Published online Sep 16, 2021. doi: 10.4253/wjge.v13.i9.426
Peer-review started: May 29, 2021
First decision: June 11, 2021
Revised: June 12, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: September 16, 2021
Processing time: 103 Days and 16.1 Hours
Accurate diagnosis of the depth of gastric cancer invasion is crucial in clinical practice. The diagnosis of gastric cancer depth is often made using endoscopic characteristics of the tumor and its margins; however, evaluating invasion depth based on endoscopic background gastritis remains unclear.
To investigate predicting submucosal invasion using the endoscopy-based Kyoto classification of gastritis.
Patients with gastric cancer detected on esophagogastroduodenoscopy at Toyoshima Endoscopy Clinic were enrolled. We analyzed the effects of patient and tumor characteristics, including age, sex, body mass index, surveillance endoscopy within 2 years, current Helicobacter pylori infection, the Kyoto classification, and Lauren’s tumor type, on submucosal tumor invasion and curative endoscopic resection. The Kyoto classification included atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness. Atrophy was characterized by non-reddish and low mucosa. Intestinal metaplasia was detected as patchy whitish or grayish-white flat elevations, forming an irregular uneven surface. An enlarged fold referred to a fold width ≥ 5 mm in the greater curvature of the corpus. Nodularity was characterized by goosebump-like multiple nodules in the antrum. Diffuse redness was characterized by uniform reddish non-atrophic mucosa in the greater curvature of the corpus.
A total of 266 gastric cancer patients (mean age, 66.7 years; male sex, 58.6%; mean body mass index, 22.8 kg/m2) were enrolled. Ninety-three patients underwent esophagogastroduodenoscopy for surveillance within 2 years, and 140 had current Helicobacter pylori infection. The mean Kyoto score was 4.54. Fifty-eight cancers were diffuse-type, and 87 cancers had invaded the submucosa. Multivariate analysis revealed that low body mass index (odds ratio 0.88, P = 0.02), no surveillance esophagogastroduodenoscopy within 2 years (odds ratio 0.15, P < 0.001), endoscopic enlarged folds of gastritis (odds ratio 3.39, P = 0.001), and Lauren’s diffuse-type (odds ratio 5.09, P < 0.001) were independently associated with submucosal invasion. Similar results were obtained with curative endoscopic resection. Among cancer patients with enlarged folds, severely enlarged folds (width ≥ 10 mm) were more related to submucosal invasion than mildly enlarged folds (width 5-9 mm, P < 0.001).
Enlarged folds of gastritis were associated with submucosal invasion. Endoscopic observation of background gastritis as well as the lesion itself may help diagnose the depth of cancer invasion.
Core Tip: We investigated predicting submucosal invasion using the endoscopy-based Kyoto classification of gastritis. We analyzed the effects of patient and tumor characteristics, including the Kyoto classification, on submucosal tumor invasion. Two hundred sixty-six gastric cancer patients were enrolled. Multivariate analysis revealed that low body mass index, no surveillance esophagogastroduodenoscopy within 2 years, endoscopic enlarged folds of gastritis, and Lauren’s diffuse-type were independently associated with submucosal invasion. Among cancer patients with enlarged folds, severely enlarged folds (width ≥ 10 mm) were more related to submucosal invasion than mildly enlarged folds (width 5-9 mm). Enlarged folds of gastritis were associated with submucosal invasion.
- Citation: Toyoshima O, Yoshida S, Nishizawa T, Toyoshima A, Sakitani K, Matsuno T, Yamada T, Matsuo T, Nakagawa H, Koike K. Enlarged folds on endoscopic gastritis as a predictor for submucosal invasion of gastric cancers. World J Gastrointest Endosc 2021; 13(9): 426-436
- URL: https://www.wjgnet.com/1948-5190/full/v13/i9/426.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i9.426
Gastric cancer is the third most common cause of cancer mortality worldwide, making it an important disease[1,2]. The depth of gastric cancer invasion is associated with lymph node metastasis[3,4], recurrence[5], and survival[6,7] and has a great influence on therapeutic strategy[8-10]. This means that the diagnosis of invasion depth is crucial.
At present, the diagnosis of gastric cancer depth is often made using the endoscopic characteristics of the tumor and its margins. For example, an irregular surface, marked marginal elevation, and clubbing/abrupt cutting/fusion of converting folds are useful for the diagnosis of submucosal invasion[11]. Similarly, using nodular mucosal changes, deep depression, and fold convergence for the diagnosis of signet ring cell carcinoma with submucosal invasion[12], and the non-extension sign[13], size > 30 mm, margin elevation, uneven surface[14], remarkable redness[14,15], and abrupt cutting converging folds[15] for the diagnosis of deeper submucosal invasion (SM2: ≥ 500 µm in depth) have also been reported. For the last decade, the depth of gastric cancer has been predicted using magnifying narrow-band imaging, which is an image-enhanced endoscopy, in addition to conventional white-light imaging[16]. Findings such as non-structure, scattering, or multi-caliber vessels[17], D-vessels[18], and the vessel plus surface classification[19] were found to be useful for depth diagnosis. Furthermore, various modalities, including endoscopic ultrasonography[20] and computed tomography[21], have been found to assist in depth diagnosis. Thus, research on the depth of invasion is being vigorously conducted.
On the other hand, artificial intelligence is now overwhelming human intelligence. Artificial intelligence defeated the world champion in chess in 1997 and in the East Asian game of go in 2017. The style of play used by artificial intelligence was of a different dimension unimaginable to humans. Recently, artificial intelligence has been used for endoscopic diagnosis[22]. In the future, artificial intelligence may be used to diagnose the depth of invasion based not only on the tumor itself but also on background gastritis. However, there are few reports on the evaluation of invasion depth based on endoscopic background gastritis. Therefore, we decided to investigate predictions for submucosal invasion using the endoscopy-based Kyoto classification of gastritis, for which evidence has been accumulated recently[23-25].
This study involved those patients who underwent esophagogastroduodenoscopy (EGD) between January 2008 and August 2020 at Toyoshima Endoscopy Clinic, in whom gastric cancers were detected. Exclusion criteria were cancer located in the esophagogastric junction or in the residual stomach after surgery, or unavailable EGD images. We also excluded patients with unavailable Helicobacter pylori (H. pylori) status. In this study, curative endoscopic resection of gastric cancer was performed according to the guidelines of the Japanese Gastric Cancer Association[26].
This retrospective study was approved by the Certificated Review Board, Hattori Clinic on September 4, 2020 (approval No. S2009-U04). Written informed consent was obtained from all participants. All clinical evaluations were conducted in accordance with the ethical guidelines of the Declaration of Helsinki. This study had no financial support.
The Japan Gastroenterological Endoscopy Society advocated the endoscopy-based Kyoto classification of gastritis in 2013 with the aim of matching endoscopic findings and pathology. The Kyoto classification of gastritis comprises atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness. Endoscopic atrophy is characterized by non-reddish and low mucosa, identified by an atrophic border, according to the Kimura-Takemoto classification[27]. Endoscopic intestinal metaplasia is detected as patchy whitish or grayish-white flat elevations, forming an irregular uneven surface[28]. An enlarged fold refers to a fold with width ≥ 5 mm in the greater curvature of the corpus, which is not flattened or only partially flattened by stomach insufflation. Endoscopic nodularity is characterized by goosebump-like multiple nodules that appear mainly in the antrum and represent a collection of lymphoid follicles. Diffuse redness is characterized by uniform reddish non-atrophic mucosa located mainly in the greater curvature of the corpus and representing superficial gastritis.
The Kyoto score is the sum of the following five parameters: atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness score and ranges from 0 to 8. Kimura-Takemoto classification gradings of C0 and CI are defined as an atrophy score of 0, CII and CIII have an atrophy score of 1, and OI to OIII have an atrophy score of 2. Absence of intestinal metaplasia was defined as an intestinal metaplasia score of 0, intestinal metaplasia limited to the antrum was given 1, and intestinal metaplasia extending into the corpus received an intestinal metaplasia score of 2. The absence and presence of enlarged folds were defined as enlarged fold scores of 0 and 1, respectively. The absence and presence of nodularity were defined as nodularity scores of 0 and 1, respectively. Diffuse redness scores were defined as 0, 1, and 2 for no diffuse redness, mild redness, and severe redness, respectively. The Kyoto score has been proven to be associated with the presence of gastric cancer[23], the risk of gastric cancer[25], and H. pylori infection[24].
In this study, enlarged folds were divided into two groups: severely enlarged folds with widths ≥ 10 mm and mildly enlarged folds with widths of 5-9 mm[29,30]. Fold width was measured by placing a closed or opened forceps, which has a width of 2 mm or 7mm, against enlarged folds.
One expert endoscopist retrospectively reviewed the EGD images and evaluated the Kyoto score. Surveillance EGD was defined as such only if the patients had undergone a previous EGD at our institution within the last 2 years[31].
The depth of the tumor was diagnosed using the resected specimen or if unresectable, from computed tomography images. Tumor type was evaluated according to the Lauren classification (diffuse- or intestinal-type)[32].
We divided the H. pylori infection status into two groups: current infection and negative for current infection. The current infection group included patients in whom H. pylori eradication therapy had failed. The group of negative for current infection included H. pylori-uninfected patients and H. pylori-past infected patients who had undergone successful eradication therapy or in whom H. pylori had spontaneously disappeared[33].
The T-File System (STS-Medic Inc., Tokyo, Japan) was used to file the endoscopic images and for documentation of the endoscopic findings. We collected data on age, sex, interval from previous EGD, and endoscopic images from the T-File System, and data on body mass index (BMI), H. pylori status, treatment for the cancer, and Lauren type of the tumor from electronic medical records.
Univariate and multivariate analyses for the effect on submucosal invasion and curative endoscopic resection were performed using a binomial logistic regression model. Variables with a P value < 0.1 in the univariate analysis were entered into the multivariate analysis and calculated using the all-possible-regressions procedure. We used a complete analysis for missing data. We evaluated the frequency of submucosal invasion among patients with negatively enlarged folds and mildly and severely enlarged folds using the Cochran-Armitage trend test.
Statistical significance was indicated by a P value of < 0.05. Calculations were performed using the statistical software Ekuseru-Toukei 2015 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
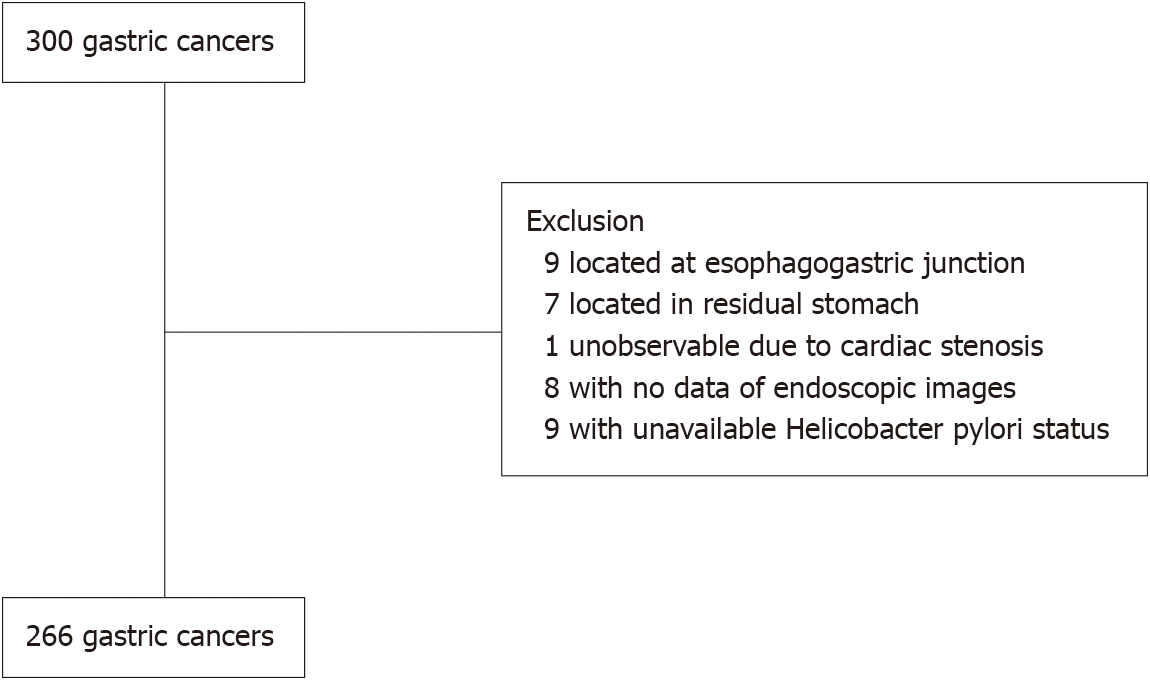
A total of 300 patients with gastric adenocarcinomas were observed at the Toyoshima Endoscopy Clinic during the study period. We excluded nine cancers located at the esophagogastric junction, seven cancers located in the residual stomach after surgery, nine cancers with unavailable EGD images, and nine cancers with unavailable H. pylori status. Finally, 266 gastric cancers were enrolled. Figure 1 presents the patient flowchart of this study.
Table 1 shows the patient characteristics of the study. The mean age was 66.7 (range, 37-89) years. Of the patients, 58.6% were male. The mean BMI was 22.8 kg/m2. Ninety-three patients (35.0%) underwent EGD for surveillance within 2 years. Current H. pylori infection was identified in 52.6% (including 129 patients without past eradication therapy and 11 patients with failed eradication therapy) of the study patients. Cases negative for current H. pylori infection included 13 uninfected and 113 past-infected patients. The mean Kyoto score was 4.54 (atrophy score, 1.75; intestinal metaplasia, 1.32; enlarged folds, 0.24; nodularity, 0.08; diffuse redness score, 1.15). The proportion of diffuse-type adenocarcinoma on the Lauren classification was 21.8%. With respect to the depth of gastric cancer, 179 (67.3%) were in the mucosa, 51 (19.2%) were in the submucosa, and 36 (13.5%) were in the muscularis propria or deeper.
| Patient characteristics | |
| n | 266 |
| Age, mean (SD), yr | 66.7 (12.1) |
| Male sex | 58.6% |
| Body mass index, mean (SD), kg/m2 | 22.8 (3.3) |
| Surveillance endoscopy within 2 yr | 35.0% |
| Current Helicobacter pylori infection | 52.6% |
| Endoscopic findings | |
| Atrophy score, mean (SD) | 1.75 (0.54) |
| Intestinal metaplasia score, mean (SD) | 1.32 (0.84) |
| Enlarged folds score, mean (SD) | 0.24 (0.43) |
| Nodularity score, mean (SD) | 0.08 (0.27) |
| Diffuse redness score, mean (SD) | 1.15 (0.92) |
| Kyoto score, mean (SD) | 4.54 (1.84) |
| Lauren’s diffuse-type | 21.8% |
| Depth of gastric cancer, M/SM/MP or deeper, n | 179/51/36 |
We analyzed the effects on submucosal invasion of gastric cancer using univariate and multivariate analyses (Table 2). Multivariate analysis showed that low BMI (odds ratio 0.88, P = 0.02), non-surveillance EGD (odds ratio 0.15, P < 0.001), enlarged folds (odds ratio 3.39, P = 0.001), and Lauren’s diffuse-type adenocarcinoma (odds ratio 5.09, P < 0.001) were associated with submucosal invasion.
| Univariate analysis | Multivariate analysis | ||||
| Odds ratio | P value | Regression coefficient | Odds ratio (95% confidence interval) | P value | |
| Age | 0.96 | < 0.001 | 0.003 | 1.00 (0.97-1.03) | 0.82 |
| Male sex | 1.17 | 0.56 | |||
| Body mass index | 0.85 | < 0.001 | -0.130 | 0.88 (0.79-0.98) | 0.02 |
| Surveillance endoscopy within 2 yr | 0.12 | < 0.001 | -1.913 | 0.15 (0.06-0.38) | < 0.001 |
| Current Helicobacter pylori infection | 2.55 | < 0.001 | -0.387 | 0.68 (0.21-2.24) | 0.52 |
| Endoscopic findings | |||||
| Atrophy score | 0.58 | 0.11 | |||
| Intestinal metaplasia score | 0.71 | 0.03 | -0.014 | 0.99 (0.65-1.49) | 0.95 |
| Enlarged folds score | 4.76 | < 0.001 | 1.222 | 3.39 (1.61-7.14) | 0.001 |
| Nodularity score | 1.57 | 0.33 | |||
| Diffuse redness score | 1.48 | 0.01 | -0.020 | 0.98 (0.54-1.78) | 0.95 |
| Kyoto score | 1.14 | 0.08 | |||
| Lauren’s diffuse-type | 7.61 | < 0.001 | 1.627 | 5.09 (2.22-11.64) | < 0.001 |
Next, we analyzed the effects on patients who underwent curative treatment with endoscopic resection without surgery. In addition to the mucosal depth of gastric cancer, patients who underwent curative endoscopic resection were associated with high BMI, surveillance EGD, no enlarged folds, and Lauren’s intestinal-type adenocarcinoma (Supplementary Table 1).
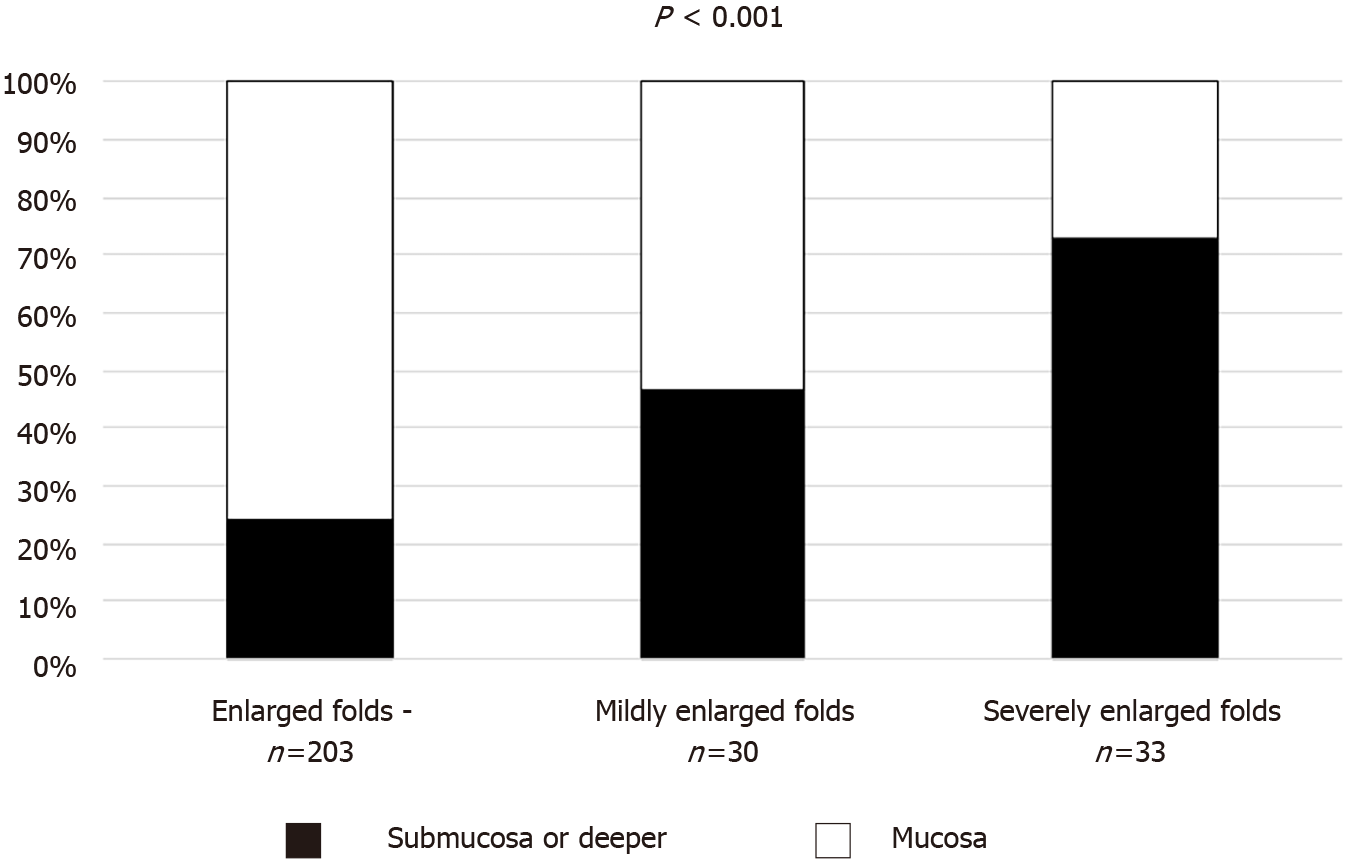
We divided gastric cancer patients with enlarged folds into two categories: mildly and severely enlarged folds. Submucosal invasion was observed in 49 of 203 cancers without enlarged folds, 14 of 30 cancers with mildly enlarged folds, and 24 of 33 cancers with severely enlarged folds. Figure 2 shows the proportions of submucosal invasion based on the severity of the enlarged folds. The severity of the enlarged folds was related to the depth of the tumor (P < 0.001, Cochran-Armitage trend test).
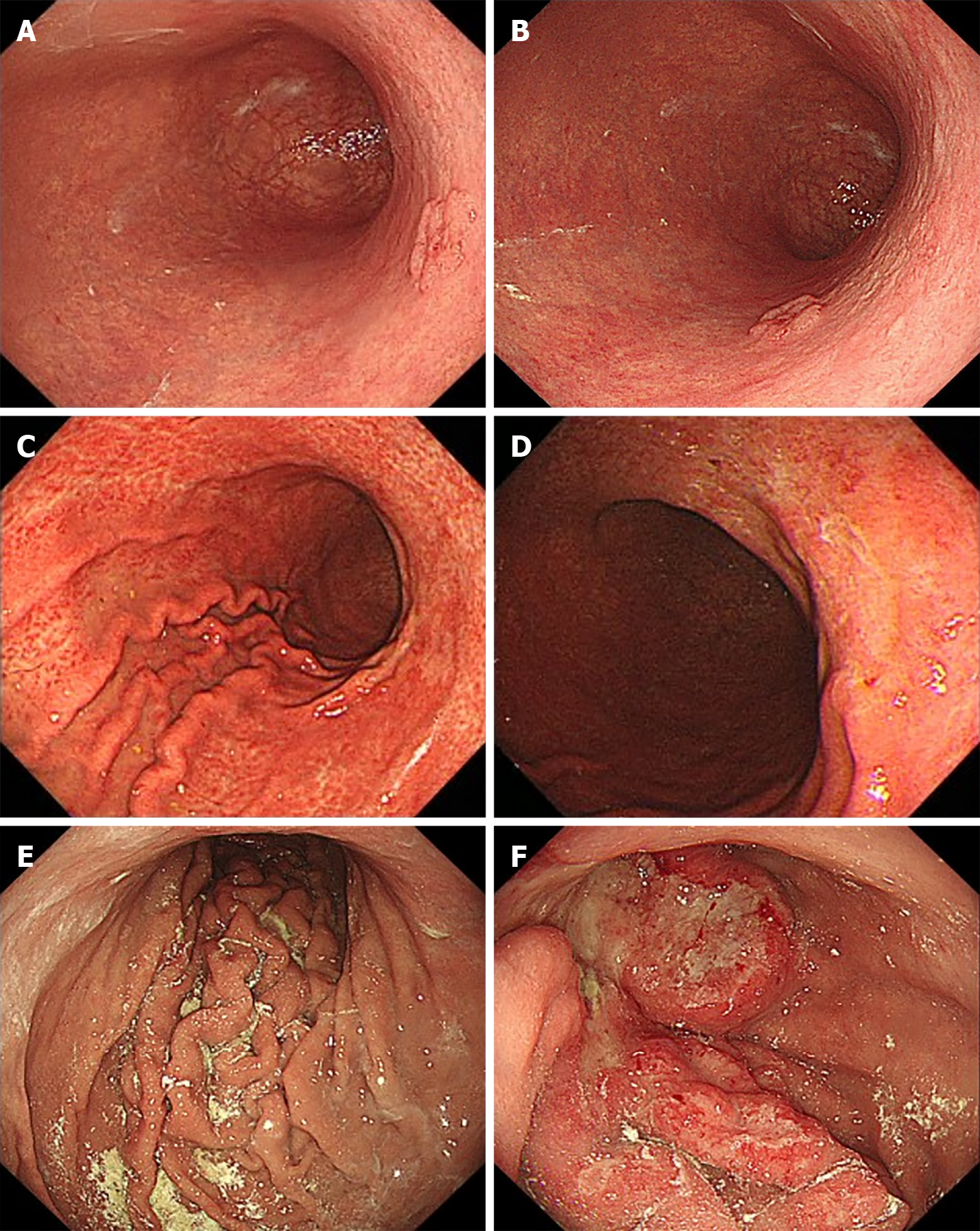
Representative images of enlarged fold gastritis and coexisting gastric cancer are shown in Figure 3.
In this study, we found that the enlarged folds of background gastritis were related to submucosal invasion of gastric cancer. Furthermore, the severity of the enlarged folds was associated with the depth of the tumor. We showed that cancer invasion may be predicted based on background gastritis. The strength of this study is that background gastritis, under the new criterion of the Kyoto classification, is related to the depth of invasion and not limited to observation of the lesions themselves. However, comprehensive endoscopic diagnosis is required in clinical practice because of advances in technology such as artificial intelligence.
Enlarged folds have been well studied for their biological characteristics. Enlarged folds have been shown to be associated with the tumor necrosis factor-alpha gene polymorphism as a genetic predisposition[34]. Genome wide hypomethylation and regional hypermethylation have been shown to occur in enlarged folds[35,36]. The production of interleukin 1 beta and hepatocyte growth factor caused by H. pylori infection reportedly contributes to fold enlargement in the stomach by stimulating epithelial cell proliferation and inhibiting acid secretion[37,38]. Morphological changes in parietal cells associated with H. pylori infection have been reported to be functionally related to the inhibition of acid secretion seen in patients with enlarged folds[39]. In addition, enlarged folds are strongly associated with H. pylori infection and have been shown to improve with eradication[24,29,34]. Enlarged folds are considered to be at high risk of gastric cancer, especially diffuse cancer, which is closely related to highly active inflammation[36,40]. These biological behaviors of the enlarged folds may be attributed to the depth of the cancer.
Yasunaga et al[29] divided enlarged folds into two categories (severe and mild) and found that severely enlarged folds suppressed acid secretion and had higher serum gastrin, pepsinogen I, and pepsinogen II levels compared to mildly enlarged folds[30]. Such differences may contribute to active inflammation of the mucosa and depth of cancer.
Invasion depth has already been reported to be associated with Lauren’s histological type[41], surveillance endoscopy[31], and BMI[42]. Consistent with these previous reports, the multivariate analysis of the present study demonstrated that submucosal invasion was associated with pathology, surveillance, and BMI.
This study has some limitations. First, this was a single-institute retrospective study. However, the quality of the data was well-controlled. In the future, a prospective, multicenter design is needed. Second, because the number of events was small, the variables that could be entered into multivariate analysis were limited. It is desirable to increase the number of events and investigate factors such as family history, drinking and smoking history, and aspirin use. Third, we did not endoscopically evaluate the tumor itself. Comprehensive analyses of the tumor itself and background gastritis are warranted.
Endoscopy-based enlarged folds of gastritis were associated with submucosal invasion of the tumor. Endoscopic observation of background gastritis as well as the lesion itself may help diagnose the depth of cancer invasion in clinical practice. Therefore, further comprehensive investigations are required.
The diagnosis of gastric cancer depth is often made using endoscopic characteristics of the tumor and its margins.
In the future, artificial intelligence may be used to diagnose the depth of invasion based not only on the tumor itself but also on background gastritis.
We investigated predicting submucosal invasion based on endoscopic background gastritis.
Patients with gastric cancer detected on esophagogastroduodenoscopy were enrolled. We analyzed the effects of patient and tumor characteristics including the Kyoto classification.
Endoscopic enlarged folds of gastritis (odds ratio 3.39, P = 0.001) was independently associated with submucosal invasion. Among cancer patients with enlarged folds, severely enlarged folds (width ≥ 10 mm) were more related to submucosal invasion than mildly enlarged folds (width 5-9 mm, P < 0.001).
Enlarged folds of gastritis were associated with submucosal invasion.
Endoscopic observation of background gastritis as well as the lesion itself may help diagnose the depth of cancer invasion.
Manuscript source: Unsolicited manuscript
Corresponding Author’s Membership in Professional Societies: The Japanese Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Dai DL, Kotelevets SM, Shinohara H S-Editor: Wu YXJ L-Editor: Filipodia P-Editor:Guo X
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 2. | Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 307] [Cited by in RCA: 298] [Article Influence: 49.7] [Reference Citation Analysis (3)] |
| 3. | Miyahara K, Hatta W, Nakagawa M, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Aoyagi H, Shimosegawa T. The role of an undifferentiated component in submucosal invasion and submucosal invasion depth after endoscopic submucosal dissection for early gastric cancer. Digestion. 2018;98:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kim SM, Min BH, Ahn JH, Jung SH, An JY, Choi MG, Sohn TS, Bae JM, Kim S, Lee H, Lee JH, Kim YW, Ryu KW, Kim JJ. Nomogram to predict lymph node metastasis in patients with early gastric cancer: a useful clinical tool to reduce gastrectomy after endoscopic resection. Endoscopy. 2020;52:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Lee IS, Yook JH, Kim TH, Kim HS, Kim KC, Oh ST, Kim BS. Prognostic factors and recurrence pattern in node-negative advanced gastric cancer. Eur J Surg Oncol. 2013;39:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Qu JL, Qu XJ, Li Z, Zhang JD, Liu J, Teng YE, Jin B, Zhao MF, Yu P, Shi J, Fu LY, Wang ZN, Liu YP. Prognostic model based on systemic inflammatory response and clinicopathological factors to predict outcome of patients with node-negative gastric cancer. PLoS One. 2015;10:e0128540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological variation of lauren classification in gastric cancer. Pathol Oncol Res. 2016;22:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 9. | Chu YN, Yu YN, Jing X, Mao T, Chen YQ, Zhou XB, Song W, Zhao XZ, Tian ZB. Feasibility of endoscopic treatment and predictors of lymph node metastasis in early gastric cancer. World J Gastroenterol. 2019;25:5344-5355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Pellino A, Riello E, Nappo F, Brignola S, Murgioni S, Djaballah SA, Lonardi S, Zagonel V, Rugge M, Loupakis F, Fassan M. Targeted therapies in metastatic gastric cancer: Current knowledge and future perspectives. World J Gastroenterol. 2019;25:5773-5788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 11. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Kang SH, Moon HS, Sung JK, Jeong HY, Kim SH, Kim KB, Youn SJ, Kim SM, Song KH, Lee SW, Lee DS, Cho YS, Chung IK, Bang KB. Endoscopic prediction of tumor invasion depth in early gastric signet ring cell carcinoma. Dig Dis. 2019;37:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kato M, Uedo N, Nagahama T, Yao K, Doyama H, Tsuji S, Gotoda T, Kawamura T, Ebi M, Yamamoto K, Akasaka T, Takatori H, Handa O, Akamatsu T, Nishikawa J, Hikichi T, Yamashina T, Imoto A, Kitamura Y, Mikami T, Koike T, Ohara S, Kitamura S, Yamaguchi T, Kinjo T, Inoue T, Suzuki S, Kaneko A, Hirasawa K, Tanaka K, Kotachi T, Miwa K, Toya Y, Kayaba S, Ikehata A, Minami S, Mizukami K, Oya H, Ara N, Fukumoto Y, Komura T, Yoshio T, Morizono R, Yamazaki K, Shimodate Y, Yamanouchi K, Kawata N, Kumagai M, Sato Y, Umeki K, Kawai D, Tanuma T, Kishino M, Konishi J, Sumiyoshi T, Oka S, Kono M, Sakamoto T, Horikawa Y, Ohyauchi M, Hashiguchi K, Waseda Y, Kasai T, Aoyagi H, Oyamada H, Shoji M, Kiyotoki S, Asonuma S, Orikasa S, Akaishi C, Nagami Y, Nakata S, Iida F, Nomura T, Tominaga K, Oka K, Morita Y, Suzuki H, Ozeki K, Kuribayashi S, Akazawa Y, Sasaki S, Miki G, Sano T, Satoh H, Nakamura M, Iwai W, Tawa H, Wada M, Yoshimura D, Hisanaga Y, Shimokawa T, Ishikawa H. Self-study of the non-extension sign in an e-learning program improves diagnostic accuracy of invasion depth of early gastric cancer. Endosc Int Open. 2019;7:E871-E882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Abe S, Oda I, Shimazu T, Kinjo T, Tada K, Sakamoto T, Kusano C, Gotoda T. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer. 2011;14:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Cheng J, Wu X, Yang A, Jiang Q, Yao F, Feng Y, Guo T, Zhou W, Wu D, Yan X, Lai Y, Qian J, Lu X, Fang W. Model to identify early-stage gastric cancers with deep invasion of submucosa based on endoscopy and endoscopic ultrasonography findings. Surg Endosc. 2018;32:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Teh JL, Shabbir A, Yuen S, So JB. Recent advances in diagnostic upper endoscopy. World J Gastroenterol. 2020;26:433-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (2)] |
| 17. | Kobara H, Mori H, Fujihara S, Kobayashi M, Nishiyama N, Nomura T, Kato K, Ishihara S, Morito T, Mizobuchi K, Iwama H, Masaki T. Prediction of invasion depth for submucosal differentiated gastric cancer by magnifying endoscopy with narrow-band imaging. Oncol Rep. 2012;28:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kikuchi D, Iizuka T, Hoteya S, Yamada A, Furuhata T, Yamashita S, Domon K, Nakamura M, Matsui A, Mitani T, Ogawa O, Watanabe S, Kaise M. Usefulness of magnifying endoscopy with narrow-band imaging for determining tumor invasion depth in early gastric cancer. Gastroenterol Res Pract. 2013;2013:217695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Yao K, Nagahama T, Matsui T, Iwashita A. Detection and characterization of early gastric cancer for curative endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Kuroki K, Oka S, Tanaka S, Yorita N, Hata K, Kotachi T, Boda T, Arihiro K, Chayama K. Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer. 2021;24:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Cho I, Kwon IG, Guner A, Son T, Kim HI, Kang DR, Noh SH, Lim JS, Hyung WJ. Consideration of clinicopathologic features improves patient stratification for multimodal treatment of gastric cancer. Oncotarget. 2017;8:79594-79603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Yoon HJ, Kim S, Kim JH, Keum JS, Oh SI, Jo J, Chun J, Youn YH, Park H, Kwon IG, Choi SH, Noh SH. A lesion-based convolutional neural network improves endoscopic detection and depth prediction of early gastric cancer. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the kyoto classification of gastritis in identifying patients at high risk for gastric cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Kinoshita K, Torii A, Yamada A, Suzuki H, Koike K. Helicobacter pylori eradication improved the Kyoto classification score on endoscopy. JGH Open. 2020;4:909-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Toyoshima O, Nishizawa T, Yoshida S, Sakaguchi Y, Nakai Y, Watanabe H, Suzuki H, Tanikawa C, Matsuda K, Koike K. Endoscopy-based Kyoto classification score of gastritis related to pathological topography of neutrophil activity. World J Gastroenterol. 2020;26:5146-5155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (4)] |
| 26. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1332] [Article Influence: 333.0] [Reference Citation Analysis (2)] |
| 27. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 742] [Article Influence: 43.6] [Reference Citation Analysis (3)] |
| 28. | Yip HC, Uedo N, Chan SM, Teoh AYB, Wong SKH, Chiu PW, Ng EKW. An international survey on recognition and characterization of atrophic gastritis and intestinal metaplasia. Endosc Int Open. 2020;8:E1365-E1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yasunaga Y, Shinomura Y, Kanayama S, Yabu M, Nakanishi T, Miyazaki Y, Murayama Y, Bonilla-Palacios JJ, Matsuzawa Y. Improved fold width and increased acid secretion after eradication of the organism in Helicobacter pylori associated enlarged fold gastritis. Gut. 1994;35:1571-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Yasunaga Y, Bonilla-Palacios JJ, Shinomura Y, Kanayama S, Miyazaki Y, Matsuzawa Y. High prevalence of serum immunoglobulin G antibody to Helicobacter pylori and raised serum gastrin and pepsinogen levels in enlarged fold gastritis. Can J Gastroenterol. 1997;11:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Sakitani K, Nishizawa T, Arita M, Yoshida S, Kataoka Y, Ohki D, Yamashita H, Isomura Y, Toyoshima A, Watanabe H, Iizuka T, Saito Y, Fujisaki J, Yahagi N, Koike K, Toyoshima O. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter. 2018;23:e12503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4321] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 33. | Glover B, Teare J, Patel N. A systematic review of the role of non-magnified endoscopy for the assessment of H. pylori infection. Endosc Int Open. 2020;8:E105-E114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Ohyama I, Ohmiya N, Niwa Y, Shirai K, Taguchi A, Itoh A, Hirooka Y, Wakai K, Hamajima N, Mori N, Goto H. The association between tumour necrosis factor-alpha gene polymorphism and the susceptibility to rugal hyperplastic gastritis and gastric carcinoma. Eur J Gastroenterol Hepatol. 2004;16:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Yamamoto E, Toyota M, Suzuki H, Kondo Y, Sanomura T, Murayama Y, Ohe-Toyota M, Maruyama R, Nojima M, Ashida M, Fujii K, Sasaki Y, Hayashi N, Mori M, Imai K, Tokino T, Shinomura Y. LINE-1 hypomethylation is associated with increased CpG island methylation in Helicobacter pylori-related enlarged-fold gastritis. Cancer Epidemiol Biomarkers Prev. 2008;17:2555-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Tahara T, Tahara S, Horiguchi N, Kato T, Shinkai Y, Okubo M, Terada T, Yoshida D, Funasaka K, Nagasaka M, Nakagawa Y, Kurahashi H, Shibata T, Tsukamoto T, Ohmiya N. Prostate Stem Cell Antigen Gene Polymorphism Is Associated with H. pylori–related Promoter DNA Methylation in Nonneoplastic Gastric Epithelium. Cancer Prev Res (Phila). 2019;12:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Yasunaga Y, Shinomura Y, Kanayama S, Higashimoto Y, Yabu M, Miyazaki Y, Kondo S, Murayama Y, Nishibayashi H, Kitamura S, Matsuzawa Y. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996;39:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Yasunaga Y, Shinomura Y, Kanayama S, Higashimoto Y, Yabu M, Miyazaki Y, Murayama Y, Nishibayashi H, Kitamura S, Matsuzawa Y. Mucosal interleukin-1 beta production and acid secretion in enlarged fold gastritis. Aliment Pharmacol Ther. 1997;11:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Yasunaga Y, Nishibayashi H, Yamamori K, Higashimoto Y, Matsuzawa Y. Morphological and functional restoration of parietal cells in helicobacter pylori associated enlarged fold gastritis after eradication. Gut. 1999;45:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T, Iguchi M, Tamai H, Utsunomiya H, Yamamichi N, Fujishiro M, Iwane M, Tekeshita T, Mohara O, Ushijima T, Ichinose M. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Kanesaka T, Nagahama T, Uedo N, Doyama H, Ueo T, Uchita K, Yoshida N, Takeda Y, Imamura K, Wada K, Ishikawa H, Yao K. Clinical predictors of histologic type of gastric cancer. Gastrointest Endosc. 2018;87:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Feng F, Zheng G, Guo X, Liu Z, Xu G, Wang F, Wang Q, Guo M, Lian X, Zhang H. Impact of body mass index on surgical outcomes of gastric cancer. BMC Cancer. 2018;18:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |