Published online Jun 16, 2021. doi: 10.4253/wjge.v13.i6.189
Peer-review started: February 1, 2021
First decision: March 1, 2021
Revised: March 12, 2021
Accepted: May 21, 2021
Article in press: May 21, 2021
Published online: June 16, 2021
Processing time: 128 Days and 21 Hours
Primary aorto-enteric fistula (PAEF) is a rare condition, traditionally treated in the acute, bleeding phase with open surgery or endovascular repair. However, these approaches have high morbidity and mortality, indicating a need for new methods. With advances in endoscopic techniques and equipment, haemoclipping of fistulas has now become feasible. Therefore, we present a systematic review of the English literature and a rare case of a PAEF successfully treated by endoscopic haemoclipping.
A 74-year-old man with an abdominal aortic aneurysm presented with symptoms of haemorrhagic shock and bloody stools. An oesophago-gastro-duodenoscopy was performed with haemoclipping of a suspected PAEF in the third part of the duodenum. Afterward, a computed tomography-angiography showed a contrast filled protrusion from the abdominal aortic aneurysm. Based on the clinical presentation and the combined endoscopic and radiographic findings, we argue that this is a case of a PAEF.
Endoscopic therapy appears capable of achieving haemodynamic stabilisation in patients with bleeding PAEF, serving as a bridge to final therapy.
Core Tip: Primary aorto-enteric fistula is a rare condition with high mortality. The current acute phase treatment is surgical or endovascular and is followed by high morbidity and mortality. The aim of this systematic review and case report was to put forward endoscopic haemoclipping as a new treatment option in the acute bleeding phase of an aorto-enteric fistula and, in a systematic manner, to search the literature for any evidence behind this therapy, including other reported cases.
- Citation: Berner-Hansen V, Olsen AA, Brandstrup B. Endoscopic treatment of primary aorto-enteric fistulas: A case report and review of literature. World J Gastrointest Endosc 2021; 13(6): 189-197
- URL: https://www.wjgnet.com/1948-5190/full/v13/i6/189.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i6.189
A primary aorto-enteric fistula (PAEF) is a communication between the native aorta and the enteric system without prior surgery on the aorta[1]. Traditionally, treatment of a PAEF with acute bleeding includes open emergency surgical repair with in situ reconstruction or an extra-anatomic bypass of the aorta combined with repair of the bowel lesion. Over the last decades, endovascular aortic stenting has been introduced as a minimally invasive treatment option for abdominal aneurysms[2-4]. Closure of enterovascular fistulas from the vascular side is associated with better early survival than open surgery. A review of literature by Saers et al[5] reported an overall 30-d mortality rate for patients with PAEF of 44% and a mortality of 34% for patients who have had surgical treatment. By comparison, they found a 30-d mortality of 14% in a small group of patients who had endovascular repair. However, most of the benefit of lower mortality rates for patients treated with endovascular aortic stent was lost during long-term follow-up due to recurrent infection or recurrent AEF[6,7].
An aorto-duodenal fistula often presents itself with upper gastrointestinal bleeding and circulatory shock, which are symptoms identical to those of the much more common bleeding duodenal or gastric ulcers. Oesophago-gastro-duodenoscopy (OGD) is recommended as the first diagnostic choice in patients with signs of upper gastrointestinal haemorrhage[8,9]. OGD is, in most cases, successful in achieving haemostasis in patients with bleeding ulcers, and dual therapy with injection of epinephrine, electrocoagulation, or haemoclipping of the bleeding site or vessel is the state of art technique[10]. Sometimes, however, the bleeding is not caused by an ulcer but by a Dieulafoy lesion, a Cameron lesion, or bleeding from varicose veins among other reasons. Endoscopic treatment is also effective in achieving haemostasis and preventing rebleeding in these cases[11]. With advances in endoscopic techniques and equipment, large clips for the closure of vascular-enteric fistulas have come forward[12]. Per the literature and in our institution, such clips are successfully used for endoscopic closure of full-thickness gastrointestinal wall defects as well as interenteric or enterocutaneous fistulas[13,14]. Thus, clipping of fistulas has now become feasible, and the clipping of aorta-duodenal fistulas might be the next step forward.
The aim of this systematic review and case report was to put forward endoscopic haemoclipping as a new treatment option in the acute bleeding phase of an AEF and in a systematic manner to search the literature for any evidence behind this therapy, including other reported cases.
This case report adheres to the SCARE criteria[15]. The systematic review follows the PRISMA guidelines[16]. The protocol was registered in Prospero (number CRD42019142202) before the literature search was commenced. Included were all original studies, including case reports describing symptomatic PAEFs treated with therapeutic endoscopy. A PAEF was defined as a fistula from the thoracic or abdominal aorta to the intestines, including the duodenum, ileum, jejunum, and all segments of the colon. Endoscopic treatment was defined as all therapeutic endoscopic procedures. Excluded were papers describing treatment of secondary fistulas, fistulas from other branches of the aorta and other segments of the gastrointestinal tract, and papers not using endoscopy for treatment. The language was limited to English.
We performed an electronic literature search in PubMed, Embase, and The Cochrane Library for articles published between January 1999 and April 2020. The search was built as a “text word” search combining synonyms for “aortoenteric fistula” and “primary” and limited to English literature. The first author screened all titles and abstracts of the retrieved studies and performed full-text assessments to determine the inclusion. In any case of doubt, the second author assessed the study. Disagreements were resolved through discussion and consultation of the supervisor (BB).
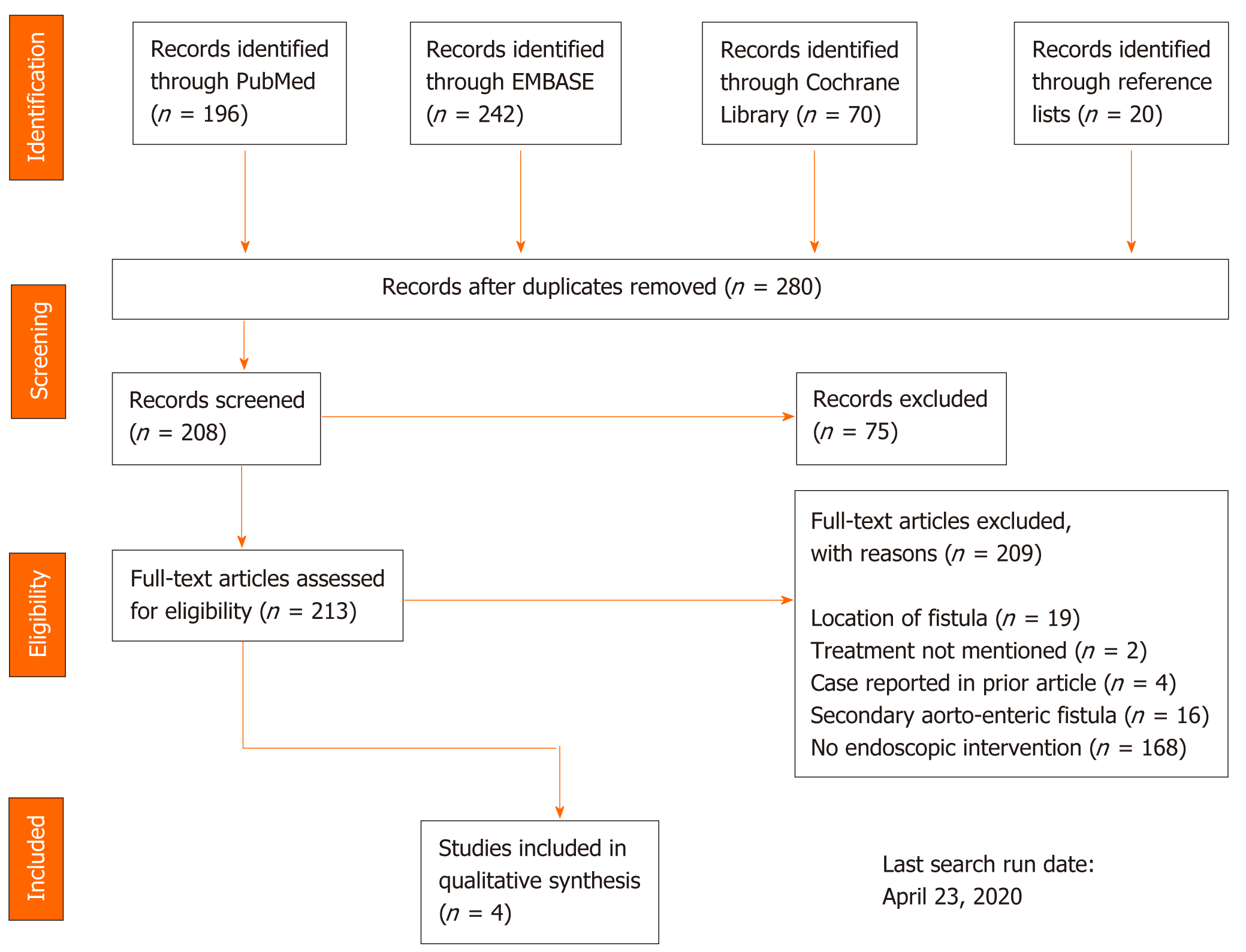
Additional articles were found by screening reference lists of studies included after full-text assessment. To assess the risk of bias, the included studies were analysed according to the assessment tool provided by Murad et al[17]. Figure 1 shows the PRISMA search strategy and the inclusion of studies. The data extracted were patient age, gender, anatomic location of the fistula, endoscopic finding, endoscopic treatment modality, subsequent treatment, follow-up length, and outcome. Because we did not expect to find any randomized clinical trials or high-quality cohort studies, we did not plan for any statistical analysis.
A 74-year-old man was admitted to our department with fresh bloody stools and melena during the evening.
Earlier on the same day, the patient was discharged after 4 d of admission due to gastrointestinal bleeding. During the earlier admission, an OGD to the second part of the duodenum revealed two small (8.0 mm) fibrin-covered ulcers in the duodenum with no sign of bleeding. Because this was the only pathological finding and considering the patient’s history, they were treated with dual therapy (injection of diluted epinephrine and electrocoagulation). Also, the patient received blood transfusions, intravenous fluid, and pantoprazole. During the following 4 d, the patient had minor episodes of dark stools but no fresh bleeding, and the haemoglobin levels increased.
The patient had several comorbidities including oropharynx cancer, chronic obstructive pulmonary disease, paroxysmal atrial fibrillation, and essential arterial hypertension. Also, the patient had an infrarenal abdominal aortic aneurysm with a diameter of 6.3 cm and a long neck of 2.2 cm in diameter, and an aneurysm on the right common iliac artery measuring 5.0 cm. The patient had regular check-ups for his aorta aneurysm at a vascular surgical department in another hospital.
At admission, the patient was pale but alert with a blood pressure of 95/70 mmHg and a heart rate of 68 beats per minute. On physical examination, auscultation of heart and lungs was normal, the abdomen was soft and without tenderness, but a pulsating filling could be felt to the left of the umbilicus. The rectal examination showed melena with fresh blood.
The plasma haemoglobin was 4.0 mmol/L.
The final diagnosis of the presented case is, in our opinion, PAEF, based on the combined endoscopic and CT-angiographic findings.
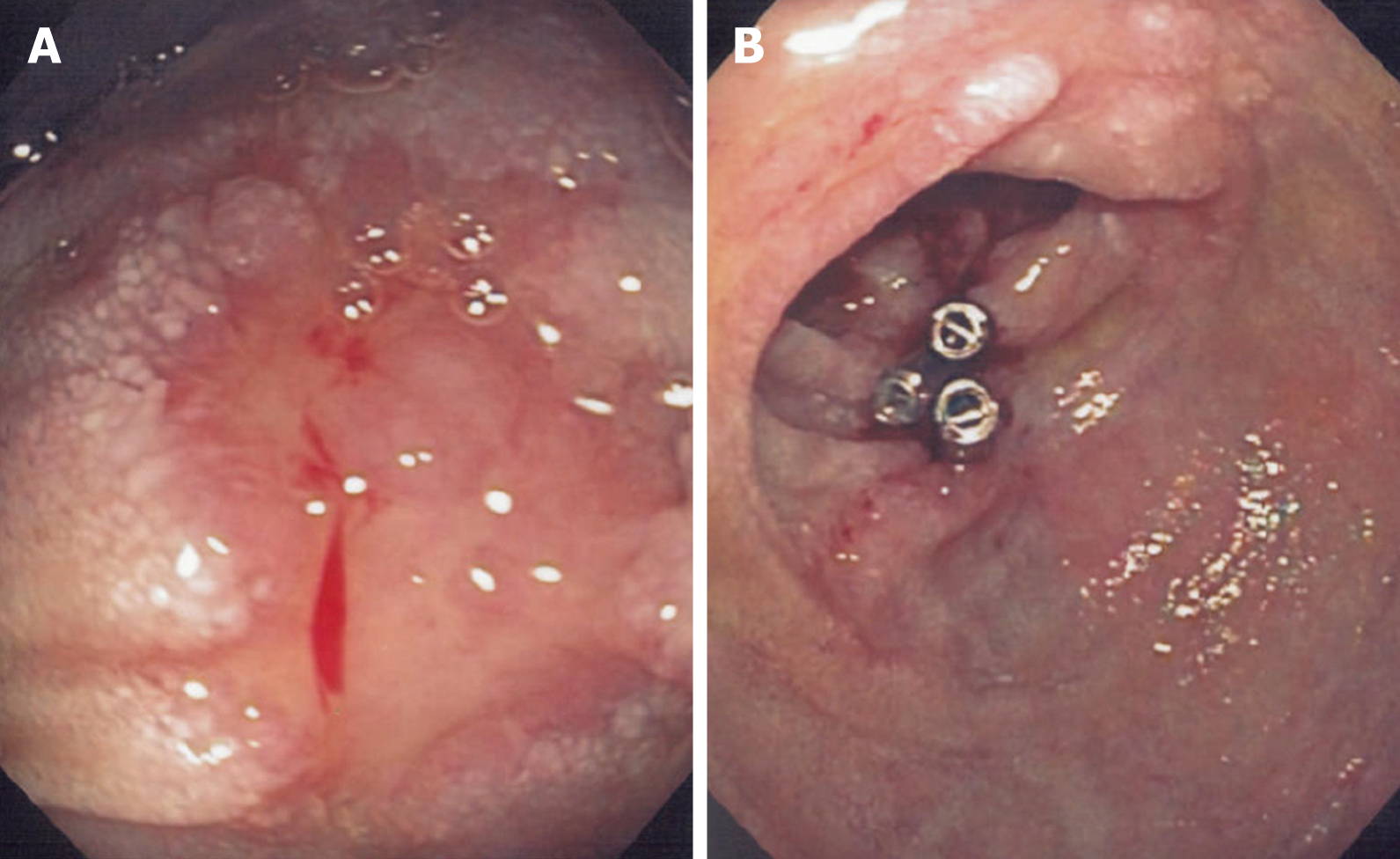
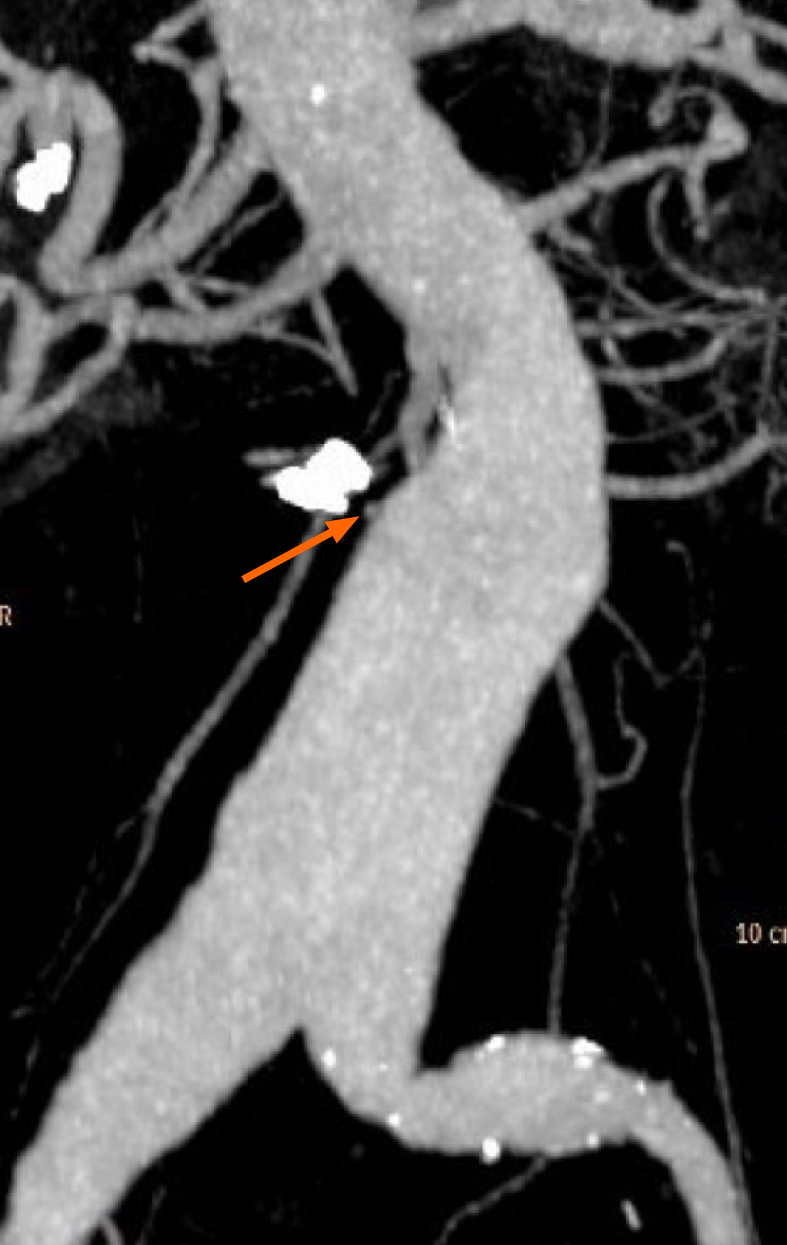
The patient was readmitted with the abovementioned symptoms. An emergency OGD was performed, and the nonbleeding small ulcers in the duodenum were visualized without signs of bleeding. However, this time the endoscope was advanced further, and an erosion of the mucosa in the third part of the duodenum (measuring 5.0 mm) with a blood clot and a visible pulsating aorta underneath was found (Figure 2A). A PAEF was suspected, and haemostasis was achieved with three haemoclips placed deep in the wall of the gut, covering the fistula (Figure 2B). Following this procedure, no subsequent haemorrhage was identified. To support the hypothesis of an aorto-duodenal fistula, contrast-enhanced computed tomography (CT) with arterial and venous phase was performed the following day. The CT showed a contrast-filled protrusion from the abdominal aortic aneurysm towards the haemoclips adjacent to the aneurysm sack, separated by a mural thrombus (Figure 3). For possible vascular intervention, we contacted two vascular surgical departments. However, based on the patient’s cancer disease and the CT-angiography, they did not find vascular therapy indicated and did not find it proven that a PAEF caused the bleeding. To our knowledge, the endoscopic pictures were not included in the decision-making.
The patient was discharged in his habitual condition after 10 d, without any signs of infection or rebleeding. Fourteen months later, the patient succumbed due to respiratory problems and terminal cancer without any incidences of gastrointestinal bleeding. The death was expected, and an autopsy was not sought.
The English literature identified only four case reports[18-21] fulfilling the inclusion criteria (Table 1). The anatomical location of the PAEF varied, and the endoscopic findings included pigmented protuberances, lesions/erosions, and oozing bleeding[22,23]. In two of the cases, the treatment was dual, as recommended for bleeding ulcers
| Tsai et al[18] | Lee et al[19] | Kim et al[20] | Mok et al[21] | Present case | |
| Age in year, Sex | 69, male | 86, female | 63, male | 67, male | 74, male |
| Anatomic location | Third part of duodenum | Sigmoid colon | Second part of duodenum | Oesophago-jejunal anastomosis | Third part of duodenum |
| Cause of PAEF | Aortic aneurysm | Aortic aneurysm | Aortic aneurysm | Infectious thoracic pseudo-aneurysm; oesophago-jejunal anastomotic leak | Aortic aneurysm |
| Endoscopic finding | Vessel-like bleeding with blood clot adhesion | Nodular lesion with central dimpling (small, depressed area) | Active bleeding | Oozing blood and blood clot | Mucosal erosion/nodular lesion with central depression and blood clot; Pulsating aorta underneath |
| Endoscopic treatment | Epinephrine and haemoclips | Electro-coagulation and haemoclips | Haemoclips alone | Fibrin sealant | Haemoclips alone |
| Endoscopic outcome | Successful haemostasis | Initial haemostasis, rebleeding | Successful haemostasis | Successful closure of fistula | Successful haemostasis |
| Following treatment | Open surgical repair of the aneurysm and bowel | Angiographic embolization | Endovascular stent repair with angiographic embolization and open surgical repair of the bowel | Endovascular stent repair with drainage of aneurysm sac (performed before endoscopic treatment) | None |
| Outcome | Alive at 24 mo follow-up | Died during the angiographic procedure | Alive at 12 mo follow-up | Alive at 14 mo follow-up | Died 14 mo after endoscopy |
More than 50% of PAEF are situated in the duodenum because of its close anatomical relation to the aorta. Other locations include the oesophagus, stomach, ileum, jejunum, and colon[5,24]. One review found an incidence of PAEF as the cause of gastrointestinal haemorrhage in 0.18% of cases[25]. Another study, asking 180 surgeons if they have ever treated a patient with a PAEF during their career, suggested the incidence to be greater[26]. The classic clinical presentation includes abdominal pain, signs of upper gastrointestinal bleeding, and an abdominal pulsating mass. Before the diagnosis, patients often have a “herald bleeding” that may cause exsanguination and death. However, only 6%-28% of the cases present all three symptoms. Thus, the majority of the patients present as “atypical”[5,24,27,28]. An AEF is therefore important to consider in patients with gastrointestinal haemorrhages and an aortic aneurysm.
A contrast-enhanced CT scan is regarded as the best diagnostic tool for the detection of AEF[28-31]. However, in a patient without a history of vascular surgery presenting with upper gastrointestinal bleeding, an AEF is not the first diagnosis that comes to mind. Hughes et al[31] investigated the signs found on CT scans. They showed that CT scans have an overall specificity of 100%, but the sensitivity was only 50%. The presence of ectopic bowel gas or extravasation of contrast into the bowel lumen increased the sensitivity to 100%. The finding of a branch from the aortic wall had a sensitivity of 80% and specificity of 75%. These numbers are in agreement with the findings of others[5,24,31,32]. Thus, in the diagnosis of an AEF, a positive CT result is useful but a negative CT finding does not yield reliable information. Because ulcers are common, OGD is the gold standard for the diagnosis and treatment of upper gastrointestinal bleeding. An OGD identified the AEF or showed other abnormalities in only 25%-50% of confirmed AEF cases, likely due to a typical OGD often omitting the examination of the third part of the duodenum[5,33]. In four of the cases described in this study, active bleeding was seen at the sight of the fistula during OGD[18,20,21]. In other cases, where OGD was the diagnostic tool, the AEF was described as a submucosal tumour-like lesion and being “pulsating”[23,34]. Sometimes oozing bleeding, the presence of a blood clot or the sense of pulsation may be the only thing that differentiate the descriptions given of the AEF from a description of other enteric fistulas[35].
No study on the diagnostic specificity and sensitivity for the endoscopic detection of PAEF exists. In our opinion, the negative CT finding in the case presented here does not necessarily rule out an AEF, especially following endoscopic haemostasis. On the contrary, the CT-angiography with three-dimensional reconstruction showed a contrast-filled branch from the abdominal aorta aneurysm in close relation to the duodenum, a finding with a specificity of 75% according to the literature[31]. In our opinion, the endoscopic and the CT findings together strengthen the diagnosis.
In all the cases described in the literature, final surgery (endovascular or open) followed the endoscopic treatment, and the endoscopically achieved haemostasis successfully formed a bridge to surgery. The patient presented herein was the only patient who did not have surgery after endoscopic haemostasis was achieved. The reason for this was due to the patient’s multiple comorbidities, including incurable cancer, a limited life expectancy, and a high risk of postoperative adverse outcomes. However, the haemoclipping was immediately lifesaving and was an effective long-term treatment.
Haemoclips can remain in situ for a long period, but we found no literature describing the life span or the mean in situ time for a haemoclip. Olmez et al[36] described a haemoclip in situ for more than 2 years. In our experience, a well-placed haemoclip for haemostasis after polypectomy is often found in situ at the adenoma control endoscopy after 6-12 mo, but no studies were found to support this. However, the clip functions as a ligation, and the nature of the fistula determines whether a ligation is sufficient to close it. Open vessels are closed by ligation, as are fistulas following anastomotic leakage of the gut. It is unknown whether a PAEF can be closed by simple ligation (clipping).
When a PAEF is clipped, the clip may function as a marker for the fistula site before final therapy. A clip can mark a lesion in the gastrointestinal tract[37] and increases the success rate of angiographic embolization of bleeding arteries in ulcers[38]. Based on our knowledge of the more common secondary AEFs, we know that infection is a common problem when the fistula is closed from the vascular side only. In a trial comparing closure of secondary AEFs using a vascular stent to open surgical repair, Kakkos et al[6] found that vascular stent repair can achieve immediate haemostasis and has a better short-term outcome. However, an infection of the stent and sepsis often follow the procedure, losing most of the benefit during long-term follow-up. The open communication to the bacteria-filled enteric side likely causes the infection. Therefore, closure of the enteric lesion by open surgery is recommended to follow vascular stenting[6,39].
The option of two minimally invasive procedures combined have not been investigated. It may be possible to clip (or stent in the oesophagus) the fistula from the enteric side followed by stenting from the vascular side. A successful case of secondary AEF initially closed from the vascular side immediately followed by endoscopic closure from the enteric side has been reported[40].
The small number of endoscopically treated PAEFs described in the literature limits this study. This small number increases the difficulty of creating clinical recommendations. However, bleeding from an AEF is a life-threatening condition, and every effort to stop the loss of blood as soon as possible is desirable. By creating a bridge to surgery and achieving haemostasis, critical time is bought for the patient. This time may mean that the experienced surgeon is present or that the patient can be transferred to another facility for optimal care. Thus, any modality creating immediate haemostasis for these patients represents a therapeutic improvement. It is unknown whether endoscopic treatment such as haemoclipping or epinephrine injection can aggravate ongoing bleeding from an AEF.
Endoscopy is the first-choice modality for the diagnosis and treatment of upper gastrointestinal bleeding including a PAEF, which is a rare finding. Endoscopic therapy including haemoclipping can establish lifesaving, immediate haemostasis from a bleeding PAEF, thus stabilizing the patient without hampering subsequent endovascular therapy or surgery. Endoscopic therapy might be useful as a bridge to surgery.
We thank the patient’s next of kin for the permission to use this clinical story to bring new methods of medical therapy forward. We thank the Department of Radiology at Holbaek Hospital for the three-dimensional simulations performed and included as figures here.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Danish Medical Association, No. 40008782.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawabata H S-Editor: Liu M L-Editor: A P-Editor: Zhang YL
| 1. | Antoniou GA, Koutsias S, Antoniou SA, Georgiakakis A, Lazarides MK, Giannoukas AD. Outcome after endovascular stent graft repair of aortoenteric fistula: A systematic review. J Vasc Surg. 2009;49:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Xiromeritis K, Dalainas I, Stamatakos M, Filis K. Aortoenteric fistulae: present-day management. Int Surg. 2011;96:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Baril DT, Carroccio A, Ellozy SH, Palchik E, Sachdev U, Jacobs TS, Marin ML. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg. 2006;44:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Spanos K, Kouvelos G, Karathanos C, Matsagkas M, Giannoukas AD. Current status of endovascular treatment of aortoenteric fistula. Semin Vasc Surg. 2017;30:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg. 2005;92:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Kakkos SK, Bicknell CD, Tsolakis IA, Bergqvist D; Hellenic Co-operative Group on Aortic Surgery. Editor's Choice - Management of Secondary Aorto-enteric and Other Abdominal Arterio-enteric Fistulas: A Review and Pooled Data Analysis. Eur J Vasc Endovasc Surg. 2016;52:770-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 7. | Danneels MI, Verhagen HJ, Teijink JA, Cuypers P, Nevelsteen A, Vermassen FE. Endovascular repair for aorto-enteric fistula: a bridge too far or a bridge to surgery? Eur J Vasc Endovasc Surg. 2006;32:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 496] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 9. | Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Early DS, Evans JA, Fanelli RD, Foley K, Fukami N, Jain R, Jue TL, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha S, Saltzman J, Sharaf R, Shergill AK, Dominitz JA, Cash BD; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7:33-47; quiz 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (38)] |
| 11. | Nelms DW, Pelaez CA. The Acute Upper Gastrointestinal Bleed. Surg Clin North Am. 2018;98:1047-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Schneider A, Fessner H. Interventional flexible endoscopy. In: Jackman A, editor. Biomedical engineering in gastrointestinal surgery. 1st ed. United Kingdom: Mara Conner, 2017: 329-349. |
| 13. | Iabichino G, Eusebi LH, Palamara MA, Arena M, Pellicano R, Consolo P, Fagoonee S, Amato L, Opocher E, Barabino M, Luigiano C. Performance of the over-the-scope clip system in the endoscopic closure of iatrogenic gastrointestinal perforations and post-surgical leaks and fistulas. Minerva Gastroenterol Dietol. 2018;64:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Mosquera-Klinger G, Torres-Rincón R, Jaime-Carvajal J. Endoscopic closure of gastrointestinal perforations and fistulas using the Ovesco Over-The-Scope Clip system at a tertiary care hospital center. Rev Gastroenterol Mex. 2019;84:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP; SCARE Group. The SCARE 2018 statement: Updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. 2018;60:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2071] [Article Influence: 295.9] [Reference Citation Analysis (0)] |
| 16. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13305] [Article Influence: 831.6] [Reference Citation Analysis (0)] |
| 17. | Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1529] [Article Influence: 218.4] [Reference Citation Analysis (0)] |
| 18. | Tsai TJ, Yu HC, Lai KH, Lo GH, Hsu PI, Fu TY. Primary aortoduodenal fistula caused by tuberculous aortitis presenting as recurrent massive gastrointestinal bleeding. J Formos Med Assoc. 2008;107:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lee W, Jung CM, Cho EH, Ryu DR, Choi D, Kim J. Primary aortoenteric fistula to the sigmoid colon in association with intra-abdominal abscess. Korean J Gastroenterol. 2014;63:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Kim WC, Ferrada P, Rodas E, Levy M, Aboutanos MA, Anand RJ. Making the Diagnosis-Acute Primary Aortoduodenal Fistula Bleeding Presenting in a Blunt Trauma Patient. Am Surg. 2019;85:e326-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Mok VW, Ting AC, Law S, Wong KH, Cheng SW, Wong J. Combined endovascular stent grafting and endoscopic injection of fibrin sealant for aortoenteric fistula complicating esophagectomy. J Vasc Surg. 2004;40:1234-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Busuttil SJ, Goldstone J. Diagnosis and management of aortoenteric fistulas. Semin Vasc Surg. 2001;14:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Cho YP, Han MS, You CH, Kim SY, Jang HJ, Kim JS, Kim YH, Lee SG. Role of endoscopy in the diagnosis of aneurysm-duodenal fistula. Hepatogastroenterology. 2003;50 Suppl 2:cclxvi-cclxviii. [PubMed] |
| 24. | Song Y, Liu Q, Shen H, Jia X, Zhang H, Qiao L. Diagnosis and management of primary aortoenteric fistulas--experience learned from eighteen patients. Surgery. 2008;143:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Voorhoeve R, Moll FL, de Letter JA, Bast TJ, Wester JP, Slee PH. Primary aortoenteric fistula: report of eight new cases and review of the literature. Ann Vasc Surg. 1996;10:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 26. | Voorhoeve R, Moll FL, Bast TJ. The primary aortoenteric fistula in The Netherlands--the unpublished cases. Eur J Vasc Endovasc Surg. 1996;11:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Ramanujam S, Shiels A, Zuckerman G, Prakash C. Unusual presentations of aorto-enteric fistula. Gastrointest Endosc. 2004;59:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Debonnaire P, Van Rillaer O, Arts J, Ramboer K, Tubbax H, Van Hootegem P. Primary aorto enteric fistula: report of 18 Belgian cases and literature review. Acta Gastroenterol Belg. 2008;71:250-258. [PubMed] |
| 29. | Mellnick VM, Heiken JP. The Acute Abdominal Aorta. Radiol Clin North Am. 2015;53:1209-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics. 2009;29:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Hughes FM, Kavanagh D, Barry M, Owens A, MacErlaine DP, Malone DE. Aortoenteric fistula: a diagnostic dilemma. Abdom Imaging. 2007;32:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Fasel JHD, Ignjatovic D. 3D reconstruction of a primary aortoenteric fistula - centerline calculation and measurements. Curr Med Imaging Rev. 2015;11:127-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Deijen CL, Smulders YM, Coveliers HME, Wisselink W, Rauwerda JA, Hoksbergen AWJ. The Importance of Early Diagnosis and Treatment of Patients with Aortoenteric Fistulas Presenting with Herald Bleeds. Ann Vasc Surg. 2016;36:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Honjo K, Okada H, Sumii K, Kobatake T, Fujioka S, Kumagai I, et al. Endoscopy is one of the valuable diagnostic methods for primary aorto-enteric fistula. Dig Endosc. 2004;16:353-355. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 35. | Kothari TH, Haber GB. Use of otsc device system for closure of fistulas in the alimentary tract – a case series. Video J Encycl GI Endosc. 2014;1:647-650. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Olmez S, Ozaslan E, Avcioglu U. Hemoclip retained for more than 2 years. Endoscopy. 2012;44 Suppl 2:E323-E324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 38. | Song JS, Kwak HS, Chung GH. Nonvariceal upper gastrointestinal bleeding: the usefulness of rotational angiography after endoscopic marking with a metallic clip. Korean J Radiol. 2011;12:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Rodrigues dos Santos C, Casaca R, Mendes de Almeida JC, Mendes-Pedro L. Enteric repair in aortoduodenal fistulas: a forgotten but often lethal player. Ann Vasc Surg. 2014;28:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Nayak RD, Paul M, Valooran GJ, Varghese R. Emergent endovascular stent grafting for saccular arch aneurysm complicated by aorto-esophageal fistula. Indian Heart J. 2015;67 Suppl 3:S60-S63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |