Published online Dec 16, 2021. doi: 10.4253/wjge.v13.i12.659
Peer-review started: April 24, 2021
First decision: June 13, 2021
Revised: June 25, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: December 16, 2021
Processing time: 233 Days and 16.3 Hours
Low-volume preparations for colonoscopy have shown similar efficacy compared to high-volume ones in randomized controlled trials (RCT). However, most RCTs do not provide data about clinical outcomes including lesions detection rate. Moreover, real-life comparisons are lacking.
To compare efficacy (both in terms of adequate bowel preparation and detection of colorectal lesions) and tolerability of a high-volume (HV: 4 L polyethylene glycol, PEG) and a low-volume (LV: 2 L PEG plus bisacodyl) bowel preparation in a real-life setting.
Consecutive outpatients referred for colonoscopy were prospectively enrolled between 1 December 2014 and 31 December 2016. Patients could choose either LV or HV preparation, with a day-before schedule for morning colonoscopies and a split-dose for afternoon procedures. Adequate bowel preparation according to Boston Bowel Preparation Scale (BBPS), clinical outcomes including polyp detection rate (PDR), adenoma detection rate (ADR), advanced adenoma detection rate (AADR), sessile/serrated lesion detection rate (SDR) and cancer detection rate and self-reported tolerability of HV and LV were blindly assessed.
Total 2040 patients were enrolled and 1815 (mean age 60.6 years, 50.2% men) finally included. LV was chosen by 52% of patients (50.8% of men, 54.9% of women). Split-dose schedule was more common with HV (44.7% vs 38.2%, P = 0.005). High-definition scopes were used in 33.4% of patients, without difference in the two groups (P = 0.605). HV and LV preparations showed similar adequate bowel preparation rates (89.2% vs 86.6%, P = 0.098), also considering the two different schedules (HV split-dose 93.8% vs LV split-dose 93.6%, P = 1; HV day-before 85.5% vs LV day-before 82.3%, P = 0.182). Mean global BBPS score was higher for HV preparations (7.1 ± 1.7 vs 6.8 ± 1.6, P < 0.001). After adjustment for sex, age and indications for colonoscopy, HV preparation resulted higher in PDR [Odds ratio (OR) 1.32, 95%CI: 1.07-1.63, P = 0.011] and ADR (OR 1.29, 95%CI 1.02–1.63, P = 0.038) and comparable to LV in AADR (OR 1.51, 95%CI 0.97-2.35, P = 0.069), SDR and cancer detection rate. The use of standard-definition colonoscopes was associated to lower PDR (adjusted OR 1.59, 95%CI: 1.22-2.08, P < 0.001), ADR (adjusted OR 1.71, 95%CI: 1.26–2.30, P < 0.001) and AADR (adjusted OR 1.97, 95%CI: 1.09-3.56, P = 0.025) in patients receiving LV preparation. Mean Visual Analogue Scale tolerability scored equally (7, P = 0.627) but a ≥ 75% dose intake was more frequent with LV (94.6% vs 92.1%, P = 0.003).
In a real-life setting, PEG-based low-volume preparation with bisacodyl showed similar efficacy and tolerability compared to standard HV preparation. However, with higher PDR and ADR, HV should still be considered as the reference standard for clinical trials and the preferred option in screening colonoscopy, especially when colonoscopy is performed with standard resolution imaging.
Core Tip: Quality of bowel preparation is one of the main factors influencing outcomes of colonoscopy. This prospective real-life study compared bowel cleansing (according to the Boston Bowel Preparation Scale), clinically relevant colonoscopy outcomes (lesions detection rate) and tolerability of a standard high-volume bowel preparation and a low-volume preparation (2 L polyethylene glycol + bisacodyl). Even if the two study groups did not show differences in terms of adequate bowel preparation, the use of the high-volume preparation was associated with higher polyp and adenoma detection rates. There were no differences in terms of advanced adenomas, sessile/serrated lesions and cancer detections. Performance of low-volume preparation seems influenced by image resolution of colonoscopes, with fewer lesions detected compared to high-volume when using standard-definition colonoscopes. The two preparations were comparable in terms of patients’ self-reported tolerability, but complete adherence to preparation was more common with the low-volume product.
- Citation: Occhipinti V, Soriani P, Bagolini F, Milani V, Rondonotti E, Annunziata ML, Cavallaro F, Vavassori S, Vecchi M, Pastorelli L, Tontini GE. Efficacy and tolerability of high and low-volume bowel preparation compared: A real-life single-blinded large-population study. World J Gastrointest Endosc 2021; 13(12): 659-672
- URL: https://www.wjgnet.com/1948-5190/full/v13/i12/659.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i12.659
The clinical performance of colonoscopy is markedly influenced by the quality of bowel preparation. In fact, inadequate bowel preparation has proved to have a detrimental effect on different clinically significant outcomes, such as complete colonoscopy rate[1-3], polyp (PDR) and adenoma detection rates (ADR)[4-6]. Moreover, inadequate preparation may require to repeat the procedure, with the subsequent increase in waiting times, risks and costs[7,8]. Large volumes (4 L) of polyethylene glycol (PEG) have been classically prescribed to achieve adequate cathartic effect. Over the past years, several low-volume preparations have been developed to increase the patients’ acceptability, compliance and willingness to repeat the procedure. Randomized controlled trials (RCTs) and some meta-analysis have shown that low-volume preparations have similar efficacy in terms of adequate bowel preparation rate compared to high-volume preparations[9-15], however two meta-analysis[16,17] reported a superiority of high-volume PEG over low-volume PEG. Moreover, the direct comparison of clinical outcomes such as ADR is available only in a minority of trials[11,12], and real-life data suggest higher detection rates with high-volume preparations[18].
Therefore, we have performed a real-life study to (1) compare efficacy of HV and LV preparations by means of adequate bowel preparation rate and detection of colonic lesions; and (2) to compare self-reported tolerability of different regimens.
We prospectively enrolled the consecutive patients referred for colonoscopy to the Digestive Endoscopy Outpatient Service of IRCCS Policlinico San Donato between 1 December 2014 and 31 December 2016. The patients enrolled in the regional colorectal cancer screening program were not included as in our Center they are all advised to use high-volume PEG-based preparation. If a patient underwent multiple colonoscopies during the study period, only the first procedure was taken into account for the study.
The exclusion criteria were: inability to give informed consent, use of cleansing products different from the recommended ones, incomplete patient forms as to the type of preparation used, incomplete colonoscopy because of a pathological stricture.
At the time of booking the examination, all the patients received written detailed instructions about the diet regimen (no fruit, legumes, or vegetables for 3 d before the procedure; light breakfast and lunch the day before colonoscopy, followed by clear liquids only) and about bowel preparation. Instructions contained an introductory paragraph underlying the importance to adhere to the prescriptions provided in order to increase the chance to achieve good diagnostic and therapeutic results and to reduce adverse events of colonoscopy. Patients were free to choose either a high-volume (HV) or a low-volume (LV) preparation. The HV preparation (SELG ESSE; Promefarm, Italy) was a PEG 4000 solution plus simethicone and electrolytes that had to be diluted in 4L still water, while the LV preparation was a combination of a PEG 4000 solution plus simethicone and electrolytes (Lovol-Esse; Alfasigma, Italy) diluted in 2 L still water and the stimulant laxative bisacodyl (Lovoldyl; Alfasigma, Italy). In the written instructions handed to the patients, the two preparations were stated as equally effective and tolerated and complete free choice was left to patients’ preferences. The preparations were listed with the HV preparation first.
For the procedures planned before 12:00 pm, the patients were instructed to take the entire quantity of the PEG solution the evening before colonoscopy, starting from 7 pm; in case of LV preparation, 4 tablets (20 mg) of bisacodyl were also taken at 3:00 pm. For afternoon procedures a split-dose regimen was prescribed: half the dose of PEG was taken in the afternoon before and half the dose at 7:00 a.m. in the morning on the day of the colonoscopy; in case of LV preparation 20 mg bisacodyl was taken at sleep time.
The study was approved by the local Ethics Committee of San Raffaele Hospital and a specific written informed consent was taken from all the study participants. The study was conducted in accordance with the Declaration of Helsinki 1975 and subsequent amendments.
All the procedures were performed under mild-to-moderate sedation (midazolam ± pethidine i.v.) by 5 experienced endoscopists (> 1000 colonoscopies overall, > 300/year), well-trained in the use of bowel preparation rating scales and blinded to the content of the patient form and to the preparation taken. The indication for colonoscopy was collected by the endoscopist matching medical prescription and pre-colonoscopy interview, following the standard clinical protocol. The endoscopes used were either standard-definition (SD) or high-definition (HD) scopes by Pentax (Tokyo, Japan).
On the morning of colonoscopy, the patients were asked to fill a specific questionnaire covering the kind of bowel preparation used (HV or LV), amount of PEG solution taken (the 75% threshold was chosen to define the PEG intake as “full”), time of the exam, demographics, morphometrics, social circumstances (living alone, instruction level) and clinical data. The questionnaire included a specific section about personal bowel habits (Bristol stool chart, frequency of bowel movements per week). Constipation was defined as Bristol stool chart type 1-2 and less than 3 bowel movements/week, and/or chronic constipation as indication for colonoscopy. The form also contained a section about general satisfaction about the used preparation [evaluated by visual analogue scale (VAS) score, from 0 = ’absolutely unsatisfied’ to 10 = ’perfectly satisfied’] and symptoms (nausea, vomit, bloating, abdominal pain) experienced during the preparation.
The quality of bowel preparation was assessed using the Boston bowel preparation scale (BBPS)[19]. Bowel preparation was defined adequate if a global score ≥ 6 with segmental scores ≥ 2 in all colonic segments was achieved. For any patients with previous bowel resection, the preparation was considerate adequate if all the segmental sub-scores were ≥ 2.
The number, size and final histology of lesions resected or biopsied during the procedures were collected. PDR, ADR, advanced adenoma (adenomas ≥ 1 cm or with villous component or harboring high-grade dysplasia) detection rate (AADR), sessile/serrated lesion detection rate (SDR, excluding hyperplastic polyps) and cancer detection rate were calculated.
Considering an expected adequate preparation rate of 87.1% with LV preparation and of 92.5% with HV preparation from a previous study[20], power of 90% with an alpha error of 0.05, we estimated that 1384 patients would be sufficient. A possible drop-out rate of 30% was considered for the study, therefore the final required sample size was 1977 patients.
The descriptive statistics were expressed as counts and percentages for categorical variables and mean ± SD or median (interquartile ranges, IQR) for continuous variables, as appropriate. Normality assumption was to be tested in continuous variables by visual inspection of the qq-plot.
The association between bowel preparation and baseline variables was investigated with the χ2 test for categorical variables; the continuous variables were compared by analysis of variance ANOVA or by the non-parametric Kruskal–Wallis test for non-normally distributed data.
Univariate and multi-variate logistic regression was used to identify if adequate bowel preparation and volume of bowel preparation were independently associated with clinical outcomes (PDR, ADR, AADR, SDR and cancer). Multivariate analysis was performed considering age (as a continuous variable), sex and indications for colonoscopy [positive fecal blood test (FBT), surveillance, symptoms or inflammatory bowel disease (IBD)]. Separate analysis was also performed considering the type of colonoscopes used (HD or SD imaging). Odds ratios (ORs) with their corresponding 95%CIs were calculated, and P values were considered statistically significant if they were less than 0.05.
Statistical analysis was carried out by computer software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Total 2040 patients were enrolled and 1815 patients (mean age 60.6 years, 50.2% male) were finally included according to exclusion criteria (study flowchart in Supplementary Figure 1). 944 patients (52%) chose a LV preparation, while 871 patients (48%) preferred a HV preparation. 750 patients (41.3%) had their colonoscopy scheduled in the afternoon and thereafter used a split-dose regimen; the use of a split-dose regimen was more common in the HV group (44.7% vs 38.2%, P = 0.0055).
Indications for colonoscopy were symptoms (altered bowel movements, anemia or bleeding, abdominal pain) in 60.6%, post-polypectomy or post-colorectal cancer surveillance in 24.0%, positive FBT in 8.3% and follow-up of known IBD in 7.1% of the cases. Positive FBT was more common in the HV group and known IBD in the LV group. The patients in the HV preparation group were more frequently male, had higher body mass index and more frequently had a cardiac disease and a low-level education. There were no statistically significant differences in terms of age and other possible risk factors for poor bowel preparation (previous abdominal/pelvic surgery, constipation, living-alone status or non-adherence to low-fiber dieting before colonoscopy). HD colonoscopes were used in 606 patients (33.4%), without difference in the two groups (P = 0.605) (Table 1).
| Characteristics | High volume (n = 871) | Low volume (n = 944) | P value1 |
| Age | 61.2 ± 14.3 | 60.1 ± 14.6 | 0.092 |
| Male sex | 463 (53.2) | 448 (47.5) | 0.0153 |
| Split-dose | 389 (44.7) | 361 (38.2) | 0.0063 |
| High-definition colonoscope | 296 (33.9) | 310 (32.8) | 0.605 |
| Indication | |||
| Symptoms | 538 (61.8) | 563 (59.6) | |
| Surveillance | < 0.0013 | ||
| Post polypectomy | 134 (15.4) | 154 (16.3) | |
| Post colonic resection for CRC | 73 (8.4) | 73 (7.7) | |
| Positive FBT | 94 (10.8) | 57 (6.1) | |
| IBD | 32 (3.6) | 97 (10.3) | |
| BMI, mean ± SD2 | 25.5 ± 4.3 | 25.0 ± 4.0 | 0.0153 |
| Previous abdominal surgery | 98 (11.3) | 96 (10.2) | 0.456 |
| Constipation | 66 (7.6) | 86 (9.1) | 0.239 |
| Comorbidities | |||
| Heart disease | 90 (10.3) | 65 (6.9) | 0.0093 |
| Diabetes | 72 (8.3) | 65 (6.9) | 0.266 |
| Stroke/dementia | 19 (2.2) | 25 (2.6) | 0.518 |
| Severe CKD | 21 (2.4) | 15 (1.6) | 0.209 |
| Cirrhosis | 12 (1.4) | 13 (1.4) | 0.999 |
| GERD | 192 (22.0) | 219 (23.2) | 0.557 |
| Waiting time > 1 mo | 485 (55.7) | 570 (60.4) | 0.0903 |
| Non-adherence to low fiber diet | 91 (10.5) | 112 (11.9) | 0.329 |
| Lives alone2 | 123 (14.8) | 149 (16.3) | 0.395 |
| Low instruction2 | 157 (19.6) | 122 (14.1) | 0.0023 |
Overall, adequate preparation was observed in 1595/1815 (87.9%) patients. Complete colonoscopy was possible in 1793 patients (98.8%). At least one polypoid lesion was found in 520/1815 colonoscopies (PDR 28.7%). Histology revealed at least one adenoma in 381/1815 colonoscopies (ADR 20.1%) and at least one sessile/serrated lesion in 28/1815 colonoscopies (SDR 1.5%). Non adenomatous/non serrated lesions were mostly hyperplastic (n = 81) or inflammatory (n = 23) polyps, with less common histology encountered in 7 cases.
Adequate bowel preparation was associated with a higher complete colonoscopy rate (99.7% vs 92.5%, OR 24.05, 95%CI: 7.82–73.92, P < 0.001), higher PDR (29.8% vs 20.1%, OR 1.69, 95%CI: 1.20–2.40, P = 0.003) and ADR (21.8% vs 15.5%, OR 1.52, 95%CI: 1.04–2.23, P = 0.033), while no significant differences were found in AADR, cancer detection and SDR (Table 2).
| Outcome | Adequate preparation (n = 1595) | Inadequate preparation (n = 220) | OR (95%CI) | P value1 |
| Complete examination | 1590 (99.7) | 203 (92.3) | 26.63 (9.72-72.96) | < 0.0012 |
| PDR | 476 (29.8) | 44 (20.1) | 1.69 (1.20-2.40) | 0.0032 |
| ADR | 347 (21.8) | 34 (15.5) | 1.52 (1.04-2.23) | 0.0332 |
| AADR | 82 (5.1) | 9(4.1) | 1.27 (0.63-2.57) | 0.505 |
| Cancer | 27 (1.7) | 7 (3.2) | 0.52 (0.23-1.22) | 0.133 |
| SDR | 26 (1.6) | 2 (0.9) | 1.81 (0.43-7.66) | 0.423 |
PDR, ADR, AADR and cancer rates were higher in the positive FBT group, followed by the surveillance, symptoms and IBD groups (Supplementary Table 1). The use of HD instruments was related to significantly higher ADR (P = 0.040) compared to standard definition instruments, without significant difference in other clinical outcomes (Supplementary Table 2).
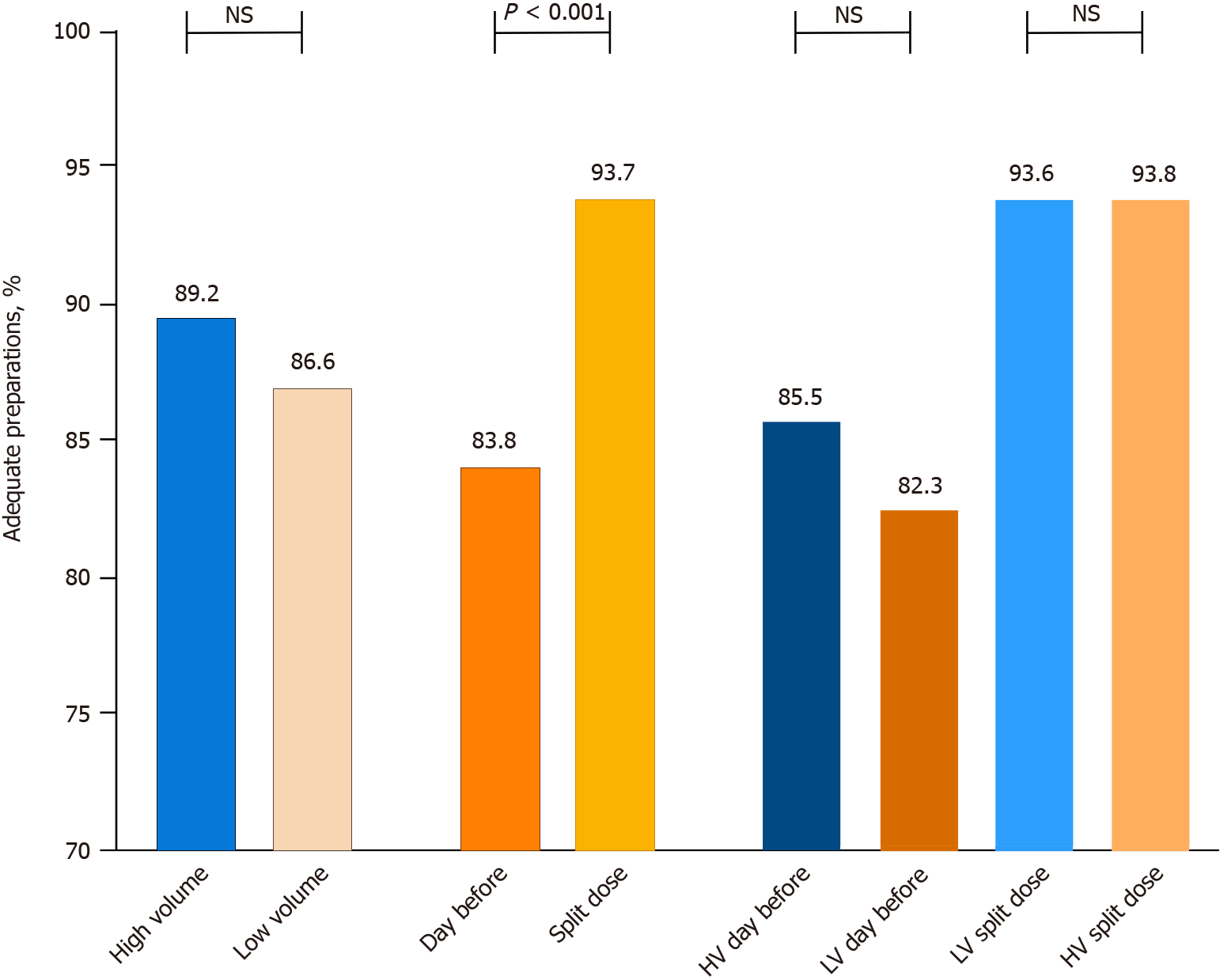
The adequacy of preparation was independent of the use of HV or LV preparations (89.2% vs 86.6%, P = 0.098). The split-dose schedule was superior to day-before for either HV (93.8% vs 85.5%, P < 0.001) or LV preparation (93.6% vs 82.3%, P < 0.001). Also considering the two different schedules, there was no difference among HV and LV preparation (HV split-dose 93.8% vs LV split-dose 93.6%, P = 1; HV day-before 85.5% vs LV day-before 82.3%, P = 0.182) (Figure 1). The efficacy of HV and LV preparations was similar in all the colonic segments (Supplementary Figure 2), irrespective of the use of the day-before or a split-dose schedule (Supplementary Figure 3).
The mean global BBPS scores were higher with HV preparations compared to LV (overall: 7.1 ± 1.7 vs 6.8 ± 1.6, P < 0.001; day-before schedule: 6.9 ± 1.7 vs 6.6 ± 1.7, P = 0.003; split-dose schedule: 7.5 ± 1.6 vs 7.2 ± 1.5, P = 0.019).
As compared to LV preparation, HV preparation was associated with higher PDR (32.5% vs 25.1%, OR 1.43, 95%CI: 1.17–1.76, P < 0.001), higher ADR (24.1% vs 18.1%, OR 1.44, 95%CI: 1.14-1.80, P = 0.002) and higher AADR (6.4% vs 3.7%, OR 1.79, 95%CI: 1.16–2.75, P = 0.009) without differences in cancer detection and SDR. After adjustment for age, sex and indication for colonoscopy, the difference remained statistically significant for PDR (adjusted OR 1.320, 95%CI: 1.07-1.63, P = 0.011) and for ADR (adjusted OR 1.29, 95%CI: 1.02-1.63, P = 0.038) but not for AADR (adjusted OR 1.51, 95%CI: 0.97–2.35, P = 0.069) (Table 3).
| Outcome | High volume (n = 871) | Low volume (n = 944) | OR (95%CI) | P value1 | Adjusted2 OR (95%CI) | P value2 |
| PDR | 283 (32.5) | 237 (25.1) | 1.43 (1.17–1.76) | < 0.0013 | 1.32 (1.07–1.63) | 0.0113 |
| ADR | 210 (24.1) | 171 (18.1) | 1.44 (1.14–1.80) | 0.0023 | 1.29 (1.02–1.63) | 0.0383 |
| AADR | 56 (6.4) | 35 (3.7) | 1.79 (1.16–2.75) | 0.0093 | 1.51 (0.97–2.35) | 0.069 |
| Cancer | 19 (2.2) | 15 (1.6) | 1.38 (0.70–2.74) | 0.354 | ||
| SDR | 16 (1.8) | 12 (1.3) | 1.45 (0.68–3.09) | 0.331 |
HV and LV preparations were associated to comparable PDR, ADR, AADR, SDR and cancer detection when colonoscopy was performed under HD endoscopic imaging (Table 4). On the contrary, the use of HV preparation was linked to significantly higher PDR, ADR and AADR compared to LV preparation in patients receiving colonoscopy with SD imaging, after adjustment for age, sex and indications for colonoscopy (Table 5).
| Outcome | High volume (n = 296) | Low volume (n = 310) | OR (95% CI) | P value1 |
| PDR | 97 (32.7) | 93 (30.0) | 1.13 (0.81–1.60) | 0.462 |
| ADR | 70 (23.6) | 74 (23.9) | 0.99 (0.68–1.44) | 0.948 |
| AADR | 21 (7.1) | 17 (5.5) | 1.31 (0.68–2.54) | 0.415 |
| Cancer | 5 (1.7) | 5 (1.6) | 1.05 (0.30–3.66) | 0.941 |
| SDR | 4 (1.4) | 4 (1.3) | 1.05 (0.26–4.23) | 0.947 |
| Outcome | High volume (n = 575) | Low volume (n = 634) | OR (95%CI) | P value1 | Adjusted2 OR (95%CI) | P value2 |
| PDR | 186 (32.3) | 144 (22.7) | 1.63 (1.26–2.10) | < 0.0013 | 1.59 (1.22–2.08) | < 0.0013 |
| ADR | 140 (24.3) | 97 (15.3) | 1.78 (1.34–2.38) | < 0.0013 | 1.71 (1.26–2.30) | < 0.0013 |
| AADR | 35 (6.1) | 18 (2.8) | 2.23 (1.24–3.96) | 0.0073 | 1.97 (1.09–3.56) | 0.0253 |
| Cancer | 14 (2.4) | 10 (1.6) | 1.56 (0.69–3.53) | 0.289 | ||
| SDR | 12 (2.1) | 8 (1.3) | 1.67 (0.68–4.11) | 0.266 |
The use of the split-dose schedule was not linked with significantly better clinical outcomes as compared to day-before for either HV or LV preparations (Table 6).
| Outcome | High volume day before (n = 482) | High volume split-dose (n = 389) | P value1 | Low volume day before (n = 583) | Low volume split-dose (n = 361) | P value1 |
| PDR | 149 (30.9) | 134 (34.4) | 0.277 | 145 (24.9) | 92 (25.5) | 0.833 |
| ADR | 108 (22.4) | 102 (26.2) | 0.191 | 103 (17.7) | 68 (18.8) | 0.650 |
| AADR | 30 (6.2) | 26 (6.7) | 0.783 | 20 (3.4) | 15 (4.2) | 0.567 |
| Cancer | 11 (2.3) | 8 (2.1) | 0.827 | 6 (1.0) | 9 (2.5) | 0.088 |
| SDR | 5 (1.0) | 11 (2.8) | 0.050 | 8 (1.4) | 4 (1.1) | 1.000 |
Overall, HV and LV preparations were equally well tolerated (median VAS score 7, interquartile range 5-9 for both preparations). Total 860 patients (47.4%) reported gastrointestinal symptoms during preparation: nausea (26.5%) and bloating (19.9%) were the most frequently self-reported symptoms. The occurrence of nausea, vomiting and abdominal pain was more frequent among the patients in the LV group (Table 7). Self-reported incomplete (i.e., ≤ 75%) intake of the PEG solution was more common in the HV group (7.9% vs 5.4%, P = 0.003). For the HV preparation the split-dose regimen was related to better tolerability (higher VAS score) as compared to day-before, even if with no differences in terms of reported symptoms. For the LV preparation, the split-dose regimen was related to lower incidence of symptoms (in particular nausea and bloating) (Table 8).
| Total (n = 1815) | High volume (n = 871) | Low volume (n = 944) | P value1 | |
| Global tolerance, VAS score2, median (interquartile range) | 7 (5-9) | 7 (5-9) | 7 (5-9) | 0.627 |
| Incomplete preparation (< 75% of PEG assumed) | 116 (6.6) | 67 (7.9) | 49 (5.4) | 0.0323 |
| Any symptom during preparation | 860 (47.4) | 369 (42.4) | 491 (52) | < 0.0013 |
| Bloating | 363 (20) | 183 (21) | 180 (19.1) | 0.301 |
| Nausea | 480 (26.5) | 187 (21.5) | 293 (31) | < 0.0013 |
| Vomiting | 174 (9.6) | 55 (6.3) | 119 (12.6) | < 0.0013 |
| Abdominal pain | 281 (15.5) | 104 (11.9) | 177 (18.8) | < 0.0013 |
| High volume one-day (n = 482) | High volume split dose (n = 389) | P value1 | Low volume one-day (n = 583) | Low volume split dose (n = 361) | P value1 | |
| Global tolerance, VAS score2, median (interquartile range) | 7 (5-8) | 7 (5-9) | 0.0063 | 7 (5-9) | 7 (5-9) | 0.033 |
| Incomplete preparation | 37 (7.9) | 30 (7.9) | 0.994 | 31 (5.5) | 18 (5.2) | 0.840 |
| Any symptom during preparation | 211 (43.8) | 158 (40.6) | 0.384 | 324 (55.6) | 167 (46.3) | 0.0053 |
| Bloating | 103 (21.4) | 80 (20.6) | 0.772 | 126 (21.6) | 54 (14.9) | 0.0113 |
| Nausea | 112 (23.2) | 75 (19.3) | 0.158 | 196 (33.6) | 97 (26.9) | 0.0293 |
| Vomiting | 33 (6.9) | 22 (5.7) | 0.473 | 73 (12.5) | 46 (12.7) | 0.921 |
| Abdominal pain | 54 (11.2) | 50 (12.9) | 0.455 | 105 (18.0) | 72 (19.9) | 0.459 |
The standard high-volume PEG-based preparation is safe and effective, but even in clinical studies a significant proportion of patients is unable to take all the prescribed dose[21] with detrimental effect on its efficacy. RCTs and some meta-analyses have shown a comparable efficacy of different low-volume preparations compared to high-volume PEG[9,10,13-15,22], and the use of these preparations is now recommended in both the European[23] and North American[24] guidelines. However, robust comparisons in RCTs between HV and LV preparations in terms of clinically relevant outcomes (such as ADR) are missing, in particular for the two most recently introduced LV preparations: 2 L PEG plus citrate and 1L PEG plus ascorbate. The former has been compared to HV preparation in a RCT[14] in terms of adequate bowel preparation rate and tolerability but not in terms of lesions detection rates, while the latter has been compared in RCTs[25-27] only to other low-volume preparations. Moreover, real-life data are scarce and conflicting: a recent real-life direct comparison of 1 L PEG plus ascorbate and HV preparation[28] has showed higher cleansing success and tolerability in the LV group, but did not analyze lesions detection. Lesions detection rates were not reported also in a recently presented abstract comparing HV and 2 L PEG plus ascorbate and sodium sulfate[29]. In addition, a recent prospective observational study has shown better cleansing results and higher ADR and AADR with 4 L PEG compared to lower volume preparations[18].
In our real-life setting, we confirmed that the low-volume PEG plus bisacodyl preparation is equally effective than HV in all the colonic segments (while some studies have shown worse performances of low-volume preparations in the right colon[30]) and irrespective of the intake schedule, with split-dose regimens largely superior to day-before ones. In particular, it is to note that the split LV preparation was as effective as the split HV preparation, confirming the results achieved in a recent meta-analysis[22], in opposition to previous ones[16,17].
Overall, 87.9% of our patients achieved adequate preparation. This result is in line or superior to the results reported in the literature[31,32], even if slightly inferior to the 90% target proposed by the European Society of Gastrointestinal Endoscopy in 2019[33]. We confirmed the importance of bowel preparation in terms of relevant outcomes such as complete colonoscopy rate, PDR and ADR, while we did not find differences in terms of AADR, SDR and cancer detection. Advanced adenomas and cancers are usually bigger lesions, easier to find even in a not well-prepared colon[6], while the SDR result can be explained by their low prevalence in our population.
Quite surprisingly, only a slight majority of patients (52%) preferred the LV preparation over the standard HV. This may be partially explained by the order in which the two preparations were listed in the instructions handed to the patients (HV preparation listed first). Even if stated equally effective in the instructions given, it is also possible that the patients perceived more effective a high-volume preparation and leaned towards that choice, especially for “strong” indications such as positive FBT. In fact, we have observed a different distribution of indications for colonoscopy in the two study groups. While FBT-positive patients chose more frequently the HV preparation, the large majority (75.2%) of IBD patients chose LV preparation. Women also used more frequently the LV preparation, while we did not find any age-related difference. Interestingly, 52% of patients with colonoscopy planned in the afternoon chose the HV preparation. This may suggest that the possibility to reduce the volume of PEG was not felt so compelling once given the possibility to split its assumption.
Quite surprisingly, despite similar efficacy in terms of bowel cleansing, the use of the HV preparation was related to higher PDR, ADR and AADR compared to the LV preparation. To remove confounding factors due to the absence of randomization, we adjusted the OR considering three main characteristics related to the prevalence of colorectal lesions such as age, sex and indication. Even after this adjustment, the HV preparation showed better results, with a statistically significant difference for PDR (adjusted OR 1.32, P = 0.011) and ADR (adjusted OR 1.29, P = 0.038). This result is unlikely to be explained by the more frequent use of split-dose in the HV group, considering that we did not find differences in lesions detection among split and day-before schedules. The type of colonoscopes used seems to have a relevant role in our study. HD colonoscopes, that have shown better diagnostic performances compared to SD ones[34], were used in a similar proportion of patients in the two groups. However, while we did not observe a difference in performance in the two preparations with HD instruments, performance of LV preparation was significantly inferior to HV in terms of lower PDR, ADR and AADR when SD imaging colonoscopy was adopted. This is likely to be linked to the lower mean BBPS score observed in patients using LV preparation. We hypothesize that the persistence of some fluids in the bowel lumen may reduce visibility of lesions, especially when SD scopes are used. Our results suggest that the use of SD definition colonoscopes in patients prepared with LV preparation should be avoided because of an increased risk of missed lesions.
About tolerability, LV preparations[10,14] and in particular 2 L PEG plus bisacodyl[9] were found to be better tolerated as compared to high-volume PEG in previous RCTs. On the contrary, we have observed more self-reported gastrointestinal symptoms such as nausea, vomiting and abdominal pain in the LV group. This result can be explained by the real-life observational design of our study, rather than reflecting an intrinsic lower tolerability of the LV preparation. Nonetheless, these GI symptoms affected neither the patients’ adherence nor tolerability. In fact, the LV preparation was judged as tolerable as the HV preparation according to the VAS scale, and it was more frequently taken completely. The use of a split-dose regimen increased the reported tolerability of both the HV (higher VAS score) and the LV (less frequent symptoms) preparations, as previously shown in RCTs and meta-analyses[17,35].
We recognize that our study has several limitations. The most important limitation is the adoption of day-before schedule for morning procedures; day-before preparations are not recommended by guidelines because of its inferior efficacy when compared to split-dose, as confirmed by our results. Due to the extension of the metropolitan area served by our center, however, we decided to maintain the possibility to choose a day-before regimen. In fact, living far from the endoscopic centers has been demonstrated to be a significant limitation for adherence to split dose regimen, especially for early morning scheduled colonoscopy[36]. Secondly, the opportunity to leave the choice of the preparation to the patient may be debatable. However, both the preparations used in this study are equally recommended by international guidelines[23,24] and clinical criteria to prefer a specific preparation over another in a specific patient are lacking. Thirdly, as compared to RCTs, the real-life “patients-determined” allocation among different study groups could result in an unbalanced distribution of risk factors. Even if most of the baseline characteristics were comparable in the two study groups, the higher number of male and FBT-positive patients in the HV group could lead to overestimation of performances of HV preparation. However, we performed multivariate analysis considering these factors to provide reliable adjusted odds ratio for lesions detection rates in the two study groups. Fourthly, in our study HD scopes were used only in approximately one-third of cases. We recognize that the use of HD colonoscopes is preferable over SD because of better mucosal visualization. However, SD colonoscopes are still widely used in many centers worldwide. For this reason, we think that our real-life observation that LV preparations could be less effective combined with SD scopes may be of particular interest. Lastly, the single-center observational design implies the risk of sub-optimal reproducibility. However, the large sample size and the prospective nature of this study support our results. On the other hand, additional strengths of our study consist in the blindness of the endoscopists to the type of preparation taken, the use of a well-validated bowel preparation scale and the available histology for all the resected lesions.
To resume, this large prospective single-blinded real-life study reveals that adequate bowel cleansing can be equally achieved by means of either HV or LV preparation, showing better result with split dosage. However, in the real-life setting the HV preparation is associated with higher PDR and ADR as compared to the LV preparation, due to reduced performances of LV preparation when SD colonoscopes are used. Our results suggest that the HV preparation should still be proposed as one of the preferred options in screening colonoscopy, and that the use of LV preparations should be avoided in average-to-high risk patients if HD scopes are not available. Looking forward to large multi-center real-life studies, we believe that 4L PEG should be still considered the reference standard for new RCTs assessing both the bowel cleansing and the ADR in screening colonoscopy.
Colonoscopy is a key procedure for the diagnosis of several colorectal pathologies and for prevention of colorectal cancer. The diagnostic yield of colonoscopy is strongly influenced by quality of bowel preparation. In the last years, several low-volume (LV) preparations have been introduced with the aim to improve patients’ adherence and compliance.
LV preparations have demonstrated similar cleansing effects compared to standard, high-volume (HV) preparation in randomized controlled trials. However, few real-life studies have compared these two types of preparation in terms of clinically relevant outcomes such as lesions detection.
Primary aim of our study was to compare the real-life efficacy of a standard HV preparation (4 L polyethylene glycol) and of a LV preparation (2 L polyethylene glycol with bisacodyl), either in terms of adequate bowel preparation rate (defined as Boston Bowel Preparation Scale score ≥ 2 in all bowel segments) or in terms of lesions detection. Secondary aim was to compare patients’ self-reported adherence and tolerability.
A prospective study was conducted from 1 December 2014 to 31 December 2016, enrolling all the consecutive outpatients referred for colonoscopy in a single endoscopy center in Italy. Patients were free to choose one of the two proposed preparations (HV or LV). A questionnaire was administered to the patients to collect comorbidities, type of preparation chosen, adherence to preparation and tolerability. Indications for colonoscopy, type of scope used (high-definition, HD, or standard-definition, SD), Boston Bowel Preparation Scale (BBPS) score for each colonic segment, histology of all the lesions resected or biopsied were collected.
LV was chosen by 52% of patients (50.8% of men, 54.9% of women). HD scopes were used in 33.4% of patients, without difference in the two groups (P = 0.605). There was no difference between HV and LV preparations in terms of adequate bowel preparation, even if mean global BBPS score was higher for HV preparation when compared to LV. Compared to LV, HV preparation resulted higher in polyp detection rate (PDR) but not in advanced adenoma detection rate (AADR) and cancer detection rate. Considering the type of colonoscope used, we observed lower PDR, adenoma detection rate (ADR) and AADR with LV preparation with SD colonoscopes, without differences between the two preparations with HD instruments.
Despite similar adequate bowel preparation rate among the two preparations compared, we observed higher PDR, ADR and AADR with HV preparation compared to LV. The difference is mainly observed when SD endoscopes are used. The two preparations were stated as equally tolerated by the patients, but self-reported adherence was higher with LV.
In the last years we have observed an increasing trend towards the use of LV preparations to increase patients’ satisfaction. However, primary aim of bowel preparation is to minimize the risk of missing colorectal lesions. Further studies, either randomized controlled trials or real-life studies, are warranted to compare efficacy in lesions detection of new LV products to standard HV preparation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Serban ED S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Aslinia F, Uradomo L, Steele A, Greenwald BD, Raufman JP. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am J Gastroenterol. 2006;101:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Radaelli F, Meucci G, Sgroi G, Minoli G; Italian Association of Hospital Gastroenterologists (AIGO). Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Hsu CM, Lin WP, Su MY, Chiu CT, Ho YP, Chen PC. Factors that influence cecal intubation rate during colonoscopy in deeply sedated patients. J Gastroenterol Hepatol. 2012;27:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, Mayr M, Mroß M, Scheel M, Schröder A, Gerber K, Stange G, Roll S, Gauger U, Wiedenmann B, Altenhofen L, Rosch T. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3). Gut. 2013;62:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Clark BT, Rustagi T, Laine L. What level of bowel prep quality requires early repeat colonoscopy: systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am J Gastroenterol. 2014;109:1714-23; quiz 1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11:e0154149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 7. | Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Kingsley J, Karanth S, Revere FL, Agrawal D. Cost Effectiveness of Screening Colonoscopy Depends on Adequate Bowel Preparation Rates - A Modeling Study. PLoS One. 2016;11:e0167452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Clark RE, Godfrey JD, Choudhary A, Ashraf I, Matteson ML, Bechtold ML. Low-volume polyethylene glycol and bisacodyl for bowel preparation prior to colonoscopy: a meta-analysis. Ann Gastroenterol. 2013;26:319-324. [PubMed] |
| 10. | Xie Q, Chen L, Zhao F, Zhou X, Huang P, Zhang L, Zhou D, Wei J, Wang W, Zheng S. A meta-analysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS One. 2014;9:e99092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Moon CM, Park DI, Choe YG, Yang DH, Yu YH, Eun CS, Han DS. Randomized trial of 2-L polyethylene glycol + ascorbic acid versus 4-L polyethylene glycol as bowel cleansing for colonoscopy in an optimal setting. J Gastroenterol Hepatol. 2014;29:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Zorzi M, Valiante F, Germanà B, Baldassarre G, Coria B, Rinaldi M, Heras Salvat H, Carta A, Bortoluzzi F, Cervellin E, Polo ML, Bulighin G, Azzurro M, Di Piramo D, Turrin A, Monica F; TriVeP Working Group. Comparison between different colon cleansing products for screening colonoscopy. A noninferiority trial in population-based screening programs in Italy. Endoscopy. 2016;48:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | van Lieshout I, Munsterman ID, Eskes AM, Maaskant JM, van der Hulst R. Systematic review and meta-analysis: Sodium picosulphate with magnesium citrate as bowel preparation for colonoscopy. United European Gastroenterol J. 2017;5:917-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Spada C, Cesaro P, Bazzoli F, Saracco GM, Cipolletta L, Buri L, Crosta C, Petruzziello L, Ceroni L, Fuccio L, Giordanino C, Elia C, Rotondano G, Bianco MA, Simeth C, Consalvo D, De Roberto G, Fiori G, Campanale M, Costamagna G. Evaluation of Clensia®, a new low-volume PEG bowel preparation in colonoscopy: Multicentre randomized controlled trial versus 4L PEG. Dig Liver Dis. 2017;49:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Yang HJ, Park SK, Kim JH, Im JP, Yeom DH, Seo GS, Park DI. Randomized trial comparing oral sulfate solution with 4-L polyethylene glycol administered in a split dose as preparation for colonoscopy. J Gastroenterol Hepatol. 2017;32:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Enestvedt BK, Tofani C, Laine LA, Tierney A, Fennerty MB. 4-Liter split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2012;10:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology. 2015;149:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Waldmann E, Penz D, Majcher B, Zagata J, Šinkovec H, Heinze G, Dokladanska A, Szymanska A, Trauner M, Ferlitsch A, Ferlitsch M. Impact of high-volume, intermediate-volume and low-volume bowel preparation on colonoscopy quality and patient satisfaction: An observational study. United European Gastroenterol J. 2019;7:114-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 924] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 20. | DiPalma JA, Wolff BG, Meagher A, Cleveland Mv. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol. 2003;98:2187-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 21. | Kelly NM, Rodgers C, Patterson N, Jacob SG, Mainie I. A prospective audit of the efficacy, safety, and acceptability of low-volume polyethylene glycol (2 L) versus standard volume polyethylene glycol (4 L) versus magnesium citrate plus stimulant laxative as bowel preparation for colonoscopy. J Clin Gastroenterol. 2012;46:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Spadaccini M, Frazzoni L, Vanella G, East J, Radaelli F, Spada C, Fuccio L, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Van Hooft JE, Dumonceau JM, Marmo C, Alfieri S, Chandrasekar VT, Sharma P, Rex DK, Repici A, Hassan C. Efficacy and Tolerability of High- vs Low-Volume Split-Dose Bowel Cleansing Regimens for Colonoscopy: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2020;18:1454-1465.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Fuccio L, Awadie H, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Vanella G, Mangas-Sanjuan C, Frazzoni L, Van Hooft JE, Dumonceau JM. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:775-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (4)] |
| 24. | Saltzman JR, Cash BD, Pasha SF, Early DS, Muthusamy VR, Khashab MA, Chathadi KV, Fanelli RD, Chandrasekhara V, Lightdale JR, Fonkalsrud L, Shergill AK, Hwang JH, Decker GA, Jue TL, Sharaf R, Fisher DA, Evans JA, Foley K, Shaukat A, Eloubeidi MA, Faulx AL, Wang A, Acosta RD; ASGE Standards of Practice Committee. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81:781-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 25. | DeMicco MP, Clayton LB, Pilot J, Epstein MS; NOCT Study Group. Novel 1 L polyethylene glycol-based bowel preparation NER1006 for overall and right-sided colon cleansing: a randomized controlled phase 3 trial versus trisulfate. Gastrointest Endosc. 2018;87:677-687.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 26. | Schreiber S, Baumgart DC, Drenth JPH, Filip RS, Clayton LB, Hylands K, Repici A, Hassan C; DAYB Study Group. Colon cleansing efficacy and safety with 1 L NER1006 versus sodium picosulfate with magnesium citrate: a randomized phase 3 trial. Endoscopy. 2019;51:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Bisschops R, Manning J, Clayton LB, Ng Kwet Shing R, Álvarez-González M; MORA Study Group. Colon cleansing efficacy and safety with 1 L NER1006 versus 2 L polyethylene glycol + ascorbate: a randomized phase 3 trial. Endoscopy. 2019;51:60-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | Maida M, Sinagra E, Morreale GC, Sferrazza S, Scalisi G, Schillaci D, Ventimiglia M, Macaluso FS, Vettori G, Conoscenti G, Di Bartolo C, Garufi S, Catarella D, Manganaro M, Virgilio CM, Camilleri S. Effectiveness of very low-volume preparation for colonoscopy: A prospective, multicenter observational study. World J Gastroenterol. 2020;26:1950-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Bushyhead D, Tiritilli A, Dominitz JA. Comparison of Low Versus High Volume Bowel Preparation Efficacy and Tolerability for Colonoscopy: A Quality Improvement Study. Gastroenterology. 2020;159:E25-6. [DOI] [Full Text] |
| 30. | Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol. 2010;45:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Gandhi K, Tofani C, Sokach C, Patel D, Kastenberg D, Daskalakis C. Patient Characteristics Associated With Quality of Colonoscopy Preparation: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:357-369.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 33. | Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, Jover R, Suchanek S, Ferlitsch M, Anderson J, Roesch T, Hultcranz R, Racz I, Kuipers EJ, Garborg K, East JE, Rupinski M, Seip B, Bennett C, Senore C, Minozzi S, Bisschops R, Domagk D, Valori R, Spada C, Hassan C, Dinis-Ribeiro M, Rutter MD. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 483] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 34. | Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA, Wallace MB. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Bucci C, Rotondano G, Hassan C, Rea M, Bianco MA, Cipolletta L, Ciacci C, Marmo R. Optimal bowel cleansing for colonoscopy: split the dose! Gastrointest Endosc. 2014;80:566-576.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 36. | Radaelli F, Paggi S, Repici A, Gullotti G, Cesaro P, Rotondano G, Cugia L, Trovato C, Spada C, Fuccio L, Occhipinti P, Pace F, Fabbri C, Buda A, Manes G, Feliciangeli G, Manno M, Barresi L, Anderloni A, Dulbecco P, Rogai F, Amato A, Senore C, Hassan C. Barriers against split-dose bowel preparation for colonoscopy. Gut. 2017;66:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |