Published online Oct 16, 2021. doi: 10.4253/wjge.v13.i10.502
Peer-review started: April 28, 2021
First decision: June 17, 2021
Revised: July 8, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 16, 2021
Processing time: 169 Days and 0.2 Hours
Colon capsule endoscopy (CCE), which became clinically applicable in 2006, is a simple and noninvasive procedure to evaluate colonic diseases; the accuracy of second-generation CCE, introduced in 2009, has dramatically improved. Currently, CCE is used as an alternative method for colorectal cancer screening, as well as for evaluating the mucosal lesions of inflammatory bowel disease, in cases where performing colonoscopy (CS) is difficult. However, the outcomes of CCE are uncertain.
To investigate the outcomes of Japanese patients with negative findings (no polyps or colorectal cancer) on initial CCE.
This retrospective, single-center study was conducted at the Endoscopic Center at Aishinkai Nakae Hospital. This study included patients who underwent continuous CCE between November 2013 and August 2019, that exhibited no evidence of polyps or colorectal cancer at the initial CCE, and could be followed up using either the fecal immunochemical test (FIT), CS, or CCE. The observational period, follow-up method, presence or absence of polyps and colorectal cancer, pathological diagnosis, and number of colorectal cancer deaths were evaluated.
Thirty-one patients (mean age, 60.4 ± 15.6 years; range, 28–84 years; 14 men and 17 women) were enrolled in this study. The reasons for performing the first CCE were screening in 12, a positive FIT in six, lower abdominal pain in nine, diarrhea in two, and anemia in two patients. The mean total water volume at the time of examination was 3460 ± 602 mL (2250–4800 mL), and a total CS was performed in 28 patients (90%). The degree of cleanliness was excellent in 15 patients and good in 16, and no poor cases were observed. No adverse events, such as retention or capsule aspiration, were observed in any of the patients. The mean follow-up period was 3.1 ± 1.5 years (range, 0.3–5.5 years). Follow-up included FIT in nine, CS in 20, and CCE in four patients (including duplicate patients). The FIT was positive in two patients, while CS revealed five polyp lesions (three in the ascending colon, one in the transverse colon, and one in the descending colon), with sizes ranging between 2 mm and 8 mm. Histopathological findings revealed a hyperplastic polyp in one patient, and adenoma with low grade dysplasia in four patients; colorectal cancers were not recognized. In the follow-up example by CCE, polyps and colorectal cancer could not be recognized. During the follow-up period, there were no deaths due to colorectal cancer in any of the patients.
We determined the outcomes in patients with negative initial CCE findings.
Core Tip: Colon capsule endoscopy is becoming popular as a screening test for colorectal cancer in patients where colonoscopy is difficult. Its accuracy is comparable to that of colonoscopy; however, the outcomes are unknown. This study evaluated the follow-up methods, presence or absence of polyps and colorectal cancer, and cancer deaths after follow-up in Japanese patients with negative capsule endoscopy findings.
- Citation: Nakaji K, Kumamoto M, Yodozawa M, Okahara K, Suzumura S, Nakae Y. Follow-up outcomes in patients with negative initial colon capsule endoscopy findings . World J Gastrointest Endosc 2021; 13(10): 502-509
- URL: https://www.wjgnet.com/1948-5190/full/v13/i10/502.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i10.502
The number of patients with colorectal cancer has been increasing in Japan[1], compared with the United States. It is the primary cause of cancer death in women, and third most common cause in men[1]. In Japan, fecal occult blood testing using the two-day method is performed for colorectal cancer screening in patients aged 40 years or older, while colonoscopy (CS) is performed in patients with at least one positive fecal immunochemical test (FIT)[1]. Still, although CS is the gold standard for colorectal cancer screening, the frequency of CS following a positive FIT is approximately 60%[1]. This may be due to fear of perforation and hemorrhage caused by the invasive nature of CS. Colon capsule endoscopy (CCE) is noninvasive and convenient; additionally, during the coronavirus disease 2019 (COVID-19) pandemic, CCE has drawn attention as a home-based test that does not pose a risk of severe acute respiratory syndrome coronavirus 2 infection[2]. Second-generation CCE has dramatically improved accuracy by incorporating a wide field of view and adaptive frame rate (adjusting 4–35 images/s to accommodate the capsule movement)[3], and is now regarded a noninvasive method for colorectal cancer screening in patients where CS is difficult[4]. Since 2020 in Japan, the indications have been expanded to include patients with the physical burdens associated with CS, such as hypertension, diabetes, and chronic obstructive pulmonary disease; the number of examinations is therefore expected to increase in the future.
Conversely, there are concerns regarding CCE overlooking colorectal polyps and cancers during long-term follow-up that CS would otherwise have been detected in patients who present negative initial CCE results; intermediate cancers and cancer deaths may have been caused as a result. To the best of our knowledge, there are no reports regarding the long-term follow-up of patients screened for colorectal cancer with initial negative initial CCE results; therefore, we evaluated the efficacy of initial CCE results through the follow-up of patients without polyps or colorectal cancer.
This retrospective, single-center study included consecutive patients who underwent CCE at the outpatient unit of Aishikai Nakae Hospital for colorectal cancer screening between November 2013 and August 2019 due to difficulty performing CS (either the colonoscope could not be inserted into the cecum, or CS was expected to be challenging to perform due to postoperative adhesions). Of these patients, those without findings on initial CCE (defined as those without polyps of any size and/or cancerous lesions) were followed up. Inclusion criteria for the study were patients who underwent follow-up with either FIT, CS, or CCE; patients were excluded if they had inflammatory bowel disease or were previously found to have a polyp or colorectal cancer. Exclusion criteria for performing CCE included dysphagia, pacemaker placement, and possible pregnancy. This study was conducted under the Declaration of Helsinki and was approved by the ethics committee of Aishinkai Nakae Hospital on February 12, 2021 (No. 015). Informed consent was obtained in the form of opt-out on the bulletin board in the hospital. Those who were withdrew were excluded from the study.
Follow-up from initial CCE was defined as patients reexamined over 3-month intervals after the first CCE, either by the FIT, CS, or CCE. The FIT was performed on two separate days; one positive test was considered positive, and two negative tests were considered negative.
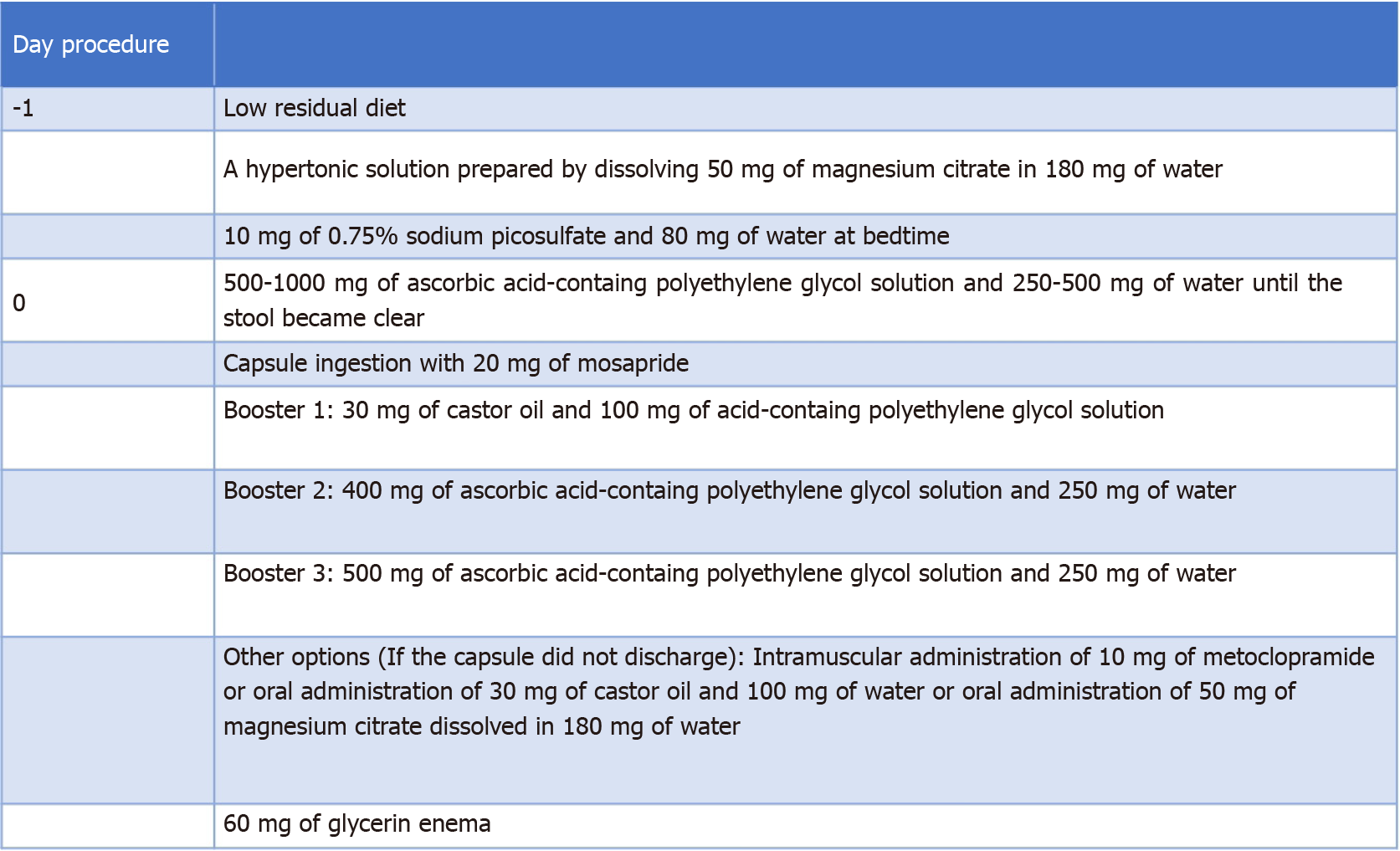
PillCamCOLON2 (Medtronic, Minneapolis, United States) was used for all patients. Pretreatment began the day before the examination. The patients ingested a low-residue diet test meal at home for breakfast, lunch, and dinner, and at 19:00, they drank a hypertonic solution by dissolving 50 g of magnesium citrate (Magcolol P; Horii Pharmaceutical Co., Ltd., Osaka) in 180 mL of water. Before bedtime, they had 10 mg of 0.75% sodium picosulfate with 100 mL of water. On the day of the examination, the patients fasted during the morning, after which they drank 1000 mL of ascorbic acid-containing hypertonic polyethylene glycol solution (Asc-PEG; Mobiprep; EA Pharma, Tokyo) and 500 mL of water. The patients’ stool frequency and properties were checked, and stool was required for a clear liquid state. Thereafter, the sensor array was fitted, and the capsule was swallowed after taking 20 mg of mosapride with 100 mL of water. Metoclopramide (10 mg) was injected intramuscularly when the small intestine did not reach 60 min after capsule swallowing. An additional 10 mg of metoclopramide was administered if the capsule did not reach the small intestine after 120 min). Once in the small intestine, 30 mL of aromatic castor oil and 100 mL of Asc-PEG were added. After reaching the large intestine, patients ingested 400 mL of Asc-PEG and 250 mL of water over 30 min. Subsequently, 500 mL of Asc-PEG and 250 mL of water were taken (over 30 min) to expel the capsule. After the capsules reached the small intestine, exercises-such as walking and stair ascending and descending exercises-were encouraged. If capsules were not expelled by 5 p.m. of the same day, the following options were considered: (1) An intramuscular injection of 10 mg metoclopramide; (2) Oral administration of 30 mg castor oil and 100 mg water; (3) Oral administration of 50 g of magnesium citrate dissolved in 180 mg of water, or (4) Administration of 60 mg of glycerin enema if there was no discharge of the colon capsule (Figure 1).
After completing the study, the data recorder was downloaded to a workstation equipped with dedicated interpretation software (RAPID software v8.0 or v8.3). The following parameters were examined: laxative dose, intestinal transit time (time from the capsule reaching the duodenum to the end of the ileum), colonic transit time (time from capsule reaching the cecum to exit the anus), total colic observation rate (when the capsule emptying through the anus or dentate line can be confirmed), and intestinal lavage rate. Intestinal cleanliness was graded on a 4-point Leighton-Rex scale[5] by five segments of the large intestine, defined as "excellent" (only a tiny amount of stool), "good" (small amounts of stool or cloudy fluid, but not sufficient to interfere with interpretation), "fair" (cloudy fluid if it completely precluded reliable examination), and "poor" (a large amount of stool). The cleanliness of the entire colon was evaluated as appropriate by adopting the lowest rating for each segment. The findings were read by a Japanese Society for Capsule Endoscopy certified support technician and one or more experienced physicians.
Adverse events were defined as the retention of capsules (stay in the intestine with the inability to confirm anal emptying of the capsule for at least 14 d) and consequent intestinal obstruction, Mallory-Weiss syndrome, intestinal perforation, vomiting due to oral laxatives, and aspiration pneumonia. In this study, we investigated the following data in patients: (1) Observation period; (2) Follow-up method; (3) Presence or absence of polyps and colorectal cancer; (4) Final pathologic diagnosis; (5) Presence or absence of adverse events, and (6) Cancer-related deaths.
All continuous variables are presented as means and standard deviations. Statistical analysis was performed using IBM SPSS Statistics for Windows (SPSS Inc., Chicago, IL, United States).
During the study, 208 patients underwent CCE for colorectal cancer screening; 82 patients were found to be negative for polyps and/or cancerous lesions after the first CS capsule. Of these, 31 patients were followed up via either FIT, CS, or CCE; the remaining 51 patients were not followed-up via either FIT, CS, or CCE since their initial CCE. The characteristics of patients with negative CCE results are shown in Table 1. The mean age of the cohort was 60.4 years, and 45.2% (n = 14) were male. The most common reason for performing CCE was screening results (n = 12; patients aged over 40 years, with no symptoms). No adverse events, such as retention or capsule aspiration, were observed.
| Total number of patients | n = 31 | |
| Gender (n) | ||
| Female | 17 | |
| Male | 14 | |
| Age (yr, range) | 60.4 ± 15.6 (28 - 84) | |
| Reasons (n) | ||
| Screening | 12 | |
| Fecal immunochemical test positive (n) | 6 | |
| Lower abdominal pain (n) | 9 | |
| Diarrhea (n) | 2 | |
| Anemia (n) | 2 | |
| Indication (n) | ||
| Incomplete colonoscopy (n) | 0 | |
| Anticipated difficulty of total colonoscopy (n) | 31 | |
| CCE completion | 28 (90) | |
| Cleanliness (n) | Excellent, good, fair, poor | 15, 16, 0, 0 |
| Total water content | 3460 ± 602 mL (2250-4800 mL) | |
| Adverse events (n) | 0 | |
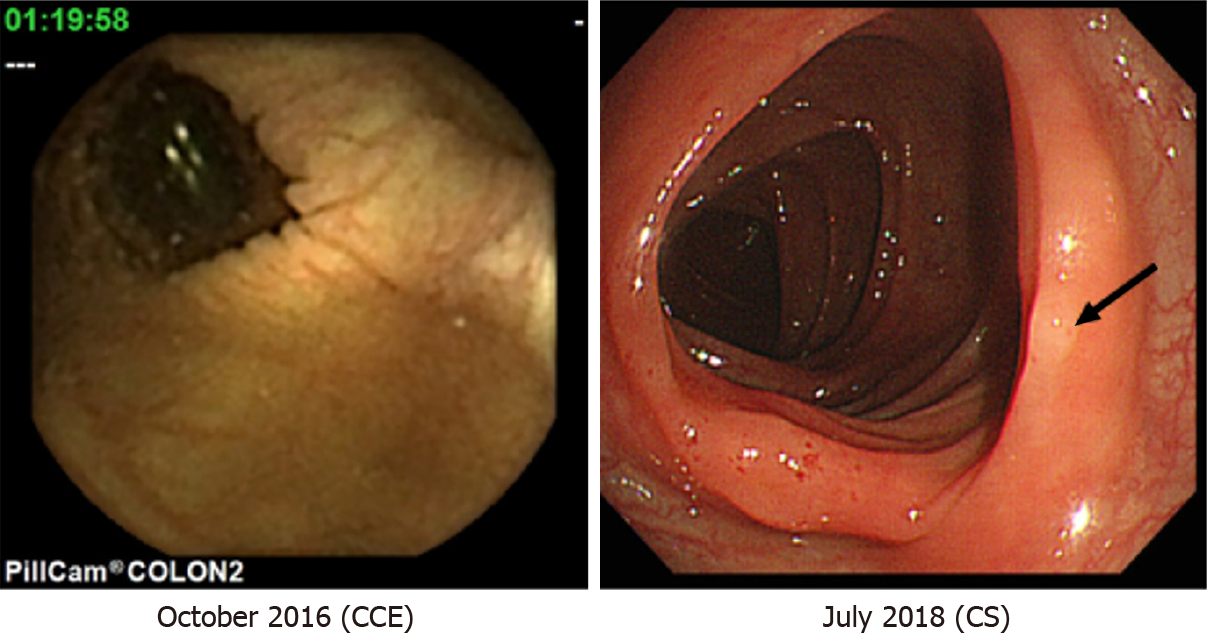
The characteristics of colonic polyps found during the follow-up period of patients with negative CCE results are shown in Table 2; the mean follow-up period was 3.1 years. CS was the most common method of follow-up after initial CCE (n = 20). Five colonic polyps (three in the ascending colon, one in the transverse colon, and one in the descending colon) were identified through follow-up CS; based on the Narrow-band imaging International Colorectal Endoscopic classification[6], these were classified as type 1 and 2 polyps. Histopathological findings included a hyperplastic polyp in one patient, and adenoma with low grade dysplasia in four patients, while in cases followed-up by CCE, colonic polyps and colorectal cancer could not be identified. Excluding symptomatic patients, screening was followed by CS in seven, FIT in three, and CCE in two patients for an average of 2.8 years; no polyps or colorectal cancers were found through either method. During the follow-up period, no deaths due to colorectal cancer occurred in any of the patients. Representative images of follow-up on CS are presented in comparison with the initial CCE findings (Figure 2).
| Number | Size (mm) | Shape | Histology | Intervals (years) | |
| Cecum | 0 | - | - | - | - |
| Ascending colon | 3 | 4, 4, 2 | Semipedunculated type | Tubular adenoma with low grade dysplasia | 5, 5, 1.8 |
| Transverse colon | 1 | 8 | Semipedunculated type | Tubular adenoma with low grade dysplasia | 2.4 |
| Descending colon | 1 | 3 | Semipedunculated type | Hyperplastic polyp | 1.8 |
| Sigmoid colon | 0 | - | - | - | - |
| Rectum | 0 | - | - | - | - |
To the best of our knowledge, this is the first follow-up study of negative initial CCE findings in Japanese patients. Colorectal cancer was not observed in any of the cases, while only small polyps were detected during the follow-up period. The widespread use of screening tests for colorectal cancer screening with FIT is expected to increase the frequency of CSs in the future; however, the number of skilled physicians performing CS is limited. Additionally, as the COVID-19 pandemic continues in the future, conventional endoscopic education becomes difficult[7]; the number of skillful physicians performing CS may not be expected to increase accordingly[7]. To compensate for this situation, noninvasive and straightforward CCE screening for colorectal cancer has been and should continue to be examined. However, the diagnostic reading of CCE is challenging. It usually requires a reading of 50000–60000 frames, may have only one or a few frames of essential findings, and is always at risk of overlooking an interpreter's findings[8]; thus, initial reviews by other clinical staff (for example, endoscopic nurses) are required[9]. Additionally, while interpretive assistance using artificial intelligence has been studied[10], it is not yet a widely established method in routine clinical practice at the research stage. Follow-up of CCE is therefore necessary-including examination of interval cancers-without overlooking significant polyp findings observed during the initial CCE that would have been detected by CS.
In the guidelines for colorectal cancer screening[11], sigmoidoscopy, multitargeted stool DNA testing (FIT-DNA), computed tomography colonography (CTC), and CCE are recommended for patients aged 50–75 years when FIT or CS is not desirable. At these intervals for follow-up, FIT is recommended annually, CS every 10 years, FIT-DNA every 3 years, sigmoidoscopy every 5 years, CTC every 5 years, and CCE every 5 years. In our review of CCE, no advanced neoplasia was found at approximately 5-year intervals; colorectal screening with CCE every 5 years was therefore considered appropriate for Japanese patients in this study.
In a review of other modalities with negative imaging, Heisser et al[12] reported in a meta-analysis of CS studies that when stratified according to negative CS results from 1–5 years, 5–10 years, or more than 10 years, the detection of polyps was 20.7%, 23.0%, and 21.9%, respectively; advanced neoplasia, including cancer, was observed in 2.8%, 3.2%, and 7.0% of cases, respectively. In a retrospective study of negative CTC results from a single institution, Pickhardt et al[13] reported that 12.1% of the patients had polyps 6 mm or larger in diameter, while 0.1% had advanced neoplasia-including cancer-in 10 years of follow-up. Although direct comparison is difficult due to differences regarding the number of patients, the definition of negative findings, and the duration of observation compared with this study, the 5-year follow-up results of their study demonstrated that 12.9% of all polyp lesions, 3.2% of polyps 6 mm or more, 0% of advanced neoplasia including cancer, and the other negative results were better than the other modalities.
In this study, CS was the most common method used for follow-up after the first CCE, followed by FIT and CCE. The widespread use of CS in Japan and the high cost of CCE may have contributed to this observation. At present, there is a report regarding improvement of the capsule discharge rate using castor oil as a booster[14]. Our study demonstrated that polyp lesions found after the first CCE were more frequent in the ascending colon. Evaluation of negative CS and CTC results indicated that many cases of polyps were found in the right-sided colon during the follow-up period. Although the cause is unknown, it is believed that in our case, the lesions were often overlooked as the capsule had passed quickly in the ascending colon.
This study has several limitations. First, this was a single-center retrospective study with a small number of cases; however, as a single-center study, follow-up of the same patient was possible. Second, the observational period was considerably short; additional long-term follow-up is necessary in the future. Third, the follow-up method was not standardized; this is a limitation of retrospective studies, and it is of particular concern that all patients who underwent the FIT were negative at follow-up in the present study. Still, there have been reports of colorectal cancer in FIT-negative patients[15]; thus, the possibility of colorectal cancer inclusion in these cases cannot be ruled out. It is necessary to follow up in CS in these cases. Fourth, there is a possibility that lesions could be overlooked during interpretation of the first CCE; however, in this study, we thoroughly reviewed the entire image. Further progress regarding the interpretation of CCE by artificial intelligence will help to provide more accurate interpretations. Finally, because CCE moves back and forth, the possibility of overcounting polyp lesions and flat polyp lesions has not been investigated in this study and should be considered in the future.
In the present study, follow-up of patients with negative initial CCE results revealed no colorectal cancer; only small polyps were found.
Colon capsule endoscopy (CCE) is a noninvasive and easy procedure for detecting colorectal lesions when difficult to perform colonoscopy (CS). The incidence of CCE has been increasing due to its noninvasive nature and low risk of infection during the Covid-19 pandemic; however, its follow-up on efficacy remains unknown.
Currently, guidelines recommend that patients with no significant findings on initial CCE should repeat CCE every five years, or follow up with another screening test. However, there is limited evidence in clinical practice.
The study’s main objective was to investigate the follow-up outcomes in Japanese patients without polyp and colonic cancer at the initial CCE.
Thirty-one consecutive Japanese patients negative for polyp and cancer lesions on initial CCE were analyzed.
We propose that researchers conduct a multicenter, prospective, long-term follow-up of initial CCE screening results.
Our study determined the outcomes of Japanese patients with negative CCE results.
The mean follow-up period was 3.1 years; CS was determined to be the most common method of follow-up after the initial CCE (n = 20). Five colonic polyps (three in the ascending colon, one in the transverse colon, and one in the descending colon) were identified through follow-up CS; based on the Narrow-band imaging International Colorectal Endoscopic classification, these were classified as type 1 and 2 polyps. Histopathological findings included a hyperplastic polyp in one patient, and adenoma with low grade dysplasia in four patients; no deaths due to colorectal cancer, or severe adverse events, were observed in any patient during follow-up.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeruc J, Sulbaran MN S-Editor: Liu M L-Editor: A P-Editor: Liu JH
| 1. | Hotta K, Matsuda T, Kakugawa Y, Ikematsu H, Kobayashi N, Kushima R, Hozawa A, Nakajima T, Sakamoto T, Mori M, Fujii T, Saito Y. Regional colorectal cancer screening program using colonoscopy on an island: a prospective Nii-jima study. Jpn J Clin Oncol. 2017;47:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | MacLeod C, Wilson P, Watson AJM. Colon capsule endoscopy: an innovative method for detecting colorectal pathology during the COVID-19 pandemic? Colorectal Dis. 2020;22:621-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Hosoe N, Limpias Kamiya KJL, Hayashi Y, Sujino T, Ogata H, Kanai T. Current status of colon capsule endoscopy. Dig Endosc. 2021;33:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Hosoe N, Hayashi Y, Ogata H. Colon Capsule Endoscopy for Inflammatory Bowel Disease. Clin Endosc. 2020;53:550-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy. 2011;43:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Puig I, Kaltenbach T. Optical Diagnosis for Colorectal Polyps: A Useful Technique Now or in the Future? Gut Liver 2018; 12: 385-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Segal JP, Smith PJ, Verma AM. The impact of COVID-19 on endoscopy training needs to be considered in the context of a global pandemic. Gastrointest Endosc. 2020;92:1146-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Yang YJ. The Future of Capsule Endoscopy: The Role of Artificial Intelligence and Other Technical Advancements. Clin Endosc. 2020;53:387-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Shiotani A, Honda K, Kawakami M, Murao T, Matsumoto H, Tarumi K, Kusunoki H, Hata J, Haruma K. Evaluation of RAPID(®) 5 Access software for examination of capsule endoscopies and reading of the capsule by an endoscopy nurse. J Gastroenterol. 2011;46:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yamada A, Niikura R, Otani K, Aoki T, Koike K. Automatic detection of colorectal neoplasia in wireless colon capsule endoscopic images using a deep convolutional neural network. Endoscopy. 2021;53:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 458] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 12. | Heisser T, Peng L, Weigl K, Hoffmeister M, Brenner H. Outcomes at follow-up of negative colonoscopy in average risk population: systematic review and meta-analysis. BMJ. 2019;367:l6109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Pickhardt PJ, Pooler BD, Mbah I, Weiss JM, Kim DH. Colorectal Findings at Repeat CT Colonography Screening after Initial CT Colonography Screening Negative for Polyps Larger than 5 mm. Radiology. 2017;282:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Ohmiya N, Hotta N, Mitsufuji S, Nakamura M, Omori T, Maeda K, Okuda K, Yatsuya H, Tajiri H. Multicenter feasibility study of bowel preparation with castor oil for colon capsule endoscopy. Dig Endosc. 2019;31:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Hasegawa R, Yashima K, Ikebuchi Y, Sasaki S, Yoshida A, Kawaguchi K, Isomoto H. Characteristics of Advanced Colorectal Cancer Detected by Fecal Immunochemical Test Screening in Participants with a Negative Result the Previous Year. Yonago Acta Med. 2020;63:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |