Published online Dec 16, 2021. doi: 10.4253/wjge.v13.i12.673
Peer-review started: March 18, 2021
First decision: July 17, 2021
Revised: July 31, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: December 16, 2021
Processing time: 270 Days and 15.7 Hours
Conventional optical colonoscopy is considered the gold standard investigation for colorectal tract pathology including colorectal malignancy, polyps and inflammatory bowel disease. Inherent limitations exist with current generation endoscopic technologies, including, but not limited to, patient discomfort, endoscopist fatigue, narrow field of view and missed pathology behind colonic folds. Rapid developments in medical robotics have led to the emergence of a variety of next-generation robotically-augmented technologies that could overcome these limitations.
To provide a comprehensive summary of recent developments in the application of robotics in lower gastrointestinal tract endoscopy.
A systematic review of the literature was performed from January 1, 2000 to the January 7, 2021 using EMBASE, MEDLINE and Cochrane databases. Studies reporting data on the use of robotic technology in ex vivo or in vivo animal and human experiments were included. In vitro studies (studies using synthetic colon models), studies evaluating non-robotic technology, robotic technology aimed at the upper gastrointestinal tract or paediatric endoscopy were excluded. System ergonomics, safety, visualisation, and diagnostic/therapeutic capabilities were assessed.
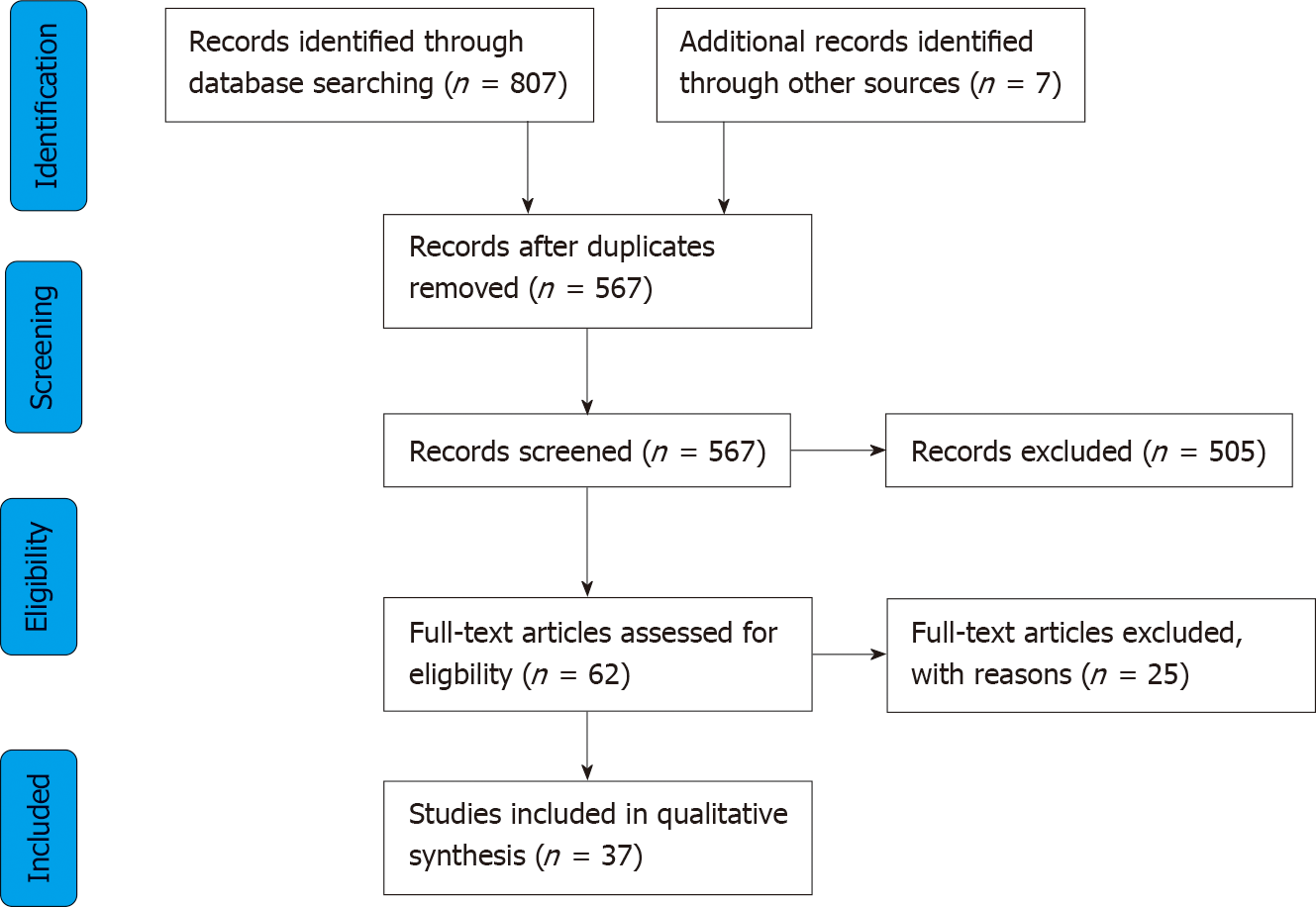
Initial literature searching identified 814 potentially eligible studies, from which 37 were deemed suitable for inclusion. Included studies were classified according to the actuation modality of the robotic device(s) as electromechanical (EM) (n = 13), pneumatic (n = 11), hydraulic (n = 1), magnetic (n = 10) and hybrid (n = 2) mechanisms. Five devices have been approved by the Food and Drug Administration, however most of the technologies reviewed remain in the early phases of testing and development. Level 1 evidence is lacking at present, but early reports suggest that these technologies may be associated with improved pain and safety. The reviewed devices appear to be ergonomically capable and efficient though to date no reports have convincingly shown diagnostic or therapeutic superiority over conventional colonoscopy.
Significant progress in robotic colonoscopy has been made over the last couple of decades. Improvements in design together with the integration of semi-autonomous and autonomous systems over the next decade will potentially result in robotic colonoscopy becoming more commonplace.
Core Tip: Robotic technologies have the potential to transform lower gastrointestinal tract endoscopy into a quicker, safer, more reliable and less painful procedure. In the long term, benefits for patients, endoscopists and the wider healthcare industry are foreseeable, though these have yet to be convincingly demonstrated in human trials. Most studies to date have employed ex vivo modelling and high quality level 1 evidence is currently lacking in this field. Robotic technologies are evolving with such rapidity at the moment, that future robo-endoscopic systems are likely to look and behave very differently to conventional master-slave systems currently in use. Exciting developments in 3D printing, soft robotics, autonomous functionality and augmented reality are likely to converge to lead to the development of truly next generation robotic endoscopy devices.
- Citation: Sekhon Inderjit Singh HK, Armstrong ER, Shah S, Mirnezami R. Application of robotic technologies in lower gastrointestinal tract endoscopy: A systematic review. World J Gastrointest Endosc 2021; 13(12): 673-697
- URL: https://www.wjgnet.com/1948-5190/full/v13/i12/673.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i12.673
Conventional optical colonoscopy represents the gold standard investigation for lower gastrointestinal (LGI) tract pathology including colorectal cancer (CRC), polyps and inflammatory bowel disease[1]. Current generation colonoscopes consist of a semi-rigid flexible scope containing fibre optic bundles with a camera at the distal end allowing visualisation of the colonic lumen. The scope tip can be manoeuvred in two directions via twin-wheels located on the control shaft of the scope, where buttons controlling air insufflation, suction and irrigation mechanisms are also located. Passage of instruments through a working channel running along the body of the scope also allows the endoscopist to perform diagnostic and therapeutic interventions. Typically, a standard scope will have a diameter of 11-13 mm with a length of approximately 160 cm[2,3]. Though this model has undergone subtle refinements in recent years, the basics of the technology remain largely unchanged. While being a familiar, well developed and effective tool for LGI tract diagnosis and therapy, current technologies in optical colonoscopy remain imperfect and are subject to a number of inherent limitations. These include the limited field of view, challenges identifying and treating mucosal lesions proximal to haustral folds, procedure-related pain, and risk of perforation. Pain during colonoscopy is multifactorial in origin, most often resulting from gas distension, looping of the scope and stretching of the mesocolon[4]. Loop formation and mucosal scope trauma have the potential to cause significant iatrogenic injury to the bowel, especially in areas affected by disease[4,5] In addition, colono
Patient discomfort during LGI endoscopy is primarily responsible for 94.6% of colonoscopies being performed under intravenous sedation in Great Britain, and 96% in the United States[9]. However sedation does not improve CIR, increases discharge times and is costly[10]. Therefore, the development of better tolerated methods for endoscopic assessment of the large bowel with reduced sedation requirements is an urgent priority. The most serious complications associated with colonoscopy are perforation and bleeding, which occur with a frequency of 3-8 per 10000 and 1.6 per 1000 colonoscopies, respectively[1]. Though these are infrequent endpoints, addressing current physical limitations with the optical colonoscope may help to further diminish their likelihood[11]. Future technologies for colorectal tract assessment would ultimately benefit from being safer and better tolerated whilst simultaneously maximising on outputs in terms of key performance indicators such as achieving CIR ≥ 95% and adenoma detection rates (ADR) of ≥ 20%[1]. Recent advances in medical robotics offer the potential to overcome the disadvantages of conventional colonoscopy, and engineers have been seeking to develop robotic prototypes capable of endoluminal exploration and visualisation since the early 1990s[12]. In particular, the concept of ‘front-wheel’ actuation, in contrast to the ‘rear wheel’ pushing mecha
The successful application of robotic devices in coronary artery bypass procedures or valvular surgery, and in advanced bronchoscopy, highlight the potential utility of this advanced technology in circumstances where the operator is performing fine tasks within a restricted working environment[15,16]. The same should apply in endoscopy, though comparatively LGI endoscopy has been slow to embrace robotic technologies potentially because of perceived cost barriers, and a lack of understanding of how the technology can improve on the existing formula. Herein we provide a comprehensive narrative review of the state-of-the-art of robotics in lower GI endoscopy.
Systemic review principles were adhered to in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines[17] An electronic literature search was undertaken using EMBASE, MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL) databases (from January 1, 2000 to January 7, 2021). The following MeSH terms were used: “robot”, “robotic”, “robot assist”, “colonoscopy”, “flexible sigmoidoscopy”, “proctoscopy”. Original work reviewing the use of robotic technology in lower GI endoscopy (colonoscopy, flexible sigmoidoscopy or proctoscopy) utilising ex vivo or in vivo studies in animal and human colons were included. There was no limitation on language and type of bowel pathology studied (polyp, CRC, inflammatory bowel disease etc.). Studies evaluating non-robotic technology, robotic technology aimed at the upper gastrointestinal tract, robotic-assisted endoscopy for minimally invasive surgery, robot assisting devices for conventional colonoscopy (such as the The EndoDrive® (ECE Medical Products, Erlangen, Germany) or the Endoscopic Operation Robot)[18] and paedia
Two authors (HKSIS and EA) independently performed literature searches and determined eligibility of studies. Once consensus was reached on studies meeting predefined inclusion criteria, the following data were extracted from included studies: First author’s name, country in which the study was performed, month and year of publication, study design, components of the robotic endoscopic platform, size/length of the endoscopic capsule or flexible scope, illumination method, visualization method, actuation method, data transmission method, aim of robot intention (visualization, diagnosis, treatment, other), degree of robot navigational assistance, type of colon model and results were collected.
A total of 814 records were identified through initial literature searching. Duplicates and obviously irrelevant abstracts were excluded at title and abstract level, leaving 62 articles, which were reviewed fully. Twenty-five articles were further excluded because they were: Review articles (n = 13); studies evaluating robotic devices using in vitro synthetic colon/other (n = 4); assessing robot assistance devices coupled to a standard colonoscope (n = 2); evaluating swallowable wireless capsules without active actuation mechanisms (n = 4); evaluating surgical rather than endoscopic platforms (n = 2). A total of 37 studies were included in the final qualitative analysis (Figure 1). For ease of interpretation of this review, studies have been classified according to mode of actuation, that is the principle active method of robotic motion for each technology. Modes of actuation were defined as EM (n = 13), pneumatic (n = 11), hydraulic (n = 1), magnetic (n = 10) and hybrid (n = 2).
EM actuation is where electrical energy is used to bring about mechanical motion. This is usually brought about by a tether (containing wires) attached to the robotic device and to an external power source. Wireless devices without a tether will require an internal battery to provide power which takes up space. The tether will provide additional weight and friction as it slides along the mucosa which the robot will need to overcome. Either way considerable power is usually required[19,20]. A summary of studies investigating this mode of actuation is provided in Table 1.
| Ref. | Design and actuation components of evaluated robotic system(s) | Endoscope and/or capsule dimensions | Mode(s) of actuation | Mode(s) of illumination and luminal visualisation | Capabilities evaluated | Degree of robot navigational assistance | Study methodology | Main findings |
| Rösch et al[21], 2008 (Germany) | InvendoscopeTM SC40 (Invendo Medical, Kissing, Germany): Colonoscope with an inverted sleave mechanism, propulsion connector, endoscope driving unit, hand-held control unit, 3.2 mm working channel | 18 mm diameter, 170-200 cm length. | Electromechanical | Three white LEDs, CMOS vision chip with a field of view of 114 degrees | Visualisation | Direct Robot control | In vivo: n = 34 Human, heathy volunteers | CIR of 82%. Pain free procedure in 92% of cases. Mean pain score 1.96/6. 0% required sedation. No complications |
| Groth et al[23], 2011 (Germany) | InvendoscopeTM SC40 (Invendo Medical, Kissing, Germany): Colonoscope with an inverted sleave mechanism, propulsion connector, endoscope driving unit, hand-held control unit, 3.2 mm working channel | 18 mm diameter, 170-200 cm length | Electromechanical | Three white LEDs, CMOS vision chip with a field of view of 114 degrees | Visualisation, Diagnosis, Treatment | Direct Robot control | In vivo: n = 61 Human, Asymptomatic individuals at average risk of CRC willing to undergo CRC screening | CIR of 98.4%. Sedation required in 4.9%. Median CIT of 15 min. Mean pain/discomfort score: 2.6. 32 of 36 polyps successfully removed with snare or forceps. 1 flat polyp required referral for conventional colonoscopy and 3 polyps seen on introduction could not be found on withdrawal |
| Eickhoff et al[24], 2007 | The NeoGuide Endoscopy System (NeoGuide Endoscopy System Inc., Los Gatos, CA United States): Scope with 16 actuator segments, steering dials to control the tip and Tip position sensor. External position sensor, support arm, 3.2 mm working channel, video processor and control unit. Computed 3D mapping of the colon | 173 cm in length, 14-20 mm in diameter | Electromechanical | Conventional CCD camera | Visualisation, safety and ease of use | Semi-autonomous | In vivo: n = 10 Humans requiring screening or diagnosis | CIR is 100%. Median CIT is 20.5 min. Adenomas successfully removed with snare or forceps. No acute colonic trauma (bleeding, perforation, submucosal petechiae). No complications at 30 d follow up. Detection and correction of looping is 100%. Physician satisfaction is 100% |
| Valdastri et al[25], 2009 (Italy) | Legged capsule consisting of two leg sets (six legs each with hooked round tips), 2 motors, bidirectional communication platform, HMI in LabVIEW | 11 mm diameter by 25 mm long | Electromechanical | No camera in this prototype | Locomotion and safety | Semi-autonomous | Ex vivo- Porcine colon between two fixtures and 140 cm porcine colon placed in an abdominal phantom | Porcine colon between two fixtures: The 12-legged capsule distended the colon in a uniform manner. Maximum pulling force of the capsule on the colon wall: 0.2 N. Porcine colon in abdominal phantom: Capsule was able to traverse the complete length of the colon, Average speed was 5 cm/min |
| Lee et al[26], 2019 (Korea) | Legged robotic colonoscope, reel controller with external motor, Bowden cable and control system. The robot has 6 legs covered with silicone | Robot: 16 mm diameter (33 mm with legs deployed) by 49 mm in length. Bowden cable: 5 mm diameter by 1 m length | Electromechanical | Not described | Locomotion and safety | Autonomous | Ex vivo: Excised porcine colon | Locomotion velocities: Straight path: 9.5 mm/s. Incline at 30 degrees: 7.1 mm/s. Incline at 60 degrees: 5.1 mm/s. No mucosal damage or perforations |
| Trovato et al[27], 2010 (Japan) | Robotic colonic endoscope consisting of a front body with a clockwise helical fin, DC motor and rear body with an anti-clockwise helical fin; Reinforcement learning algorithm (Q-learning and State-Action-Reward-State-Action) | 170 mm in length, 30 mm in diameter | Electromechanical | Not described. No Visualisation module in this prototype | Locomotion and safety | Semi-autonomous | Ex vivo: < 1 m Swine colon (6 specimens) attached to the inside of a cylindrical plastic tube. In vivo: Swine colon–10 trials, 5 min each | Ex vivo: Best travelled distance around 70 cm. Average velocity with Fixed input (15 trials): 21.47 mm/min. Average velocity with SARSA (18 trials): 40.71 mm/min (P = 0.02). Average velocity with Q-learning (21 trials): 36.05 mm/min (P = 0.039). Robot with learned algorithms are more likely to pass through bends/tight passages. In vivo: Speed 11 mm/min. Best travelled distance is 55 mm. No acute mucosal damage |
| Kim et al[28], 2010 (Korea) | Paddling-based capsule endoscope: Capsule with camera module, DC motor and 6 paddles. Tether consisting of 4 cables extend from the capsule to the external controller | Capsule: 15 mm in diameter and 43 mm in length. Tether: 2 m | Electromechanical | A camera module with 125 degree field of view and which transmits images at 10 frames per second | Locomotion and safety | Semiautonomous | Ex vivo: Porcine colon set up in 2 positions (sloped 27.5 degrees, straight length 35 cm or sloped 37.5 degrees, straight length 62 cm). In vivo: 1 pig–8 trials | Ex vivo: Velocity in sloped 27.5 degrees, straight length 35 cm colonic segment: 36.8 cm/min. Velocity in sloped 37.5 degrees, straight length 62 cm colonic segment: 37.5 cm/min. In vivo: Mean velocity: 17 cm/min over 40 cm length. Complications: Pinpoint erythema on colonic mucosa seen |
| Wang et al[29], 2006 (China) | Worm like robotic endoscope system consisting of a microrobot, controller and personal computer. The microrobot consists of a head cabin with the visualisation module and 3 mobile cells connected to the controller by an electric cable. Each mobile cell contains a linear electromagnetic driver | 9.5 mm in diameter, 120 mm in length | Electromechanical | CCD camera and lights | Locomotion | Semi-autonomous | Ex vivo: Porcine colon | Robot travels the colon length (112 cm) in 7.3 min. Robot able to move forward, backward or remain static based on controller commands |
| Wang et al[30], 2007 (China) | Worm like robotic endoscope system consisting of a microrobot, controller and personal computer. The microrobot consists of a head cabin with the visualisation module and 3 mobile cells connected to the controller by an electric cable. Each mobile cell contains a linear electromagnetic driver. Additional deflection mechanism after the head cabin controls the camera’s pose | 10 mm in diameter, 110 mm in length | Electromechanical | CCD camera and lights | Locomotion | Semi-autonomous | Ex vivo: Porcine colon | Robot travels the colon length (112 cm) in 7.3 min |
| Wang et al[31], 2017 (China) | Worm like robotic endoscope consisting of a head cabin and three independent segments; each segment is composed of a linear locomotor with micromotor, turbine-worm and wire wrapping-sliding mechanism. The robot is entirely covered by an external soft bellow | 13 mm diameter, 105 mm in length | Electromechanical | Not described | Locomotion and safety | Semi-autonomous | In vivo: Porcine colon | Greater speed in straight rather than curved paths. Speed ranges from 1.62-2.2 mm/s. Robot travels the entire colon in 119 s. Distance is not specified. No breakage or damage to the colonic mucosa |
| Naderi et al[32], 2013 (Iran) | Robot with a camera, 2 clampers, 5 discs and 15 springs allowing bending and steerability, 3 motors; Driving kit, HMI in MATLAB and Joystick | 19 mm in diameter, 180 mm in length. | Electromechanical | Camera | Locomotion and safety | Semi-autonomous | Ex vivo: Sheep colon, 2 positions: Straight or with an 84 degree bend | Velocity: Straight path: 18.4 cm/min. Curved path: 10.5 cm/min. No significant trauma |
| Lee et al[26], 2019 (Korea) | 3 elastic PTFE caterpillars with worm gear, steering module, camera module, flexible shaft with steering knobs and wires, external motor and controller | 130 mm in length, 55 mm maximum diameter | Electromechanical | LED lamps and camera | Locomotion and visualisation | Direct robot operation | Ex vivo: 1 m excised porcine colon placed in an abdominal phantom. In vivo: 1 mini pig | Ex vivo: Velocity of the robotic colonoscope: 3.0 mm/s; CIR is 50%; CIT is 8.55 min. In vivo: Failed caecal intubation with difficulty travelling through fluid and faecal material |
| Formosa et al[34], 2020 (United States) | Endoculus- treaded (4) robotic capsule endoscope consisting of an inertial measurement unit, two motors, air/water channels, a tool port, flexible tether connected to a control board and laptop with controller | 2 m tether | Electromechanical | CMOS camera with adjustable LEDs | Locomotion, visualisation and channel function | Direct robot operation | Ex vivo: 40 cm excised porcine colon. In vivo: 1 pig | Ex vivo: Able to move in forward/reverse directions at 40 mm/s and whether the colon was collapsed or inflated. Also able to pass tight haustra and make turns. In vivo: Camera, insufflation, irrigation and biopsy tools functioned as expected |
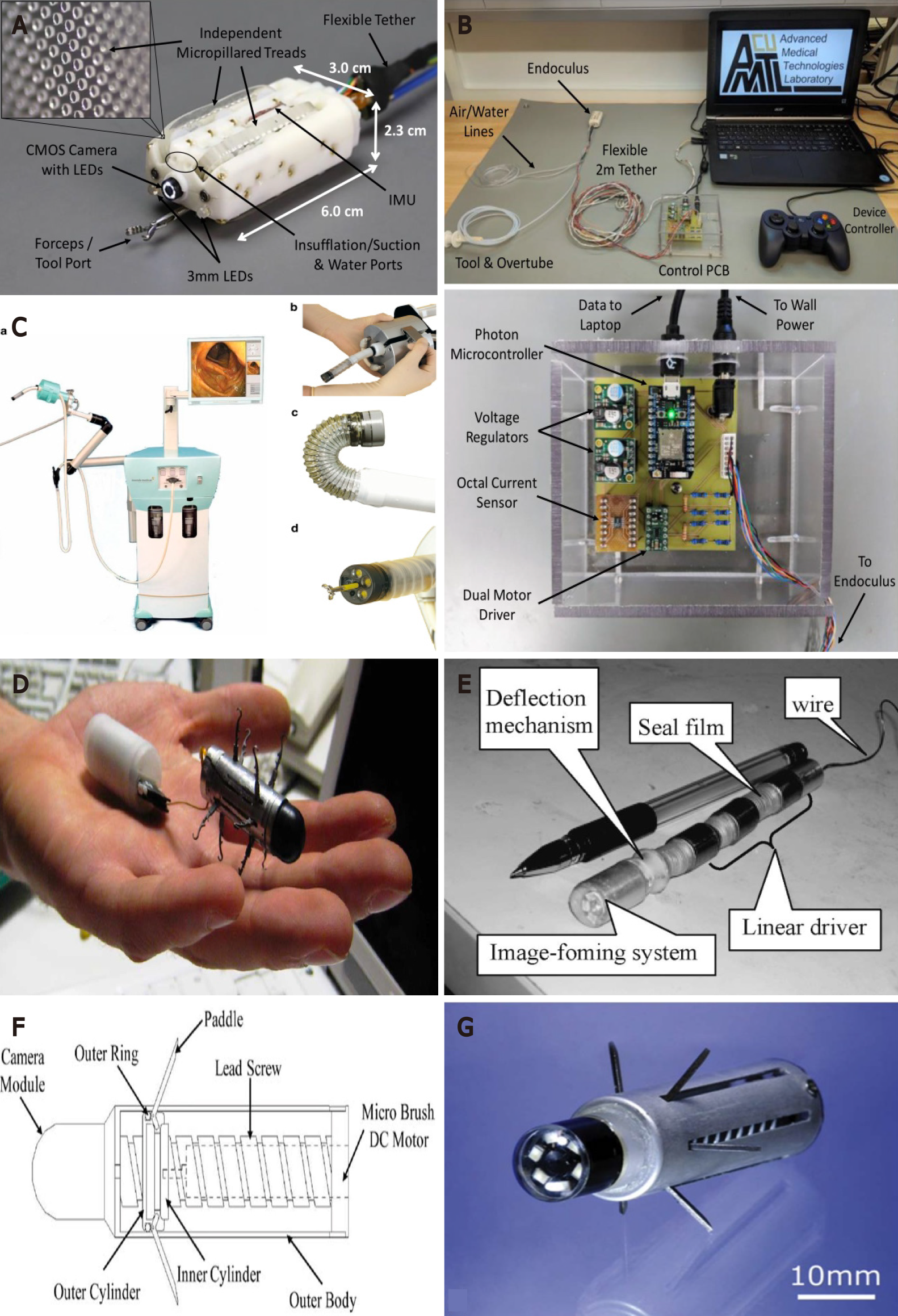
Two EM actuation robotic endoscopic systems were developed and received Food and Drug Administration (FDA) approval, though these are now no longer commercially available[19]. The Invendoscope SC40 (Invendo Medical, Kissing, Germany) is a motorised colonoscope, controlled by a joystick and actuated by an inverted sleeve mechanism and a driving unit with 8 wheels. It is 18 mm in diameter and has a visualisation module and a 3.2 mm working channel (Figure 2). Two trials on humans have been carried out to evaluate this platform. The first, in 34 healthy volunteers showed a CIR of 82%, with 92% of patients ‘pain free’ and no acute complications were reported[21]. The purported strength of this system was the combination of a highly flexible endoscope shaft with the proprietary 'inverted sleeve' technology, which the developers believed could permit potentially ‘painless’ colonoscopy, as no direct forces are applied against the intestinal walls while the device passes through narrow intestinal convolutions. Invendo medical Gmbh was acquired by Ambu A/S with plans to release a single use robotic colonoscope in 2021[19,22]. Another study in 61 asymptomatic individuals with an average risk of CRC willing to undergo CRC screening found a CIR of 98.4%, with a median caecal intubation time (CIT) of 15 min. Only 4.9% of patients required sedation[23]. The Neoguide Endoscopy System (Neoguide Endoscopy System Inc., Los Gatos, CA United States) has a scope diameter of 14-20 mm and consists of 16 actuator segments under EM control to bring about movement. It also contains a tip position sensor, an external position sensor and a 3.2 mm working channel. A trial on 10 individuals undergoing CRC screening or routine diagnostic colonoscopy showed a CIR of 100% with a median CIT of 20.5 min. Adenomas were successfully removed with snare or forceps and there was no evidence of complications at 30 d follow up[24]. With this platform, the position and angle of the scope's tip are encoded into a computer algorithm. As the scope moves forwards, the algorithm directs each successive actuator segment to assume the same shape/position that the tip had for that given insertion depth. The insertion tube thus changes its shape at different insertion depths in a "follow-the-leader" manner, which should minimise discomfort. Neoguide Endoscopy System Inc. was acquired by Intuitive Surgical Inc. and the technology translated to robotic lung biopsy[19]. Several other non-certified EM actuation devices have been developed and below these have been categorised further based on their distinct physical properties which bring about motion.
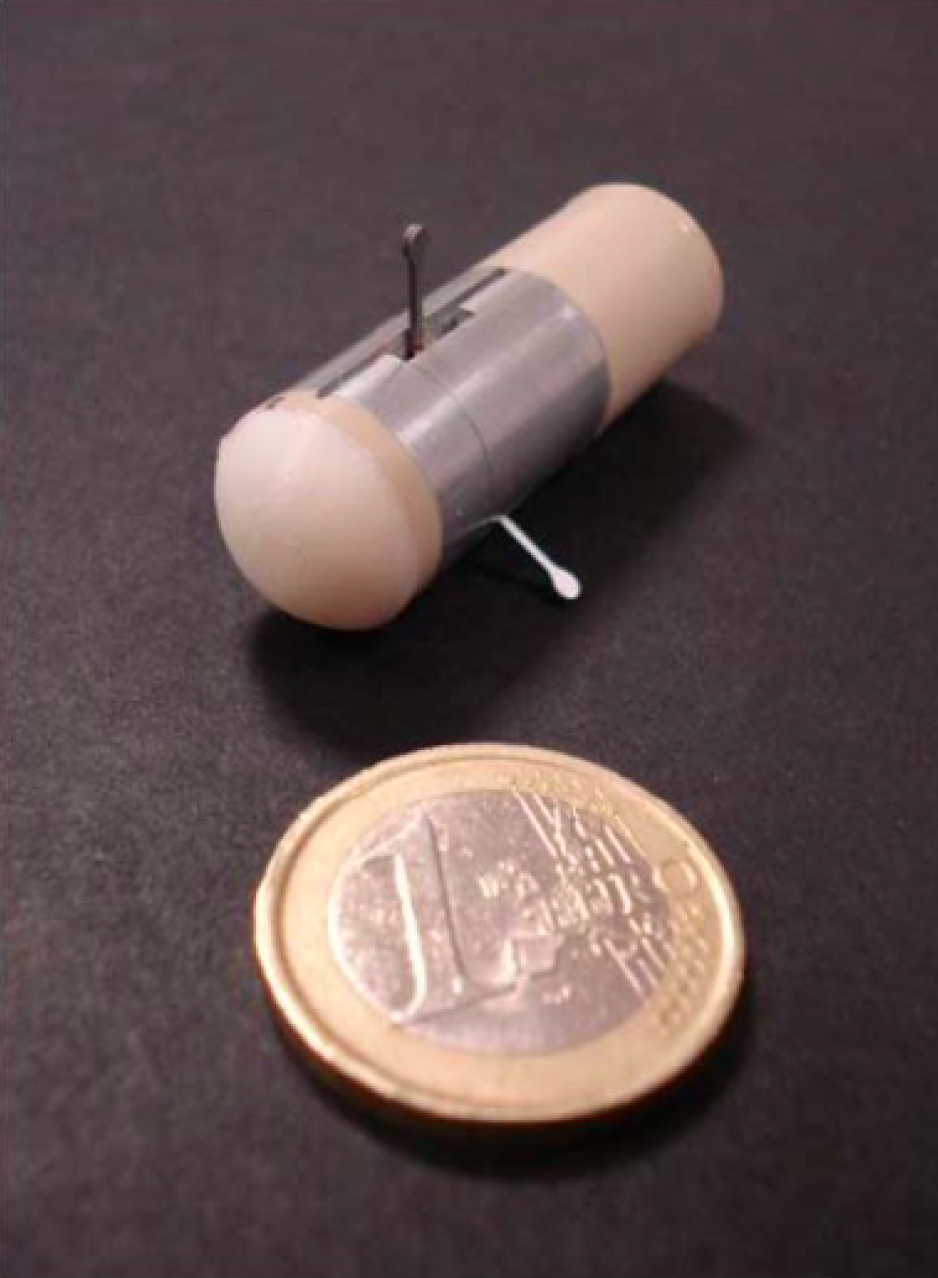
Legs: A 12-legged capsule was developed by Valdastri et al[25], comprising two motors, a bidirectional communication platform and a human machine interface (HMI) capable of semi-autonomous intrinsic EM actuation (Figure 2). The capsule measures 12.8 mm in diameter and 33.5 mm in length. The device was designed to strike a versatile balance between size and ability to traverse the bowel. The device was tested in a porcine gut model and was able to traverse the complete length of the colon (140 cm) at an average speed of 5 cm/min[25]. Though a little slower in terms of pace, this device highlights the potential for miniaturisation of devices in robotic endoscopy.
A legged colonoscope consisting of six legs covered in silicone and a Bowden cable connecting the device to an external motor and control system was tested in excised porcine colon of varying paths to determine locomotive efficacy and safety. It was able to travel at decreasing velocities of 9.5 mm/s, 7.1 mm/s and 5.1 mm/s on straight, 30 degree curved and 60 degree curved paths, respectively. No mucosal damage or perforations were observed during testing[26]. The diameter of the device is 16 mm without the legs deployed and 33 mm when they are.
Fins: A novel capsular device, 170 mm in length and 30 mm in diameter, consisting of a front body with a clockwise helical fin and rear body with an anti-clockwise helical fin was developed by a team in Japan. The bodies are connected by a DC motor and the device is computationally reinforced with learning algorithms to improve effectiveness of motion through iterative learning. It was tested in ex vivo and in vivo porcine colon models and ex vivo trials demonstrated improved movement performance with learned algorithms. In vivo trials showed an average speed of 11 mm/min with no acute mucosal damage[27].
Paddles: A tethered capsule endoscope containing a camera module, DC motor and 6 paddles measuring 15 mm in diameter was evaluated in ex vivo porcine colon as well as in an in vivo porcine model (Figure 2). At a slope of 27.5 degrees (length: 32 cm) and 37.5 degrees (straight length: 62 cm), impressive forward motion speeds of 36.8 cm/min and 37.5 cm/min were achieved. The mean velocity reached in the in vivo model over a distance of 40 cm was 17 cm/min. A degree of minor paddle-trauma was noted on the mucosa which may present a safety concern[28].
Worm-like: Wang et al[29,30] created two similar earth-worm like robotic endoscopes. The initial system consisted of a microrobot, controller and user interface. The microrobot in turn consists of a head cabin with the visualisation module and 3 mobile cells connected to the controller by an electric cable. Each mobile cell contains a linear electromagnetic driver[29,30]. The microrobot was able to travel along the porcine colon length (112 cm) in 7.3 min[29,30]. The worm-like device is pictured in Figure 2.
Later, a similar microrobot was created by the same team with two notable design adjustments: Each segment with this updated prototype is composed of a linear locomotor with its own micromotor, turbine-worm and wire wrapping-sliding mechanism, and the microrobot is entirely covered by an external soft ‘bellow’. The soft bellow acts to increase the friction gradient between the robot and the colonic mucosa which should improve locomotion ability. This device was tested in in vivo porcine experiments and demonstrated average speeds of up to 2.2 mm/s, with no mucosal damage reported[31].
A robot with a camera, two clampers, three motors, 5 discs and 15 springs was created to allow worm-like flexible movement. It could be driven using a joystick and is 19 mm in diameter and 180 mm long. Motion ability and safety were tested in sheep colon in a straight or curved (84 degree bend) path. The device travelled at 18.4 cm/min and 10.5 cm/min in straight and curved colonic segments, respectively. No mucosal trauma was seen[32]. Overall worm-like devices appear safe with a variable speed.
Caterpillars: A robot with 3 elastic caterpillars, designed to expand the colonic lumen while causing little trauma was able to travel at 3 mm/s and achieve caecal intubation 50% of the time at 8.55 min in porcine colon placed within a human abdominal phantom[33]. Unfortunately, in an in vivo experiment, the robot failed to achieve caecal intubation as it had difficulty travelling through fluid and faeces[33].
Treads: A treaded (4 treads) robotic capsule with two motors, connected via a flexible tether to a control printed circuit board and laptop (Figure 2) was tested in excised porcine colon and was able to move in forward and reverse directions at 40 mm/s even with the bowel wall collapsed[34]. The treads allow traction between the device and the colonic mucosa to allow effective locomotion. It was also able to pass tight haustra and make turns due to the presence of the second motor and resulting increased degrees of locomotion freedom. The device also had a visualisation module and channels for air, water and tools. Camera, insufflation, irrigation and biopsy tools all functioned effectively during in vivo porcine testing[34].
Electropneumatic (EP) actuation involves the use of pressurised gas to bring about motion. The Aer-o-scope (GI View Ltd, Ramat Gan, Israel), Endotics [ERA Endoscopy S.r.l., Peccioli (Pisa), Italy] and Sightline Colonosight systems (Stryker GI, Dallas, Tex, Haifa, Israel) are all examples of FDA approved EP robotic systems with a visualisation module and channels for insufflation, suction and irrigation.
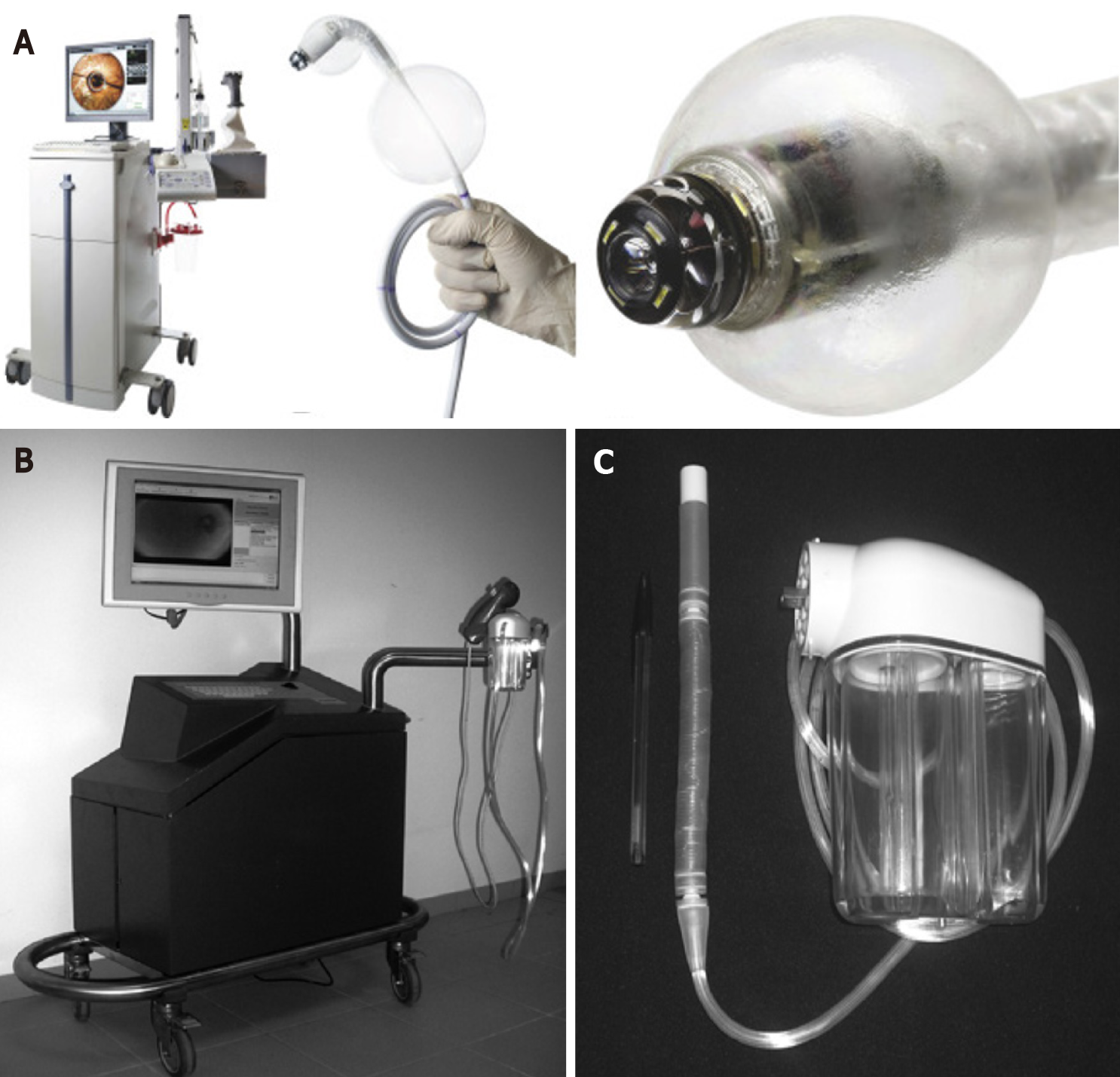
The Aer-o-scope system works by generating a gas (carbon dioxide) pressure gradient between a rectal balloon inflated in the anus and a balloon located at the tip of the scope. Safety mechanisms ensure that the pressure in the colon does not exceed 54 m bar. The scope is only 5.5 mm in diameter (Figure 3). In vivo studies on healthy human volunteers (n = 12) or those requiring CRC screening (n = 56) have reported CIR ranging from 83%-98%, average CIT of 23 min and no acute complications other than mild mucosal petechiae in some instances[35,36]. Four of twelve patients required sedation[35]. In those undergoing CRC screening, the polyp detection rate was 87.5% and mucosal visualisation was rated as ‘excellent’ by participating endoscopists[30]. The Aer-o-scope provides a 360 panoramic vision system in addition to a complementary metal-oxide-semiconductor (CMOS) camera which allows improved visualisation. In an In vivo study with 12 anaesthetised pigs with surgically simulated colonic ‘polyps’ the Aer-o-scope visualised 94.9% of polyps compared to 86.8% achieved with standard optical colonoscopy (P = 0.002)[37].
The Endotics system consists of a flexible probe with a head, body and tail, EP connector and a workstation (Figure 3). Two clampers located at the proximal and distal ends of the probe aid movement. Ex vivo testing using porcine colon has suggested that the stress exerted on the colonic wall using this device is 90% less than in standard colonoscopy[38]. This should in theory translate into a reduced need for analgesia and sedation. In fact, two human trials showed that Endotics was less painful on a scale of 1 to 10 (0.9 vs 6.9)[38] and did not require any sedation (0% vs 19.7%, P < 0.001)[39] compared to conventional colonoscopy[38,39]. This device can achieve CIR as high as 92.7% within 29 min[40]. Diagnostically, in individuals with a family history of CRC and/or polyps, the Endotics System showed a sensitivity, specificity, positive predictive value and negative predictive value of 93.3%, 100%, 100% and 97.7%, respectively[39]. The Endotics system has also demonstrated a short learning curve: Two blocks of consecutive patients underwent LGI endoscopy using the Endotics platform with improvements in CIR (85.2% vs 100%), intubation time (55 min vs 22 min, P = 0.0007) and withdrawal time (21 min vs 16 min)[40]. Importantly, in an evaluation of 102 patients previously having undergone failed colonoscopy, 95 patients (93.2%) underwent successful caecal intubation with the Endotics system[41].
The Sightline ColonoSight system consists of a reusable scope covered by a disposable sleeve and connected to an air pressure engine[42]. Shike and colleagues evaluated the performance of this system in 178 human study participants and reported a CIR of 90% with a mean CIT of 11.2 min. Scope advancement with this device is facilitated by self-propulsion of the instrument affected by an air-pressure-powered engine and LED illumination eliminates the need for fiber optics and an external light source.
Other non-certified EM actuation robotic devices include the “EndoCrawler” which consists of longitudinal and circumferential rubber bellow pneumatic actuators joined in four segments with a bending tube to allow steering between the first two segments[43]. When pressurised air enters the bellow, it extends longitudinally. It has a central hollow cavity for insufflation, irrigation, suction and instrument channels as well as charged coupled device cables to pass through. It has undergone ex vivo testing in human cadaveric colon which demonstrated clear visualisation capabilities and an average speed of 200 mm/min. In vivo assessment using a live porcine model also demonstrated some encouraging findings, though difficulties were encountered when attempting to negotiate sharp bends. These issues notwithstanding, this early prototype again demonstrates the potential for self-propulsive, remotely controlled robotic devices for endoluminal assessment[43].
In 2017, a simple colonoscopy robot consisting of the robot (tip with camera, latex tubing and anal fixture) with an external pneumatic circuit was developed. Locomotion feasibility and safety was tested in porcine colon. The device was able to traverse the entire length of the colon in 71.4% of trials, able to traverse the entire length of colon with additional bends in 90.9% of trials, had an average speed of 28 mm/s with an average CIT of 54.2 s. The maximum propulsive force was 6 N i.e., an acceptable pressure on the colonic mucosa however balloon rupture led to damage including tearing of the porcine colon[44].
A further pneumatic device consisting of three segments, each containing two soft pneumatic balloons and two rigid connectors was developed and tested in excised pig colon. The balloons are twisted in the proximal and distal gripper segments but linear in the middle propulsion segment. A camera and channels for air flow and instruments are built in. The unactuated device is 22 mm in diameter. The robot travelled at 1 mm/s and was able to clearly visualise the colonic mucosa[45].
A summary of all studies evaluating robotic EM actuation systems for LGI endoscopy is provided in Table 2.
| Ref. | Design and actuation components of evaluated robotic system(s) | Endoscope and/or capsule dimensions | Mode(s) of actuation | Mode(s) of illumination and luminal visualisation | Capabilities evaluated | Degree of robot navigational assistance | Study methodology | Main findings |
| Vucelic et al[35], 2006 (Israel) | Aer-O-scope (GI View Ltd, Ramat Gan, Israel): Workstation and Disposable unit consisting of a rectal introducer, supply cable, scanning balloon, scope and rectal balloon. The supply cable connects the disposable unit to the workstation with its joystick and is able to transmit air, water and suction | 5.5 mm diameter, 2.5 m length | Pneumatic | White LED, 360 panoramic vision system with CMOS camera with a field of view of 57 degrees | Visualisation and safety | Semi-autonomous | In vivo: n = 12 Human, healthy volunteers | CIR is 83%. Median CIT is 14 min with an average procedure duration of 23 min. Analgesia required in 2 patients. 4 patients had submucosal petechial lesions. No complications at 30 d follow up |
| Gluck et al[36], 2016 (Israel) | Aer-O-scope (GI View Ltd, Ramat Gan, Israel): Workstation and Disposable unit consisting of a rectal introducer, supply cable, scanning balloon, scope and rectal balloon. The supply cable connects the disposable unit to the workstation with its joystick and is able to transmit air, water and suction | 5.5 mm diameter, 2.5 m length | Pneumatic | White LED, 360 panoramic vision system with CMOS camera with a field of view of 57 degrees | Visualisation and safety | Semi-autonomous | In vivo: n = 56 Human, CRC screening | CIR is 98.2%. Mean withdrawal time is 14 min. Polyp detection rate of 87.5%. 0 patients had submucosal damage. No complications at 48 h follow up. Rated as excellent visualisation by endoscopists |
| Gluck et al[37], 2015 (Israel) | Aer-O-scope (GI View Ltd, Ramat Gan, Israel): Workstation and Disposable unit consisting of a rectal introducer, supply cable, scanning balloon, scope and rectal balloon. The supply cable connects the disposable unit to the workstation with its joystick and is able to transmit air, water and suction | 5.5 mm diameter, 2.5 m length | Pneumatic | White LED, 360 panoramic vision system with CMOS camera with a field of view of 57 degrees | Visualisation and detection | Semi-autonomous | In vivo: n = 12 pigs with surgically simulated colonic ‘polyps’ | A total of 36 Aer-O-scope and 24 colonoscopy procedures were performed. The Aer-o-scope visualised 94.9% of polyps compared to 86.8% with colonoscopy. This was significant (P = 0.002). Miss rates for polyps was 5.1% with Aer-O-scope and 13.2% (P = 0.002) with conventional colonoscopy. This significant difference is true for > 6 mm polyps |
| Cosentino et al[38], 2009 (Italy) | Endotics System [ERA Endoscopy S.r.l., Peccioli (Pisa), Italy]: Workstation with console and disposable flexible probe. The probe has 2 clampers to aid locomotion and a head (contains the camera, LEDs and channels for suction, irrigation and insufflation) a body and a tail | 23-37 cm in length, 17 mm in diameter | Pneumatic | LED light source and CMOS camera with a field of view of 110 degrees | Visualisation and Safety | Semi-autonomous | Ex vivo: n = 1 porcine colon fixed to a human adult abdominal phantom. In vivo: n = 40 Humans, with a family Hx of CRC, known previous polyps and FOB positive requiring investigation | Ex vivo: The stress pattern was 90% less than with colonoscopy. In vivo: CIR was 27% for the endotics system compared to 82% with colonoscopy. The mean CIT was 57 min. The endotics system was described as less painful (0.9 vs 6.9). The endotics system has a higher diagnostic accuracy as it detected 2 polyps and 2 angiodysplastic lesions not identified with colonoscopy |
| Tumino et al[39], 2010 (Italy) | Endotics System (ERA Endoscopy S.r.l., Peccioli (Pisa), Italy): Workstation with console and disposable flexible probe. The probe has 2 clampers to aid locomotion and a head (contains the camera, LEDs and channels for suction, irrigation and insufflation) a body and a tail | 25-43 cm in length, 17 mm in diameter | Pneumatic | LED light source and CMOS camera with a field of view of 110 degrees | Visualisation, sensitivity and specificity | Semi-autonomous | In vivo: n = 71 Humans, with a family Hx of CRC or polyps | Endotics system versus colonoscopy: CIR: 81.6% vs 94.3%. The average time for procedure completion: 45 min vs 23 min (P < 0.001). Patients requiring sedation: 0% vs 19.7% (P < 0.001). Endotics system for detecting polyps: Sensitivity: 93.3%; Specificity: 100%; Positive predictive value: 100%; Negative predictive value: 97.7% |
| Trecca et al[40], 2020 (Italy) | Endotics System [ERA Endoscopy S.r.l., Peccioli (Pisa), Italy]: Second generation system- Workstation with console and disposable flexible probe. The probe has 2 clampers to aid locomotion and a head (contains the camera, LEDs, chromoendoscopy and channels for suction, irrigation and insufflation) a body and a tail | 23-37 cm in length, 17 mm in diameter | Pneumatic | LED light source, chromoendoscopy and CMOS camera with a field of view of 140 degrees | Learning curve, visualisation and diagnostic accuracy, safety | Semi-autonomous | In vivo: n = 55 Humans, requiring diagnosis, CRC screening or surveillance. Training progress was evaluated by comparing two consecutive blocks of patients i.e. group A (first 27) and group B (last 28) | CIR is 92.7%. Median CIT is 29 min. Median withdrawal time is 18 min. Polyp detection rate: 40%; Adenoma detection rate: 26.7%; Advanced neoplasm: 0%; Complication: 1.8%-bleeding with polypectomy; Successful polypectomy and hot biopsy coagulation for bleeding. Mean VAS pain/discomfort: 1.8. Learning curve assessment, Group A vs Group B: CIR: 85.2% vs 100%. Median CIT: 55 min vs 22 min (P = 0.0007). Median withdrawal time: 21 min vs 16 min |
| Tumino et al[41], 2017 (Italy) | Endotics System (ERA Endoscopy S.r.l., Peccioli (Pisa), Italy): Workstation with console and disposable flexible probe. The probe has 2 clampers to aid locomotion and a head (contains the camera, LEDs and channels for suction, irrigation and insufflation) a body and a tail | 25-43 cm in length, 17 mm in diameter | Pneumatic | LED light source and CMOS camera with a field of view of 110 degrees | Visualisation and performance | Semi-autonomous | In vivo: n = 102 Humans, previously failed caecal intubation on colonoscopy | CIR was 93.1% and therefore had a 95% performance. Mean CIT was 51 min |
| Shike et al[42], 2008 (Italy/Israel/United States) | Sightline ColonoSight (Stryker GI, Dallas, Tex, Haifa, Israel): A reusable scope with LEDs and camera at the tip and steering dials proximally. Tips is covered by a disposable sleeve with 3 working channels for suction, irrigation, insufflation and instruments. Electropneumatic unit, control unit and video monitor | Not described | Pneumatic | LED light source and camera | Visualisation, diagnosis and treatment | Semi-autonomous | In vivo: 2 pigs–To assess safety in terms of bacterial transmission to the reusable scope with a disposable sleeve covering. In vivo: 178 Humans, healthy volunteers and various clinical indications for colonoscopy | In vivo, Pigs: E.coli and E. Fergusonii from scope handle, shaft and tip before the procedure: Nil growth. E.coli and E. Fergusonii from scope handle, shaft and tip after the procedure: Nil growth. E.coli and E. Fergusonii from sheath covering after the procedure: Heavy growth. In vivo, Humans: CIR is 90%. Mean CIT is 11.2 min. Diagnoses of diverticulosis, polyps, colitis, haemorrhoids, normal or other was given. Successful polypectomy, biopsy and argon plasma coagulation. No complications at 2 wk follow up |
| Ng et al[43], 2000 (Singapore) | EndoCrawler: Longitudinal and circumferential rubber bellow actuators joined in four segments with a bending tube to allow steering between the first two segments and vision module; Central hollow cavity for instruments, insufflation, irrigation and suction channels and CCD cables. These exit the proximal end as a flexible cable similar to a colonoscope; LabWindows user interface and joystick | 28 mm in diameter, 420 mm length | Pneumatic | CCD camera and light source | Locomotion and visualisation | Direct robot operation | Ex vivo- Cadaveric colon. In vivo-Pig | Ex vivo: Clear visualisation of colonic wall. Speed: 200 mm/min however required external pushing and couldn’t progress beyond bends unless the head was deflected away from the colonic wall. In vivo: ‘Red out’ images throughout most of the robot’s journey. Average speed: 150 mm/min with external pushing. Unable to progress beyond an acute bend |
| Dehghani et al[44], 2017 (United States) | Pneumatically driven colonoscopy robot consisting of the robot (tip with camera, latex tubing, tethered camera and anal fixture) and external pneumatic circuit and electric circuit with laptop | Not described | Pneumatic | Camera | Locomotion feasibility and safety | Semi-autonomous | Ex vivo: 1.5 m porcine colon in human phantom. Tests repeated 5-14 times depending on analysis performed | Able to traverse the entire length 71.4% (10/14 trials). Able to traverse the entire length with additional bends 90.9% (10/11 trials). Robot speed of 28 mm/s (5 trials). Average CIT is 54.2 s. (5 trials). Maximum propulsive force is 6 N (44 mmHg) which is less than the safe intraluminal pressure of 80 mmHg. Balloon rupture led to damage including tearing of the porcine colon |
| Chen et al[45], 2019 (China) | Soft endoscopic device which consists of two gripper segments and one propulsion segment. Each segment contains two soft pneumatic balloons and two rigid connectors. The balloons are twisted in the gripper segments but linear in the propulsion segment. The connectors contain inner channels for air flow and instruments; Lab view interface. Air compressor with regulators, pressure sensors, valves and air pipes connected to the endoscopic device and a power source | The unactuated device is 95 mm in length and 22 mm in diameter. | Pneumatic | CCD camera | Locomotion and visualisation capability | Semi-autonomous | Ex vivo: Pig colon-one end fixed to a pipe, the other free. Colon placed in a horizontal position | Velocity to traverse the colon: 1 mm/s. Clear visualisation of the colonic mucosa |
| Coleman et al[47], 2016 (United Kingdom) | Hydraulic colonoscope system: A CV connected to extra-corporeal pumps and valves via a tether. The CV contains a magnetic tracker and is surrounded by a balloon which is flexible and may be inflated or deflated. The pump system is used to pump water into the colon behind the CV; Anal port and control system on HMI | CV dimensions not described. Tether: 1.8 m long, 6 mm in diameter | Hydraulic | No camera in this prototype however a dummy with a diameter if 11 mm and length of 25 mm is incorporated to simulate its presence | Comparison of CV locomotion under manual control or automatic control to colonoscopy | Direct or semi-autonomous | Ex vivo: Two 120 cm porcine colon placed in human abdominal phantom–6 trials per manual control, automatic control and colonoscopy | 100% CV reached the caecum. CV vs colonoscopy: CIT: 3.95 vs 4.91 min (P = 0.43). Maximum force to the colon: 0.63 vs 2.2 N (P = 0.004). Maximum anal pressure: 1.53 vs 4.53 kPa (P = 1 × 10-7). Mean anal pressure: 0.65 vs 1.5 kPa (P = 0.0003). No difference in maximum or mean caecal pressure. Manual CV versus Auto CV: CIT: 2.11 vs 5.79 min (P = 0.02). Mean anal pressure: 1.86 vs 1.31 kPa (P = 0.03). No difference maximal anal pressure and maximum or mean caecal pressure |
Hydraulic actuation uses a pressurised fluid medium such as water to progress through the colon. A meta-analysis of randomised controlled trials has previously shown that water immersion colonoscopy does significantly decrease pain scores and sedation rates without affecting the diagnostic quality or completeness of colonoscopy when compared with air intubation[46].
The “Hydraulic Colonoscope” system consists of a colonic vehicle (CV) connected to external pumps and valves via a tether. The CV contains a magnetic tracker and is surrounded by a balloon which may be inflated or deflated to create an appropriate seal with the colonic wall. The pump system is used to pump water into the colon behind the CV. An anal port prevents water from escaping the colon. Motion ability was trialled in porcine colon and compared to conventional colonoscopy. The device was able to reach the caecum in all attempts. There was no difference in the CIT or caecal pressure between the device and colonoscopy. However, significant differences were found in the maximum force exerted on the colon (0.63 N vs 2.2 N, P = 0.004), maximum anal pressure (1.53 kPa vs 4.53 kPa, P = 1 × 10-7) and mean anal pressure (0.05 kPa vs 1.5 kPa, P = 0.0003) between the device and conventional colonoscopy, respectively[47] (Table 2).
Magnetic actuation is brought about externally through magnetic fields created either by an external permanent magnet (EPM) or electromagnetic coils[48]. Control of this field is crucial for locomotion as controlling the field allows movement of the device in a particular direction and orientation. The main advantage of external magnetic actuation is that it allows a ‘front-wheel’ motion without the need for large internal actuating motors. When an EPM is used, small internal permanent magnets (IPMs) incorporated into the luminal robot are required to generate the magnetic field. A power supply is generally not required. The resulting device is therefore less bulky and more likely to reduce pain and the need for sedation. Additionally, there is more scope to incorporate other subsystems. The EPMs can be moved manually and the magnetic field controlled directly by the user to cause luminal device movement. However, movement is non-linear and therefore complex. Other disadvantages include the ongoing need for insufflation and the continuous contact between the device and the colonic mucosa due to the continuous attraction between the EPM and IPM[48]. The magnetic fields generated may also interfere with nearby equipment as they are permanent and cannot be turned on or off[3]. Electromagnetic coils can improve control over the magnetic field however they do require a power supply[19]. We have further classified these devices into whether or not they are wireless or tethered.
A swallowable wireless capsule with the aim of therapeutic control of bleeding was developed[49]. It consists of a surgical clip, 4 IPMs and a bidirectional communication platform and is able to actively locomote via a magnetic link generated by its interaction with an EPM. The EPM is mounted on a passive hydraulic arm that is moved manually by the user. A HMI under direction by the controller controls clip deployment. When tested 10 times in ex vivo porcine colon, the clip release occurred 100% of the time and was instantaneous. Moving the capsule was effective and fast although it took 2-3 min to align it appropriately against the mucosa to be clipped. In vivo in a pig, ‘good’ movement and positioning of the device with the EPM was observed. The clip was released successfully onto the desired target and it remained
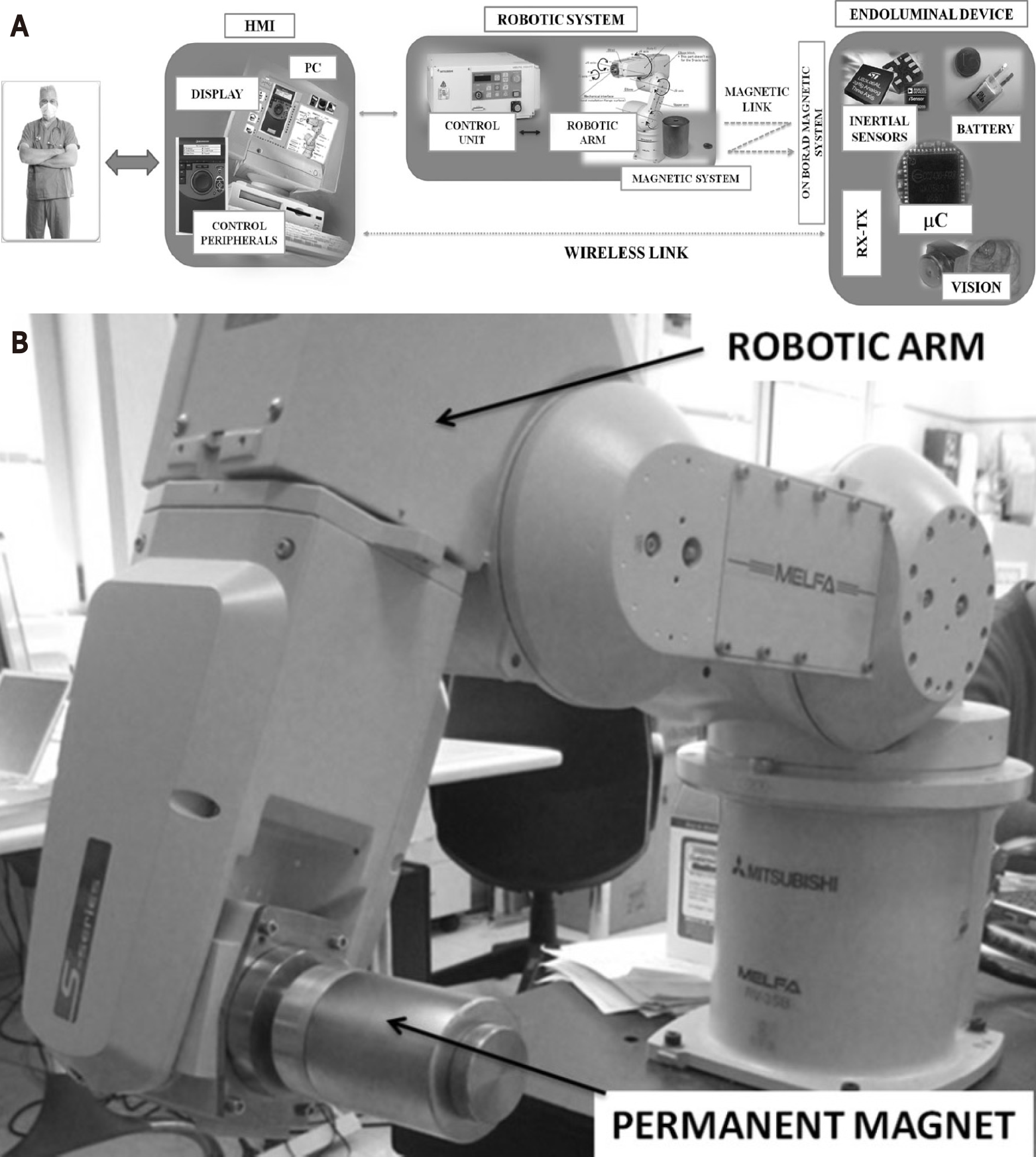
Another wireless capsule with a set of IPMs, inertial and vision sensors and vision module, with an EPM mounted on a robotic arm was created[50]. The robotic arm was moved intelligently via closed loop steering and the HMI (Figure 4). Visualisation, motion feasibility and learning curve were tested in insufflated or collapsed porcine colon (500 mm) placed in a human abdominal phantom. In the collapsed colon, the device was only able to travel very short distances whereas there was a 100% success rate in traversing the whole length when the colon was insufflated with air. The average time required was 10 min. Novice medical doctors were able to drive the EPM in an effective way within 40 trials[50]. Using a robotic arm to steer the EPM was shown to provide better manoeuvrability and lesion detection rates compared to manual steering of the EPM[51].
Carpi and colleagues used the readily available PillCam capsule created by Given Imaging Ltd, Israel to visualise the small bowel and covered it in a magnetic shell to create a simple wireless capsule capable of magnetic actuation. The magnetic link was created between the shell and two EPMs controlled by a magnetic navigation system (Niobe, Stereotaxis, Inc, United States). This navigation system is already clinically in use in the field of robotic cardiology. A remote computer workstation and mouse was used to navigate the capsule. In vivo testing in a pig showed simply that such a capsule is capable of travelling through the colon without causing damage[52].
The magnetic controlled capsule endoscopy (MCCE) system (Chongqing Jinshan Science & Technology Group Co, Ltd) consists of an ingestible colon capsule with IPM and battery, an external magnetic manipulator with EPM, and an image transmission system. The capsule measures 27.9 mm in length and 13.0 mm in diameter. It was tested in 52 volunteers for CRC screening. The average time to reach the caecum was 3.63 h. Manoeuvrability of the capsule was good (94.3%) or moderate (5.77%). It was capable of providing good-quality pictures and identified 6 positive findings (polyps, diverticulum) which were confirmed by colonoscopy. All volunteers were able to swallow the capsule and excreted the capsule within 2 d. Complications included 7 mild adverse events (abdominal discomfort, nausea, and vomiting) lasting 24 h only[53].
Using the technology from[51] the “Magnetic Air Capsule”, a device consisting of a capsule like frontal unit and a compliant multi-lumen tether was created[13]. The incorporation of the multi-lumen tether allows for intervention in addition to basic colonoscopy functions. The frontal unit contains a vision module, an IPM, a magnetic field sensor, and two channels, one for lens cleaning and the other for insufflation/suction/irrigation or instrument passage. The capsule is 11 mm in diameter, 26 mm in length and the tether is 2 m in length. 12 trials in 850 mm porcine colon placed in a human abdominal phantom with attached coloured beads (5 mm) mimicking polyps showed an 85% detection rate. 100% of which were successfully removed with a polypectomy snare. The mean completion time (inspection of the colon as well as removal of the ‘polyps’) was 11.3 min. Six trials in anaesthetised pigs showed device ability to navigate around bends and folds, retroflexion capability and successful operation of the working channels without a loss of magnetic link. In addition, there was no mucosal damage[13]. Using a similar prototype (Figure 5), visualisation and diagnostic ability was assessed 22 times in 850 mm of porcine colon and compared to that of colonoscopy. CIR for both was 100%. Compared to colonoscopy, pin detection rate was lower (80.9% with vs 85.8%) and procedure completion time (visualisation and diagnosis) was significantly longer [556 s vs 194 s (P = 0.0001)]. There was no difference in intuitiveness score[54].
Further advancement led to the “Magnetic flexible endoscope” (MFE). This tethered robot has a standard visualisation module and working channels for instruments, irrigation and insufflation. Additionally, it has a unique retroflexion control algorithm to improve this repetitive but technically challenging skill. Autonomous retroflexion ability was examined 30 times in an anaesthetised pig. Successful retroflexion manoeuvres with a mean time of 11.3 s were performed 100% of the time. No acute tissue trauma or perforation was seen[55]. A comparison of different degrees of locomotion autonomy was performed recently using the MFE in two pigs[14]. Completion times for Direct robot operation vs teleoperation vs semi-autonomous operation vs colonoscopy showed similar results over distances of 45 cm (9 min 4 s vs 2 min 20 s vs 3 mins 9 s vs 1 min 39 s) and 85 cm (unable to reach marker vs 8 min 6 s vs 9 min 39 s vs 3 min 29 s). Intelligent and semi-autonomous control had NASA Task Load Index[56] mean ratings lower/less demanding than colonoscopy or direct robot operation[14].
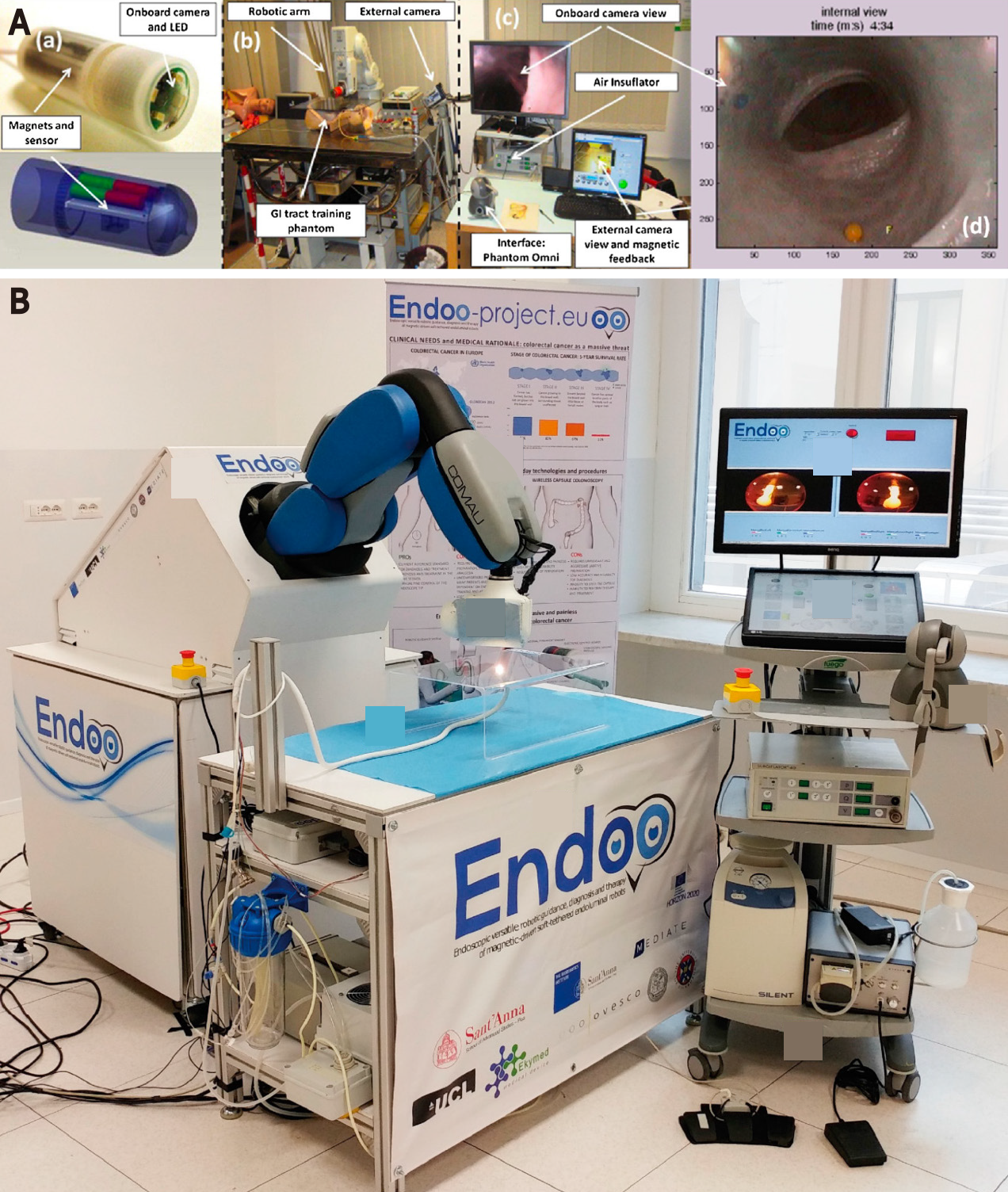
An Endoo capsule with a permanent magnet, visualisation module, tether with 4 working channels for suction, insufflation, irrigation and instruments was developed in Italy within a European H2020 project[57]. The system consists of an external robot with EPM, a localisation system and medical workstation with a joystick. The workstation and joystick allow the user to control all functions of the Endoo capsule (Figure 5). In addition, the vision system contains 4 green/blue UV-LEDs. Compared to colonoscopy CIR was 67% vs 100% at 9.5 min vs 3.5 min respectively. Interaction forces between the Endoo capsule and colonic wall as well as polyp detection rates was lower than colonoscopy [1.17 N vs 4.12 N; 87% vs 91% (P = 0.16)]. The magnetic link was lost an average of 1.28 times per complete procedure, but it was restored in 100% of cases[57]. All studies are summarised in Table 3.
| Ref. | Design and actuation components of evaluated robotic system(s) | Endoscope and/or capsule dimensions | Mode(s) of actuation | Mode(s) of illumination and luminal visualisation | Capabilities evaluated | Degree of robot navigational assistance | Study methodology | Main findings |
| Valdastri et al[49], 2008 (Italy) | Swallowable wireless capsule with surgical clip, electromagnetic motor, 4 IPMs and a bidirectional communication platform. The EPM on a passive hydraulic arm is controlled manually by the user. A HMI controls clip deployment | Diameter of 12.8 mm and a length of 33.5 mm | Magnetic | No camera in this prototype however 300 mm3 space was left for future integration. Throughout the experiments the capsule was monitored with a flexible endoscope | Therapeutic clip application for bleeding | Direct robot operation | Ex vivo- Porcine colon placed in a model of the abdomen–10 trials. In vivo-1 pig | Ex vivo: Clip release: 100%; Clip release occurred instantly, and moving of the capsule was effective and fast. It took 2-3 min to position the capsule against the mucosa to be clipped. In vivo: Good locomotion and positioning with the EPM. The clip was released successfully onto the desired target. The clip remained in situ. The amount of tissue grasped was satisfactory |
| Ciuti et al[50], 2010 (Italy) | Magnetic wireless capsule with inertial and vision sensors and a set of IPM; External robotic arm with EPM and human machine interface. The working distance is 150 mm. The HMI is used to control the robotic arm and receives input from the capsule | Capsule: 40 mm in length, 18 mm in diameter | Magnetic | CMOS camera and 4 white LEDs | Visualisation, locomotion and learning curve | Intelligent teleoperation | Ex vivo: 500 mm porcine colon in human phantom model–40 trials (some insufflated and collapsed colons) | Insufflated colon: 100% of success rate in traversing the entire colon. Short learning curve (descriptive analysis) to drive the robotic arm. The average time required to traverse the colon was approximately 10 min. Collapsed colon: Capsule was able to travel only really short distances and manual assistance was required |
| Ciuti et al[51], 2009 (Italy) | Wired capsule with 3 IPMs and vision module; EPM either controlled manually or robotically via a robotic arm controlled by a HMI and controller. The working distance is 150 mm | 14 mm in diameter and 38 mm in length | Magnetic | CMOS camera with illumination system | Robotic versus manual steering | Direct or Intelligent teleoperation | Ex vivo: 480 mm porcine colon in human phantom model–10 trials each for robot and manual arm steering. In vivo: 2 Pigs–5 trials each for robot and manual arm steering | Ex vivo: Robot versus manual steering: The mean completion time: 423 s vs 201 s (P < 0.01). The mean percentage of ‘4 mm white spherical targets’ reached: 87% versus 37% (P < 0.01). In vivo: Manual steering was usually faster, whereas manoeuvrability was better with robotic movement of the EPM (Descriptive analysis) |
| Carpi et al[52], 2011 (Italy/United States) | PillCam (Given Imaging Ltd, Israel) capsule covered in a magnetic shell; Two EPMs, a magnetic navigation system (Niobe, Stereotaxis, Inc, United States), a remote computer work-station and mouse. Fluoroscopic images were continuously acquired by means of a digital scanner to provide visual feedback regarding capsule manoeuvres | 13 mm in diameter and length | Magnetic | Not described | Steering and localisation capability | Intelligent teleoperation | In vivo: Pig (Number of pigs and trials not described) | The capsule was freely moved within the colon. No complications |
| Gu et al[53], 2017 (China) | The MCCE system (Chongqing Jinshan Science & Technology Group Co, Ltd): Ingestible colon capsule with IPM and battery, an external magnetic manipulator with an EPM, and an image transmission system | Capsule measures 27.9 mm in length by 13.0 mm in diameter | Magnetic | Not described | Manoeuvrability, visualisation, diagnosis and safety | Direct robot operation | In vivo: n = 52 Human, CRC screening volunteers. Capsule movement was visualised via colonoscopy 5 h after ingestion | Average CIT: 3.63 h. Maneuverability of the capsule was good (94.3%) or moderate (5.77%). MCCE provided good-quality pictures and identified 6 positive findings (polyps, diverticulum) which were confirmed by colonoscopy. 78% reached the rectosigmoid colon in 25 min. All 57 volunteers were able to swallow the capsule and excreted the capsule within 2 d. Complications: 7 mild adverse events (abdominal discomfort, nausea, and vomiting) lasting 24 h. No complications at one week follow up |
| Valdastri et al[13], 2012 (Italy) | MAC consists of capsule-like frontal unit and a compliant multi-lumen tether. The frontal unit contains a vision module, an IPM, a magnetic field sensor, and two channels, one for lens cleaning and the other for insufflation/suction/irrigation or instrument passage. The IPM is controlled by an EPM mounted on a robotic platform. A control device allows the user to directly control the position of the EPM. The working distance is 150 mm. The tether connects to an external control box | Capsule: 11 mm diameter, 26 mm in length. Tether: 5.4 mm diameter, 2 m length | Magnetic | CCD camera with 120 degree field of view and 4 white LEDs | Diagnostic and treatment ability, safety, usability | Intelligent teleoperation | Ex vivo: 850 mm porcine colon in human phantom model–12 trials. In vivo: 2 Pigs–3 trials each | Ex vivo: Mean percentage of 5 mm coloured beads (polyps) detected was 85%. 100% successful removal (polypectomy loop) of identified beads. Mean completion time (inspection and bead removal) was 678 s. Mean bead removal time was 18 s. Good manoeuvrability, low friction from the tether on the colon wall and reliable feedback from the vision module. In vivo: No mucosal damage or perforation. Able to navigate around bends and folds, retroflexion of the camera and successful operation of the tools (loop, forceps, retrieval basket, grasper) without loss of magnetic link |
| Arezzo et al[54], 2013 (Italy) | Robotic arm with EPM controlled by HMI and controller; Wired capsule with 3 IPMs, camera, LEDs and magnetic sensor. The working distance is 150mm. The wired sheath allows transmission from the vision module and electric energy | Capsule: 13.5 mm in diameter and 29.5 mm in length. Wired sheath: 2 mm in diameter | Magnetic | CCD camera with 120 degree view and 6 white LEDs | Visualisation and diagnostic ability compared to colonoscopy | Intelligent teleoperation | Ex vivo: 850 mm porcine colon in human phantom model–22 trials each for capsule and colonoscope | Robot vs colonoscopy: CIR: 100% for both. Pin detection rate: 80.9% vs 85.8%. Procedure completion time (visualisation and diagnosis): 556 s vs 194 s (P = 0.0001). No difference in intuitiveness score |
| Slawinski et al[55], 2018 (United States/United Kingdom) | MFE with IPM, camera, illumination module, working channel for instruments, channel for irrigation and insufflation, EPM on robotic arm and HMI. Additional sensing, retroflexion and software control systems | Tip: 20.6 mm in diameter and 18.1 mm in length. Body: 6.5 mm in diameter | Magnetic | Camera and illumination module | Retroflexion ability | Intelligent teleoperation with task autonomy | In vivo: 1 Pig–30 trials | 100% successful retroflexion manoeuvres with a mean time of 11.3 s. No acute tissue trauma or perforation |
| Martin et al[14], 2020 (United Kingdom) | MFE with an IPM, camera, an insufflation channel, irrigation channel, working channel for instruments and localisation circuit; A robotic arm with EPM; Robot operating system and joystick | Capsule: 20.6 mm in diameter and 18.1 mm in length. Tether: 6.5 mm in diameter | Magnetic | Camera and LED | Comparison of different degrees of autonomy for locomotion and novice usability | Direct robot or intelligent teleoperation or semi-autonomous | In vivo: 2 Pigs–3 trials for each MFE control and colonoscopy in the first pig and 4 trials for each in the second pig | First porcine model–colon distance of 45 cm: Task completion times for direct robot operation, teleoperation, semi-autonomous operation and conventional colonoscopy were 9 min 4 s, 2 min 20 s and 3 min 9 s and 1 min 39 s, respectively. Second porcine model-colon distance of 85 cm: Task completion times for, teleoperation, semi-autonomous operation and conventional colonoscopy were 8 min 6 s, 9 min 39 s and 3 min 29 s, respectively. It was not possible to reach the marker with direct robotic operation. Intelligent and semi-autonomous had NASA task force mean Index ratings lower/less demanding than colonoscopy or direct robot operation |
| Verra et al[57], 2020 (Italy) | Endoo system: An Endoo capsule with a IPM, soft tether connection with 4 working channels for suction, insufflation, irrigation and instruments; An external robot with EPM, force-torque sensor and movable platform, localisation system and medical workstation with a joystick complete the system. The robot with EPM is controlled via the workstation but can also be steered manually. The localisation system provides information on the capsule position and orientation | Tether: 160 cm long | Magnetic | Two CMOS cameras with 170 degree field of view, 4 white LEDs and 4 green/blue UV-LEDs | Visualisation, locomotion, diagnosis and safety | Semi-autonomous | Ex vivo: 100-120 mm porcine colon in human phantom model | Ex vivo Endoo alone: 100% success rate in operating channel (use of polypectomy snares, biopsy forceps and needles). 100% success rate for target approach tests (using these instruments to target a polyp). Ex vivo Endoo (21 trials) vs colonoscopy (13 trials): Completion rate: 67% vs 100%. Interaction forces: 1.17 N vs 4.12 N. Polyp detection rate: 87% vs 91% (P = 0.16). Mean CIT: 9.5 min vs 3.5 min. The magnetic link was lost an average of 1.28 times per complete procedure, but it was restored in 100% of cases |
| Simi et al[58], 2010 (Italy) | Wireless endocapsule with legged mechanism (3 legs), DC motor, battery, small IPMs which interacts with an EPM. LabVIEW HMI is present and is also compatible with voice commands | 14 mm in diameter, 44 mm in length. | Hybrid- Electromechanical and Magnetic | No camera in this prototype however 450 mm3 space was left for future integration. Throughout the experiments the capsule was monitored with a gastroscope | Locomotion and lumen dilatation | Semiautonomous | Ex vivo: 20 cm porcine colon–10 trials. In vivo: 4 pigs–10 trials. Capsule was placed 40 cm from the anus and expected to travel towards the anus | Ex vivo: Ability to travel 20 cm in 10 min: 70%. Average time to traverse 20 cm and number of leg activations: 4 min and 5 mechanism activations. Average speed: 5 cm/min. In vivo: Ability to travel 40 cm in 20 min: 60%. Average time to traverse 40 cm and number of leg activations: 5 min and 5 activations. Average speed: 8 cm/min |
| Nouda et al[59], 2018 (Japan) | Self-propelling capsule endoscope (SPCE) consisting of a silicon resin fin with micro-magnet connected to the PillCam SB2 capsule; External magnetic field generating controller (Minimermaid System), human interface with joystick | 45 mm in length and 11 mm in diameter | Hybrid- Mechanical and Magnetic | Camera with 156 degree field of view | Locomotion and safety | Semi-autonomous | In vivo: 1 Human | The SPCE could swim smoothly in forward and backward directions but had difficulty bypassing bends. No acute complications |
Hybrid actuation involves the combination of different propulsive mechanisms to achieve motion. A wireless endocapsule consisting of a 3 legged mechanism, DC motor, battery and small IPMs was created and tested by Simi and colleagues (Figure 6)[58]. Magnetic and EM mechanisms are combined here: The IPMs interact with an EPM to primarily move and orient the capsule while the legged mechanism is used to extract the capsule out of collapsed areas of the colon when it might otherwise get trapped. Motion feasibility was examined 10 times on 20 cm porcine colon and in 4 anaesthetised pigs. In the ex vivo trials, the average time taken to travel 20 cm and number of times the legs were activated was 4 min with 5 activations. The average speed was 5 cm/min. In the in vivo trials, the average time taken to travel 40 cm and number of times the legs were activated was 5 min with 5 activations. The average speed was 8 cm/min. The colon was not insufflated with air[58]. In Japan, a self-propelling capsule was created by attaching a silicon resin fin with micro-magnet to the commercially available Pillcam SB2 capsule (Covidien, Dublin, Ireland). In the presence of a magnetic field and water, the fin vibrates and propels the capsule. When placed in the rectum and descending colon of a human subject, it was shown to be able to swim forwards and backwards without causing damage to the mucosa however it had difficulty by-passing the bend of the sigmoid colon[59] (Table 3).
Medical robotics is realising its potential in a variety of healthcare disciplines, and the last couple of decades have seen increasing demand for robotic platforms designed specifically for endoscopy. In terms of LGI tract ‘robo-endoscopy’, significant strides have been made over this period, with five devices receiving FDA approval. These devices represent a heterogeneous group in terms of actuation modality (EM or pneumatic), and many studies have been performed using ex vivo models. These models, while able to demonstrate proof of concept, cannot effectively capture data on in vivo motion ability, pain perception or device safety. Nevertheless, the human data that is available suggests that the evaluated robo-endoscopic systems are able to locomote effectively (i.e., achieve CIR > 90%[23,24,36,40-42]), to locomote safely (i.e., be associated with mild if any mucosal disruption or complications[21,24,35,36,40,42])and to achieve endoscopic tasks with minimal associated pain[21,23,35,39]. Reducing discomfort associated with LGI endoscopy represents a key directive in robotic endoscopy and in two trials, human participants gave the Invendoscope an average pain score of 1.96/6 and 2.6/6, which translated into 0% and 4.9% requiring sedation, respectively[21,23]. When compared to colonoscopy, pain scores and sedation rates were also significantly lower with the Endotics system[38,39]. Early data suggest that the Endotics system may even have superior diagnostic capabilities compared with conventional colonoscopy as indicated by its ability to detect lesions missed on colonoscopy[38]. These reports are certainly encouraging, though overall it is important to appreciate that most devices presented in this review remain in the relatively early phases of translational application, and few have met the goal of clinical deployment outside of academic institutions. An inherent limitation lies in the fact that most systems provide primarily diagnostic functionality, with large scale trials evaluating therapeutic robotic LGI endoscopy currently lacking.
Improved reproducibility, enhanced procedural efficiency and a shorter learning curve have all been suggested as possible areas where robotic endoscopy could make a positive impact. In addition, they may offer a more comfortable system for the user, which may have potential to minimise fatigue and injury and ultimately this may equate to more years of professional service. More intuitive control and visualisation systems have the potential to shorten learning curves. For example, one trial evaluating a robotic endoscopic system suggested that only an average of 30 procedures was required for the user to achieve CIR, CIT and scope withdrawal time comparable to standard colonoscopy performed by an ‘expert’[40].
From a broader perspective, it is important to acknowledge that this review has focused entirely on the specific application(s) of robotic systems in LGI endoscopy. However, robotic advances in this area are not made in isolation from advances in other luminal organs such as the upper GI tract or in natural orifice transluminal endoscopic surgery. Thus, it is likely that advances in one field will complement another.
One can anticipate that in the future, as the technology becomes more sophisticated, it should be possible to exploit the ‘computational interface’ that robotic endoscopy provides further, with the potential for integration of AI based algorithms and novel augmented reality systems for ‘smart’ therapeutics. It is doubtful whether these next-generation technologies will work to their full capabilities if operating within anything other than a robotic system. It is an exciting time in medical robotics with recent reports confirming the potential for the development of ‘soft’ robotic systems with in-built autonomic functionality[60]. Such systems are likely to represent the long-term direction of luminal robotics. In the near- to mid-term, the goal will be to continue to stimulate strong collaborative links between GI physicians and medical engineers in order to continue to refine design and functionality.
Robotic technologies have the potential to transform LGI endoscopy into a quicker, safer, more reliable and less painful procedure. In the long term, benefits for patients, endoscopists and the wider healthcare industry are foreseeable, though these have yet to be convincingly demonstrated in human trials. Most studies to date have employed ex vivo modelling and high quality level 1 evidence is currently lacking in this field. Robotic technologies are evolving with such rapidity at the moment, that future robo-endoscopic systems are likely to look and behave very differently to conventional master-slave systems currently in use. Exciting developments in 3D printing, soft robotics, autonomous functionality and augmented reality are likely to converge over the coming decade to lead to the development of truly next generation robotic endoscopy devices.
Inherent limitations exist with conventional colonoscopy which may be overcome by a variety of next-generation robotically-augmented technologies.
Robotic technologies have the potential to transform lower gastrointestinal (LGI) tract endoscopy with long term, benefits for patients, endoscopists and the wider healthcare industry. High quality evidence is currently lacking in this field.
This review provides a comprehensive summary of recent developments in the application of robotics in LGI tract endoscopy.
A systematic review of the literature was performed. Studies reporting on the use of robotic endoscopic technology in ex vivo colon models or in vivo animal and human experiments were included.
Of 37 studies were included of varying actuation modality. Five devices have been approved by the Food and Drug Administration, however the majority remain in the early phases of testing and development. Level 1 evidence is lacking at present, but early reports suggest that these technologies may be associated with improved pain and safety.
Significant progress in robotic colonoscopy has been made over the last couple of decades. The reviewed devices appear to be ergonomically capable and efficient though to date no reports have convincingly shown diagnostic or therapeutic superiority over conventional colonoscopy.
Future improvements in design together with the integration of semi-autonomous and autonomous systems over the next decade will potentially result in robotic colonoscopy becoming more commonplace.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosoe N, Yang Y S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, Haslam N; British Society of Gastroenterology, the Joint Advisory Group on GI Endoscopy, the Association of Coloproctology of Great Britain and Ireland. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016;65:1923-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Slatkin AB, Burdick J, Grundfest W. The development of a robotic endoscope. In: Khatib O., Salisbury J.K. (eds) Experimental Robotics IV. Lecture Notes in Control and Information Sciences. Springer, Berlin, Heidelberg 1997: 223. |
| 3. | Sliker LJ, Ciuti G. Flexible and capsule endoscopy for screening, diagnosis and treatment. Expert Rev Med Devices. 2014;11:649-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Bianchi F, Ciuti G, Koulaouzidis A, Arezzo A, Stoyanov D, Schostek S, Oddo CM, Menciassi A, Dario P. An innovative robotic platform for magnetically-driven painless colonoscopy. Ann Transl Med. 2017;5:421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Lohsiriwat V. Colonoscopic perforation: incidence, risk factors, management and outcome. World J Gastroenterol. 2010;16:425-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 173] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (4)] |
| 6. | Ward ST, Mohammed MA, Walt R, Valori R, Ismail T, Dunckley P. An analysis of the learning curve to achieve competency at colonoscopy using the JETS database. Gut. 2014;63:1746-1754. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Benson M, Lucey M, Pfau P. Caecal intubation rates and colonoscopy competency. Gut. 2015;64:359. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Yung DE, Banfi T, Ciuti G, Arezzo A, Dario P, Koulaouzidis A. Musculoskeletal injuries in gastrointestinal endoscopists: a systematic review. Expert Rev Gastroenterol Hepatol. 2017;11:939-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Sporea I, Popescu A, Sandesc D, Salha CA, Sirli R, Danila M. Sedation during colonoscopy. Rom J Gastroenterol. 2005;14:195-198. [PubMed] |
| 10. | Aljebreen AM, Almadi MA, Leung FW. Sedated vs unsedated colonoscopy: a prospective study. World J Gastroenterol. 2014;20:5113-5118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kassim I, Phee L, Ng WS, Gong F, Dario P, Mosse CA. Locomotion techniques for robotic colonoscopy. IEEE Eng Med Biol Mag. 2006;25:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | De Groen PC. History of the Endoscope [Scanning Our Past]. IEEE. 2017;105:1987-1995. [DOI] [Full Text] |
| 13. | Valdastri P, Ciuti G, Verbeni A, Menciassi A, Dario P, Arezzo A, Morino M. Magnetic air capsule robotic system: proof of concept of a novel approach for painless colonoscopy. Surg Endosc. 2012;26:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Martin JW, Scaglioni B, Norton JC, Subramanian V, Arezzo A, Obstein KL, Valdastri P. Enabling the future of colonoscopy with intelligent and autonomous magnetic manipulation. Nat Mach Intell. 2020;2:595-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Chen AC, Pastis NJ Jr, Mahajan AK, Khandhar SJ, Simoff MJ, Machuzak MS, Cicenia J, Gildea TR, Silvestri GA. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest. 2021;159:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 16. | Diodato MD Jr, Damiano RJ Jr. Robotic cardiac surgery: overview. Surg Clin North Am. 2003;83:1351-1367, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15872] [Article Influence: 1587.2] [Reference Citation Analysis (1)] |
| 18. | Li Z, Chiu PW. Robotic Endoscopy. Visc Med. 2018;34:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Ciuti G, Skonieczna-Żydecka K, Marlicz W, Iacovacci V, Liu H, Stoyanov D, Arezzo A, Chiurazzi M, Toth E, Thorlacius H, Dario P, Koulaouzidis A. Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Manfredi L, Natale G. New Robotic Technologies in Cancer Colon Screening. Clin Cancer Drug. 2018;6:17. [DOI] [Full Text] |
| 21. | Rösch T, Adler A, Pohl H, Wettschureck E, Koch M, Wiedenmann B, Hoepffner N. A motor-driven single-use colonoscope controlled with a hand-held device: a feasibility study in volunteers. Gastrointest Endosc. 2008;67:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | AMBU. Familiar tools with new benefits. [cited 15 March 2021]. Available from: https://www.ambu.com/endoscopy/gastroenterology. |
| 23. | Groth S, Rex DK, Rösch T, Hoepffner N. High cecal intubation rates with a new computer-assisted colonoscope: a feasibility study. Am J Gastroenterol. 2011;106:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Eickhoff A, van Dam J, Jakobs R, Kudis V, Hartmann D, Damian U, Weickert U, Schilling D, Riemann JF. Computer-assisted colonoscopy (the NeoGuide Endoscopy System): results of the first human clinical trial ("PACE study"). Am J Gastroenterol. 2007;102:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Valdastri P, Webster RJ, Quaglia C, Quirini M, Menciassi A Dario P. A New Mechanism for Mesoscale Legged Locomotion in Compliant Tubular Environments. IEEE Transac Robot. 2009;25:1047-1057. [RCA] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Lee D, Joe S, Kang H, An T, Kim B. A reel mechanism-based robotic colonoscope with high safety and maneuverability. Surg Endosc. 2019;33:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Trovato G, Shikanai M, Ukawa G, Kinoshita J, Murai N, Lee JW, Ishii H, Takanishi A, Tanoue K, Ieiri S, Konishi K, Hashizume M. Development of a colon endoscope robot that adjusts its locomotion through the use of reinforcement learning. Int J Comput Assist Radiol Surg. 2010;5:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Kim HM, Yang S, Kim J, Park S, Cho JH, Park JY, Kim TS, Yoon ES, Song SY, Bang S. Active locomotion of a paddling-based capsule endoscope in an in vitro and in vivo experiment (with videos). Gastrointest Endosc. 2010;72:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Wang KD, Yan GZ. An earthworm-like microrobot for colonoscopy. Biomed Instrum Technol. 2006;40:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Wang K, Yan G. Micro robot prototype for colonoscopy and in vitro experiments. J Med Eng Technol. 2007;31:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Wang K, Ma J, Wang F, Wang Z, Yan G, Zhou Y. Full-driving soft robotic colonoscope in compliant colon tissue. J Med Eng Technol. 2017;41:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Naderi N, Najarian S, Hosseinali A, Karevan H. Modeling and dynamic analysis of the worm-like part of an innovative robot applicable in colonoscopy. Int J Med Robot. 2013;9:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Dongkyu L, Seonggun J, Junwoo C, Bo-In L, Byungkyu K. An elastic caterpillar-based self-propelled robotic colonoscope with high safety and mobility. Mechatronics. 2016;39:54-62. [DOI] [Full Text] |
| 34. | Formosa GA, Prendergast JM, Edmundowicz SA, Rentschler ME. Novel Optimization-Based Design and Surgical Evaluation of a Treaded Robotic Capsule Colonoscope. IEEE. 2020;36:545-552. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Vucelic B, Rex D, Pulanic R, Pfefer J, Hrstic I, Levin B, Halpern Z, Arber N. The aer-o-scope: proof of concept of a pneumatic, skill-independent, self-propelling, self-navigating colonoscope. Gastroenterology. 2006;130:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Gluck N, Melhem A, Halpern Z, Mergener K, Santo E. A novel self-propelled disposable colonoscope is effective for colonoscopy in humans (with video). Gastrointest Endosc. 2016;83:998-1004.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Gluck N, Fishman S, Melhem A, Goldfarb S, Halpern Z, Santo E. A novel colonoscope with panoramic visualization detected more simulated polyps than conventional colonoscopy in a live swine model. Endosc Int Open. 2015;3:E642-E645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Cosentino F, Tumino E, Passoni GR, Morandi E, Capria A. Functional evaluation of the endotics system, a new disposable self-propelled robotic colonoscope: in vitro tests and clinical trial. Int J Artif Organs. 2009;32:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Tumino E, Sacco R, Bertini M, Bertoni M, Parisi G, Capria A. Endotics system vs colonoscopy for the detection of polyps. World J Gastroenterol. 2010;16:5452-5456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Trecca A, Catalano F, Bella A, Borghini R. Robotic colonoscopy: efficacy, tolerability and safety. Preliminary clinical results from a pilot study. Surg Endosc. 2020;34:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Tumino E, Parisi G, Bertoni M, Bertini M, Metrangolo S, Ierardi E, Cervelli R, Bresci G, Sacco R. Use of robotic colonoscopy in patients with previous incomplete colonoscopy. Eur Rev Med Pharmacol Sci. 2017;21:819-826. [PubMed] |
| 42. | Shike M, Fireman Z, Eliakim R, Segol O, Sloyer A, Cohen LB, Goldfarb-Albak S, Repici A. Sightline ColonoSight system for a disposable, power-assisted, non-fiber-optic colonoscopy (with video). Gastrointest Endosc. 2008;68:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Ng WS, Phee SJ, Seow C, Davies BL. Development of a Robotic Colonoscope. Dig Endosc. 2000;12:131-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Dehghani H, Welch CR, Pourghodrat A, Nelson CA, Oleynikov D, Dasgupta P, Terry BS. Design and preliminary evaluation of a self-steering, pneumatically driven colonoscopy robot. J Med Eng Technol. 2017;41:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Chen Z, Liu J, Wang S, Zuo S. A bio-inspired self-propelling endoscopic device for inspecting the large intestine. Bioinspir Biomim. 2019;14:066013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Lin S, Zhu W, Xiao K, Su P, Liu Y, Chen P, Bai Y. Water intubation method can reduce patients' pain and sedation rate in colonoscopy: a meta-analysis. Dig Endosc. 2013;25:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Coleman SA, Tapia-Siles SC, Pakleppa M, Vorstius JB, Keatch RP, Tang B, Cuschieri A. A hydraulically driven colonoscope. Surg Endosc. 2016;30:4515-4524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Pittiglio G, Barducci L, Martin JW, Norton JC, Avizzano CA, Obstein KL, Valdastri P. Magnetic Levitation for Soft-Tethered Capsule Colonoscopy Actuated With a Single Permanent Magnet: A Dynamic Control Approach. IEEE Robot Autom Lett. 2019;4:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Valdastri P, Quaglia C, Susilo E, Menciassi A, Dario P, Ho CN, Anhoeck G, Schurr MO. Wireless therapeutic endoscopic capsule: in vivo experiment. Endoscopy. 2008;40:979-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Ciuti G, Valdastri P, Menciassi A, Dario P. Robotic magnetic steering and locomotion of capsule endoscope for diagnosis and surgical endoluminal procedures. Robotica. Cambridge University Press. 2010;28:199-207. [RCA] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Ciuti G, Donlin R, Valdastri P, Arezzo A, Menciassi A, Morino M, Dario P. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010;42:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Carpi F, Kastelein N, Talcott M, Pappone C. Magnetically controllable gastrointestinal steering of video capsules. IEEE Trans Biomed Eng. 2011;58:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Gu H, Zheng H, Cui X, Huang Y, Jiang B. Maneuverability and safety of a magnetic-controlled capsule endoscopy system to examine the human colon under real-time monitoring by colonoscopy: a pilot study (with video). Gastrointest Endosc. 2017;85:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Arezzo A, Menciassi A, Valdastri P, Ciuti G, Lucarini G, Salerno M, Di Natali C, Verra M, Dario P, Morino M. Experimental assessment of a novel robotically-driven endoscopic capsule compared to traditional colonoscopy. Dig Liver Dis. 2013;45:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Slawinski PR, Taddese AZ, Musto KB, Sarker S, Valdastri P, Obstein KL. Autonomously Controlled Magnetic Flexible Endoscope for Colon Exploration. Gastroenterology. 2018;154:1577-1579.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Agency for Healthcare Research and Quality (AHRQ). NASA Task Load Index. [cited 15 March 2021]. Available from: https://digital.ahrq.gov/health-it-tools-and-resources/evaluation-resources/workflow-assessment-health-it-toolkit/all-workflow-tools/nasa-task-load-index. |
| 57. | Verra M, Firrincieli A, Chiurazzi M, Mariani A, Lo Secco G, Forcignanò E, Koulaouzidis A, Menciassi A, Dario P, Ciuti G, Arezzo A. Robotic-Assisted Colonoscopy Platform with a Magnetically-Actuated Soft-Tethered Capsule. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Simi M, Valdastri P, Quaglia C, Menciassi A, Dario P. Design, Fabrication, and Testing of a Capsule With Hybrid Locomotion for Gastrointestinal Tract Exploration. IEEE/ASME Trans Mechatron. 2010;15:170-180. [RCA] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Nouda S, Ota K, Higuchi K. Retrograde colon capsule endoscopy with the self-propelling capsule endoscope: The first human trial (with videos). Dig Endosc. 2018;30:117-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Wehner M, Truby RL, Fitzgerald DJ, Mosadegh B, Whitesides GM, Lewis JA, Wood RJ. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature. 2016;536:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 796] [Article Influence: 88.4] [Reference Citation Analysis (0)] |