Published online Mar 16, 2021. doi: 10.4253/wjge.v13.i3.72
Peer-review started: January 8, 2021
First decision: January 23, 2021
Revised: January 23, 2021
Accepted: February 18, 2021
Article in press: February 18, 2021
Published online: March 16, 2021
Processing time: 59 Days and 15.7 Hours
Computed tomography colonography (CTC) has become a key examination in detecting colonic polyps and colorectal carcinoma (CRC). It is particularly useful after incomplete optical colonoscopy (OC) for patients with sedation risks and patients anxious about the risks or potential discomfort associated with OC. CTC's main advantages compared with OC are its non-invasive nature, better patient compliance, and the ability to assess the extracolonic disease. Despite these advantages, ionizing radiation remains the most significant burden of CTC. This opinion review comprehensively addresses the radiation risk of CTC, incorporating imaging technology refinements such as automatic tube current modulation, filtered back projections, lowering the tube voltage, and iterative reconstructions as tools for optimizing low and ultra-low dose protocols of CTC. Future perspectives arise from integrating artificial intelligence in computed tomography machines for the screening of CRC.
Core Tip: Computed tomography colonography (CTC) is an important imaging technique with significant advantages over optical colonoscopy in terms of less invasiveness, better compliance, and assessment of extracolonic structures. Ionizing radiation is the most significant burden of this technique. This opinion review comprehensively addresses the radiation risk in CTC with imaging technology refinements that should be used to lower radiation doses.
- Citation: Popic J, Tipuric S, Balen I, Mrzljak A. Computed tomography colonography and radiation risk: How low can we go? World J Gastrointest Endosc 2021; 13(3): 72-81
- URL: https://www.wjgnet.com/1948-5190/full/v13/i3/72.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i3.72
Computed tomography colonography (CTC), also referred to as a virtual colonoscopy (VC), was introduced in 1994 by Vining et al[1]. They were the first to describe this modified computed tomography (CT) examination of the large intestine as a diagnostic test for colorectal carcinoma (CRC) and polyps[2]. Since then, CTC has become an examination of crucial importance in imaging polyps and potential CRC in patients not amenable to optical colonoscopy (OC). CTC has advantages over OC because of its less invasive nature, better patient compliance, and the ability to detect extracolonic disease[3]. Hence, CTC is an accepted screening test for CRC and is growing in its utilization. We have to be aware that no CTC findings allow us to distinguish adenomas from non-neoplastic polypoid lesions such as hyperplastic or inflammatory polyps, making the histological study necessary in all instances. One of the drawbacks of CTC is usually missed flat lesions such as a flat polyp. Images that can be misinterpreted and can mimic polyps include untagged stool, partially distended haustra, or focally thickened folds[4].
On the other hand, OC is often associated with anxiety, fear, and discomfort compared to CTC, and carries a risk of being incomplete, especially in elderly patients[5]. Despite these advantages of CTC, ionizing radiation is the most significant burden of this technique (Table 1). However, imaging technology refinements, favorable cost analyses, and the impact of extracolonic findings make this method a suitable alternative to OC for CRC screening[3].
| Advantage | Limitation |
| Minimally invasive procedure | Exclusively diagnostic method |
| Safe procedure | Ionizing radiation |
| No need for sedation | Fecal residue simulate pathology |
| Short examination time | Laxative residue simulate pathology |
| Assess to extracolonic disease | Flat lesions |
| Three dimensional view | |
| View of the entire colonic surface | |
| Access to post-obstructed bowel | |
| “Second look“ |
One of the unanimously accepted CTC indications is to complete a colonic workup after an incomplete OC. Some 10% of colonoscopies cannot be completed for different causes: Neoplastic stenosis, diverticulosis, adhesions, loops, or redundant colon[6-9]. A study revealed that 4.3% of neoplasms were missed by incomplete colonoscopy and were found in additional imaging studies[6]. Moreover, the proximal colon study is particularly important in neoplastic stenosis, as the percentage of synchronous cancer is high (4%-5%)[10]. In some patients, OC can be technically challenging, with the inability to achieve cecal intubation, resulting in inadequate visualization of the entire colon, hence a potential risk of undetected colon cancer and polyps[11,12] Except radiology practices with an active screening program, incomplete OC examinations likely account for the vast majority of CTC requests[13]. Factors previously shown to contribute to the risk of incomplete OC include; increasing patient age, low body mass index, female gender, history of prior abdominal and pelvic surgeries, presence of severe diverticular disease, poor bowel preparation, the experience of the endoscopist, tumorous obstruction of the entire lumen and anesthesia-related complications[7].
There are two primary strategies regarding the timing of CTC following incomplete OC. The first and most common is same-day CTC utilizing the prior OC prep, often supplemented with oral contrast after recovery from OC[14]. This is often the more convenient option for the patient as they do not have to undergo further bowel preparation (assuming bowel prep for OC was adequate) and return on a separate day. CTC is usually performed 2–3 h later. Another option is to have the patient return for CTC at a later date utilizing a standard CTC bowel regimen with an osmotic cathartic and dual agent tagging protocol. CTC should be delayed if an endoscopic resection has been performed during OC[15].
Most population-based screening programs for CRC target the age range from 50 to 74 years old and include indirect screening, such as fecal occult blood testing or direct visualization with flexible sigmoidoscopy or OC[16]. The most common is the stool test-based screening [guaiac fecal occult blood test (FOBt) or fecal immunochemical test (FIT)] due to its low cost, availability, safety, and easy transport (via post). If positive, FOBt and FIT are usually followed by OC to confirm neoplasia or suspect polyps[5].
Since CTC has become an available alternative option to OC, more patients choose CTC as a more desirable option. In a multicenter survey of 1417 individuals, 68% chose CTC over OC due to its less invasive nature, and 47% chose CTC to avoid the risks associated with OC[17]. Another Dutch study showed that 93% of patients would choose another CTC after the initial one[18].
The CRC screening potential of CTC has been investigated in three European randomized trials: COCOS study in the Netherlands (CTC vs OC)[19], SAVE[20], and PROTEUS[21] studies in Italy.
The SAVE study compared reduced preparation and full-preparation CTC, FIT, and OC, while the PROTEUS study compared CTC vs sigmoidoscopy. The participation rates, positivity rate, and CTC detection rates were similar amongst the studies. The participation rate for screening CTC was higher than that for an OC, with a slightly lower detection rate, but with comparable yield per invitee. The participation rate for screening CTC was much lower than that for FIT, but its detection rate was three-fold that of one FIT round. CTC and sigmoidoscopy showed similar participation and detection rate. These results encourage CTC implementation in screening programs for CRC[22].
CTC's main disadvantage is ionizing radiation, especially since CTC has been considered a CRC screening tool. Radiation dose significantly determines CT image quality, its diagnostic accuracy, and clinical utility. Strategies for lowering radiation dose are utilized to maintain and improve image quality. The dose should only be reduced if one can preserve the diagnostic image quality for the specific pathology. It is essential to understand the relation between image quality and radiation dose to optimize the radiation dose in CTC[23].
CTC dose is lower than the conventional CT examination, about one half of the dose, because of high natural contrast between the soft tissue of the colonic wall, luminal gas, and tagged fecal residue and fluids[6].
To give the proper insight, it is meaningful to compare the doses of different diagnostic procedures with the chest X-ray dose or years of exposure to natural background radiation, ranging from 1 to 3 mSv/year, depending on the geographical region. Thus, mammography has a dose of 0.13 mSv, which corresponds to 6 chest X-rays or 14 days of background radiation. An average abdominal CT has 5-25 mSv, which corresponds to 250-1250 chest X–rays or 2-11.5 years of background radiation, depending on the number of phases that have to be scanned to confirm the suspect diagnosis[24] (Table 2).
| Examination | Ionizing radiation dose [mSv] |
| X-ray lung | 0, 1 |
| X-ray abdomen | 1 |
| Barium enema fluoroscopy exam | 9 |
| CT abdomen and pelvis (w/o contrast) | 10 |
| CTC (2 series) | 20 |
| CTC ultra low-dose protocol | 2 |
During the last few decades, physicists, radiologists, and technologists have studied CT technology to find ways to reduce radiation doses for specific "diagnosis-related" CT examinations. Currently, we have well-established "diagnosis-related" protocols such as "low-dose" kidney stone dedicated protocol, "low-dose" lung cancer screening protocol, etc.
Dose reduction can be achieved in two ways. Firstly it is crucial to appropriately target image quality for a specific diagnostic test, not demanding lower noise or higher spatial resolution than necessary. For instance, in a high-contrast setting, as in the detection of colon polyps from a background of air and contrast-tagged stool[25,26], it allows high noise level and relatively low radiation dose without sacrificing the diagnostic confidence. Detection and characterization of low-contrast lesions present in CT imaging of hepatobiliary and brain pathology require a relatively low noise level and higher radiation dose. Consensus agreement on image quality requirements exists in guidelines and standards[27], but precise quantitative requirements exist only for several examinations[28].
There are many ways to adjust scanning parameters in order to lower the dose. One way to reduce the dose is to change the technical exposure parameters of scanning: The tube current or the voltage depending on the tissue density and contrast, scanning region, and the patients' body shape and size[29].
Modern CT equipment can automatically modulate the X-ray tube current after obtaining a scanned region’s initial topogram, known as automatic tube current modulation (ATCM). ATCM adjusts the X-ray tube current (mAs) according to the size and the attenuation of the examined body part. It has been recommended to use ATCM for CTC[5,20,21].
Each time the scanning parameters are changed, it influences the image's quality, namely spatial and/or contrast resolution, which are important for detecting specific pathologies. Spatial resolution relates to sharp boundaries of the tissues, organs, or structures, while contrast resolution involves the difference in contrast of various tissues (e.g., normal or pathologically altered). Low dose protocols have a higher image noise due to altered (lower) electrical conditions. Spatial or contrast resolution is sacrificed, and the radiologist has to get the same information from granulated images. Therefore, it is important to balance the dose by adjusting electrical conditions and maintaining image quality. The image quality needs to be good enough to distinguish pathologic lesions from normal structures. Thus, it is crucial to find a delicate balance between the lowest dose and acceptable image quality, making it possible for a radiologist to discern pathologic structures[5]. This is also referred to as the As Low As Reasonably Achievable principle, well established in the area of radiation protection[23]. In addition to altering exposure parameters, software options have been developed to make less image noise by keeping the tube current as low as possible. These software reconstructions techniques are Sinogram-Affirmed Iterative Reconstruction (SAFIRE) and a conventional filtered back projection. These techniques allowed the use of even lower doses of radiation than the conventional low dose (LD) protocol named ultra-low dose (ULD) with maintained image quality[5,24,30]. In 2018, a study evaluating the ULD protocol's diagnostic value in detecting polyps[31] showed that the ULD protocol lowers the effective dose up to 63.2% compared to LD protocol (0.98 mSv for ULD and 2.69 mSv for LD). Image noise measurements with ULD were slightly lower (28.6) than with LD (29.8) (P = 0.09). Image quality was not different between 2D and 3D with either ULD and LD. A special 3D software option must be used to navigate the large bowel and when interpreting CTC to help detect intraluminal lesions. In contrast, the 2D option is the routine CT examination technique. Polyp detection was also comparable, with no significant difference in detection rate and polyp measurement for LD and ULD protocols[30]. Therefore if iterative reconstruction methods (the software option in almost all modern CT scanners) were included during the scanning, there was no significant image quality degradation with ULD-CTC compared with LD-CTC.
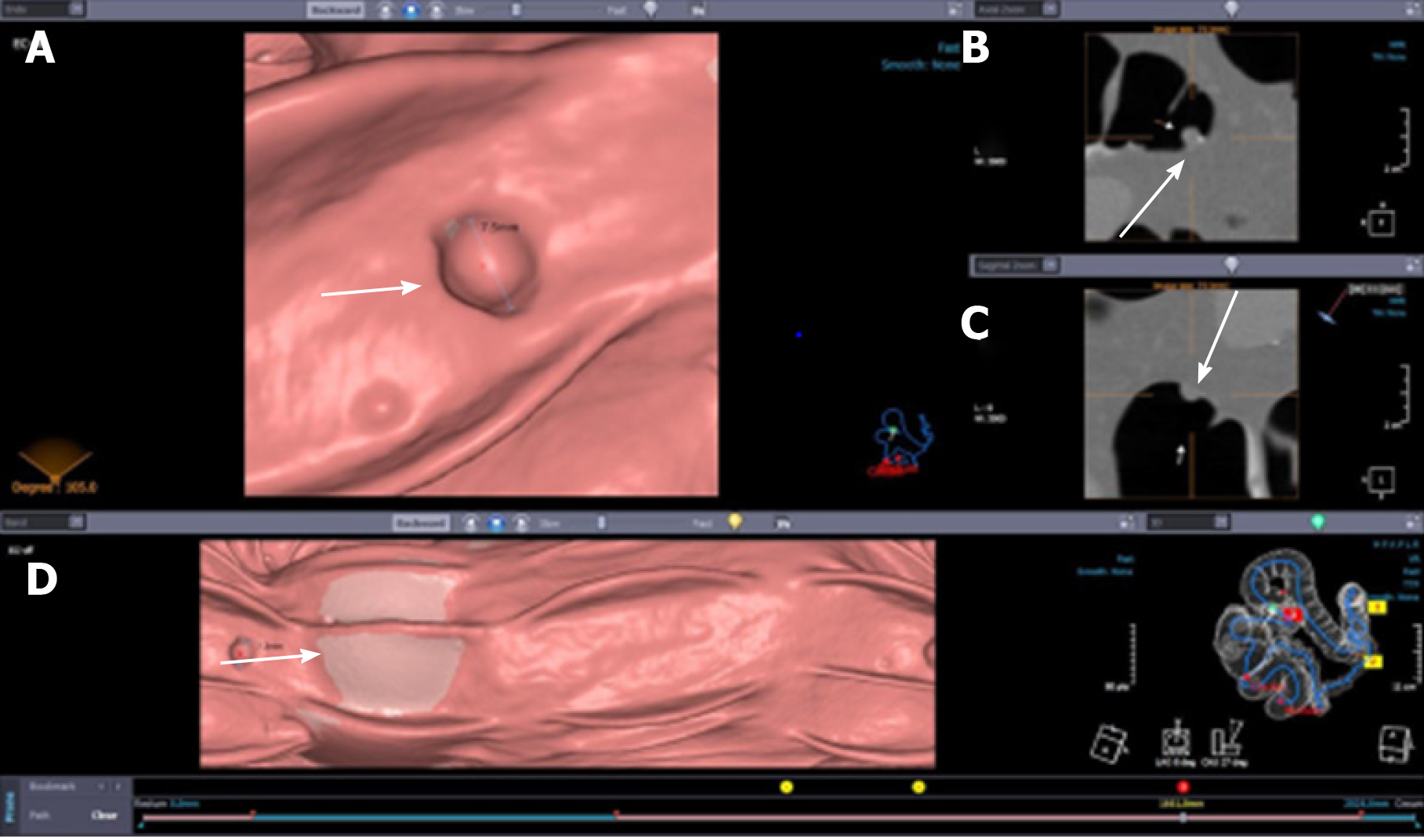
Advantages of specific computer software for CTC interpretation, which enables dynamic viewing of two-dimensional axial images, multi-planar reformats, and three-dimensional renderings, require radiologists' interactive training. The radiologist can use either 2D axial images or 3D renderings for CTC's primary interpretation, with the alternate method reserved for problem-solving specific questions related to a potential lesion. 3D reading is an additional software option that enhances polyp detection and decreases the interpretation time without increasing the patient dose (Figure 1).
Skilled usage of these techniques acquired by comprehensive training correlate with polyp detection sensitivity[31]. Primary 2D interpretation is rendered from magnified colonic axial images gained in supine and prone positions. Compared to primary 3D interpretation, it shortens the assessment time of lesion density and homogeneity.
Sessile polyps have round or ovoid morphology and are of soft tissue density. They remain fixed in location on the colon wall in both the supine and prone images. The stool can be differentiated from polyps since it is typically mixed density and shifts location when the patient changes position. Pedunculated polyps can shift in location when the patient moves from supine to prone positions, but the stalk is typically easily identified on 2D and 3D images. Multiplanar reformats and 3D images are useful for evaluating lesion morphology and confirming polyps[32].
In addition to widely used techniques of lowering radiation dose such as automatic tube dose modulation (automatic adjustment after the initial topogram), lowering the tube current, and applying iterative reconstruction (IR), lowering tube voltage can be useful. This option is rarely used for routine CT scanning because it impairs X-ray penetration through the scanned region. However, during the CTC, the bowel has a high contrast due to intraluminal gas; therefore, high voltage is not needed. If there is an option for IR, we can lower the voltage and turn on IR. The iterative reconstruction software option will fix the image noise which arises from the lower voltage[29].
The data suggest that low tube voltage with IR results in a 27 % radiation reduction while maintaining the image quality and detection (100kVp vs 80kVp)[33]. In addition, new IR such as SAFIRE could lower the voltage even more[30].
Recent studies show that both hybrid and iterative model reconstruction techniques are suitable for sub-milliSievert ultralow-dose CTC without sacrificing the study's diagnostic performance[34].
Several operational factors typically result in higher doses. Repeated CT scanning, such as multiphase examinations, increases the radiation dose. For example, suppose diagnostic CTC is being performed in a patient with suspected colorectal carcinoma. In that case, intravenous contrast may be necessary, and CT acquisition parameters will typically require higher mAs. If the patient is undergoing CTC as a screening examination, then intravenous contrast is not routinely used.
Patient’s hight and/or length also influences the radiation dose. Longer scan length results in radiation exposure to a greater anatomic region and hence higher radiation dose. For some reason, for a detailed analysis, radiologist could request thinner images that provide better image resolution and improved visibility of small objects. However, beam intensity needs to be increased to reduce the noise in these thinner images, which concurrently increases the radiation dose[35].
Since the whole abdomen is visible during CTC screening, many abnormalities outside of the colon can be picked up. Several US screening studies collected the data on clinically significant extracolonic findings that required further imaging. The proportion of patients with follow-up CT scans to investigate these findings was in the range of 5-10%[36,37]. The most common follow-up scan were; an abdomen CT scan and abdomen/pelvis and chest CT scans. The dose from an abdomen/pelvis CT scan performed with and without contrast is about 20 mSv[38], which will result in a radiation risk that is about twice as high as the risk from CTC. However, as only a small proportion (e.g., 10%) of the screening population will receive these additional scans, it is unlikely that they will increase the average risk to the whole screening population by more than 20%.
The standard American College of Radiology (ACR) CTC protocol[39-42] specifies that the patient be scanned in both the supine and prone positions to allow complete evaluation of the colon with the dependent shifting of luminal fluid and complementary distention of non-dependent colonic segments. In a minority of cases, the same colonic segments will be collapsed on the standard positions, necessitating a third series to achieve full diagnostic evaluation. The sigmoid and/or descending colon account for most non-diagnostic segments, necessitating a right lateral decubitus series to complete the examination[43,44].
The frequency for performing a decubitus series at CTC varies considerably according to study indication, practice site, patient age, BMI, and over time. It is critical to note that the CT technologist is primarily responsible for determining the need for a decubitus series–not the radiologist. These results have important implications for clinical practice, including the need for improved training and feedback for CT technologists[45].
Furthermore, practice regarding ancillary imaging before a CTC and after incomplete OC should be discussed as this can also increase radiation dose; for example, some centers perform a scout/topogram or non-contrast CT abdomen following incomplete OC, in order to exclude a perforation; although there is evidence to suggest this is unnecessary.
Perforation is a recognized complication of colonoscopy. Reported perforation rates range from one case in 3115 procedures (0.032%) to one case in 510 procedures (0.196%)[46-49]. The short time between incomplete colonoscopy and same-day or next-day CTC may not be adequate to allow some perforations to become clinically apparent. Because of the risk of exacerbating a clinically unsuspected perforation during insufflation at CTC, which can increase sepsis risk, screening for the presence of extraluminal gas before insufflation for CTC may benefit occult perforation among these patients. Colonic perforation after colonoscopy can be clinically occult. Recent studies have shown that some findings justify performing low-dose diagnostic CT before rectal tube insertion and gas insufflation in all patients referred for same-day or next-day CTC after incomplete colonoscopy to minimize the risks associated with exacerbating perforation[50].
Effects of radiation and its risk are usually estimations based on the linear extrapolation of the cancer risks associated with ultra-high doses from Hiroshima and Nagasaki atomic bomb survivor studies[51]. Still, there is no unambiguous evidence of cancer induction at low dose levels, and the issue remains highly controversial.
In 2016, the Health Physics society published that radiation lower than 100mSv did not impact the human body[52]. Assuming that the CTC dose is on average 5mSv, that means that the theoretical cancer risk would be 0.04% in 50-year-old patients and 0.02% in 70-year-old patients after initial screening[51]. Keeping in mind that a lifetime risk for developing colon cancer is around 5%, CTC's benefits outweigh its estimated radiation risk. CTC doses are, currently, in many institutions, even lower than 3mSv, the dose which is comparable to annual radiation exposure in some countries such as the United States[53].
Since the age for screening for CRC is above the age of 50, exposure is decreased significantly, and therefore the radiation-related cancer risk is even lower. Since the proportion of dividing human cells decreases with age, this further raises CTC's safety in the older population it mainly serves[54].
It is important to consider the average frequency of each examination in the population and the average radiation dose with each technique to understand the radiation dose of CTC in the context of other ionizing techniques. However, all examination-based techniques (radiography, fluoroscopy, CT, positron emission tomography-CT, scintigraphy, and interventional cardiology) constitute 34 % of the total annual population dose[53,55].
It is important to emphasize that CTC is quite different from the usual CT examination. Inherently high contrast between the air-filled lumen of the colon and the soft-tissue attenuation of the colonic wall allows a relevant dose reduction without loss of diagnostic accuracy[54].
In addition to CTC’s high safety profile, slightly better patient compliance, ability to detect extracolonic disease and comparable polyp and cancer detection rate to OC, CTC can be performed with a minimal radiation dose that poses no risk of cancer to the patient.
CTC "good practice" should include individualizing the scanning technique according to the patient's attenuation level and using suitable tube potential selected by advanced automatic exposure control techniques that adjust the tube current. Implementation of iterative reconstruction in everyday clinical practice can bring significant image quality improvement and radiation dose reduction over conventional filtered back-projection-based reconstruction algorithms.
Modern CT equipment allows us to scan CTC at much lower doses ranging from 1 to 5 mSv. These doses are comparable with 1-2 Lung radiograms and are on the annual radiation background level in some countries. Since screening programs mostly include two readers (two experienced radiologists) and "double-blinded" reading, the new perspectives arise from the integration of artificial intelligence in CT machines, which could be used for screening CTC instead of a "second reader".
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: O'Shea A, Ricci ZJ, Sun X S-Editor: Zhang H L-Editor: A P-Editor: Wang LL
| 1. | Vining DJ, Gelfand DW. Noninvasive colonoscopy using helical CT scanning, 3D reconstruction, and virtual reality. Presented at the 1994 meeting of the Society of Gastrointestinal Radiologists, Maui, Hawaii; February 13-18, 1994. |
| 2. | Pickhardt PJ, Yee J, Johnson CD. CT colonography: over two decades from discovery to practice. Abdom Radiol (NY). 2018;43:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Obaro AE, Plumb AA, Fanshawe TR, Torres US, Baldwin-Cleland R, Taylor SA, Halligan S, Burling DN. Post-imaging colorectal cancer or interval cancer rates after CT colonography: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Pagés Llinás M, Darnell Martín A, Ayuso Colella JR. [CT colonography: what radiologists need to know]. Radiologia. 2011;53:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Maupoey Ibáñez J, Pàmies Guilabert J, Frasson M, Boscà Robledo A, Giner Segura F, García-Granero Ximénez E. Accuracy of CT colonography in the preoperative staging of colon cancer: a prospective study of 217 patients. Colorectal Dis. 2019;21:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Neerincx M, Terhaar sive Droste JS, Mulder CJ, Räkers M, Bartelsman JF, Loffeld RJ, Tuynman HA, Brohet RM, van der Hulst RW. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010;42:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Copel L, Sosna J, Kruskal JB, Raptopoulos V, Farrell RJ, Morrin MM. CT colonography in 546 patients with incomplete colonoscopy. Radiology. 2007;244:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Hanson ME, Pickhardt PJ, Kim DH, Pfau PR. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol. 2007;189:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Iafrate F, Hassan C, Zullo A, Stagnitti A, Ferrari R, Spagnuolo A, Laghi A. CT colonography with reduced bowel preparation after incomplete colonoscopy in the elderly. Eur Radiol. 2008;18:1385-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Finan PJ, Ritchie JK, Hawley PR. Synchronous and 'early' metachronous carcinomas of the colon and rectum. Br J Surg. 1987;74:945-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 359] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Pullens HJ, van Leeuwen MS, Laheij RJ, Vleggaar FP, Siersema PD. CT-colonography after incomplete colonoscopy: what is the diagnostic yield? Dis Colon Rectum. 2013;56:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Duszak R Jr, Kim DH, Pickhardt PJ. Expanding utilization and regional coverage of diagnostic CT colonography: early Medicare claims experience. J Am Coll Radiol. 2011;8:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Chang KJ, Rekhi SS Jr, Anderson SW, Soto JA. Fluid tagging for CT colonography: effectiveness of a 2-hour iodinated oral preparation after incomplete optical colonoscopy. J Comput Assist Tomogr. 2011;35:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Laghi A. Virtual colonoscopy: clinical application. Eur Radiol. 2005;15 Suppl 4:D138-D141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Bevan R, Rutter MD. Colorectal Cancer Screening-Who, How, and When? Clin Endosc. 2018;51:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 17. | Pooler BD, Baumel MJ, Cash BD, Moawad FJ, Riddle MS, Patrick AM, Damiano M, Lee MH, Kim DH, Muñoz del Rio A, Pickhardt PJ. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. AJR Am J Roentgenol. 2012;198:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | de Wijkerslooth TR, de Haan MC, Stoop EM, Bossuyt PM, Thomeer M, Essink-Bot ML, van Leerdam ME, Fockens P, Kuipers EJ, Stoker J, Dekker E. Burden of colonoscopy compared to non-cathartic CT-colonography in a colorectal cancer screening programme: randomised controlled trial. Gut. 2012;61:1552-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, van de Vijver MJ, Biermann K, Thomeer M, van Leerdam ME, Fockens P, Stoker J, Kuipers EJ, Dekker E. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Sali L, Mascalchi M, Falchini M, Ventura L, Carozzi F, Castiglione G, Delsanto S, Mallardi B, Mantellini P, Milani S, Zappa M, Grazzini G; SAVE study investigators. Reduced and Full-Preparation CT Colonography, Fecal Immunochemical Test, and Colonoscopy for Population Screening of Colorectal Cancer: A Randomized Trial. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Regge D, Iussich G, Segnan N, Correale L, Hassan C, Arrigoni A, Asnaghi R, Bestagini P, Bulighin G, Cassinis MC, Ederle A, Ferraris A, Galatola G, Gallo T, Gandini G, Garretti L, Martina MC, Molinar D, Montemezzi S, Morra L, Motton M, Occhipinti P, Pinali L, Soardi GA, Senore C. Comparing CT colonography and flexible sigmoidoscopy: a randomised trial within a population-based screening programme. Gut. 2017;66:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sali L, Regge D. CT colonography for population screening of colorectal cancer: hints from European trials. Br J Radiol. 2016;89:20160517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Yu L, Liu X, Leng S, Kofler JM, Ramirez-Giraldo JC, Qu M, Christner J, Fletcher JG, McCollough CH. Radiation dose reduction in computed tomography: techniques and future perspective. Imaging Med. 2009;1:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Roguin A, Nair P. Radiation during cardiovascular imaging. Br J Cardiol. 2007;14:289-292. |
| 25. | Fletcher JG, Johnson CD, Welch TJ, MacCarty RL, Ahlquist DA, Reed JE, Harmsen WS, Wilson LA. Optimization of CT colonography technique: prospective trial in 180 patients. Radiology. 2000;216:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 216] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Callstrom MR, Johnson CD, Fletcher JG, Reed JE, Ahlquist DA, Harmsen WS, Tait K, Wilson LA, Corcoran KE. CT colonography without cathartic preparation: feasibility study. Radiology. 2001;219:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Spada C, Hassan C, Bellini D, Burling D, Cappello G, Carretero C, Dekker E, Eliakim R, de Haan M, Kaminski MF, Koulaouzidis A, Laghi A, Lefere P, Mang T, Milluzzo SM, Morrin M, McNamara D, Neri E, Pecere S, Pioche M, Plumb A, Rondonotti E, Spaander MC, Taylor S, Fernandez-Urien I, van Hooft JE, Stoker J, Regge D. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline - Update 2020. Endoscopy. 2020;52:1127-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Kalender WA, Buchenau S, Deak P, Kellermeier M, Langner O, van Straten M, Vollmar S, Wilharm S. Technical approaches to the optimisation of CT. Phys Med. 2008;24:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | International Atomic Energy Agency. Dose Reduction in CT while Maintaining Diagnostic Confidence: A Feasibility/Demonstration Study. Radiation Safety and Monitoring Section, International Atomic Energy Agency, Vienna International Centre. September 2009. [Cited 21 December 2020]. Available from: https://www-pub.iaea.org/MTCD/publications/PDF/te_1621_web.pdf. |
| 30. | Cianci R, Delli Pizzi A, Esposito G, Timpani M, Tavoletta A, Pulsone P, Basilico R, Cotroneo AR, Filippone A. Ultra-low dose CT colonography with automatic tube current modulation and sinogram-affirmed iterative reconstruction: Effects on radiation exposure and image quality. J Appl Clin Med Phys. 2019;20:321-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Heresbach D, Djabbari M, Riou F, Marcus C, Le Sidaner A, Pierredon-Foulogne MA, Ponchon T, Boudiaf M, Seyrig JA, Laumonier H, Luet D, Giraud-Cohen M, Pelletier AL, Charachon A, Ramaholimihaso F, Bouillet P, Veyrac M, Ficarelli S, Vahedi K, Keruhel J, Lamouliatte H, Ridereau-Zins C, Bouhnik Y, Tissier M, Diris B, Zagdanski AM, Josselin JM, Hamonic S, Gandon Y. Accuracy of computed tomographic colonography in a nationwide multicentre trial, and its relation to radiologist expertise. Gut. 2011;60:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG, Fidler JL, Zimmerman P, Horton KM, Coakley K, Iyer RB, Hara AK, Halvorsen RA Jr, Casola G, Yee J, Herman BA, Burgart LJ, Limburg PJ. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 706] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 33. | Shin CI, Kim SH, Lee ES, Lee DH, Hwang EJ, Chung SY, Lee JM, Han JK, Choi BI. Ultra-low peak voltage CT colonography: effect of iterative reconstruction algorithms on performance of radiologists who use anthropomorphic colonic phantoms. Radiology. 2014;273:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Lambert L, Ourednicek P, Briza J, Giepmans W, Jahoda J, Hruska L, Danes J. Sub-milliSievert ultralow-dose CT colonography with iterative model reconstruction technique. PeerJ. 2016;4:e1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Berrington de Gonzalez A, Kim KP, Yee J. CT colonography: perforation rates and potential radiation risks. Gastrointest Endosc Clin N Am. 2010;20:279-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Pickhardt PJ, Hanson ME, Vanness DJ, Lo JY, Kim DH, Taylor AJ, Winter TC, Hinshaw JL. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Gluecker TM, Johnson CD, Wilson LA, Maccarty RL, Welch TJ, Vanness DJ, Ahlquist DA. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology. 2003;124:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1364] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 39. | Yee J, Kumar NN, Hung RK, Akerkar GA, Kumar PR, Wall SD. Comparison of supine and prone scanning separately and in combination at CT colonography. Radiology. 2003;226:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Chen SC, Lu DS, Hecht JR, Kadell BM. CT colonography: value of scanning in both the supine and prone positions. AJR Am J Roentgenol. 1999;172:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | American College of Radiology. ACR Practice Parameter for the Performance of Computed Tomography (CT) Colonography in Adults. Reston, VA: American College of Radiology; 2006. [Cited 17 January 2021]. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/ct-colonog.pdf. |
| 42. | Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR Am J Roentgenol. 2006;186:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdom Imaging. 2011;36:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 46. | Misra T, Lalor E, Fedorak RN. Endoscopic perforation rates at a Canadian university teaching hospital. Can J Gastroenterol. 2004;18:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Farley DR, Bannon MP, Zietlow SP, Pemberton JH, Ilstrup DM, Larson DR. Management of colonoscopic perforations. Mayo Clin Proc. 1997;72:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Cobb WS, Heniford BT, Sigmon LB, Hasan R, Simms C, Kercher KW, Matthews BD. Colonoscopic perforations: incidence, management, and outcomes. Am Surg. 2004;70:750-7; discussion 757. [PubMed] |
| 49. | Hough DM, Kuntz MA, Fidler JL, Johnson CD, Petersen BT, Kofler JM, Fletcher JG. Detection of occult colonic perforation before CT colonography after incomplete colonoscopy: perforation rate and use of a low-dose diagnostic scan before CO2 insufflation. AJR Am J Roentgenol. 2008;191:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Chang KJ, Yee J. Dose reduction methods for CT colonography. Abdom Imaging. 2013;38:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Position Statement of the Health Physics Society PS010-4: Radiation Risk in Perspective. Health Phys. 2020;118:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6212] [Cited by in RCA: 5800] [Article Influence: 322.2] [Reference Citation Analysis (3)] |
| 53. | Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR report to the general assembly, with scientific annexes. New York: United Nations; 2008. |