Published online Dec 16, 2021. doi: 10.4253/wjge.v13.i12.607
Peer-review started: March 19, 2021
First decision: May 4, 2021
Revised: May 17, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 16, 2021
Processing time: 269 Days and 17.2 Hours
Endoscopic retrograde cholangiopancreatography (ERCP) with stenting is the treatment modality of choice for patients with benign and malignant bile duct obstruction. ERCP could fail in cases of duodenal obstruction, duodenal diverticulum, ampullary neoplastic infiltration or surgically altered anatomy. In these cases percutaneous biliary drainage (PTBD) is traditionally used as a rescue procedure but is related to high morbidity and mortality and lower quality of life. Endoscopic ultrasound-guided biliary drainage (EUS-BD) is a relatively new interventional procedure that arose due to the development of curvilinear echoendoscope and the various endoscopic devices. A large amount of data is already collected that proves its efficacy, safety and ability to replace PTBD in cases of ERCP failure. It is also possible that EUS-BD could be chosen as a first-line treatment option in some clinical scenarios in the near future. Several EUS-BD techniques are developed EUS-guided transmural stenting, antegrade stenting and rendezvous technique and can be personalized depending on the individual anatomy. EUS-BD is normally performed in the same session from the same endoscopist in case of ERCP failure. The lack of training, absence of enough dedicated devices and lack of standardization still makes EUS-BD a difficult and not very popular procedure, which is related to life-threatening adverse events. Developing training models, dedicated devices and guidelines hopefully will make EUS-BD easier, safer and well accepted in the future. This paper focuses on the technical aspects of the different EUS-BD procedures, available literature data, advantages, negative aspects and the future perspectives of these modalities.
Core Tip: Endoscopic retrograde cholangiopancreatography is the current standard of care for bile duct obstruction but is not always possible. The traditional rescue modality is percutaneous transhepatic biliary drainage which has many disadvantages. Endosonography-guided biliary drainage is a new promising interventional technique, showing many advantages over percutaneous biliary drainage and is able to fully replace it when the expertise is available. Developing new devices, training models and guidelines is expected to make this procedure easier, safe and widely accepted in the near future.
- Citation: Karagyozov PI, Tishkov I, Boeva I, Draganov K. Endoscopic ultrasound-guided biliary drainage-current status and future perspectives. World J Gastrointest Endosc 2021; 13(12): 607-618
- URL: https://www.wjgnet.com/1948-5190/full/v13/i12/607.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i12.607
Endoscopic retrograde cholangiopancreatography (ERCP) is a first-line treatment option for patients with biliary obstruction. The success rate is between 90% and 97% and the adverse event rate is less than 10%[1,2]. Some clinical situations: surgically altered anatomy, inaccessible papilla, unsuccessful cannulation require alternative approaches. Percutaneous transhepatic biliary drainage (PTC-D) is a widely accepted alternative after failed ERCP. Despite a high technical success rate (over 95%), the reported mortality remains high. The possible adverse events (AE) are bleeding, infection, drain dislodgement, tract seeding, bile leak, external fistula with a cumulative rate of 30%[2,3]. Contraindications for PTC-D performance are ascites, liver metastasis and obesity. PTB-D is related to the quality of life deterioration[4]. The palliative derivation surgery is related to high morbidity and mortality (35%-50% and 10%-15%)[5] and remains the last choice option for selected cases.
With the implementation of curvilinear-array echoendoscope, various interventional procedures have been made possible, including endoscopic ultrasound-guided biliary drainage (EUS-BD). The first successful EUS-BD was described by Giovannini et al[6] in 2001, which indicates the beginning of a new era for mini-invasive biliary drainage.
Currently, three EUS-based techniques are available- EUS-guided rendezvous technique (RV), EUS-guided antegrade stenting (AS), EUS-guided transmural stenting, EUS-guided hepaticogastrostomy (HGS), EUS-guided choledochoduodenostomy (CDS), and EUS-guided hepaticoduodenostomy. These procedures offer same-session internal drainage in cases of ERCP failure. EUS-BD includes complex and risky procedures which are performed in highly specialized centers by a very skilled endoscopist. The widely accepted indications include ERCP failure, duodenal obstruction due to tumor infiltration, duodenal diverticulum, bile duct tortuosity and previous duodenal stent placement or presence of altered anatomy.
The technique was first introduced in 2003. In current times, this is a single-step procedure and consists of a transhepatic puncture of the biliary system and the creation of a stable fistula between the gastrointestinal lumen and the bile ducts.
This approach is preferred when the papilla cannot be reached endoscopically (duodenal obstruction or surgically altered anatomy). The most common indications for HGS are palliative therapy of hilar obstruction or distal obstruction when the papilla is not accessible. In rare cases, HGS is used for the creation of a temporary tract to the biliary tree in order to manage benign stricture or lithiasis. Sufficient intrahepatic bile duct dilation is needed for the HGS performance. The major contraindications are tumor infiltration of the gastric wall at the site of puncture, massive ascites, and coagulopathy[7].
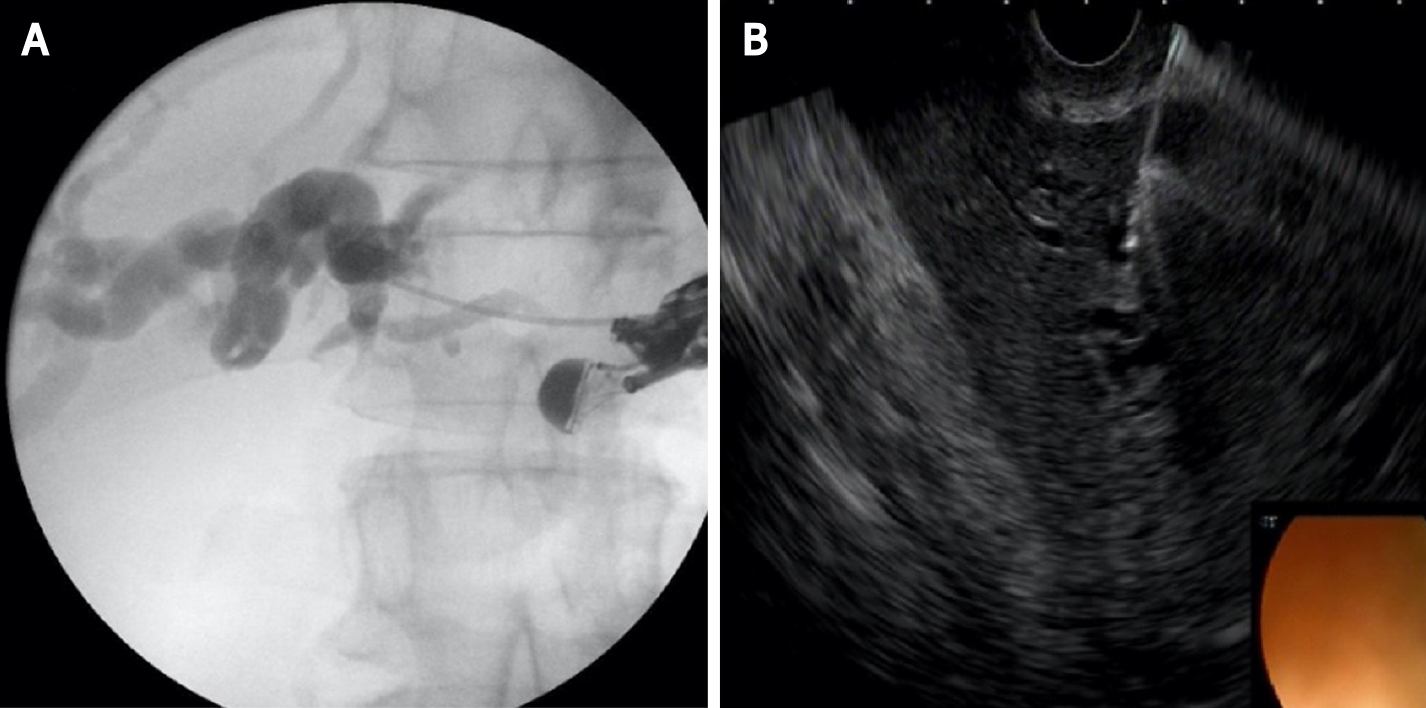
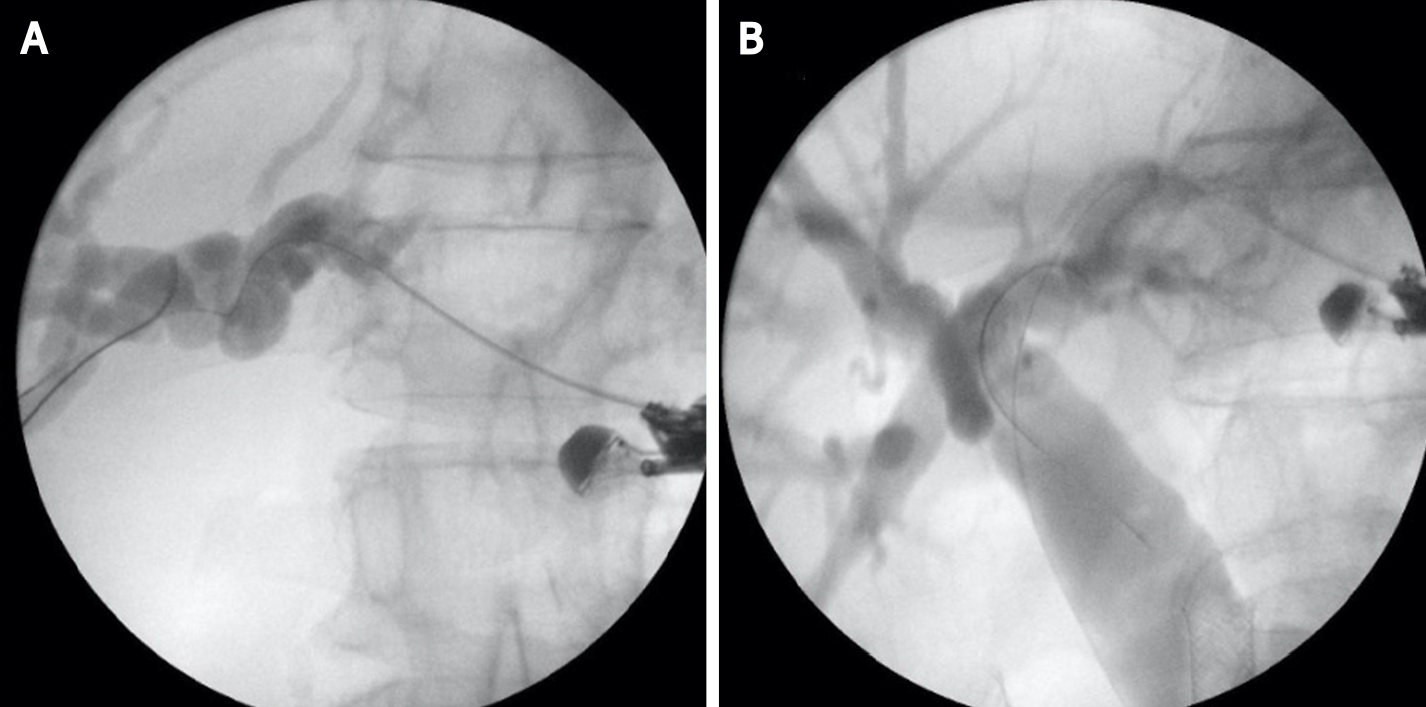
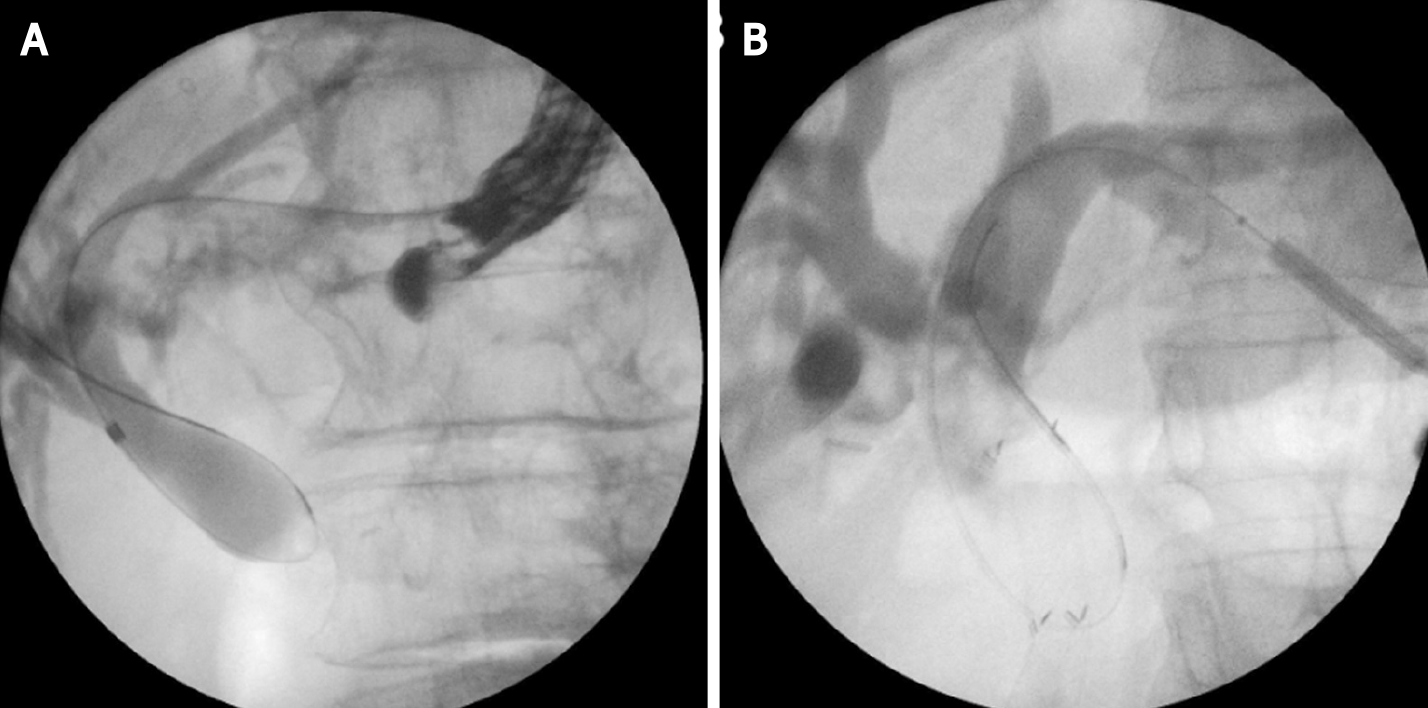
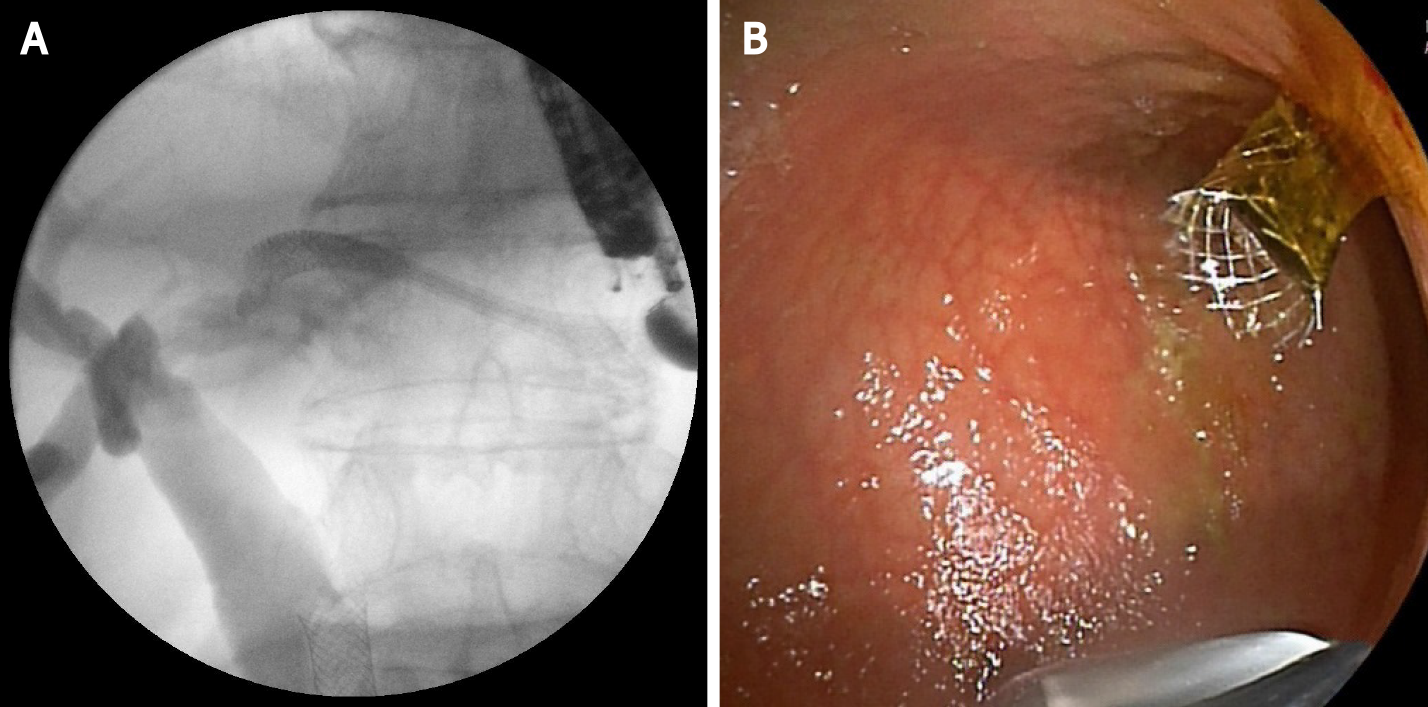
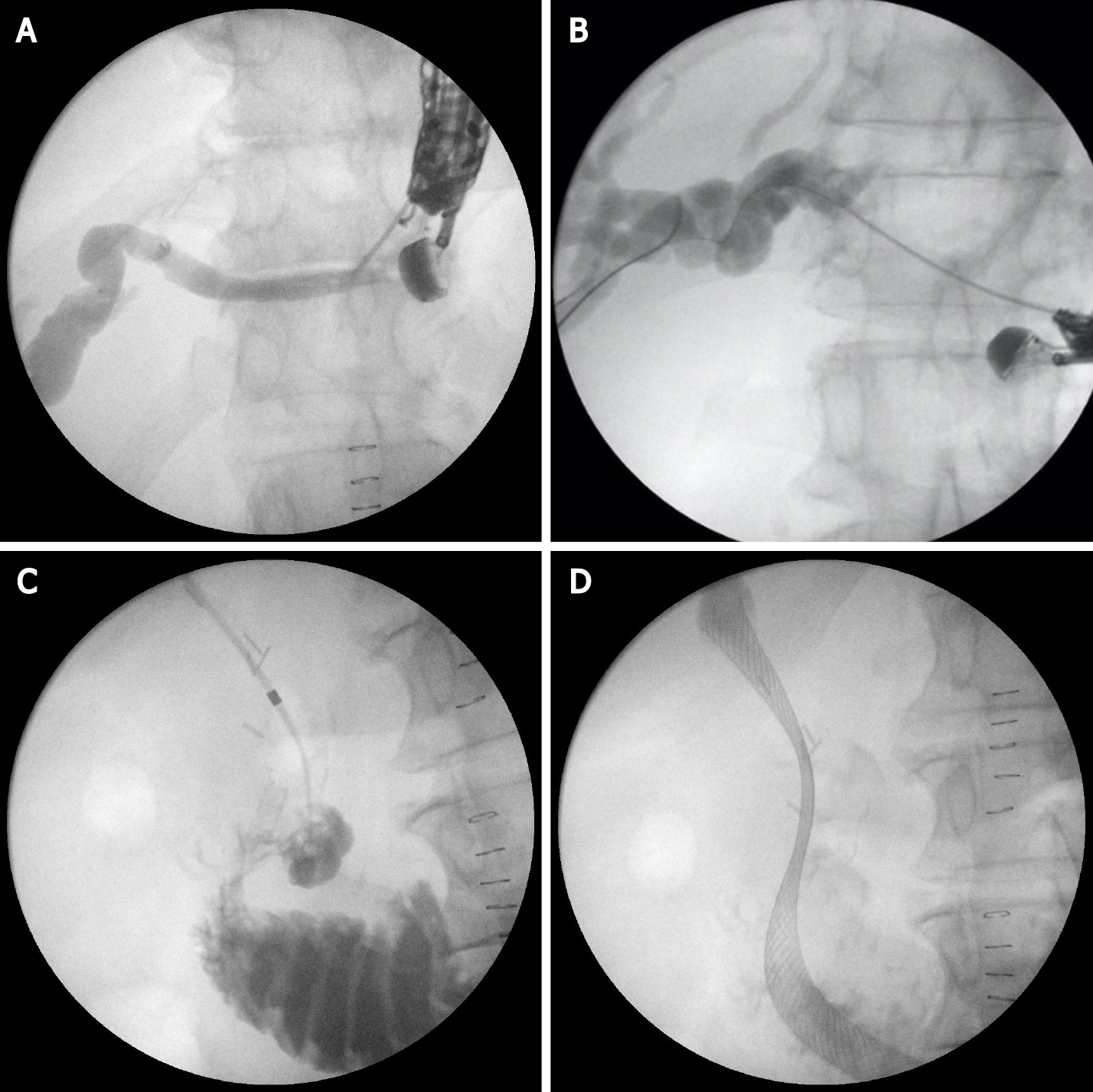
This technique is not standardized. The tip of the echoendoscope is positioned in the stomach body at the lesser curvature. The dilated left hepatic duct can be seen (Segment III). Segment II is not a preferred approach to avoid transesophageal puncture and risk of mediastinitis. The puncture is performed using 19G needle and after bile aspiration contrast medium is injected (Figure 1). The procedure is performed under combined endosonographic and fluoroscopic guidance. A hydrophilic guidewire (0.025-inch or 0.035-inch) is inserted through the needle and manipulated in the bile ducts (Figure 2). Large caliber needles reduce the risk of shearing off the guidewire coating. A special needle was developed-19G EchoTip Access Needle (Cook Ireland Ltd., Limerick, Ireland) to avoid shearing off the guidewire coating and leaving a part in the liver. The needle is smooth with a sharp stylet, used to puncture the gastric wall and the liver. After removing the stylet, the guidewire manipulation is more easily compared with the standard FNA needle and reduces the risk of wire stripping. The most important step is the creation of a stable fistula and the proper technique is the prerequisite to avoid major complications like bile peritonitis, bleeding and perforation. The needle is exchanged over the guidewire with a 6 French cystotome and electrocautery-enhanced tract dilation is performed. Biliary dilation catheters or balloons could also be used (Figure 3). The procedure is finished by placing a stent (Figure 4). Especially dedicated HGS stents [Giobor stent TAEWOONG, proximal covered (NC) stent, HANARO] are commonly used for this technique. These are specially designed partially covered metallic stents with a proximal uncovered part to prevent blockage of segmental bile duct branches and a distal covered part to reduce the risk of bile leakage. Fully covered stents can be used in benign obstruction, but are related to increased risk of focal cholangitis, liver abscess, and migration. Plastic stents are not a reasonable option due to unacceptable high risk of bile peritonitis. An alternative to Giobor stents is the so-called “stent in stent technique” with transgastric placement of two metallic stents- a first one uncovered 8 or 10 cm to prevent bile duct blockage and a second 6 cm fully covered to secure the transmural tract[8,9].
The procedure was first described by Nguyen-Tang et al[10] in 2010 and offers a possibility of physiological bile flow in cases of an inaccessible papilla or failed bile duct cannulation during ERCP. The authors report about 5 cases with malignant bile duct obstruction and endoscopically inaccessible biliary orifice. At the time of failed ERCP they performed transhepatic or transbulbar bile duct puncture and self-expandable metal stent (SEMS) deployment in an antegrade fashion without any AE and concluded that EUS-AS is an efficient technique for palliation of bile duct obstruction when standard ERCP has failed[10].
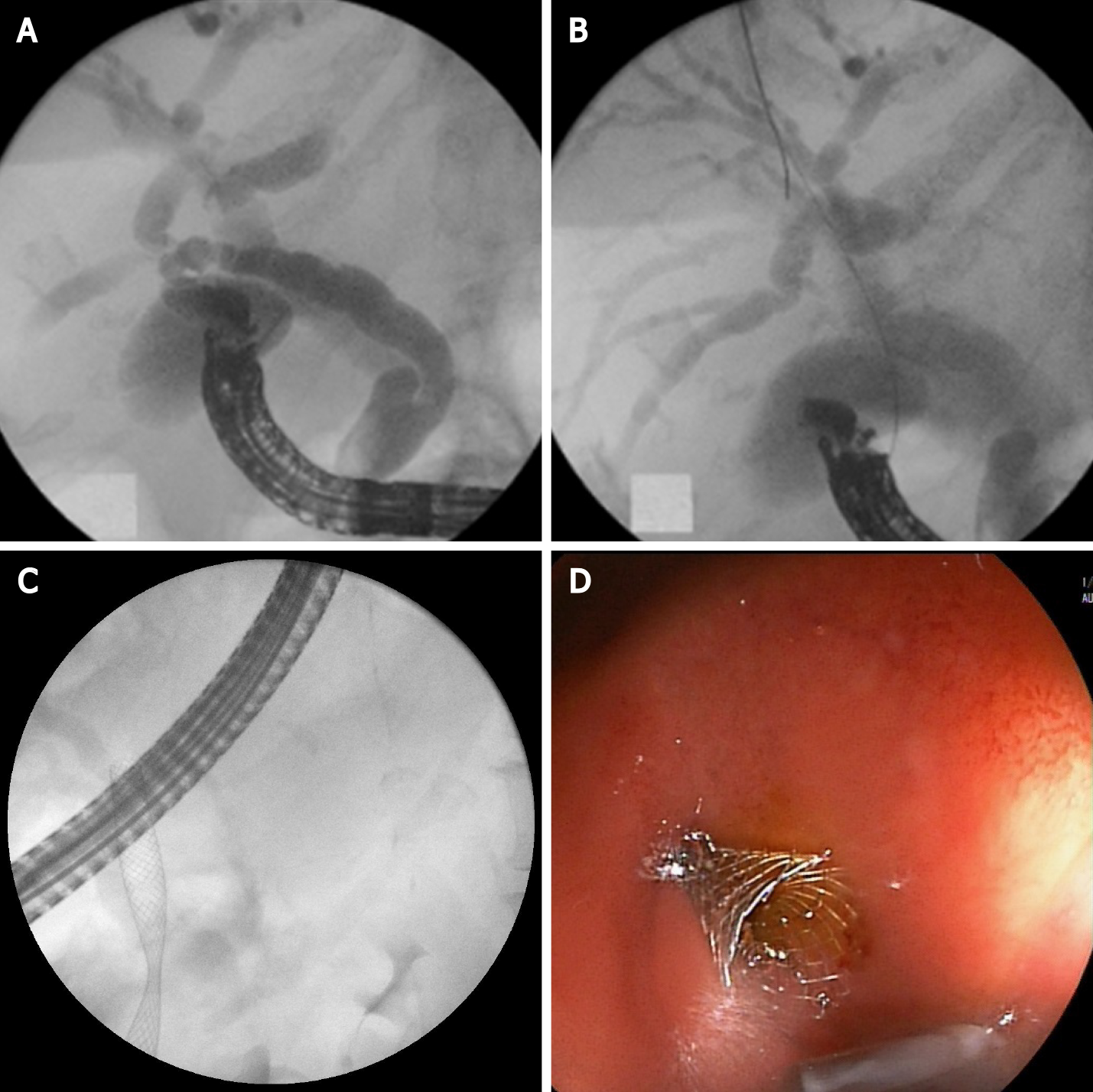
The initial steps of the intervention are the same as HGS-bile duct puncture, guidewire manipulation and tract dilatation. The procedure consists of transgastric left intrahepatic bile duct puncture with 19-gauge needle under EUS visualization. Color Doppler imaging is used to exclude intervening blood vessels and to prevent intra-and postprocedural bleeding. After bile aspiration contrast medium is injected to obtain cholangiogram. The guidewire is inserted through the needle and manipulated and advanced through the stricture and transpapillary in the duodenum or through a biliary anastomosis in the small intestine. After needle tract dilatation using ERCP catheter and mechanical dilators, a stent is placed at the stricture site and most commonly through the papilla of Vater in an antegrade fashion (Figure 5).
There is an increased risk of bile leakage at the puncture site and in cases of stent dysfunction reintervention could be extremely difficult or impossible. For that reason, some authors combine antegrade stenting with HGS. Placing a transenteric metallic stent simultaneously with the antegrade SEMS placement at the stricture site reduces the risk of leakage and bile peritonitis and makes reinterventions through the transhepatic tract possible[11].
The procedure is usually performed in cases of malignant distal bile duct obstruction when standard cannulation has failed or when endoscopic access to the papilla is not possible. The technique was first described by Giovannini et al[6] in 2001.
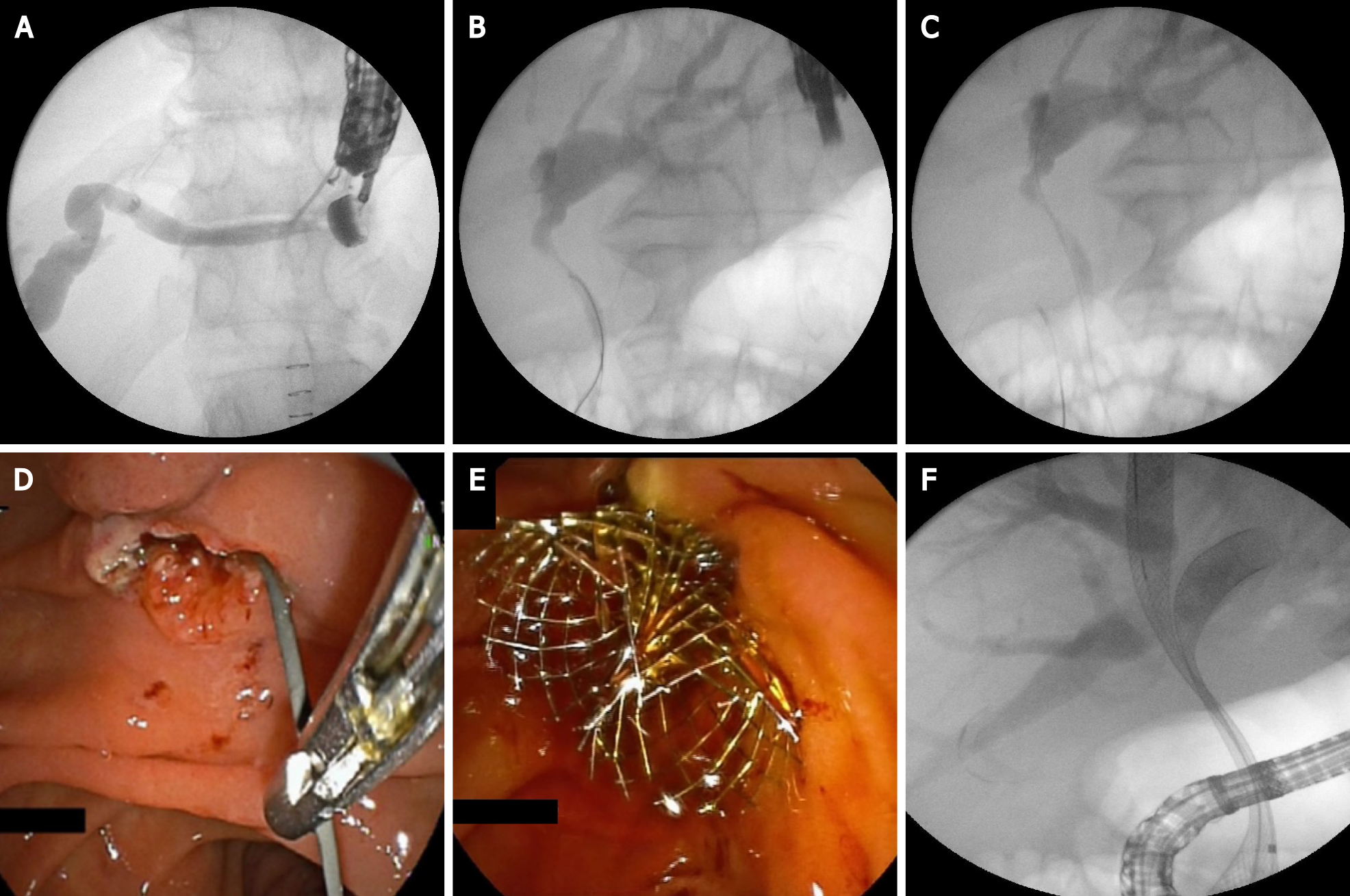
The tip of the echoendoscope is positioned in the duodenal bulb (or in the antrum) where the common bile duct (CBD) is very close to the duodenal or gastric wall. Before puncture, fluoroscopy is used to align the direction of the needle tip towards the liver hilum. The CBD is punctured with a 19-gauge needle. After the bile aspiration guidewire is inserted and manipulated in the direction of the intrahepatic bile ducts, the needle is exchanged over the wire with a 6 French cystotome, biliary catheter or a small (4 mm) dilation balloon to dilate the tract. Most commonly a fully covered SEMS is placed (Figure 6). Using plastic stent or a recently developed lumen-apposing metal stent (LAMS) is also possible[9,12].
EUS-RV was first reported in 2004. The technique is considered when the papilla of Vater is endoscopically accessible but selective bile duct cannulation with ERCP has failed[13].
The procedure consists of intra- or extrahepatic bile duct puncture under EUS guidance with a 19-gauge needle. Contrast is injected through the needle and after obtaining a cholangiogram, a guidewire is inserted and manipulated to negotiate the stricture and to pass across the papilla in the duodenum in an antegrade manner. To maintain a stable position, several loops of the guidewire in the duodenum should be made. Then, the linear echoendoscope is exchanged by duodenoscope. Retrograde cannulation is performed alongside the guidewire or over the guidewire by grasping it with a rath tooth forceps or a snare and pulling it in the duodenoscope working channel. The procedure seems to be the safest of all EUS-guided bile duct approaches. The most common reasons for failure is the inability to manipulate the guidewire across the stricture and the papilla or to reach the bile duct orifice endoscopically (Figure 7). The need for the exchange of two endoscopes and the fact that the procedure is not feasible in cases of altered anatomy are limiting factors for this intervention[12,14].
A large amount of data that has been collected demonstrates the fast improvement in the technical and clinical success of EUS-BD[15-18]. A recently published systematic review, including 42 studies with 1192 patients, reports about a 94.7% technical success and 91.7% clinical success with a 23.3% adverse even rate. These data indicate that EUS-BD is an acceptable alternative in cases when ERCP has failed or is not possible. The morbidity is high but most of the reported AE are mild, self-limited and respond to conservative therapy. The most commonly reported AE are bleeding (4%), bile leakage (4%), pneumoperitoneum (3%), stent migration (2.7%), cholangitis (2.4%), peritonitis (1.3%), abdominal pain (1.5%)[19].
The important point here is that these results are reported from high-volume centers and the procedures were performed by highly experienced endoscopists. “Real-world” data could be much worse and the AE rate-unacceptably high. A national survey in Spain, including 106 patients who have EUS-BD performed, reports 67.2% technical success and a 63.2% clinical success. Improving the safety and reducing the complexity of EUS- BD are the main issues regarding this procedure[20].
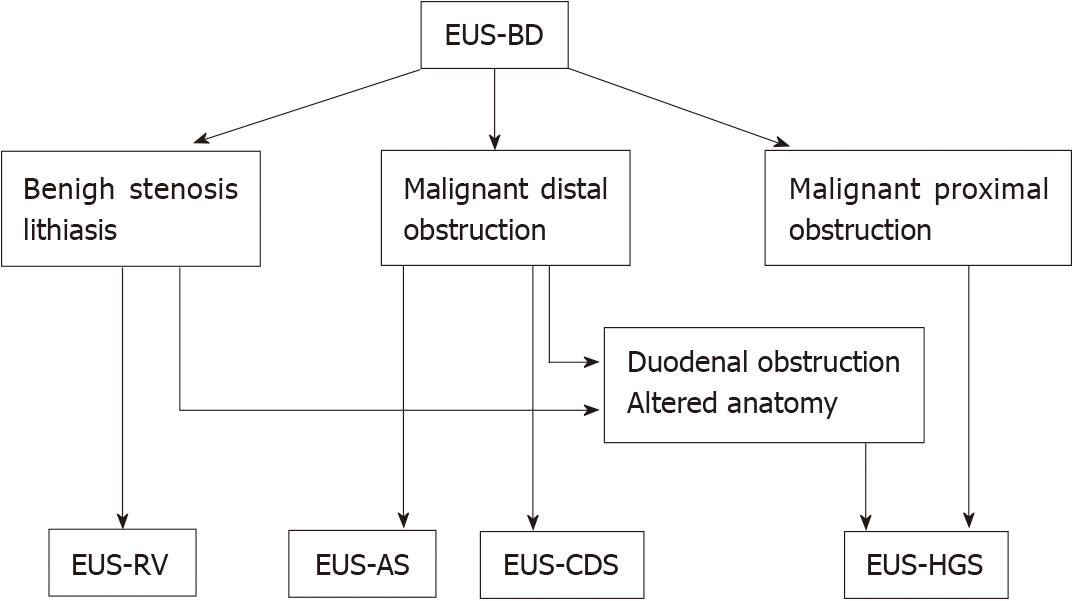
Algorithms for the EUS-BD approach, based on the nature of obstruction and anatomy of the patient were developed. The patients with a dilated intrahepatic bile duct on cross-sectional imaging should be approached intrahepatically and antegrade stenting should be attempted. When antegrade stenting fails or is not possible, HGS is a suitable option. When the intrahepatic approach fails, conversion to an extrahepatic approach is advisable. In cases without intrahepatic bile duct dilatation, the extrahepatic approach is the method of choice. After transbulbar or transantral puncture of CBD, rendezvous technique is advised. In case of failure, CDS should be performed[21].
According to the published data, there is no significant difference between the EUS-BD techniques in terms of technical, clinical success and AE. Khashab et al[22] compared the outcomes of HGS and CDS in a multicenter comparative trial. The technical and clinical success was similar in both groups[22].
EUS-BD is still used mostly when ERCP is not successful or not feasible. A retrospective multicenter analysis comparing ERCP with EUS-BD, however, indicated that both techniques have similar efficacy[23]. The growing expertise and the advances in specially dedicated equipment have led to better clinical results with success rates over 90% and comparable AE rates[24,25].
Many clinical situations (altered anatomy, periampullary tumors, presence of duodenal stent covering the ampulla) suggest difficult biliary cannulation. Extended procedural time and numerous cannulation attempts are related to increased AE, consisting mainly in post-ERCP pancreatitis. On the other hand, tumor ingrowth/ overgrowth is the major reason indicating the need for re-intervention. Both disadvantages could be overcome by resorting to a EUS-BD procedure[26,27].
Several prospective randomized trials and meta-analyses, published over the last 2 years, have compared the two techniques as a first-choice option for biliary drainage (Table 1).
| Ref. | Type of evidence | Patients, n (%) | Technical success, EUS-BD–ERCP, n (%) | Clinical success, EUS-BD-ERCP, n (%) | AE, EUS-BD-ERCP, n (%) |
| Dhir et al[23], 2015 | Multicenter retrospective analysis | 208 | 94.23-93.26 (98/104-97/104) | N/A | 8.65-8.65 (N/A) |
| Kawakubo et al[27], 2016 | Retrospective study | 82 | N/A | 96.2-98.2 (25/26-55/56) | 26.9-35.7 (7/26-20/56) |
| Park et al[29], 2018 | Prospective randomized controlled study | 30 | 92.9-100.0 (13/14-14/14) | 92.9-100.0 (13/14-14/14) | 0.0-0.0 (0/14-0/14) |
| Paik et al[30], 2018 | Multicenter randomized trial | 125 | 93.8-90.2 (60/64-55/61) | 84.4-85.2 (54/64-52/61) | 10.9-39.3 (7/64-24/61) |
| Bang et al[28], 2018 | Prospective randomized trial | 125 | 90.9-94.1 (30/33-32/34) | 97.0-100.0(32/33-34 /34) | 21.2-14.7 (7/33-5/34) |
| Logiudice et al[34], 2019 | Meta-analysis | 222 | 91.96-91.81 (N/A) | 84.81-85.53 (N/A) | N/A (4/79–25/76) |
In a single-center randomized trial Bang et al[28] compared EUS-CDS (n = 33) and ERCP (n = 34) as primary treatment for malignant distal biliary obstruction. There was no significant difference in the rates of technical success (90.9% vs 94.1%), clinical success and rate of reinterventions. AE rate was reported in 21.2% in the first and 14.7% in the second group (P = 0.49). The authors highlight the potency of EUS to ensure diagnostics (FNA, FNB), and palliative therapy (biliary drainage, celiac plexus neurolysis) in a single endoscopic session. Additionally in this study, the CDS performance did not affect the surgical technique in the operable cases[28].
In another prospective randomized controlled study Park et al[29] compared the EUS-BD and ERCP as a primary treatment modality for malignant extrahepatic bile duct obstruction. The authors (n = 30) suggest that EUS-BD has equivalent efficacy to ERCP. No severe AE were observed in both groups. In the ERCP group, four cases were reported with tumor ingrowth, and in the EUS group, two cases were reported with food impaction and another two with stent migration. In cases of stent migration in the EUS-BD group reintervention was not needed because the iatrogenic choledocho-duodenal fistula, created during the procedure provided sufficient bile drainage[29].
In a multicenter randomized trial including 125 patients, Paik et al[30] aim to compare EUS-BD (either CDS or HGS) with ERCP-BD for palliative drainage of distal malignant stenosis. The study confirms the similar efficacy and safety of the two techniques. EUS-BD was found to have lower AEs, including post-procedural pancreatitis, also lower re-intervention rate[30].
A meta-analysis (10 studies and 756 patients) from 2019[24] comparing EUS-BD with ERCP as a primary treatment modality of malignant distal bile duct obstruction reports equivalent clinical and technical success in both groups (over 90%), with similar rates of AE (15.5% for EUS-BD and 18.6% for ERCP). The EUS drainage demonstrated longer stent patency and lower rates of reinterventions, but without statistical significance. The most common AE in the EUS-BD group was bile peritonitis, while in the ERCP group, pancreatitis[24].
Another systematic review and meta-analysis by Jin et al[26] published in the same year announce similar results in terms of technical and clinical success, AE, reinterventions, procedure duration, stent patency and overall survival for both techniques. EUS-BD was associated with lower rates of stent dysfunction and tumor in/ overgrowth[26].
A meta-analysis comparing EUS-BD with ERCP-drainage for primary management of malignant biliary obstruction regardless of stricture site from 2020 by Kakked et al[31] demonstrated identical technical and clinical success and AE rates. Patients after ERCP required significantly more re-interventions[31].
A meta-analysis, published in 2019[32] and involving 222 patients, reports comparable procedure time, technical and clinical success and complication rate. In conclusion, the authors report a significantly lower rate of stent dysfunction in the EUS-BD group and distinguish EUS as a reasonable option of the first choice for patients with malignant obstruction[32].
A final meta-analysis, published by Lou et al[33] includes 428 patients, (EUS-BD n = 215, ERCP n = 213). No significant difference was reported concerning procedure duration, technical and clinical success. EUS-BD, however, was associated with a lower rate of re-intervention and fewer procedure-related AE regarding pancreatitis and cholangitis[33].
In summary, given the comparable results in terms of AE and treatment outcomes, EUS is likely to become a feasible alternative to ERCP for primary biliary decompression.
Over the last decade, enough data have been collected to allow comparative analyses between EUS-BD and percutaneous biliary drainage (PTBD). Several advantages of EUS-BD over PTBD have been proved over time: It could provide drainage of intra- and extrahepatic ducts, according to the obstruction level; it is less invasive and eliminates the need for an external catheter. The latter spare the possibility for catheter-related complications like bleeding, infection, dislocation and bile leak.
The first meta-analysis comparing EUS-BD and PTBD in terms of efficacy and safety is published by Sharaiha et al[34] in 2017. Nine studies with 483 patients were included. No difference in technical success and length of hospital stay was found, but EUS-BD was found to have better clinical success, fewer post-procedure AE, lower rate of re-interventions and was more cost-effective[35].
In conclusion, published data suggest that EUS-BD is better compared with PTBD, reducing the risk of AE, hospital stay, the need for re-interventions and offers a better quality of life for the patients[36]. In cases of ERCP failure, whenever an experienced endoscopy team is available EUS-BD should be performed instead of PTBD.
At the moment, EUS-BD is primarily used as a rescue procedure following a failed ERCP. According to the published data, EUS-BD demonstrates some clinical advantages over ERCP but further randomized studies will determine the real place of EUS as therapy in cases of malignant biliary obstruction. We suggest a simple scheme summarizing the current role of EUS in endoscopic biliary drainage therapy (Figure 8).
There are many questions in consideration before the adoption of EUS as a standard first-line therapeutic option. Despite the promising results, published in the literature, these procedures remain difficult and are not routine outside a few expert centers. The reasons are lack of training, lack of procedure standardization, and few available dedicated devices. Although the similar rate of AE for both procedures, according to some authors, EUS complications are more severe and difficult to be managed. Most of the published data comes from experienced endoscopists in high volume expert centers and it remains unclear if these results can be achieved in smaller centers[36]. On the other hand, EUS-BD is rarely indicated and expertise acquisition is difficult.
The low case volume limits the training opportunities and the existing training models are not able to simulate all the difficulties encountered when performing these procedures. Developing training models is a key step to understand, learn and perform more safely EUS-BD. Dhir et al[37] created and evaluated a hybrid model consisting of pig esophagus and stomach and synthetic duodenum and biliary system and concluded that it replicates real situations encountered during EUS-RV and EUS-BD and training and mentoring using this model improves the chances of success performing these procedures[37].
Taking into consideration the above-mentioned limitations, important steps were made to improve safety, reduce complexity, and standardize these procedures. The creation of the dedicated devices, training models, and guidelines presume a promising future of EUS-BD.
The development of dedicated devices is an important step toward making EUS-BD easier, reducing procedure time, and improving safety. The introduction of cautery-enhanced LAMS and their implementation for EUS-CDS is a step forward to make the procedure less complex and to reduce the number of AE. Significant progress has been made by the development of dedicated stents for EUS-HGS (Giobor-TaeWoong; Proximally covered SEMS-Hanarostent). This has led to a substantial reduction of severe AE like cholangitis, stent migration and bile peritonitis. Cautery-enhanced HGS- stents and “one step delivery” stents without the need for tract dilation are on the way and hopefully will make EUS-HGS a more popular, easy and safe intervention. There is a real perspective of full replacement of PTBD and surgery in malignant bile duct disease and ERCP failure cases. Gaining experience and widely spread expertise for the technique could lead to further expansion of indications and new treatment opportunities.
In an attempt to standardize EUS-BD the Asian EUS group published the first guideline on the optimal management in interventional EUS procedures. Fifteen statements address the indications, technical aspects, pre-and post-procedural management, management of complications, competency and training of EUS-BD[38].
EUS-BD is a new, promising mini-invasive biliary drainage modality, offering many advantages over traditional interventional methods and surgery. The accepted indications are ERCP failure, duodenal obstruction or biliary diseases in patients with surgically altered anatomy. EUS-BD includes several techniques which could be adapted to the unique patient anatomy and condition such as EUS-guided rendezvous technique, antegrade stenting or transmural drainage. A large amount of data suggests that EUS-BD should be preferred over PTBD if required expertise is available.
Provenance and peer review: Invited manuscript; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 154505; European Society of Gastrointestinal Endoscopy, No. 45909537.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pizzicannella M, Salerno R S-Editor: Fan JR L-Editor: Filipodia CL P-Editor: Fan JR
| 1. | Peng C, Nietert PJ, Cotton PB, Lackland DT, Romagnuolo J. Predicting native papilla biliary cannulation success using a multinational Endoscopic Retrograde Cholangiopancreatography (ERCP) Quality Network. BMC Gastroenterol. 2013;13:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 771] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 3. | Saad WE, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, Neu B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol. 2015;49:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Giovannini M, Bories E. EUS-Guided Biliary Drainage. Gastroenterol Res Pract. 2012;2012:348719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Park DH. Endoscopic ultrasound-guided biliary drainage of hilar biliary obstruction. J Hepatobiliary Pancreat Sci. 2015;22:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Minaga K, Takenaka M, Ogura T, Tamura T, Kuroda T, Kaku T, Uenoyama Y, Noguchi C, Nishikiori H, Imai H, Sagami R, Fujimori N, Higuchi K, Kudo M, Chiba Y, Kitano M. Endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction with surgically altered anatomy: a multicenter prospective registration study. Therap Adv Gastroenterol. 2020;13:1756284820930964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 9. | Oh D, Park DH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Mukai S, Itoi T. EUS-guided antegrade procedures. Endosc Ultrasound. 2019;8:S7-S13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | El Chafic AH, Shah JN. Advances in Biliary Access. Curr Gastroenterol Rep. 2020;22:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sarkaria S, Lee HS, Gaidhane M, Kahaleh M. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver. 2013;7:129-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Paik WH, Park DH. Endoscopic Ultrasound-Guided Biliary Access, with Focus on Technique and Practical Tips. Clin Endosc. 2017;50:104-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008;14:6078-6082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Chen YI, Callichurn K, Chatterjee A, Desilets E, Fergal D, Forbes N, Gan I, Kenshil S, Khashab MA, Kunda R, Lam E, May G, Mohamed R, Mosko J, Paquin SC, Sahai A, Sandha G, Teshima C, Barkun A, Barkun J, Bessissow A, Candido K, Martel M, Miller C, Waschke K, Zogopoulos G, Wong C; ELEMENT trial and for the Canadian Endoscopic Research Collaborative (CERC). ELEMENT TRIAL: study protocol for a randomized controlled trial on endoscopic ultrasound-guided biliary drainage of first intent with a lumen-apposing metal stent vs. endoscopic retrograde cholangio-pancreatography in the management of malignant distal biliary obstruction. Trials. 2019;20:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7:94-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 18. | Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL, Nawras A, Ismail MK. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 19. | Artifon EL, Ferreira FC, Otoch JP, Rasslan S, Itoi T, Perez-Miranda M. EUS-guided biliary drainage: a review article. JOP. 2012;13:7-17. [PubMed] |
| 20. | Baars JE, Kaffes AJ, Saxena P. EUS-guided biliary drainage: A comprehensive review of the literature. Endosc Ultrasound. 2018;7:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Salerno R, Davies SEC, Mezzina N, Ardizzone S. Comprehensive review on EUS-guided biliary drainage. World J Gastrointest Endosc. 2019;11:354-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Khashab MA, Messallam AA, Penas I, Nakai Y, Modayil RJ, De la Serna C, Hara K, El Zein M, Stavropoulos SN, Perez-Miranda M, Kumbhari V, Ngamruengphong S, Dhir VK, Park DH. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175-E181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Dhir V, Itoi T, Khashab MA, Park DH, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Han SY, Kim SO, So H, Shin E, Kim DU, Park DH. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci Rep. 2019;9:16551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 25. | Nunes N, Flor de Lima M, Caldeira A, Leite S, Marques S, Moreira T, Moutinho-Ribeiro P, Bispo M. GRUPUGE PERSPECTIVE: Endoscopic Ultrasound-Guided Biliary Drainage. GE Port J Gastroenterol. 2021;28:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Jin Z, Wei Y, Lin H, Yang J, Jin H, Shen S, Zhang X. Endoscopic ultrasound-guided versus endoscopic retrograde cholangiopancreatography-guided biliary drainage for primary treatment of distal malignant biliary obstruction: A systematic review and meta-analysis. Dig Endosc. 2020;32:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Kawakubo K, Kawakami H, Kuwatani M, Kubota Y, Kawahata S, Kubo K, Sakamoto N. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy. 2016;48:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc. 2018;88:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 29. | Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Paik WH, Lee TH, Park DH, Choi JH, Kim SO, Jang S, Kim DU, Shim JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018;113:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (1)] |
| 31. | Kakked G, Salameh H, Cheesman AR, Kumta NA, Nagula S, DiMaio CJ. Primary EUS-guided biliary drainage versus ERCP drainage for the management of malignant biliary obstruction: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:298-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Logiudice FP, Bernardo WM, Galetti F, Sagae VM, Matsubayashi CO, Madruga Neto AC, Brunaldi VO, de Moura DTH, Franzini T, Cheng S, Matuguma SE, de Moura EGH. Endoscopic ultrasound-guided vs endoscopic retrograde cholangiopancreatography biliary drainage for obstructed distal malignant biliary strictures: A systematic review and meta-analysis. World J Gastrointest Endosc. 2019;11:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Lou X, Yu D, Li J, Feng S, Sun JJ. Efficacy of endoscopic ultrasound-guided and endoscopic retrograde cholangiopancreatography-guided biliary drainage for malignant biliary obstruction: a systematic review and meta-analysis. Minerva Med. 2019;110:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904-914. [RCA] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (2)] |
| 35. | Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI, Kalloo AN. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Hathorn KE, Bazarbashi AN, Sack JS, McCarty TR, Wang TJ, Chan WW, Thompson CC, Ryou M. EUS-guided biliary drainage is equivalent to ERCP for primary treatment of malignant distal biliary obstruction: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E1432-E1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 37. | Dhir V, Itoi T, Pausawasdi N, Khashab MA, Perez-Miranda M, Sun S, Park DH, Iwashita T, Teoh AYB, Maydeo AP, Ho KY. Evaluation of a novel, hybrid model (Mumbai EUS II) for stepwise teaching and training in EUS-guided biliary drainage and rendezvous procedures. Endosc Int Open. 2017;5:E1087-E1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Teoh AYB, Dhir V, Kida M, Yasuda I, Jin ZD, Seo DW, Almadi M, Ang TL, Hara K, Hilmi I, Itoi T, Lakhtakia S, Matsuda K, Pausawasdi N, Puri R, Tang RS, Wang HP, Yang AM, Hawes R, Varadarajulu S, Yasuda K, Ho LKY. Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |