited for severe disease. Cryotherapy on the other hand has shown efficacy in patients with refractory chronic radiation proctitis[59]. Utilization of APC as compared to cryotherapy can be very time consuming, require bowel preparation to reduce the risk of perforation and may require multiple sessions. Cryotherapy can also be carried out with little or no sedation[55]. Larger studies need to be conducted to validate these findings and to determine the role of cryotherapy in acute and chronic radiation proctitis.
Treatment of symptoms: Dysphagia can be a debilitating symptom in patients with inoperable esophageal carcinoma. Further, it can lead to malnutrition and significant decrease in overall quality of life. Currently the two most common palliative tre
In a case series of 49 patients with inoperable malignant dysphagia, 120 liquid nitrogen cryotherapy sessions were conducted, and overall dysphagia scores had improved. Minor AEs were seen in 5% with one patient developing a dilation-related perforation[60]. Cryotherapy may be an alternative treatment option for improving dysphagia with minimal side effects in esophageal carcinoma, however larger studies are needed.
Treatment of EAC and squamous cell cancer: Globally, squamous cell carcinoma of the esophagus comprises 80% of all esophageal carcinomas. These patients have a poor prognosis, however, if diagnosed at the stage of squamous cell neoplasia, then curative endoscopic therapy can be performed. Currently, there is limited data assessing its overall effectiveness.
Cryoballoon focal ablation with liquid nitrous oxide for esophageal cancer: In a prospective trial from China of 80 patients, CbFAS was utilized in patients with one flat intraepithelial neoplasm that was less than 6 cm. Complete eradication occurred in 90% after a single treatment. At the one-year mark, 97% had complete eradication and one had a persistent moderate grade intraepithelial neoplasia. Self-limiting lacerations of the mucosa occurred in 3 patients and no strictures developed[61]. Cryotherapy with CbFAS seems promising, but further studies are needed.
Liquid nitrogen cryotherapy for esophageal cancer: Cash et al[62] had described a 73-year-old male with stage 3 squamous cell carcinoma of the esophagus who was not a candidate for radiation therapy or surgery, and he achieved complete remission for 24 mo after treatment with liquid nitrogen cryotherapy. The patient did develop a signi
Tsai et al[63] conducted a prospective study utilizing liquid nitrogen cryotherapy in patients with EAC. Eighty-eight patients were analyzed with stages T1a-T2. Complete eradication rates in patients with T1a and T2 were 76.3% and 6.7%, respectively. The most common side effect was stricture and developed in 13.6% of patients. Cryo
Another study done by Ramay et al[64] utilized liquid nitrogen cryotherapy for palliation in patients with both invasive adenocarcinoma and squamous esophageal carcinoma. At fifty months, 50% (26) of patients remained alive after treatments. There were few AEs including hematemesis in one patient and stricture formation in 3 with 2 requiring dilations. Overall this method may be a viable treatment palliative treatment option, however larger scale studies are needed.
Survival benefits in metastatic disease: Beyond treatment, another study assessed the impact on overall survival in patients with metastatic esophageal carcinoma. This study retrospectively studied 83 patients with stage IV metastatic esophageal cancer. Thirty-nine patients received chemotherapy alone and 44 patients received chemotherapy and palliative liquid nitrogen cryotherapy. All patients that underwent treatment with cryotherapy had malignant dysphagia. The median overall survival was 19.2 in cryotherapy with chemotherapy and 9.5 mo in with chemotherapy alone. This study demonstrated that cryotherapy might have survival benefits for patients with metastatic esophageal cancer. While the etiology for this is unknown, the authors of the study postulated that cryotherapy can improve dysphagia and thus nutritional status[65].
The role for cryotherapy in palliative treatment of esophageal carcinoma and symptomatic improvement is promising however larger scale studies are needed.
Liquid nitrogen cryotherapy followed by dilation (cryodilation) has been utilized in benign tracheal strictures and stenoses by pulmonologists and thoracic surgeons with improved airway narrowing. Recently a case report described its use in a patient with a benign refractory esophageal stricture who had previously undergone an eso
Endoscopic cryotherapy is a promising and growing field. First demonstrated in BE, the use now spans from cancer treatment to symptomatic improvement in GAVE. Most studies done have been on small populations. Large scale randomized control studies are needed to determine the overall effectiveness and utility of endoscopic cryotherapy in treatment of various GI disorders. The ease of use and the ability for relatively safe and noninvasive procedures makes it a very promising modality for the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gunay S, Xi K S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, Nishioka NS, Yachimski P, Johnston MH, Shaheen NJ, Zfass AM, Smith JO, Gill KR, Burdick JS, Mallat D, Wolfsen HC. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21:S187-S191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 663] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | Clarke DM, Robilotto AT, Rhee E, VanBuskirk RG, Baust JG, Gage AA, Baust JM. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007;6:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Hanai A, Yang WL, Ravikumar TS. Induction of apoptosis in human colon carcinoma cells HT29 by sublethal cryo-injury: mediation by cytochrome c release. Int J Cancer. 2001;93:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95:1187-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Kahlenberg MS, Volpe C, Klippenstein DL, Penetrante RB, Petrelli NJ, Rodriguez-Bigas MA. Clinicopathologic effects of cryotherapy on hepatic vessels and bile ducts in a porcine model. Ann Surg Oncol. 1998;5:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Weber SM, Lee FT. Cryoablation: history, mechanism of action, and guidance modalities. Tumor Ablat. 2005;250-265. [DOI] [Full Text] |
| 9. | Weber SM, Lee FT Jr, Chinn DO, Warner T, Chosy SG, Mahvi DM. Perivascular and intralesional tissue necrosis after hepatic cryoablation: results in a porcine model. Surgery. 1997;122:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Shepherd JP, Dawber RP. Wound healing and scarring after cryosurgery. Cryobiology. 1984;21:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Hu SNY, Adler DG. Endoscopic Cryotherapy: Indications and Efficacy. Pract Gastroenterol. 2015;39:19-45. |
| 13. | Muguruma N, Marcon NE. Technique and emerging role of cryotherapy. Tech Gastroint Endos. 2010;12:44-48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | ASGE Technology Committee; Parsi MA, Trindade AJ, Bhutani MS, Melson J, Navaneethan U, Thosani N, Trikudanathan G, Watson RR, Maple JT. Cryotherapy in gastrointestinal endoscopy. VideoGIE. 2017;2:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Halsey KD, Greenwald BD. Cryotherapy in the management of esophageal dysplasia and malignancy. Gastrointest Endosc Clin N Am. 2010;20:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kaul V, Bittner K, Ullah A, Kothari S. Liquid nitrogen spray cryotherapy-based multimodal endoscopic management of dysplastic Barrett's esophagus and early esophageal neoplasia: retrospective review and long-term follow-up at an academic tertiary care referral center. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Chen AM, Pasricha PJ. Cryotherapy for Barrett's esophagus: Who, how, and why? Gastrointest Endosc Clin N Am. 2011;21:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Dumot JA, Greenwald BD. Cryotherapy for Barrett's esophagus: does the gas really matter? Endoscopy. 2011;43:432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Greenwald BD, Lightdale CJ, Abrams JA, Horwhat JD, Chuttani R, Komanduri S, Upton MP, Appelman HD, Shields HM, Shaheen NJ, Sontag SJ. Barrett's esophagus: endoscopic treatments II. Ann N Y Acad Sci. 2011;1232:156-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Raju GS, Ahmed I, Xiao SY, Brining D, Bhutani MS, Pasricha PJ. Graded esophageal mucosal ablation with cryotherapy, and the protective effects of submucosal saline. Endoscopy. 2005;37:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Johnston CM, Schoenfeld LP, Mysore JV, Dubois A. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc. 1999;50:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Booth CL, Thompson KS. Barrett's esophagus: A review of diagnostic criteria, clinical surveillance practices and new developments. J Gastrointest Oncol. 2012;3:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 980] [Article Influence: 70.0] [Reference Citation Analysis (1)] |
| 24. | Sharma P, Shaheen NJ, Katzka D, Bergman JJGHM. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology. 2020;158:760-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 25. | Becq A, Camus M, Rahmi G, de Parades V, Marteau P, Dray X. Emerging indications of endoscopic radiofrequency ablation. United European Gastroenterol J. 2015;3:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Dumot JA, Greenwald BD. Argon plasma coagulation, bipolar cautery, and cryotherapy: ABC's of ablative techniques. Endoscopy. 2008;40:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Frederiks CN, Canto MI, Weusten BLAM. Updates in Cryotherapy for Barrett's Esophagus. Gastrointest Endosc Clin N Am. 2021;31:155-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Johnston MH, Eastone JA, Horwhat JD, Cartledge J, Mathews JS, Foggy JR. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc. 2005;62:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Greenwald BD, Dumot JA, Horwhat JD, Lightdale CJ, Abrams JA. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus. 2010;23:13-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Ramay FH, Cui Q, Greenwald BD. Outcomes after liquid nitrogen spray cryotherapy in Barrett's esophagus-associated high-grade dysplasia and intramucosal adenocarcinoma: 5-year follow-up. Gastrointest Endosc. 2017;86:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Jovani M, Lee M, Hur C, Stump N, Chan AT, Nishioka NS. Mo1123 Cryotherapy (Trufreeze™) vs. Radio Frequency Ablation (Barrx™) for the Treatment of Barrett’S Esophagus with High-Grade Dysplasia and/or Early Adenocarcinoma: Ad Interim Results of a Non-Inferiority Randomized Clinical Trial. Gastrointest Endosc. 2018;87:AB408. [DOI] [Full Text] |
| 32. | Solomon SS, Kothari S, Smallfield GB, Inamdar S, Stein P, Rodriguez VA, Sima AP, Bittner K, Zfass AM, Kaul V, Trindade AJ. Liquid Nitrogen Spray Cryotherapy is Associated With Less Postprocedural Pain Than Radiofrequency Ablation in Barrett’s Esophagus: A Multicenter Prospective Study. J Clin Gastroenterol. 2019;53:e84-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Thota PN, Arora Z, Dumot JA, Falk G, Benjamin T, Goldblum J, Jang S, Lopez R, Vargo JJ. Cryotherapy and Radiofrequency Ablation for Eradication of Barrett's Esophagus with Dysplasia or Intramucosal Cancer. Dig Dis Sci. 2018;63:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Fasullo M, Shah T, Patel M, Mutha P, Zfass A, Lippman R, Smallfield G. Outcomes of Radiofrequency Ablation Compared to Liquid Nitrogen Spray Cryotherapy for the Eradication of Dysplasia in Barrett's Esophagus. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Canto MI, Shin EJ, Khashab MA, Molena D, Okolo P, Montgomery E, Pasricha P. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett's esophagus. Endoscopy. 2015;47:591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Verbeek RE, Vleggaar FP, Ten Kate FJ, van Baal JW, Siersema PD. Cryospray ablation using pressurized CO2 for ablation of Barrett’s esophagus with early neoplasia: early termination of a prospective series. Endosc Int Open. 2015;3:E107-E112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Alshelleh M, Raphael KL, Inamdar S, McKinley MJ, Trindade AJ. Cryoballoon and Cryospray Ablation Therapies are Equivalent for Eradication of Barrett's Esophagus. Tech Innovations Gastrointest Endosc. 2021;23:110-112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Sullivan R, Mulki R, Peter S. The role of ablation in the treatment of dysplastic Barrett's esophagus. Ther Adv Gastrointest Endosc. 2021;14:26317745211049967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Sawas T, Visrodia K, Zakko L, Leggett C, Wang KK. A Western Experience in the Use of a Novel CryoBalloon Focal Ablation System for the Management of Barrett’s Neoplasia: 378. Am J Gastroenterol. 2018;113:S220-S221. |
| 40. | van Munster SN, Overwater A, Haidry R, Bisschops R, Bergman J, Weusten BL. 478 Cryoballoon Ablation of Dysplastic Barrett's Esophagus Causes Shorter Duration and Less Severe Post-Procedural Pain as Compared to Radiofrequency Ablation. Gastrointest Endosc. 2018;87:AB81-AB82. |
| 41. | Canto MI, Trindade AJ, Abrams J, Rosenblum M, Dumot J, Chak A, Iyer P, Diehl D, Khara HS, Corbett FS, McKinley M, Shin EJ, Waxman I, Infantolino A, Tofani C, Samarasena J, Chang K, Wang B, Goldblum J, Voltaggio L, Montgomery E, Lightdale CJ, Shaheen NJ. Multifocal Cryoballoon Ablation for Eradication of Barrett's Esophagus-Related Neoplasia: A Prospective Multicenter Clinical Trial. Am J Gastroenterol. 2020;115:1879-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Gostout CJ, Viggiano TR, Ahlquist DA, Wang KK, Larson MV, Balm R. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165-1170. [PubMed] |
| 45. | Dias de Castro F, Boal Carvalho P, Cúrdia Gonçalves T, Magalhães J, Moreira MJ, Marinho C, Cotter J. Treating Gastric Antral Vascular Ectasia - When Argon Therapy Is Not Enough. GE Port J Gastroenterol. 2016;23:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Patel AA, Trindade AJ, Diehl DL, Khara HS, Lee TP, Lee C, Sethi A. Nitrous oxide cryotherapy ablation for refractory gastric antral vascular ectasia. United European Gastroenterol J. 2018;6:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Cho S, Zanati S, Yong E, Cirocco M, Kandel G, Kortan P, May G, Marcon N. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Kantsevoy SV, Cruz-Correa MR, Vaughn CA, Jagannath SB, Pasricha PJ, Kalloo AN. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc. 2003;57:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Trindade AJ, Inamdar S, Sejpal DV. Nitrous oxide CryoBalloon therapy of refractory gastric antral vascular ectasia. Endoscopy. 2017;49:923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Do NL, Nagle D, Poylin VY. Radiation proctitis: current strategies in management. Gastroenterol Res Pract. 2011;2011:917941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Sebastian S, O'Connor H, O'Morain C, Buckley M. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J Gastroenterol Hepatol. 2004;19:1169-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Denton AS, Andreyev HJ, Forbes A, Maher EJ. Systematic review for non-surgical interventions for the management of late radiation proctitis. Br J Cancer. 2002;87:134-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Denton A, Forbes A, Andreyev J, Maher EJ. Non surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst Rev. 2002;CD003455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Sarin A, Safar B. Management of radiation proctitis. Gastroenterol Clin North Am. 2013;42:913-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Moawad FJ, Maydonovitch CL, Horwhat JD. Efficacy of cryospray ablation for the treatment of chronic radiation proctitis in a pilot study. Dig Endosc. 2013;25:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Tabaja L, Sidani SM. Management of Radiation Proctitis. Dig Dis Sci. 2018;63:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Hou JK, Abudayyeh S, Shaib Y. Treatment of chronic radiation proctitis with cryoablation. Gastrointest Endosc. 2011;73:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Leiper K, Morris AI. Treatment of radiation proctitis. Clin Oncol (R Coll Radiol). 2007;19:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 59. | Karamanolis G, Psatha P, Triantafyllou K. Endoscopic treatments for chronic radiation proctitis. World J Gastrointest Endosc. 2013;5:308-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Kachaamy T, Prakash R, Kundranda M, Batish R, Weber J, Hendrickson S, Yoder L, Do H, Magat T, Nayar R, Gupta D, DaSilva T, Sangal A, Kothari S, Kaul V, Vashi P. Liquid nitrogen spray cryotherapy for dysphagia palliation in patients with inoperable esophageal cancer. Gastrointest Endosc. 2018;88:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Ke Y, van Munster SN, Chen J, Liu F, Zhao D, Li W, He S, Zhang Y, Dou L, Liu Y. 1031 Endoscopic Cryoballoon Ablation is Safe, Well-Tolerated and Highly Effective in the Eradication of Esophageal Squamous Cell Neoplasia. Gastrointest Endosc. 2018;87:AB141-AB142. [DOI] [Full Text] |
| 62. | Cash BD, Johnston LR, Johnston MH. Cryospray ablation (CSA) in the palliative treatment of squamous cell carcinoma of the esophagus. World J Surg Oncol. 2007;5:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Tsai FC, Ghorbani S, Greenwald BD, Jang S, Dumot JA, McKinley MJ, Shaheen NJ, Habr F, Wolfsen HC, Abrams JA, Lightdale CJ, Nishioka NS, Johnston MH, Zfass A, Coyle WJ. Safety and efficacy of endoscopic spray cryotherapy for esophageal cancer. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Ramay FH, Shaheen NJ, Kaul V, Nieto J, Joshi V, Litle V, Fernando HC, Fukami N, Hoffman BJ, Bizekis C. Tu1147 Liquid Nitrogen Spray Cryotherapy for Palliation of Invasive Esophageal Carcinoma: Results from a Multicenter us Registry. Gastrointest Endosc. 2018;87:AB541. [DOI] [Full Text] |
| 65. | Prakash RK, Kachaamy T, Ambrosius M, Magat T, Shin JS, Gupta D, Vashi PG. Tu1256 Impact of Liquid Nitrogen Endoscopic Spray Cryotherapy on Overall Survival in Metastatic Esophageal Cancer. Gastrointest Endosc. 2017;85:AB603-AB604. [DOI] [Full Text] |
| 66. | Roccato MK, Duh E, Mai D, Chehade NEH, Hashimoto R, Samarasena J. CryoDilation: A Novel Treatment for Benign Esophageal Strictures Using Liquid Nitrogen Spray Cryotherapy: 1828. Am J Gastroenterol. 2019;114:S1025-S1026. [DOI] [Full Text] |