Published online Sep 16, 2020. doi: 10.4253/wjge.v12.i9.285
Peer-review started: April 9, 2020
First decision: May 26, 2020
Revised: June 3, 2020
Accepted: July 26, 2020
Article in press: July 26, 2020
Published online: September 16, 2020
Processing time: 153 Days and 12.3 Hours
Given most patients with distal malignant biliary obstruction present in the non-resectable stage, palliative endoscopic biliary drainage with fully covered metal stent (FCMS) or uncovered metal stent (UCMS) is the only available measure to improve patients’ quality of life. Half covered metal stent (HCMS) has been recently introduced commercially. The adverse effects and stent function between FCMS and UCMS have been extensively discussed.
To study the duration of stent patency of HCMS and compare it with FCMS and UCMS to optimize biliary drainage in inoperable patients with distal malignant obstruction. Secondary aims in our study included evaluation of patients’ survival and the rates of adverse events for each type of stent.
We studied 210 patients and randomized them into three equal groups; HCMS, FCMS and UCMS were inserted endoscopically.
Stent occlusion occurred in (18.6%, 17.1% and 15.7% in HCMS, FCMS and UCMS groups, respectively, P = 0.9). Stent migration occurred only in patients with FCMS (8.6% of patients). Cholangitis and cholecystitis occurred in 11.4% and 5.7% of patients, respectively, in FCMS. Tumor growth occurred only in 10 cases among patients with UCMS after a median of 140 d, sludge occurred in nine, seven and one patients in HCMS, FCMS and UCMS, respectively (P = 0.04).
Given the prolonged stent functioning time, the use of HCMS is preferred over the use of UCMS and FCMS for optimizing biliary drainage in patients with distal malignant biliary obstruction.
Core Tip: Given most patients with distal malignant biliary obstruction present in the non-resectable stage, palliative endoscopic biliary drainage with fully covered or uncovered metal stent is the only available measure to improve patients’ quality of life. Half covered metal stent has been recently introduced commercially. The adverse effects and stent function between fully covered metal stent and uncovered metal stent have been extensively discussed. Given the prolonged stent functioning time, the use of half covered metal stent is preferred to the use of uncovered metal stent and fully covered metal stent for optimizing biliary drainage in patients with distal inoperable malignant biliary obstruction.
- Citation: Elshimi E, Morad W, Elshaarawy O, Attia A. Optimization of biliary drainage in inoperable distal malignant strictures. World J Gastrointest Endosc 2020; 12(9): 285-296
- URL: https://www.wjgnet.com/1948-5190/full/v12/i9/285.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i9.285
Most patients with malignant obstructive jaundice present at the unresectable stage, when the management is restricted only to palliative measures. The common causes of distal malignant biliary strictures are cancer of the head of the pancreas and extra hepatic cholangiocarcinoma. Biliary drainage and decompression by metal stents to improve quality of life[1] is the therapeutic modality of choice by endoscopic or percutaneous routes, the percutaneous route is generally considered after many failed endoscopic trials[2] with or without chemo- or radiotherapy. At this stage, the prognosis is dismal, with 5-year survival rates of < 2%[3,4]. Endoscopic stenting by metal and plastic stents has been used to decompress biliary obstruction with varying success rates. The superiority of metal stents over plastic stents has been demonstrated in many meta-analyses’ reports[5-7]. Metal stents offer better patency and wider caliber than plastic stents and thus there is less need for subsequent endoscopic retrograde cholangio-pancreatography (ERCP) procedure and re-stenting. Moreover, metal stents compared to plastic stents are more cost effective, especially in patients with longer survival, and decrease the number of ERCP procedures in centers with heavy ERCP volume.
Many types of metal stents are commercially available, including covered and uncovered stents. However, it is still questionable which type of stent is more suitable for drainage. The uncovered stents have higher rates of tumor ingrowth with subsequent occlusion and cholangitis (16%-46%)[8-14], while fully covered stents obviate this disadvantage. In this type, however, the deposition of sludge and bacterial biofilm and tumor overgrowth may lead to occlusion and cholangitis. Stent migration as well as cholecystitis and pancreatitis are more likely to occur with the fully covered type[14].
Although several meta-analyses have compared both types of stents, no definitive results have been obtained showing the merits of one type over the other type[15-18]. Recently, another type of metal stent (named half covered metal stent) was introduced to offer the advantages of both types, in which the distal half is covered to obviate tumor ingrowth occurring in uncovered stents and the proximal half is uncovered as a protective character against migration and cholecystitis, which occur as adverse events with covered metal stents[19,20].
This study is a single center three armed prospective randomized study, which was conducted at the National Liver Institute, Egypt, a tertiary referral, government-based, well-equipped center for gastroenterology and liver disease in Egypt, between May 2015 and May 2019.
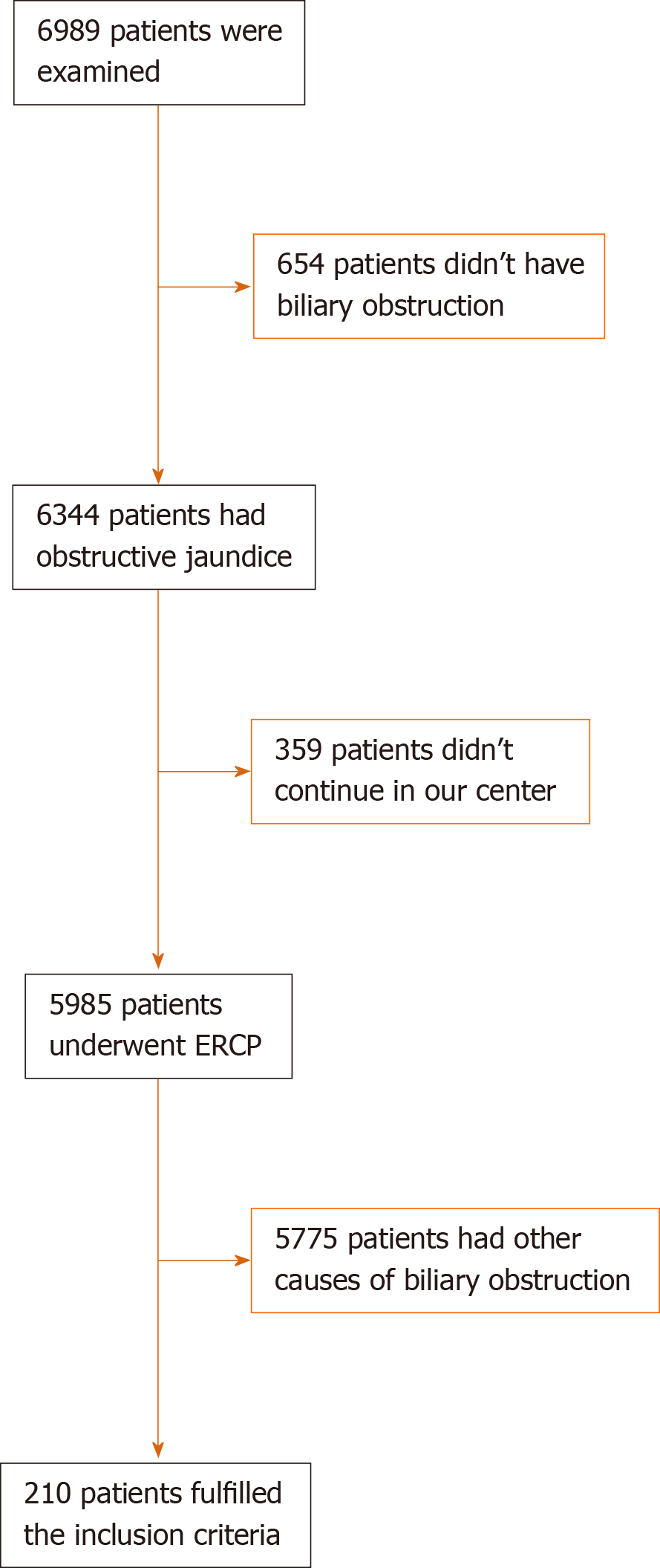
A committee of endoscopists, interventional radiologists and hepatobiliary surgeons examined 6989 patients and their medical records in obstructive jaundice clinics at the same center for evaluation of medical management plan (Figure 1): 6344 had obstructive jaundice, and 5985 patients underwent ERCP for relieving biliary obstruction. In total, 210 patients were included in the current study under the diagnosis of unresectable malignant extra hepatic biliary obstruction. Resectability was based on clinical findings, imaging and laboratory investigations.
Inclusion criteria were as follows: Patient's age ≥ 18 years; elevated serum bilirubin levels ≥ 1 gm/dL and non-resectability or inoperability based on associated comorbid conditions. All patients signed an Arabic form of written informed consent before ERCP.
We excluded patients with undiagnosed or benign strictures; anatomical changes with previous gastric bypass or patients with duodenal or pyloric strictures not allowing the scope to pass to papilla even after dilatation. We excluded patients with cardiopulmonary co-morbidity, not suitable for general sedation for endoscopy. Patients with previous metal stents after occlusion were also excluded.
ERCP and stenting were considered if there was elevated serum bilirubin and dilatation of a proximal portion of the common bile duct and/or dilated common hepatic duct.
Early or procedure-related complications were defined as adverse events that occurred within 1 mo post-procedure. Serious procedure-related complications were reported when interventions or hospitalization were required[21,22].
Clinical post-ERCP pancreatitis was considered if the patients developed elevated serum amylase > 3 fold the upper normal limits on the first-day post-ERCP in presence of associated abdominal pain. The severity of pancreatitis was classified as mild, moderate and severe if the patient was hospitalized ≤ 3 d, 4-10 d and > 10 d, respectively. Severe pancreatitis was also considered if the patient was admitted to the intensive care unit and/or if surgical intervention and radiological or endoscopic drainage were required regardless of hospitalization time.
Cholangitis was defined as persistence of jaundice and fever > 38 °C for > 24 h, leukocytosis > 15.000/dL. Cholecystitis was diagnosed when the patients developed positive Murphy’s sign and distended gall bladder in sonographer examination[22].
All studied patients were followed up clinically at day 7, day 14 and day 30 and then after 6 mo post-ERCP. Successful procedure and stent deployment were considered when there was appropriate fluoroscopic positioning of the stent across the stricture in addition to normalization of serum bilirubin or at least a drop of total bilirubin by 30% from the baseline level by day 7 post-procedure.
Thereafter, patients were followed up monthly until death. Telephone follow-up was performed when patients could not return to the hospital, those with poor clinical condition and more than 6 mo after stent placement. In patients with recurrence of jaundice, reassessment of liver function with abdominal ultrasound was performed. When bile duct and intrahepatic biliary radicle dilation were observed during ultrasound or magnetic resonance cholangio-pancreatography examinations, subsequent ERCP was immediately performed.
Prior to ERCP procedure, studied patients were randomized for allocation to receive one of three stents: Half covered metal stent (HCMS), fully covered metal stent (FCMS) or uncovered metal stent (UCMS). Using 210 sealed, numbered, opaque envelopes by an endoscopist who did not perform the ERCP, one-third contained a card labeled “HCMS”, one-third contained a card labeled “FCMS” and the final one-third contained a designed card labeled “UCMS”. The procedure of randomization was performed in the endoscopy unit by opening the sealed envelopes consecutively.
Subsequently, data were collected prospectively in previously designed form for data analysis and interpretation.
This study was conducted according to the Declaration of Helsinki, and the protocol was approved by the ethics committee (for medical research) of Würzburg University and by the Institutional Review Board of the National Liver Institute, Menoufia University (IRB number IRB00003413) in April 2015. A consent form was signed by every patient.
All stents were inserted endoscopically. After successful cannulation, small papillotomy was performed, and the metal stent was deployed from its delivery system under careful endoscopic and fluoroscopic guidance. Dilatation by balloon or Sohendera dilators was done to determine accurately the desired length and facilitate stent insertion prior to procedure, provided that the distal end of the stent was passed from the papillary orifice. Prophylactic antibiotics were routinely given.
Types of stents: Three types of metal stents were used from Hanarostent (myocardial infarction-tech, Seoul, Korea): (1) HCMS, this stent is made from wires from braided nitinol, and the distal half of stent is coated with a silicone covering membrane, the proximal half is uncovered; (2) FCMS, this stent is similar in structure to HCMS, but it is coated with covering silicone membrane through its entire length; and (3) UCMS, this stent is made from wires from braided nitinol with no covering membrane through its entire length.
Endpoints of the study were stent patency, complications and patient survival.
According to Ung et al[23], stent dysfunction is considered when the baseline level of serum bilirubin is doubled in addition to increase of serum alkaline phosphatase and/or occurrence of cholangitis. ERCP procedure was repeated in case of stent dysfunction if the general condition of the patient was fair. Stent patency was defined as the time from ERCP and stenting until stent dysfunction or death. Patients with functioning stents were censored until the end of the study or at date of their last follow-up or death.
We included 210 patients with inoperable distal malignant biliary obstruction based on the following assumptions: With the power of 80%, α = 0.05, and the ratio of exposed to inoperable distal malignant biliary obstruction to those who with inoperable proximal malignant biliary obstruction = 1:2. The required sample size was determined using Epi info software.
Statistical analysis was performed using SPSS Statistics, version 26 (Armonk, NY, United States). A 2-sided P value of < 0.05 was considered statistically significant. Simple random sampling and blind analysis were performed. Continuous variables were summarized as mean ± standard deviation or median (range) for and number (%) for categorical variables. Categorical variables associations were tested using chi-square test. Continuous variables differences were tested among the three groups by parametric test (one-way analysis of variance test) when data were normally distributed or by non-parametric test (Kruskal Wallis test, post-hoc Tamhane test). The cumulative 6 mo and 12 mo survival probabilities were estimated using Kaplan-Meier survival curve, which was used to estimate median duration of each stent patency type. In each group, the log-rank test was used for comparison between the three stent types
Between May 2015 and May 2019, 210 patients met our inclusion criteria and were randomized for biliary drainage using HCMS (70 patients), UCMS (70 patients) or FCMS (70 patients).
Patient characteristics and demographic are shown in Table 1. In the studied patients, cancer head of the pancreas represented the most cause of biliary obstruction (67.1%, 68.6% and 65.7%) in HCMS, FCMS and UCMS, respectively. Distal cholangiocarcinoma represented 12.9%, 14.3% and 17.2% in HCMS, FCMS and UCMS, respectively. Gall bladder carcinoma represented 7.1%, 5.7% and 7.1% in HCMS, FCMS and UCMS, respectively, and ampullary carcinoma represented 12.9%, 11.4% and 10% in HCMS, FCMS and UCMS, respectively.
| Number | Stent type, n = 70, n (%) | P value | ||
| HCMS | FCMS | UMS | ||
| Gender | 0.86 | |||
| Male | 45 (64.3) | 46 (65.7) | 48 (68.6) | |
| Female | 25 (33.7) | 24 (34.3) | 22 (31.4) | |
| Age, median (range) | 67 (39-84) | 65 (45-82) | 64 (42-83) | 0.37 |
| Cholecystectomy | 16 (22.9) | 17 (24.3) | 17 (24.3) | 0.97 |
| Cause of distal biliary obstruction | 0.99 | |||
| Cancer pancreas | 47 (67.1) | 48 (68.6) | 46 (65.7) | |
| Distal cholangiocarcinoma | 9 (12.9) | 10 (14.3) | 12 (17.2) | |
| Gallbladder cancer | 5 (7.1) | 4 (5.7) | 5 (7.1) | |
| Ampullary cancer | 9 (12.9) | 8 (11.4) | 7 (10.0) | |
| Laboratory investigations, mean ± SD | ||||
| Serum total bilirubin, mg/dL | 15 ± 8.7 | 14.5 ± 5.6 | 16.1 ± 7.7 | 0.43 |
| Serum direct bilirubin, mg/dL | 13 ± 6.4 | 12.9 ± 4.1 | 13.2 ± 6.8 | 0.95 |
| SGPT, UI/L | 112 ± 90 | 110.7 ± 96.5 | 120.2 ± 90.3 | 0.80 |
| SGOT, UI/L | 146 ± 110.2 | 129.3 ± 106.2 | 142.4 ± 112.1 | 0.64 |
| Albumin | 4.1 ± 1.3 | 3.9 ± 1.4 | 3.8 ± 1.6 | 0.46 |
| INR | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.94 |
| Imaging | ||||
| Liver metastasis | 2 (2.9) | 1 (1.4) | 1 (1.4) | 0.78 |
| PVT | 1 (1.4) | 1 (1.4) | 2 (2.9) | 0.78 |
| Ascites | 1 (1.4) | 2 (2.9) | 1 (1.4) | 0.78 |
| Clinical presentation | ||||
| Jaundice | 57 (81.4) | 55 (78.6) | 55 (78.6) | 0.89 |
| Pain | 34 (48.6) | 31 (44.3) | 34 (48.6) | 0.84 |
| Fever | 9 (12.9) | 8 (11.4) | 9 (12.9) | 0.96 |
| Itching | 16 (22.9) | 15 (21.4) | 18 (25.7) | 0.83 |
| Anorexia | 27 (38.6) | 26 (37.1) | 20 (28.6) | 0.41 |
| Weight loss | 44 (62.9) | 42 (60.0) | 45 (64.3) | 0.87 |
| Cholangitis | 12 (17.1) | 10 (14.3) | 13 (18.6) | 0.79 |
| Operative time, time passed from selective cannulation till the end of procedure | 4.5 ± 2.5 min | 4 ± 3 min | 5 ± 3.5 min | 0.15 |
| X-ray dose, time passed from selective cannulation till the end of procedure | 2 ± 1 min | 2 ± 1.5 min | 2 ± 1 min | NA |
| Antibiotic | 0.87 | |||
| No | 44 (62.9) | 42 (60.0) | 45 (64.3) | |
| Yes | 26 (37.1) | 28 (40.0) | 25 (35.7) | |
| Previous chemotherapy | 0.9 | |||
| No | 59 (84.3) | 58 (82.9) | 57 (81.4) | |
| Yes | 11 (15.7) | 12 (17.1) | 13 (18.6) | |
| Radiotherapy | 0.36 | |||
| No | 69 (98.6) | 70 (100) | 68 (97.1) | |
| Yes | 1 (1.4) | 0 (0.0) | 2 (2.9) | |
| Post-stenting chemotherapy | 0.91 | |||
| No | 55 (78.6) | 56 (80.0) | 57 (81.4) | |
| Yes | 15 (21.4) | 14 (20.0) | 13 (19.6) | |
Patients in all groups were matched in regard to age, gender, baseline laboratory investigations and chemotherapy. Successful deployment was achieved in all patients. The operative time (time passed from selective cannulation until the end of procedure) was 4.5 ± 2.5 min, 4 ± 1.5 min and 5 ± 3.5 min in HCMS, FCMS and UCMS, respectively. The X-ray time was 2 ± 1 min, 2 ± 1.5 min and 2 ± 1 min, respectively.
Post-procedure outcomes are shown in Table 2. There was no procedure-related major adverse morbidity or mortality (Figure 1).
| Half and half stents, n (%) | Fully covered stents, n (%) | Un-covered stents, n (%) | P value | |
| Successful deployment | 70/70 (100) | 70/70 (100) | 70 /70 (100) | |
| Procedure related complications | ||||
| Major adverse events and or mortality | 0 | 0 | 0 | 00 |
| pancreatitis | 6 (8.6 ) | 5 (7.1) | 5 (7.1) | 0.93 |
| Minor bleeding | 4 (5.7) | 3 ( 4.3) | 5 (7.1) | 0.77 |
| Post-ERCP complications | ||||
| Occlusion | 13 (18.6) | 12 (17.1) | 11 (15.7) | 0.9 |
| Early stent adverse effect, within 1 mo | 1 (1.4) | 4 (5.7) | 1 (1.4) | 0.21 |
| Sludge formation | 9 (12.9) | 7 (11.1) | 1 (1.4) | 0.04 |
| Tumor Ingrowth | 0 (0.0) | 0 (0.0) | 10 (14.3) | 0.00003 |
| Tumor Overgrowth | 4 (5.7) | 5 (7.1) | 0 (0.0) | 0.09 |
| Cholangitis due to stent occlusion | 7 (10.0) | 8 (11.4) | 7 (10.0) | 0.95 |
| Cholecystitis | 0 (0.0) | 4 (5.7) | 0 (0.0) | 0.02 |
| Migration | 0 (0.0) | 6 (8.6) | 0 (0.0) | 0.002 |
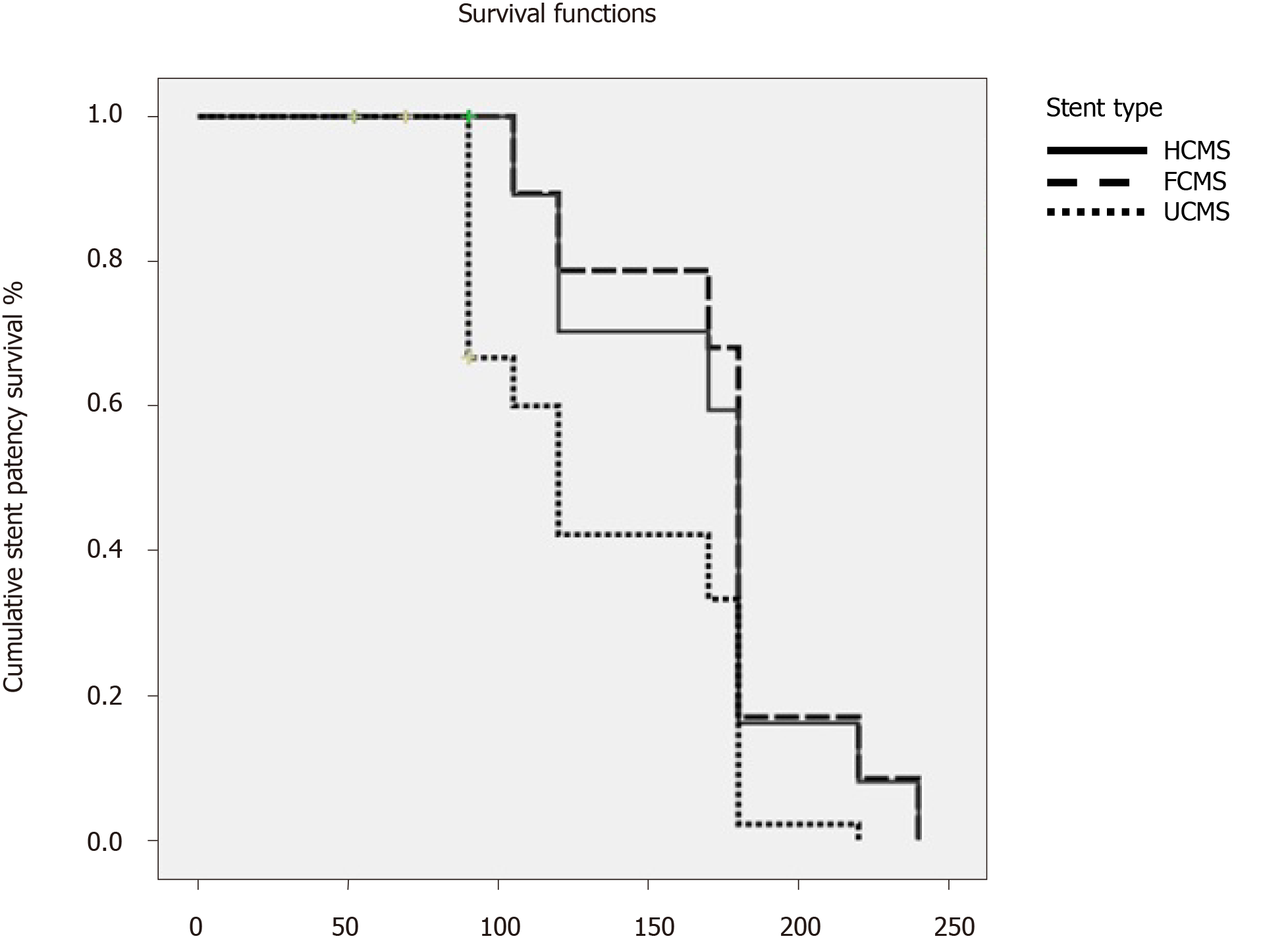
| Stent patency in d, median (95%CI) | 614 (390.6-780.1) | 256.0 (167.5-315.4)1,2 | 536 (323.1-743.9) | 0.02 |
| Follow-up in d, median (range) | 112 (5-613) | 109 (7-621) | 108 (7-541) | 0.82 |
| Overall survival in d, median (95%CI) | 129 (96.8-167.1) | 114.0 (92.7 -165.4) | 119.0 (89.9-160.1) | 0.20 |
During follow-up, stent occlusion occurred in 36 out of 210 patients (18.6%, 17.1% and 15.7% in HCMS, FCMS and UCMS, respectively, P = 0.9) (Table 2). Stent migration occurred only in patients with FCMS (8.6% of patients). Cholecystitis, probably due to mechanical obstruction of cystic duct orifice exerted by the stent, was observed in four patients with FCMS. None of the patients developed cholecystitis in the HCMS and UCMS groups. Cholangitis occurred in 5.7% of patients in the FCMS group.
Regarding procedure-related complications, pancreatitis occurred in 8.6%, 7.1% and 7.1% in HCMS, FCMS and UCM, respectively (P = 0.93); all cases were mild. Minor bleeding occurred in 5.7%, 4.3% and 7.1% in HCMS, FCMS and UCM, respectively (P = 0.77). Stent dysfunction occurred in six patients (stent migration occurred in one patient in the FCMS group, cholangitis and stent obstruction occurred in one patient in each group, cholecystitis occurred in two patients in the FCMS group).
Tumor ingrowth was found in 10 cases among patients with UCMS (six cases associated with cholangitis) after a median of 140 d (range 52-541 d). Tumor overgrowth occurred in four FCMS patients, five patients with HCMS, and none in the UCMS developed tumor overgrowth. Sludge and stent occlusion occurred in nine, seven and one patient in HCMS, CMS and UCMS, respectively, P = 0.04).
Table 2 shows follow-up, survival and stent patency (Table 2). Patients’ follow-up was 112, 109 and 108 d in HCMS, FCMS and UCMS, respectively. The median survival rates were 129 d 95% confidence interval (CI), 114 d 95%CI and 119 d 95%CI for HCMS, FCMS and UCMS, respectively, P = 0.000002).The median rate for stent patency was 614 d at 95%CI, 256 d at 95%CI and 526 d at 95%CI for HCMS, FCMS and UCMS, respectively, (P = 0.02) (Figure 2).
The newly designed HCMS was introduced commercially to get the merits of FCMS in terms of stent survival by preventing tumor ingrowth through the covering membrane. At the same time, the uncovered half of HCMS prevents the high rates stent migration and cholecystitis inherent in the FCMS.
We speculate that the technical characteristics of the anti-migration power offered by the proximal uncovered part of the HCMS might play significant roles in prevention of HCMS stent displacement either intra-ductal or distal to the duodenum. Moreover, the commercially available FCMS was characterized by inefficient radial or axial forces in addition to the inefficient covering portion, which may influence stent function or stent survival. To the best of our knowledge, this is the first randomized study comparing the three commercially available metal stents in palliation of non-resectable or inoperable malignant distal biliary strictures.
In our study, successful stent deployment was achieved in all patients, and there were no significant differences among the three types of stents in term of factors of clinical efficacy and serum bilirubin reduction. The early procedure related adverse events (pancreatitis and bleeding) were not significantly different among all groups. Early secondary re-interventions resulting from early stent dysfunction were reported in (one, four and one patients in HCMS, FCMS and UCMS respectively).
Early stent-related adverse events (within 1 mo) were reported in more patients with HCMS than those with FCMS and UCMS insertion. However, HCMS showed significantly better median patency rates than FCMS and UCMS. The main cause of HCMS and FCMS dysfunction was early occlusion with cholangitis due to sludge or overgrowth, while in UCMS, occlusion occurred due to tumor ingrowth. Stent migration was a more added cause for stent dysfunction. In our studied patients, there was no significant difference regarding procedure-related pancreatitis and bleeding among the studied groups.
Stent patency was higher among the HCMS group, given that the migration rate was less in comparison to the other two groups. Cholecystitis occurred more frequently in patients with a covered stent than the other two groups based on mechanical obstruction caused by covering membrane of the FCMS and/or the chemical injury caused by stent membrane.
Several clinical trials have discussed the advantage of each type of previously available stent (FCMS and UCMS). These studies have shown the superiority of FCMS over UCMS in term of stent patency and function in patients with malignant distal biliary obstruction[6,7,14,23-29]. Another advantage of covered stents over UCMS is the ease of removability, given its lack of adherence to tumorous tissues.
Since 2011, five meta-analysis studies comparing FCMS and UCMS have been published. However, these studies have evaluated heterogeneous cohorts with retrospective and prospective nature. Moreover, percutaneous and endoscopic approaches of deployments were used[15,23,30-32].
In an analysis by Saleem et al[30], the patency of FCMS was significantly longer than that of UCMS. Although the stent obstruction was detected at similar a rate in both groups, patients with FCMS tended to develop stent dysfunction later than patents with UCMS[30,33]. Stent migration was noticed significantly more in another meta-analysis[31] among patients with FCMS; however, patients experienced no difference in stent patency within the first 6 mo post-procedure. In another three meta-analyses[15,31,32], there was no reported difference in term of stent patency, patient survival or adverse events between both groups.
In our patients, we designed the current study to apply a closer look and to shed more light on the newly introduced stent. The HCMS has a covering membrane in its distal portion to avoid tumor ingrowth. At the same time, it has the advantage of UCMS in terms of an absence of covering membrane in its proximal part, which allows bile to flow through its mesh from the cystic duct and hence avoidance of cholecystitis associated with FCMS. At the same time, it devotes its proximal portion to anti-migration maintaining efficient axial and radial forces that in turn prevent stent displacement.
In our patients with HCMS insertion, none developed stent migration and tumor ingrowth, similar to patients with UCMS insertion. At the same time, none of them developed stent migration or cholecystitis like patients with UCMS insertion.
The discrepancies between the findings of our study and the findings of aforementioned meta-analyses studies may be due to the use of the newly introduced stent, the heterogeneity in the study designs, degree and level of stricture, the characteristics of tumors that cause biliary stricture and stent configurations and materials.
Aiming to reduce the confounding bias of our findings in correspondence to stent materials, we used three types of stents from the same manufacture. So, our findings may not be generalizable to other types of metal stents. Given the results of our study, we supposed that HCMS stents are the best choice in this group of patients due to the inherent advantages of both types of its characteristics. However, early occlusions of the HCMS and FCMS were noticed due to tumor overgrowth, and these finding were surprising and controversial, when compared with other studies[15,20,23,30-34]. Conio et al[20] have explained that this overgrowth may be due to a created tissue hyperplasia induced by inflammatory reaction caused by the membrane covering of the stents rather than a true tumor overgrowth. And so, the overgrowth material is principally due to granulation tissue rather than tumorous tissue[20].
In our study and Lee et al[29], the FCMS patency was not significantly better than UCMS. Notably, stent migration occurred more in patients with FCMS. However, with use of HCMS, the migration was similar to UCMS and less than FCMS. Similar findings regarding the absence of stent migration in patients treated with UCMS were found in previously published studies[5,23-30,33]. In our patients and in Conio et al[20], the migration rate for FCMS was significantly higher than in other studies[27,28]. Probably in these trials, the endoscopists used stents with an anti-migration system consisting of a portion of uncovered flares at both ends[24]. However, Yang et al[15] found no clinical usefulness of the anti-migration design[15,34].
Mechanical closure of the orifice of cystic and pancreatic ducts by the covering membrane of FCMS resulted in higher rates of post -RCP cholecystitis and pancreatitis[14,19,20,24,26]. To avoid post-ERCP pancreatitis with FCMS, Conio et al[20] advised not to place long FCMS. The post-ERCP cholecystitis could be avoided with use of HCMS or UCMS[20]. However, Isayama et al[14] found that the post-ERCP cholecystitis occurred only in cases with cystic duct invasion rather than with the use of FCMS. In our study, no significant difference was found among the different groups regarding pancreatitis, although Isayama et al[14] found significantly more cases of mild pancreatitis in FCMS vs UCMS (8.7% vs 1.8%)[14]. The difference in procedure-related pancreatitis may be due to the routine performance of small papillotomy in our patients to facilitate stent deployment. However, post-ERCP pancreatitis is a multifactorial adverse event.
The present study has many strengths, including its prospective and randomization design, comparison among three types of metal stents, the stent material was from the same manufacture, the relatively large number of included patients and the close follow-up period to assess stent patency and patient’s survival.
This study was limited by being a single center study with possible lack of generalizability to other centers. However, this factor is unlikely to compromise the results of our study, based on the relatively large number of included patients and the high volume of ERCP procedure conducted in our center.
The study was also limited by the heterogeneity of included patients regarding the cause of biliary obstruction, the use of pre- and post-procedure anti-cancer therapies in some patients and possible lack of possible generalizability to other types of metal stents from other manufacturers. In addition, quality of life and cost were not assessed.
In conclusion, given the prolonged stent function, in terms of longer patency and decreased rates of migration, cholecystitis and tissue ingrowth, the use of HCMS is preferred over the use of UCMS and FCMS in palliation for distal malignant biliary obstruction. Our results need to be supported by additional studies. Cost and patients’ quality of life should be addressed.
Most patients with malignant obstructive jaundice present at the unresectable stage, when the management is restricted only to palliative measures. The common causes of distal malignant biliary strictures are cancer of the head of the pancreas and extrahepatic cholangiocarcinoma. This is mostly treated with metal stent insertion placement for biliary drainage.
Many types of metal stents are commercially available, including covered and uncovered stents. However, it is still questionable which type of stent is more suitable for drainage, and half covered metal stents have been recently introduced.
To study the adverse effects and functionality of the recently introduced half covered metal stents vs fully covered and uncovered ones.
We studied 210 patients and randomized them into three equal groups to investigate the functionality and performance of the three different types of metal stents which are the newly introduced half covered vs fully and uncovered metal stents.
The half covered metal stents showed no significant difference in the incidence of occlusion while significantly less incidence of migration than uncovered stents. In addition, the half covered stents showed significantly more functioning and patency time.
The use of half covered metal stent is preferred over the use of uncovered metal stent and fully covered metal stent for optimizing biliary drainage in patients with distal inoperable malignant biliary obstruction.
Our findings will appeal to endoscopists and promote the use of half covered metal stents for biliary drainage of malignant obstructive jaundice because of the decrease in adverse events and migration and the increase in functioning time.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jaworek JM S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Martin FA. The merck manual of geriatrics, 1st Ed. William B. Abrams, Robert Berkow, Merck Sharp & Dohme, Rahway, NJ, 1990, 1267 pages. JPEN J Parenter Enteral Nutr. 1992;16:88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Cubiella J, Castells A, Fondevila C, Sans M, Sabater L, Navarro S, Fernández-Cruz L. Prognostic factors in nonresectable pancreatic adenocarcinoma: a rationale to design therapeutic trials. Am J Gastroenterol. 1999;94:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 450] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Mendonça EQ, Bernardo WM, Moura EG, Chaves DM, Kondo A, Pu LZ, Baracat FI. Endoscopic versus surgical treatment of ampullary adenomas: a systematic review and meta-analysis. Clinics (Sao Paulo). 2016;71:28-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82:256-267.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Dumonceau JM, Cremer M, Auroux J, Delhaye M, Devière J. A comparison of Ultraflex Diamond stents and Wallstents for palliation of distal malignant biliary strictures. Am J Gastroenterol. 2000;95:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Ferlitsch A, Oesterreicher C, Dumonceau JM, Deviere J, Leban T, Born P, Rösch T, Suter W, Binek J, Meyenberger C, Müllner M, Schneider B, Schöfl R. Diamond stents for palliation of malignant bile duct obstruction: a prospective multicenter evaluation. Endoscopy. 2001;33:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Ahmad J, Siqueira E, Martin J, Slivka A. Effectiveness of the Ultraflex Diamond stent for the palliation of malignant biliary obstruction. Endoscopy. 2002;34:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Shah RJ, Howell DA, Desilets DJ, Sheth SG, Parsons WG, Okolo P, Lehman GA, Sherman S, Baillie J, Branch MS, Pleskow D, Chuttani R, Bosco JJ. Multicenter randomized trial of the spiral Z-stent compared with the Wallstent for malignant biliary obstruction. Gastrointest Endosc. 2003;57:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 358] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H, Shiratori Y, Kawabe T, Omata M. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 458] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Yang Z, Wu Q, Wang F, Ye X, Qi X, Fan D. A systematic review and meta-analysis of randomized trials and prospective studies comparing covered and bare self-expandable metal stents for the treatment of malignant obstruction in the digestive tract. Int J Med Sci. 2013;10:825-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Moole H, Bechtold ML, Cashman M, Volmar FH, Dhillon S, Forcione D, Taneja D, Puli SR. Covered versus uncovered self-expandable metal stents for malignant biliary strictures: A meta-analysis and systematic review. Indian J Gastroenterol. 2016;35:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Chen MY, Lin JW, Zhu HP, Zhang B, Jiang GY, Yan PJ, Cai XJ. Covered Stents versus Uncovered Stents for Unresectable Malignant Biliary Strictures: A Meta-Analysis. Biomed Res Int. 2016;2016:6408067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Yang MJ, Kim JH, Yoo BM, Hwang JC, Yoo JH, Lee KS, Kang JK, Kim SS, Lim SG, Shin SJ, Cheong JY, Lee KM, Lee KJ, Cho SW. Partially covered versus uncovered self-expandable nitinol stents with anti-migration properties for the palliation of malignant distal biliary obstruction: A randomized controlled trial. Scand J Gastroenterol. 2015;50:1490-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Tringali A, Hassan C, Rota M, Rossi M, Mutignani M, Aabakken L. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: a systematic review and meta-analysis. Endoscopy. 2018;50:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Conio M, Mangiavillano B, Caruso A, Filiberti RA, Baron TH, De Luca L, Signorelli S, Crespi M, Marini M, Ravelli P, Conigliaro R, De Ceglie A. Covered versus uncovered self-expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: a randomized multicenter study. Gastrointest Endosc. 2018;88:283-291.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1835] [Article Influence: 122.3] [Reference Citation Analysis (1)] |
| 22. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2035] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 23. | Ung KA, Stotzer PO, Nilsson A, Gustavsson ML, Johnsson E. Covered and uncovered self-expandable metallic Hanarostents are equally efficacious in the drainage of extrahepatic malignant strictures. Results of a double-blind randomized study. Scand J Gastroenterol. 2013;48:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Kitano M, Yamashita Y, Tanaka K, Konishi H, Yazumi S, Nakai Y, Nishiyama O, Uehara H, Mitoro A, Sanuki T, Takaoka M, Koshitani T, Arisaka Y, Shiba M, Hoki N, Sato H, Sasaki Y, Sato M, Hasegawa K, Kawabata H, Okabe Y, Mukai H. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013;108:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Yoon WJ, Lee JK, Lee KH, Lee WJ, Ryu JK, Kim YT, Yoon YB. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Park DH, Kim MH, Choi JS, Lee SS, Seo DW, Kim JH, Han J, Kim JC, Choi EK, Lee SK. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: a cohort comparative analysis. Clin Gastroenterol Hepatol. 2006;4:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Telford JJ, Carr-Locke DL, Baron TH, Poneros JM, Bounds BC, Kelsey PB, Schapiro RH, Huang CS, Lichtenstein DR, Jacobson BC, Saltzman JR, Thompson CC, Forcione DG, Gostout CJ, Brugge WR. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Kullman E, Frozanpor F, Söderlund C, Linder S, Sandström P, Lindhoff-Larsson A, Toth E, Lindell G, Jonas E, Freedman J, Ljungman M, Rudberg C, Ohlin B, Zacharias R, Leijonmarck CE, Teder K, Ringman A, Persson G, Gözen M, Eriksson O. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Lee JH, Krishna SG, Singh A, Ladha HS, Slack RS, Ramireddy S, Raju GS, Davila M, Ross WA. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest Endosc. 2013;78:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321-327.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Li J, Li T, Sun P, Yu Q, Wang K, Chang W, Song Z, Zheng Q. Covered versus Uncovered Self-Expandable Metal Stents for Managing Malignant Distal Biliary Obstruction: A Meta-Analysis. PLoS One. 2016;11:e0149066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:27-37.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 33. | Nakai Y, Isayama H, Wang HP, Rerknimitr R, Khor C, Yasuda I, Kogure H, Moon JH, Lau J, Lakhtakia S, Ratanachu-Ek T, Seo DW, Lee DK, Makmun D, Dy F, Liao WC, Draganov PV, Almadi M, Irisawa A, Katanuma A, Kitano M, Ryozawa S, Fujisawa T, Wallace MB, Itoi T, Devereaux B. International consensus statements for endoscopic management of distal biliary stricture. J Gastroenterol Hepatol. 2020;35:967-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 34. | Fernandez Y Viesca M, Arvanitakis M. Early Diagnosis And Management Of Malignant Distal Biliary Obstruction: A Review On Current Recommendations And Guidelines. Clin Exp Gastroenterol. 2019;12:415-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |