Published online Sep 16, 2021. doi: 10.4253/wjge.v13.i9.416
Peer-review started: February 25, 2021
First decision: April 18, 2021
Revised: April 27, 2021
Accepted: July 21, 2021
Article in press: July 21, 2021
Published online: September 16, 2021
Processing time: 196 Days and 18 Hours
Coronavirus disease 2019 (COVID-19) significantly affected endoscopy practice, as gastrointestinal endoscopy is considered a risky procedure for transmission of infection to patients and personnel of endoscopy units (PEU).
To assess the impact of COVID-19 on endoscopy during the first European lockdown (March-May 2020).
Patients undergoing endoscopy in nine endoscopy units across six European countries during the period of the first European lockdown for COVID-19 (March-May 2020) were included. Prior to the endoscopy procedure, participants were stratified as low- or high- risk for potential COVID-19 infection according to the European Society of Gastrointestinal Endoscopy (ESGE) and the European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) joint statement, and contacted 7-14 d later to assess COVID-19 infection status. PEU were questioned regarding COVID-19 symptoms and/or infection via questionnaire, while information regarding hospitalizations, intensive care unit-admissions and COVID-19-related deaths were collected. The number of weekly endoscopies at each center during the lockdown period was also recorded.
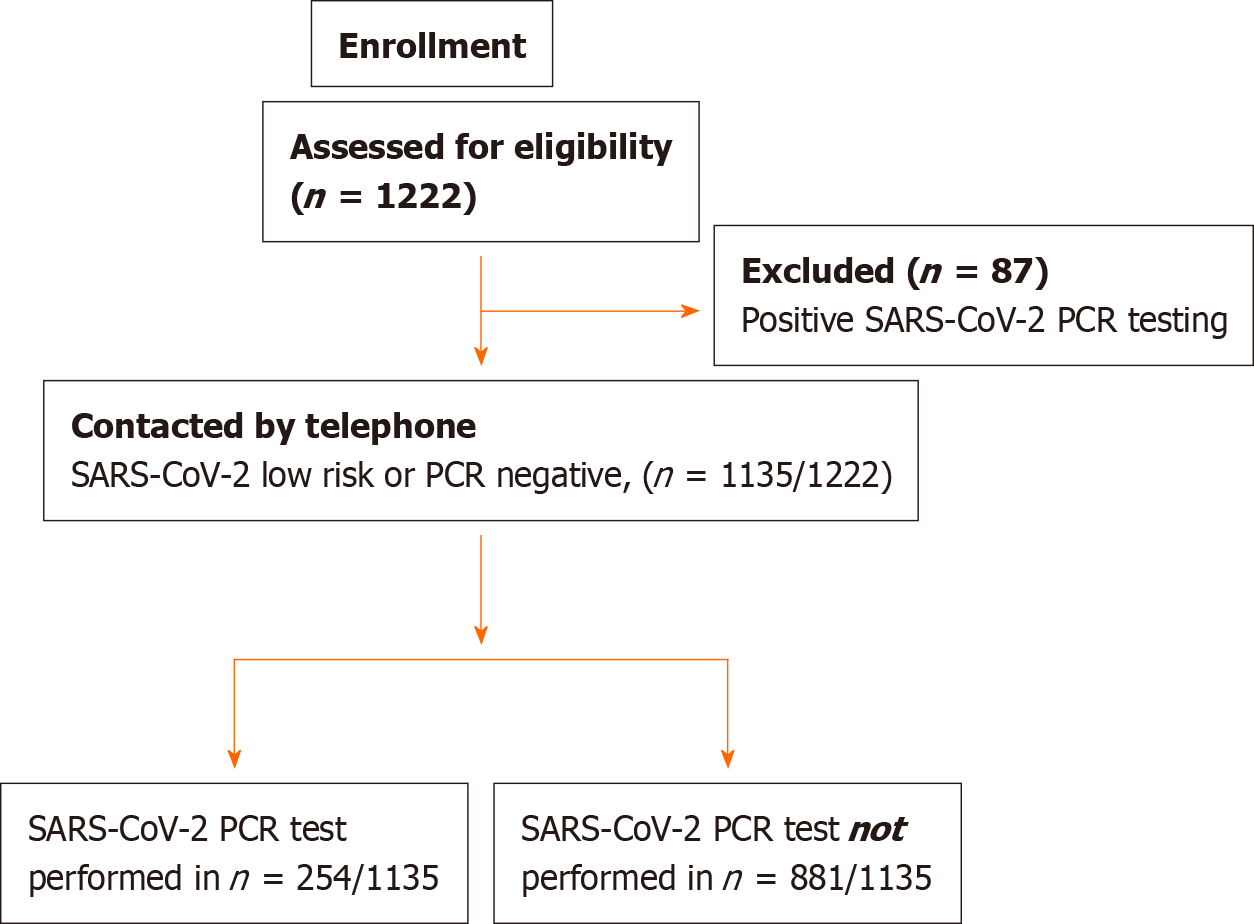
A total of 1267 endoscopies were performed in 1222 individuals across nine European endoscopy departments in six countries. Eighty-seven (7%) were excluded because of initial positive testing. Of the 1135 pre-endoscopy low risk or polymerase chain reaction negative for COVID-19, 254 (22.4%) were tested post endoscopy and 8 were eventually found positive, resulting in an infection rate of 0.7% [(95%CI: 0.2-0.12]. The majority (6 of the 8 patients, 75%) had undergone esophagogastroduodenoscopy. Of the 163 PEU, 5 [3%; (95%CI: 0.4-5.7)] tested positive during the study period. A decrease of 68.7% (95%CI: 64.8-72.7) in the number of weekly endoscopies was recorded in all centers after March 2020. All centers implemented appropriate personal protective measures (PPM) from the initial phases of the lockdown.
COVID-19 transmission in endoscopy units is highly unlikely in a lockdown setting, provided endoscopies are restricted to emergency cases and PPM are implemented.
Core Tip: The Coronavirus disease 2019 (COVID-19) pandemic outbreak caused an unprecedented disruption in everyday endoscopy practice worldwide, with recent guidelines advocating suspension of nonemergency endoscopies, implementation of strict personal protection measures (PPM) and post-endoscopy evaluation of patient COVID-19 status. This was an international multicenter study seeking to evaluate the impact of COVID-19 on endoscopy during the first European lockdown (March-May 2020). COVID-19 transmission across endoscopic units proved to be highly unlikely in lockdown circumstances as long as endoscopy performance was restricted to emergency cases and sufficient PPM are available.
- Citation: Papanikolaou IS, Tziatzios G, Chatzidakis A, Facciorusso A, Crinò SF, Gkolfakis P, Deriban G, Tadic M, Hauser G, Vezakis A, Jovanovic I, Muscatiello N, Meneghetti A, Miltiadou K, Stardelova K, Lacković A, Bourou MZ, Djuranovic S, Triantafyllou K. COVID-19 in the endoscopy unit: How likely is transmission of infection? Results from an international, multicenter study. World J Gastrointest Endosc 2021; 13(9): 416-425
- URL: https://www.wjgnet.com/1948-5190/full/v13/i9/416.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i9.416
The coronavirus disease 2019 (COVID-19) pandemic has spread throughout the world in a short period of time, rapidly affecting medical practice. Although the disease usually manifests with respiratory symptoms, gastrointestinal (GI) symptoms are not rare and, in some cases, constitute the basic clinical manifestations[1,2]. GI endoscopy is considered a risky procedure for transmission of the infection. During endoscopy, close contact of the endoscopist with the patient takes place, respiratory droplets and aerosols are generated, and contact with contaminated material, body fluids, and feces is likely to occur. Moreover, endoscopy also involves the assisting personnel of the unit (PEU). The PEU include not only the endoscopist, but also nurses and paramedical staff. In light of these considerations, specific protective measures and disinfection procedures have been recommended by scientific societies and recognized experts[3-5]. Endoscopic societies such as the European Society of Gastrointestinal Endoscopy (ESGE) and the European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) recently published a joint position statement for GI endoscopy during the COVID-19 pandemic regarding safe endoscopies for patients and PEU[3]. The statement suggests minimizing nonemergency endoscopies, implementation of personal protection measures (PPM), and post-endoscopy calls to patients 7 d and 14 d after the endoscopy to check their COVID-19 status. In a study from the heavily affected north of Italy, the number of post-endoscopy COVID-19 infections was negligible and the number of infected PEU was very small[6]. The aim of this European multicenter study was to evaluate the impact of endoscopic procedures on the risk of transmission for patients and PEU using the telephone as contact tool as suggested by ESGE and ESGENA.
This was an international, multicenter study conducted during the period of the first European lockdown for COVID-19 (March-May 2020) in nine high-volume endoscopy departments across six European countries: Athens, Greece (two centers), Foggia/Verona, Italy (two centers), Brussels, Belgium, Skopje, Republic of North Macedonia, Zagreb/Rijeka, Croatia (two centers), and Belgrade, Serbia. The centers were included based on their high volume of endoscopic procedures prior to the COVID-19 outbreak and because they represented regions with a high prevalence of the disease on one side of the spectrum (Verona and Brussels) as well as regions with a lower prevalence of COVID-19 in southern Europe. This was an analysis of retrospectively collected data within a prospectively built database.
All consecutive patients undergoing any endoscopic procedure, including upper and lower GI endoscopy (colonoscopy or rectosigmoidoscopy), endoscopic retrograde cholangiopancreatography (ERCP), or endoscopic ultrasonography (EUS) during the aforementioned period and involving each of the abovementioned PEU were considered eligible for inclusion.
Patients undergoing endoscopy: Following the triage protocol at each center, on the day of the endoscopy or the day before, all patients were questioned by the predetermined local study coordinator for symptoms and contacts that could be linked to COVID-19 and then stratified as low- or high-risk of potential COVID-19 infection, according to the ESGE/ESGENA joint statement[3]. Demographic data and procedural information regarding the endoscopy performed as well as previous performance of testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were also recorded. Following the ESGE/ESGENA joint statement recommendation regarding post-procedure risk management[3], local study coordinators contacted the patients by telephone on day 7 and day 14 after the endoscopy to inquire about any new COVID-19 diagnosis, or development of COVID-19 symptoms. The calls were carried out using a structured questionnaire that was identical across all centers (Supplementary Table 1) and filled out for each patient. Polymerase chain reaction (PCR) testing a posteriori was possible at physician’s discretion after the endoscopic procedure on a case-by-case basis, taking into account each patient’s clinical status. For those who tested positive after the endoscopic procedure, additional information regarding need for hospitalization, intensive care unit (ICU) admission for COVID-19 and COVID-19-related deaths were also collected.
PEU: The PEU were questioned regarding potential COVID-19 symptoms and/or SARS-CoV-2 infection with the use of a structured questionnaire (Supplementary Table 2). PEU included not only medical and nursing staff, but also assisting staff working in the unit who could contact patients or material potentially infected by SARS-CoV-2, i.e. cleaning personnel, transporters, and secretarial staff. For those positive for SARS-CoV-2, information regarding hospitalization, ICU admission and COVID-19-related deaths were collected. Additionally, the final part of the questionnaire recorded the total number of endoscopies conducted pre-, during and post-implementation of COVID-19-transmission preventative measures.
The primary endpoint of the study was the incidence of infection among patients who underwent endoscopy during the established time period. Secondary endpoints were: (1) Incidence and outcome of hospitalization, ICU admission for COVID-19, and COVID-19-related deaths among patients who tested positive; (2) Prevalence of COVID-19 symptoms and/or positive SARS-CoV-2 testing among PEU; (3) Incidence and outcome of hospitalization, ICU admission for COVID-19, and COVID-19-related deaths among PEU who tested positive; and (4) Percentage decrease in the overall number of endoscopies before and after implementation of lockdown measures and implementation of PPM in the study centers. For the purposes of this study, only PCR testing was deemed adequately accurate for confirmation of infection. Rapid tests, when performed, needed to be confirmed by PCR.
Categorical data were reported as numbers and percentages (%) with their 95%CIs. The distribution of quantitative data was evaluated for normality by the Kolmogorov–Smirnov statistic and reported as means ± SD or means and interquartile range (IQR) depending to their distribution. A P value < 0.05 was considered significant. A statistical review of the study was performed by a biomedical statistician (IP).
The protocol of this study was reviewed and approved by the local institutional review board (BΠΠΚ EBΔ 320/10-6-20). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with good clinical practice.
Overall, 1267 endoscopies were performed in 1222 patients during the study time period. Of those, 87 (7%) were excluded because of initial positive testing. The remaining 1135 patients were enrolled in the study (Figure 1). Baseline patient baseline characteristics and recruitment at center are presented in Table 1.
| Patients characteristics | |
| Male/female | 678 (59.7)/457 (40.3) |
| Age (mean ± SD), yr | 63.4 ± 14.5 |
| Inpatient | 506 (44.6) |
| Outpatient | 598 (52.7) |
| Referral | 31 (2.7) |
| Recruitment per center | |
| "Attikon" Hospital, Athens, Greece | 236 (20.8) |
| Aretaieio Hospital, Athens, Greece | 42 (3.7) |
| Foggia, Italy | 215 (18.9) |
| Verona, Italy | 235 (20.7) |
| Belgrade, Serbia | 19 (1.7) |
| Brussels, Belgium | 143 (12.6) |
| Skopje, Republic of North Macedonia | 149 (13.1) |
| Zagreb/Rijeka, Croatia | 96 (8.5) |
| Type of endoscopy1 | |
| Upper GI-endoscopies | 587 (46.3) |
| Colonoscopies/rectosigmoidoscopies | 444 (35.1) |
| ERCP | 178 (14.1) |
| EUS | 57 (4.5) |
Among the 1135 enrolled patients, 254 (22.4%) were retested the days following endoscopy because of the onset of new symptoms that could indicate a potential COVID-19 infection. Eight (n = 8) were eventually found positive. The incidence of infection among patients undergoing endoscopy was thus 0.7% (95%CI: 0.2-0.12). Of those eight patients, the majority had undergone upper GI endoscopy (n = 6/8, 75%). A negative pre-endoscopy PCR test was available in only 1 case. A detailed overview of the infected characteristics of the patients is presented in Table 2.
| Case | Patient, age | Endoscopy | Date of endoscopy | COVID PCR test before endoscopy | Contact of suspected or confirmed COVID 19 case after endoscopy | Symptoms | COVID PCR test after endoscopy | Outcome of those hospitalized | Case related to endoscopy |
| 1 | Female, 66 yr | Upper GI | March 12, 2020 | No | No | Fever and cough | Tested positive March 18, 2020 | Death/deceased due to COVID-19 | Cannot reasonably exclude |
| 2 | Male, 81 yr | Upper GI | April 8, 2020 | No | No | Fever, cough and sore throat since April 17 for 42 d | Hospital admission April 12, 2020, tested positive and had Pneumonia | Death May 4/deceased due to COVID-19 | Cannot reasonably exclude |
| 3 | Male, 66 yr, head/neck cancer and arterial disease | Upper GI | March 18, 2020 | No | Yes with suspected case | Fever and Diarrhea since March 27, 2020 | Tested positive March 28, 2020 | Death May 7 due to cancer | Cannot reasonably exclude |
| 4 | Male, 55 yr, cancer esophagus | Upper GI | March 18, 2020 | No | Yes with suspected case | Cough since March 16, 2020 | Tested positive March 24, 2020 | Discharge | No |
| 5 | Male, 76 yr, cancer stomach, 2, COPD | EUS | March 24, 2020 | No | Yes with suspected case | Cough since March 19, 2020 | Tested positive Apirl 23, 2020 | Became negative/remained at nursing home | No |
| 6 | Female, 66 yr, AML | Lower GI | Apirl 1, 2020 | Yes March 30, 2020negative | Yes with suspected case | Fever since April 3, 2020 for 6 d | Tested positive Apirl 10, 2020 | Death May 4 due to cancer/at home | Cannot reasonably exclude |
| 7 | Male, 48 yr | Upper GI | March 27, 2020 | No | No | Fever and cough since April 8, 2020 for 4 d | Tested positive Apirl 12, 2020 | Not hospitalized | No |
| 8 | Male, 63 yr, diabetes, lung disease, IBD | Upper GI | March 30, 2020 | No | Yes with suspected case | Fever and cough since April 22, 2020 for 2 d | Tested positive Apirl 22, 2020 | Not hospitalized | No |
Of the 8 SARS-CoV-2-positive cases, 2 (25%) presented with a very mild illness and did not require hospitalization at all; the other 6 (75%) were hospitalized at some point, with 2 of them (33.3%) ultimately dying of COVID-19. Another 2 patients (33.3%) died, but the cause of death was considered to be their underlying cancer. The remaining 2 (33.3%) were discharged to home and to a nursing residency.
Overall, the data included the COVID-19 infection status of 163 PEU from all 9 PEU. Eighty-four of the 163 (51.5%) were physicians (attendings as well as trainees), 62/163 (38%) were nurses and 17/163 (10.4%) were assisting staff working exclusively (or mostly) in the PEU (i.e. cleaning personnel, transporters, and secretarial staff of the units). Overall, 5/163 of the total PEU tested positive during the study period (2 physicians and 3 nurses), giving a 3% (95%CI: 0.4-5.7) incidence of infection. The majority of the infections (n = 4, 80%) were considered to be associated with the work environment. Those cases represent 2.3% (4/163) of the total PEU in our study and 7% and 16.6% of the PEU of their own units, respectively. None (0/5) of the infected PEU developed severe disease, none required hospitalization, and no COVID-19-related deaths occurred in the PEU who were included in our study.
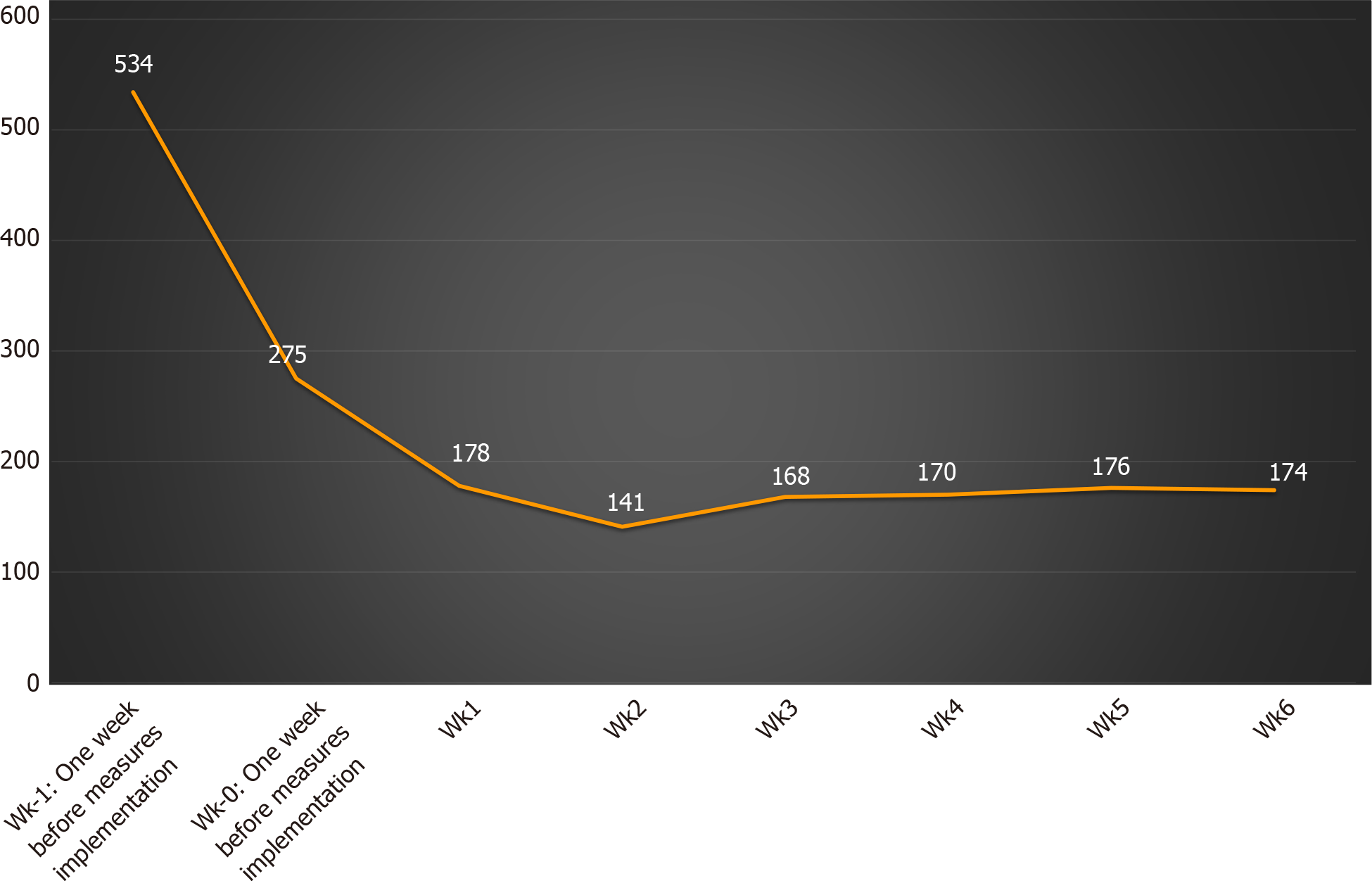
PPM in accord with the ESGE/ESGENA position statement regarding reduction of cases to focus on emergency therapies, i.e. gowns, goggles, and masks, were implemented and adhered to in all participating centers during the initial phase of the study, which continued from 9 to 23 March, 2020. Overall, a significant reduction in the number of endoscopies was evident in all the participating centers after March 2020 (Figure 2). In detail, 1 wk before implementation of the ESGE/ESGENA position statement suggestions, the total number of endoscopies across all centers was 534 (246 upper GI-endoscopies, 209 colonoscopies/rectosigmoidoscopies, 56 ERCPs and 23 EUS). During the following 6 wk, the number gradually dropped, reaching a plateau with a mean of 167 ± 14 endoscopies per week, an estimated 68.7% (95%CI: 64.8-72.7) decrease in the performance of endoscopic procedures.
Endoscopic procedures were deemed as risky procedures for bidirectional COVID-19 infection transmission[1,2,7,8]. In this analysis of retrospectively collected data within a prospectively built database conducted across nine European endoscopic facilities, we showed that the risk of COVID-19 infection for patients undergoing GI endoscopy was extremely low in a lockdown setting. The results underline the value of following ESGE/ESGENA recommendations to address the danger of COVID-19 infection in everyday, real-world clinical practice.
Although COVID-19 infection and its potential implications have been at the focal point of ongoing research worldwide, evidence regarding this risk of healthcare professional and patient infection after endoscopy remain scarce[9]. In one of the few studies, Repici et al[6] retrospectively analyzed data from 802 patients and 968 PEU in 41 hospitals in northern Italy. Their results suggested that the number of post-endoscopy patient infections was negligible, i.e. 1 infection in 802 patients for a confirmed infection rate of 0.12%. Similarly in a much smaller multicenter, retrospective study that evaluated patients who underwent stent placement for upper GI obstruction[10]; only 1 of 29 patients (3.4%) tested positive for SARS-CoV-2 after the procedure. All the medical staff involved in the stenting procedures remained COVID-19 free 14 d later. The results of our multicenter study are also in line with those, as only 8 of the 1135 patients who were deemed pre-endoscopy SARS-CoV-2 low risk or negative, became positive. The results are further corroborated by the findings of a recent cross-sectional study. In a high-volume Japanese endoscopic facility, not a single positive result was detected among 783 PCR-analyzed saliva samples from patients undergoing endoscopic procedures[11].
Regarding PEU infection after endoscopy, our study is consistent with that of Repici et al[6], who found a very low risk of PEU contamination. Indeed, the Italian study reported a very small number of infected PEU (42 cases, or only 4.3% of the PEU population in their study), with 85.7% of the infections occurring before PPM were introduced. Even for the PEU who were infected, fewer than 1% needed hospitalization and none required admission in ICU or died[6]. Outside Europe, the risk of COVID-19 infection of PEU may be higher, up to 23.9%, especially in endoscopy technicians[12]. Our study had even more impressive results, with only 5 PEU testing positive during the study period, representing a 3% of the total PEU involved in the endoscopies that were performed in the study. In only 4 of the total PEU, 1 physician and 3 nurses, was the infection considered to be linked to their work. As in the Italian study, none of the infected PEU in our study developed severe disease, required hospitalization, or died, compared with 2 COVID-19-related deaths that occurred in the 8 patients who became positive post endoscopy. Whether that was merely a random association or a result of the younger age and better health status of the PEU compared with that of our patient population, who were severely ill individuals undergoing emergency endoscopies, remains unclear. Published data suggest that PEU, when affected, experience relatively mild disease, but as the numbers were extremely small, we cannot provide further insights[5,6]. Notably, a case-by-case analysis revealed a clustering of infections, as all PEU found positive worked in a unit performing almost exclusively ERCPs. A possible explanation could be based on the longer duration of those particular examinations compared with standard upper GI-endoscopies, resulting in increased risk for transmission.
Pre-endoscopic testing for COVID-19 was available only for one-fourth of the patients of our study (326/1222, 26.7%). One might consider that to be a low percentage; however, it should be noted that this policy is in accordance with the ESGE/ESGENA recommendations that do not advocate SARS-CoV-2 tests as a prerequisite for GI endoscopy. On the contrary, they put a spotlight on appropriate triaging of nonemergency endoscopies and PPM. Our low post-endoscopy infection rates of both patients and PEU seem to justify those suggestions.
The finding that the COVID-19 pandemic led to a significant reduction in the volume of endoscopic procedures is not novel. Beyond patient stratification as low- or high-risk of COVID-19 infection, the position ESGE/ESGENA statement for GI endoscopy during the COVID-19 pandemic also clearly lists which endoscopic procedures should be definitely performed and which can be postponed. That policy was uniformly applied at all the participating centers of our study. Thus, all the endoscopies performed in our series, if not emergency, were nevertheless completely necessary; none were purely elective. Still, the optimal policy, when resumption of endoscopy services comes into question, remains to be elucidated. In that regard, a stepwise approach that takes: (1) The regional prevalence of COVID-19 with stricter guidelines in endoscopy and use of PPE in high-prevalence (> 2%) areas[13]; (2) Patient stratification for procedures that should be performed immediately or postponed, as well as low- or high-risk of infection[3]; and (3) Modifications in PEU working schedules to prevent hospital-based transmission into account seems the most appropriate[14,15].
A number of study strengths should be cited. First, this iteration is one of the few studies addressing the question of the safety of endoscopy during the COVID-19 pandemic. Second, we enrolled patients in different countries, giving a more representative overview of the impact of COVID-19 outbreak on endoscopy units. Third, our questionnaire content was guided by the ESGE/ESGENA position statement. Finally, our population was homogenous, including patients who underwent endoscopic procedures involving both the upper and lower GI tract as well as the respective participating PEU.
On the other hand, there are also limitations that merit attention. The lack of SARS-CoV-2 testing of patients presenting for endoscopy without COVID-19 symptoms and heterogeneity of PEU testing can initially be seen as such; but that practice was in accord with endoscopy society recommendations including those of the ESGE/ESGENA). The practice should therefore be considered unavoidable, but it undoubtedly had an impact on our epidemiological data, as the percentage of asymptomatic patients in our group remains unknown and hinders the complete tracking of the infection. Another shortcoming is the possibility of recall bias, given that the study data was acquired by asking patients to recall their symptoms. Again, that was unavoidable, as it complied with the ESGE/ESGENA directive stating that patients should be contacted 7 d and 14 d post endoscopy. Finally, the small number of positive cases and study design prevent a definitive causal relationship to be established. However, aim of the study was not to address issues related to potential routes of infection, but rather to investigate the actual possibility of COVID-19 transmission in endoscopy units when established guidelines are implemented.
In conclusion, COVID-19 transmission in endoscopy units is a highly unlikely event for both patients and PEU in a lockdown setting, provided endoscopies are effectively restricted to emergency cases and appropriate, stringent PPM are implemented. In the extremely rare cases of PEU infection in our series, the disease was relatively mild, with no hospitalizations or COVID-19-related deaths.
The coronavirus disease 2019 (COVID-19) outbreak significantly affected endoscopic practice, as gastrointestinal endoscopy is considered as a risky procedure for transmission of infection. The ESGE and ESGENA published a position statement for endoscopy during the COVID-19 pandemic regarding the safety of endoscopies for patients and the personnel of endoscopy units (PEU). However, the incidence and outcome of infection among patients undergoing endoscopy and PEU remains to be determined.
Currently, there is insufficient data regarding the incidence and outcomes of COVID-19 infection among patients undergoing endoscopy and in PEU.
We aimed to evaluate the impact of endoscopic procedures on the risk of transmission to patients and PEU in a European multicenter study, using telephone contact as a tool as suggested by the ESGE and ESGENA.
Patients undergoing endoscopy in nine endoscopy departments across six European countries during the period of the first European lockdown for COVID-19 (March-May 2020) were included. Participants were stratified as low- or high-risk for potential COVID-19 infection according to the ESGE/ESGENA joint statement were contacted 7 d and 14 d later to assess COVID-19 infection status. PEU were questioned regarding COVID-19 symptoms and/or infection by questionnaire. Information on hospitalizations, ICU-admissions, and COVID-19-related deaths were collected. The number of weekly endoscopies during the lockdown period was also recorded.
A total of 1267 endoscopies were performed in 1222 individuals; 87 (7%) were excluded following initial positive PCR testing. The remaining 1135 individuals were at low risk or PCR negative for COVID-19 before endoscopy, and of 254 (22.4%) who were tested post endoscopy, eight were eventually found positive, resulting in an infection rate of 0.7% (95%CI: 0.2-0.12). The majority, (6/8, 75%) had undergone esophagogastroduodenoscopy. Data were available for 163 PEU, and 5 (3%; 95%CI: 0.4-5.7) tested positive during the study period. In 4 of the 5, or 2% of the total, the infection was deemed relevant to their work environment. A decrease of 68.7% (95%CI: 64.8-72.7) in the number of endoscopies was recorded.
This study showed that COVID-19 transmission in endoscopic units was highly unlikely during a lockdown setting, provided endoscopies were restricted to emergency cases and PPM were implemented.
More robust data are definitely warranted to identify various clinical factors that contribute to an increased risk of endoscopy-related COVID-19 infection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Arabey AA, Kamran M, Sánchez de Medina LH F, Şehirli AÖ S-Editor: Liu M L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 949] [Article Influence: 189.8] [Reference Citation Analysis (1)] |
| 2. | Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, Gao XZ, Qu T, Wang MY. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 3. | Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellisè M, Arvanitakis M, Bhandari P, Bisschops R, Van Hooft JE, Kaminski MF, Triantafyllou K, Webster G, Pohl H, Dunkley I, Fehrke B, Gazic M, Gjergek T, Maasen S, Waagenes W, de Pater M, Ponchon T, Siersema PD, Messmann H, Dinis-Ribeiro M. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52:483-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 4. | Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, Carrara S, Fugazza A, Di Leo M, Galtieri PA, Pellegatta G, Ferrara EC, Azzolini E, Lagioia M. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 5. | Lui RN. Safety in Endoscopy for Patients and Healthcare Workers During the COVID-19 Pandemic. Tech Innov Gastrointest Endosc. 2021;23:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Repici A, Aragona G, Cengia G, Cantù P, Spadaccini M, Maselli R, Carrara S, Anderloni A, Fugazza A, Pace F, Rösch T; ITALIAN GI-COVID19 Working Group. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020;69:1925-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Ang TL. Gastrointestinal endoscopy during COVID-19 pandemic. J Gastroenterol Hepatol. 2020;35:701-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 9. | Ginès À, Fernández-Esparrach G, Pellisé M, Sendino O, Balaguer F, Llach J, González-Suárez B, Saló S. Critical importance of early introduction of prevention measures for SARS-CoV-2 infection in endoscopy units. Gastrointest Endosc. 2020;92:936-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Rodrigues-Pinto E, Ferreira-Silva J, Fugazza A, Capogreco A, Repici A, Everett S, Albers D, Schumacher B, Gines A, Siersema PD, Macedo G. Upper gastrointestinal stenting during the SARS-CoV-2 outbreak: impact of mitigation measures and risk of contamination for patients and staff. Endosc Int Open. 2021;9:E76-E86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Miyake S, Ashikari K, Kato S, Takatsu T, Kuwashima H, Kaneko H, Nagai K, Watari I, Sato T, Yamaoka Y, Yamamoto T, Ryo A, Maeda S, Nakajima A, Higurashi T. Severe acute respiratory syndrome coronavirus 2 prevalence in saliva and gastric and intestinal fluid in patients undergoing gastrointestinal endoscopy in coronavirus disease 2019 endemic areas: Prospective cross-sectional study in Japan. Dig Endosc. 2021 epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kumar Goenka M, Bharat Shah B, Goenka U, Das SS, Afzalpurkar S, Mukherjee M, Patil VU, Jajodia S, Ashokrao Rodge G, Khan U, Bandopadhyay S. COVID-19 prevalence among health-care workers of Gastroenterology department: An audit from a tertiary-care hospital in India. JGH Open. 2021;5:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Bhandari P, Subramaniam S, Bourke MJ, Alkandari A, Chiu PWY, Brown JF, Keswani RN, Bisschops R, Hassan C, Raju GS, Muthusamy VR, Sethi A, May GR, Albéniz E, Bruno M, Kaminski MF, Alkhatry M, Almadi M, Ibrahim M, Emura F, Moura E, Navarrete C, Wulfson A, Khor C, Ponnudurai R, Inoue H, Saito Y, Yahagi N, Kashin S, Nikonov E, Yu H, Maydeo AP, Reddy DN, Wallace MB, Pochapin MB, Rösch T, Sharma P, Repici A. Recovery of endoscopy services in the era of COVID-19: recommendations from an international Delphi consensus. Gut. 2020;69:1915-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Gupta S, Shahidi N, Gilroy N, Rex DK, Burgess NG, Bourke MJ. Proposal for the return to routine endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:735-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Hennessy B, Vicari J, Bernstein B, Chapman F, Khaykis I, Littenberg G, Robbins D. Guidance for resuming GI endoscopy and practice operations after the COVID-19 pandemic. Gastrointest Endosc. 2020;92:743-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |