Published online Sep 16, 2020. doi: 10.4253/wjge.v12.i9.256
Peer-review started: May 21, 2020
First decision: June 13, 2020
Revised: July 29, 2020
Accepted: August 31, 2020
Article in press: August 31, 2020
Published online: September 16, 2020
Processing time: 111 Days and 22.9 Hours
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in coronavirus disease 2019 (COVID-19) which has affected more than 4.5 million people in 213 countries, and has been declared a pandemic by World Health Organization on March 11, 2020. The transmission of SARS-CoV-2 has been reported to occur primarily through direct contact or droplets. There have also been reports that SARS-CoV-2 can be detected in biopsy and stool specimens, and it has been postulated that there is potential for fecal–oral transmission as well. Gastrointestinal symptoms have been reported in 17.6% of COVID-19 patients and transmission can potentially occur through gastrointestinal secretions in this group of patients. Furthermore, transmission can also occur in asymptomatic carriers or patients with viral shedding during the incubation period. Endoscopic procedures hence may pose significant risks of transmission (even for those not directly involving confirmed COVID-19 cases) as endoscopists and endoscopy staff are in close contact with patients during these aerosol generating procedures. This could result in inadvertent transmission of infection at time of endoscopy.
Core Tip: Coronavirus pandemic has united the world in taking enhanced measures in the endoscopy center to limit the spread of disease. However, considerable variation exists in the society recommendations that have come out of United States, Canada, Europe, United Kingdom, Australia, Asia and Japan. We summarize these recommendations, provide an overview, and describe our practical application of endoscopy in this challenging times.
- Citation: Teng M, Tang SY, Koh CJ. Endoscopy during COVID-19 pandemic: An overview of infection control measures and practical application. World J Gastrointest Endosc 2020; 12(9): 256-265
- URL: https://www.wjgnet.com/1948-5190/full/v12/i9/256.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i9.256
Numerous organizations and societies worldwide have come up with guidelines, recommendations or position statements to optimize the practice of endoscopy during the coronavirus disease 2019 (COVID-19) pandemic[1-9]. In addition, various centers have described their experiences regarding endoscopy in COVID-19[10,11]. The guidelines by leading organizations in the United States, Canada, Europe, United Kingdom, Australia, Asia and Japan are summarized in Table 1. We aim to compare practices regarding endoscopy during COVID-19 between countries and share our experience in devising and implementing infection prevention and control measures to mitigate risk of transmission during endoscopy.
| United States Joint GI Society | United States (AGA) | United States (ASGE) | Canada (CAG) | Europe (ESGE/ESGENA) | United Kingdom (BSG/JAG) | Australia (GESA) | Asia (APSDE) | Japan (JGES) | |
| Pre-endoscopy | |||||||||
| Procedure review and stratification | Urgent: Perform, non-urgent which may need to be performed, non-urgent: Postpone | Time-sensitive (within 24 h-8 wk), not time-sensitive; - defer procedure on case-by-case basis | Urgent/emergent: Perform, elective: Postpone | Essential: Perform, not essential: Postpone | Emergent, elective: Postpone; Evaluate risk of GI disease-related vs COVID-19 related morbidity and mortality | Emergent/essential (continue), needs discussion (case-by-case basis), defer until further notice | Urgent/emergent: Perform, semi-elective: Review, elective: Postpone | Urgent: Perform, semi-urgent: Case-by-case basis, elective: Postpone | |
| Procedures to proceed | Upper GI bleeding; Lower GI bleeding (if SARS-CoV-2 PCR negative) | Upper/lower GI bleeding; Dysphagia causing decreased intake; Time-sensitive diagnosis e.g. evaluation/treatment of cancer/pre; Cancerous conditions;IBD if endoscopy may change management; GI obstruction requiring palliation;Cholangitis | GI bleeding which is life-threatening; GI obstruction (e.g. esophageal obstruction due to food bolus / foreign body); Cholangitis | Upper/lower GI bleeding with haemodynamic instability; Foreign body in esophagus or high-risk foreign body in stomach; Obstructive jaundice; Cholangitis | Upper GI bleeding likely to require therapy; Lower GI bleeding which failed radiological intervention; Foreign body; GI obstruction requiring stenting; Cholangitis, infected peri-pancreatic collection; Nutrition support: Urgent NJT/PEG | Upper GI bleeding, clinically significant;Lower GI bleeding not due to haemorrhoids; Evaluation/treatment of cancer; New diagnosis / flare of IBD in which endoscopy may change management; GI obstruction; Cholangitis, infected/symptomatic peri-pancreatic collection;Nutrition: Urgent NGT/NJT/PEG | GI bleeding; Foreign body; GI obstruction requiring stenting; Management of leakage/perforations; Biliary sepsis; Nutrition: Urgent GI access for feeding | ||
| Procedures to consider (case-by-case) | Evaluation of suspected cancer; Evaluation of significant symptoms | Conditions in which delay in diagnosis can have implications on treatment (e.g. cancer, IBD); Treatment of pre-cancerous lesions e.g. high-grade dysplasia in Barrett’s, EMR of large colon polyp | Mild dysphagia; Iron deficiency anaemia | High priority; Upper GI bleeding without instability; Severe anaemia; Dysphagia /dyspepsia with alarm symptoms;Evaluation of suspected cancer e.g. imaging evidence of mass; Treatment of early cancer/pre-cancerous lesions; Pancreatobiliary stent replacement; Low priority; Iron deficiency anaemia; Pancreatic cyst (depends on risk features) | Variceal surveillance in high risk cases (e.g. recent acute bleeding);Evaluation of malignant conditions; EUS for staging/planning of treatment of cancer; Treatment of high-risk lesions e.g. EMR/ESD | Dysphagia; Iron deficiency anaemia (except female < 50 yr) where no other likely cause on clinical exam; Marked weight loss; Evaluation of suspected cancer e.g. abnormal imaging; Treatment of pre-cancerous lesions e.g. resection of large colonic polyp; Pancreatobiliary stent replacement/ removal | High suspicion of cancer; Treatment of cancer/pre-cancerous lesions with EMR/ESD; ERCP for hepatobiliary cancers | ||
| Procedures to defer | Screening / surveillance colonoscopy | Screening / surveillance OGD or colonoscopy in asymptomatic patients (including variceal surveillance); Evaluation of non-urgent symptoms or disease states (e.g. intermediate risk pancreatic cysts) | Screening / surveillance OGD or colonoscopy | Screening / surveillance; Evaluation of dyspepsia, reflux or IBS-like symptoms with no alarm symptoms | Screening / surveillance; Assessment of disease in IBD; Low-risk follow-up scopes (e.g. esophagitis or gastric ulcer healing); EUS for biliary dilatation, possible stones, pancreatic cyst (not high risk) | Screening / surveillance; Non-specific symptoms; Evaluation of GERD, probable IBS; EUS for pancreatic cyst (low risk)/chronic pancreatitis; Asymptomatic gallstones | Screening / surveillance; Diagnostic; Therapeutic for benign disease | ||
| Postpone non-urgent procedure | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Patient pre-screening | Screen for fever, respiratory symptoms and high risk exposure | Screen for symptoms (fever, cough, shortness of breath, diarrhea) and contact history | Screen for symptoms (flu-like symptoms), contact and travel history | Screen for fever, contact history, travel history, occupational exposure | Screen for fever, symptoms (respiratory tract infection symptoms, shortness of breath, diarrhea, dysosmia/dysgeusia, fatigue), contact and travel history | ||||
| Patient assessment | Check patient's temperature on arrival | Check patient's temperature on arrival | Check patient's temperature on arrival | ||||||
| Patient precautions | Ensure patients maintain an appropriate distance (at least 6 ft) from each other | Patients should use face masks and maintain a distance (at least 1-2 m) | Ensure patients maintain an appropriate distance (2 m) from each other | Ensure patients maintain an appropriate distance from each other | |||||
| Endoscopy staff screening | Daily assessment of symptoms/signs and risk factors; Isolation and testing if symptomatic | Daily assessment of symptoms/signs and risk factors | |||||||
| Waiting area policy | Avoid bringing patients (or escorts) ≥ 65 or with 1 of the CDC recognised risks | No caregiver/relatives allowed except in special situations | No caregiver/relatives allowed except in special situations | ||||||
| During Endoscopy | |||||||||
| Type of PPE | Mask (type not specified), eye shield/goggles, face shield, gown, gloves | N95 mask (or PAPR), double gloves | N95 mask | High risk (include all upper GI procedures): N95 mask or equivalent, double gloves; Low risk: Surgical mask, gloves; Common: Goggles/face shield, gown, hairnet | Confirmed COVID-19 or high risk cases: N95 mask or equivalent, double gloves; Low risk: Surgical mask, gloves; Common: Goggles/face shield, water-proof gown, shoe covers, hairnet | Confirmed COVID-19 or high-risk (upper GI procedures): FFP3 mask, full visor, long-sleeved gown; Low risk: Surgical mask, glasses/visor, disposable apron; Common: Gloves, shoe covers, hairnet | Confirmed/suspected COVID-19 or high risk cases: N95 mask (or FFP2/3); Low risk: Surgical mask; Common: Goggles/face shield, long-sleeved waterproof gown, gloves | Confirmed/suspected COVID-19 cases: N95 mask; Low risk: N95 or surgical mask; Common: Goggles/face shield, water-resistant gown, gloves | Face mask, goggle/face shield, long-sleeved gown, gloves, cap |
| Members of endoscopy team | Only essential staff should be present in procedures | Minimise number of staff in room during endotracheal intubation (anaesthesia team only); avoid switch in staff during procedures | Only essential staff should be present in procedures | Restrict number of staff in procedures | Confirmed/at high risk of COVID-19 cases: Restrict number of staff in procedures; Low risk: Standard number of staff | 1 experienced endoscopist + 2 nurses only | |||
| Endoscopy training | Review appropriateness of trainee involvement in procedures | Modify training - encourage use of e-learning | Limit trainee involvement | Confirmed/at high risk of COVID-19 cases: No trainees; Low risk: Trainees can be involved | |||||
| Location | Confirmed/suspected COVID-19 cases: Do procedure in negative pressure rooms | Confirmed/suspected COVID-19 cases: Do procedure in negative pressure rooms | Confirmed/high-risk of COVID-19 cases: Do procedure in negative pressure rooms | Confirmed/high risk of COVID-19 cases: Do procedure in negative pressure rooms | Confirmed/suspected COVID-19 cases: Do procedure in negative pressure rooms | ||||
| Post-Endoscopy | |||||||||
| Follow-up | Consider phone follow-up at 7 and 14 d to ask about new diagnosis or development of symptoms of COVID-19 | Consider contacting patients at 7 and 14 d to ask about new diagnosis or development of symptoms of COVID-19 |
All organizations agree with the broad principle that elective cases should be individually assessed and reviewed, and elective non-urgent cases should be deferred. Depending on risk assessment, cases deemed to be of higher priority like those with suspected time-sensitive diagnosis e.g. malignancy should still proceed with endoscopic evaluation as delay may result in deleterious effect on patient outcomes. It is not a straightforward dichotomy, and the rationale underlying this approach is the need to balance medical urgency of procedure (as delay in procedure may have consequent delay in diagnosis and appropriate treatment, possibly leading to complications of disease or disease progression) with the risk of infection and utilization of potentially scarce resources. However, the definition of an elective case which should proceed differs considerably between various organizations and societies – it reflects that different areas have different incidences of COVID-19 and hence varying capacities for the performance of semi-urgent endoscopy. Our practice is that emergent cases are performed whereas outpatient elective cases are reviewed on a case-by-case basis and may be rescheduled. Direct-access endoscopy is suspended during this period.
Examples of emergent procedures which should be done: Upper or lower Gastrointestinal (GI) bleeding (BSG further recommends for upper GI bleeding to risk stratify to only for patients predicted to require endoscopic therapy, and for lower GI bleeding to limit to patients in whom interventional radiology is not possible or unsuccessful). Foreign body removal; Pancreatobiliary: Cholangitis; GI obstruction requiring palliation; Examples of elective procedures which should be deferred: Screening and surveillance oesophagogastroduodenoscopy (OGD) or colonoscopy in asymptomatic patients. Evaluation of non-urgent symptoms. Therapeutic endoscopy in benign disease.
Of note, BSG recommends that if procedures are deferred for urgent referrals, the cases should be listed on a separate urgent deferred waiting list to ensure appropriate follow-up and to prioritize endoscopy when normal activities resume.
Our experience is also that there are many patients who do not fit clearly into either emergent or elective categories, for example, patients with symptomatic iron-deficiency anemia who are not actively bleeding, but for whom delay until normal endoscopic services resume might be life threatening or have prognostic implications due to undiagnosed peptic ulcer disease or GI malignancy. After clinical review, numerous such patients would still be considered to proceed with endoscopy. Despite utilizing this approach, we have reduced the total endoscopy case load to less than 40% of the usual load in our center.
At our center, prior to endoscopy, patients are pre-screened for symptoms (fever, upper respiratory tract symptoms) and significant contact and travel history.
On arrival at the endoscopy center, patients are screened again for symptoms, and patients’ temperatures are checked. After passing through screening and temperature taking, patients will enter the waiting area. All patients should be wearing at least a surgical mask and should maintain adequate physical distance of 1-2 m from others. Asian Pacific Society for Digestive Endoscopy and ESGE suggest that no caregivers or relatives should be allowed to enter the endoscopy center.
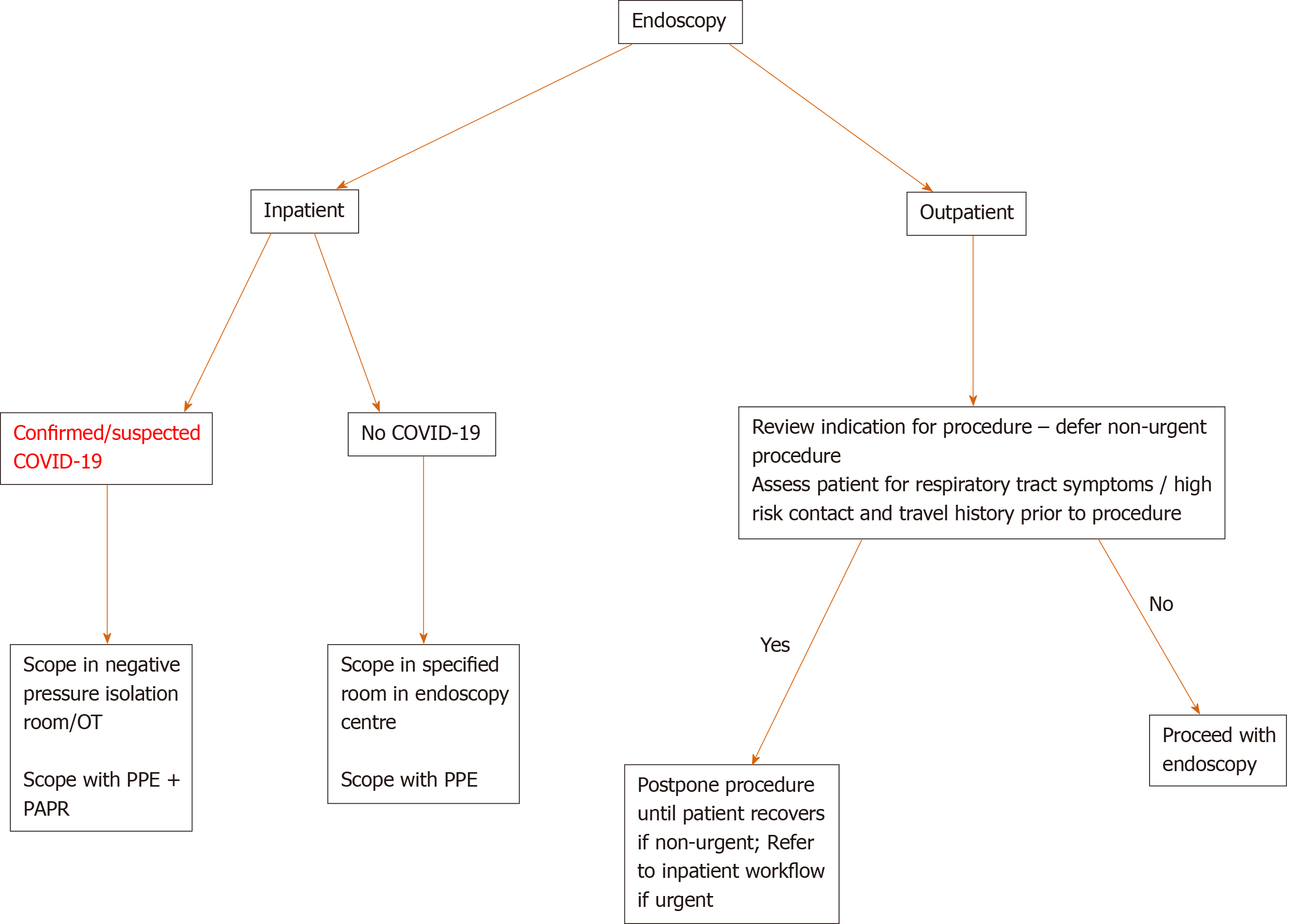
We have devised a simple algorithm to aid decision making regarding timing of procedures (Figure 1).
All organizations concur that for all procedures, all members of the endoscopy team should wear appropriate personal protection equipment (PPE) – usually consisting of N95 or surgical mask, eye shield/goggles, face shield, water-resistant gown and gloves. AGA and ASGE advise the use of N95 mask for all procedures. In contrast, ESGE, CAG and GESA limit the use of N95 mask for high-risk procedures only. AGA also specifies that 2 sets of gloves (rather than 1 set) should be used in all procedures, whereas CAG and ESGE suggest that 2 sets of gloves be used in high-risk procedures only. This minimizes contamination by reducing risk of transferring viral organisms from PPE to clothes or the rest of the body during removal of PPE. These differences probably reflect variable availability and practical rationing to conserve limited PPE resources amidst competing needs. Endoscopy staff should be trained in donning and removing PPE, and hand hygiene practices must be observed strictly.
In our center, we have enhanced our universal precautions, to N95 mask, eye goggles, water resistant gown and gloves for endoscopy in all patients. In addition, for confirmed or suspected COVID-19 cases, the endoscopist and assisting nurses wear all PPE with the addition of powered air-purifying respirators (PAPR) as an additional barrier. All confirmed or suspected COVID-19 cases should have been admitted to hospital. Suspected cases are defined as per guidance from our Ministry of Health[12] – usually suspected if they have clinical signs and symptoms suggestive of acute respiratory illness, and COVID-19 cases are confirmed with syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction test of respiratory or nasopharyngeal swabs.
In our center, endoscopy staff are assessed daily for symptoms and signs suggestive of COVID-19 infection–temperature is checked twice a day. Staff are grouped into teams of 2-3 which are segregated into separate endoscopy rooms and remain together for the whole day. This is to minimize concomitant exposure to infection and prevent potential spread of infection between teams.
The number of endoscopy staff should be curbed with only essential staff (senior endoscopist +/- trainee, maximum of 2-3 assisting nurses) allowed in the room during procedures. Particular mention should be made of different perspectives of the involvement of trainees in procedures. AGA and BSG have recommended to review and consider limiting their participation in procedures in view of constraints in PPE supply and concerns of increased procedural time. GESA has adopted a more nuanced approach and suggested that trainees should be restricted from participating in procedures involving confirmed COVID-19 cases or cases at high risk of COVID-19 but should be allowed to do procedures involving cases at low risk of COVID-19. Our center adopts this stance, valuing trainee participation in standard endoscopy but limiting their exposure to COVID-19 confirmed or suspected cases.
In our center, for confirmed or suspected patients with COVID-19, endoscopic procedures should be done in negative pressure rooms if fluoroscopy is not required, or in a designated operating theatre with negative pressure if fluoroscopy is required. In limited resource settings where negative pressure rooms are not available, AGA advises portable industrial-grade high-efficiency particulate air filters as an alternative, in line with Centers for Disease Control and Prevention guidelines. Our center has a total of 6 available endoscopy rooms-all inpatient cases are consolidated in 1-2 specified rooms, and outpatient cases are done in other available rooms. This ensures no cross-contamination between patients.
Standard cleaning and disinfection of endoscopy rooms and endoscopy equipment should continue. Endoscopes and endoscopic accessories are reprocessed with standardized reprocessing procedures. Our center’s practice is in line with the United States multi-society guidelines[13]. Endoscopes are cleaned manually–endoscope components are disassembled, and endoscope and their components are immersed in detergent which is compatible with the endoscope. All available channels are flushed and brushed to remove any residue. Endoscopes and their components are subsequently subjected to high-level disinfection with an automated endoscope reprocessing unit.
For confirmed or suspected COVID-19 cases, used endoscopes and endoscopic equipment will be cleaned on site with disinfectant. Used scopes will then be placed in biohazard bags (double bagged) into a container and transported back to the endoscopy centre for further cleaning and reprocessing, which will be done separately from other endoscopic equipment.
All endoscopy staff involved in disinfection and reprocessing of endoscopes and endoscopic equipment should be wearing PPE.
Patients can be contacted at 7-d and 14-d post-procedure to ask about new diagnosis of COVID-19 or development of symptoms of COVID-19 infection. This is suggested by the United States Joint GI Society and ESGE but not routinely practiced elsewhere. Our center conducts routine follow-up calls as per our patient feedback process but not specifically for COVID-19.
We have formulated a proposed workflow incorporating the above measures which can enhance safety of endoscopy in this period (Table 2).
| Issues | Steps |
| Pre-procedure | |
| Risk assessment; Patient/ Procedure; Patient precautions | Inpatient urgent cases are done on a case-to-case basis; Outpatient elective non-urgent cases are reviewed by physician in charge – proceed with cases with suspected significant or time-specific diagnosis, reschedule all other cases; Direct access endoscopy is suspended; Prior to endoscopy: Pre-screen patients for history of fever or upper respiratory tract symptoms (cough, sore throat, rhinorrhea), significant contact and travel history, or if they have been issued a home quarantine order or stay home notice; This includes patients who have family members or close contact with suspected or confirmed COVID-19 case, and patients with recent travel to high risk countries in the past 14 d. On day of endoscopy: Check patient’s body temperature on arrival and ensure patients are at least 2 m apart in the endoscopy centre. All patients and staff wear surgical masks while in the endoscopy centre. Hand hygiene is performed before and after patient contact; Only 1 visitor per patient will be allowed to enter the endoscopy centre. |
| Procedure | |
| Personal protection equipment (PPE) | All members of the endoscopy team wear PPE consisting of N95 mask, face shield, eye shield/goggles, long-sleeved surgical gown and gloves; For confirmed COVID-19 cases; The transfer team will wear PPE while transporting patients to and from the ward; The endoscopist and assisting nurses will wear PPE with powered air-purifying respirators (PAPR) (eye shield/goggles are not required with a PAPR) before entering the room; All endoscopy staff are trained to don and remove PPE accurately; Hand hygiene is performed before wearing and after removing PPE. Wearing of PPE follows these steps: Gown is worn first, followed by N95 mask and eye shield/goggles, then face shield, and finally gloves. Removal of PPE follows these steps: Remove gloves and gown first inside the room, then remove PAPR and N95 mask outside the room or in ante-room (if available). |
| Members of endoscopy team | Endoscopy staff are grouped into teams and segregated into separate endoscopy rooms. Endoscopy staff are advised to minimise personal contact and interaction with staff from other groups. |
| Logistics | For confirmed or suspected patients with COVID-19, endoscopic procedures are done in negative pressure rooms. If fluoroscopy is not required, endoscopy is done at bedside in negative pressure isolation room in the ward. If fluoroscopy is required, endoscopy is done in a designated major operating theatre room. The endoscopy team prepares all necessary equipment and scopes on a clean trolley before proceeding to the location. All other inpatient cases are consolidated in a specified room in the endoscopy centre. If this is not possible, the inpatient case will be scheduled as last case in the room. Outpatient elective cases are performed in other available rooms. |
| Post procedure | |
| Cleaning and disinfection | Standard cleaning and disinfection of endoscopy rooms continue. All surfaces in endoscopy rooms are cleaned, followed by disinfection. For confirmed COVID-19 cases. Used equipment will be wiped down on site with disinfectant, placed in a labeled “dirty” trolley and brought back to endoscopy centre for further cleaning and disinfection. Used scopes will be wiped down on site with disinfectant, placed in a biohazard bag (double bagged), and placed in a rigid container with lid for transportation back to the endoscopy centre for reprocessing. |
With numerous countries employing strategies such as social distancing to decrease rates of SARS-CoV-2 infection, the peak of the COVID-19 pandemic may have passed and attention now turns to how best to re-introduce normal activities and services safely. AGA and Digestive Health Physicians Association (DHPA), ASGE, and BSG have recently published guidelines on resumption of endoscopy during the COVID-19 pandemic[14-16]. Timing of resuming elective procedures should be guided by incidence of COVID-19 cases in the local community, and availability of equipment and manpower. AGA/DHPA propose to resume elective endoscopic procedures when there is a sustained decrease in rate of new COVID-19 cases in the community for at least 14 d, and the decision to resume should also take into consideration availability of resources required to ensure the safety of both healthcare staff and patients.
Resumption of elective endoscopic procedures should be done cautiously and gradually in a phased manner. Both AGA/DHPA and ASGE recommend additional measures for pre-procedure patient screening. AGA/DHPA suggest conducting SARS-CoV-2 PCR testing within 48 h before procedure; if unable to do so, to consider asking patients to keep daily temperature logs for 10 d before procedure. On the day of procedure, a symptom questionnaire will be administered to patients and their temperatures will be checked. ASGE suggests doing pre-screening with a questionnaire on symptoms, contact history, travel history, and occupational exposure within 72 h before procedure. Responses to the questionnaire should be updated on the day of procedure. The rest of the infection prevention and control measures discussed above should continue to be implemented and observed. Additionally, ASGE mentions that patients should be followed up and surveyed 1-2 wk post-procedure – they are advised to inform the endoscopy centre if they develop symptoms or are diagnosed with COVID-19 within 14 d of procedure.
The COVID-19 pandemic has united the world in taking enhanced measures in endoscopy to limit the spread of disease. However, different approaches to these measures highlight system differences in approach to care and logistic limitations. Guidelines and recommendations on endoscopy during COVID-19 are not exhaustive and not inflexible. Of note, many of these guidelines were released consecutively as the pandemic evolved in individual countries and might not necessarily reflect the current state of practice. In these challenging and rapidly evolving times, there is constant emergence of new information, and new innovations in testing and treatment. We should hence be prepared to continually adapt our practices to improve quality and safety of endoscopy during the pandemic.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 157856.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Foong TW, Köker IH, Zhang HW S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Joint GI Society Message on COVID-19. [accessed 19 May 2020]. Available from: https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/. |
| 2. | Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, Falck-Ytter Y, El-Serag HB; American Gastroenterological Association. Electronic address: ewilson@gastro.org. AGA Institute Rapid Recommendations for Gastrointestinal Procedures during the COVID-19 Pandemic. Gastroenterology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 3. | Gastroenterology Professional Society. Guidance on Endoscopic Procedures during the COVID-19 Pandemic. [accessed 19 May 2020]. Available from: https://www.asge.org/home/advanced-education-training/covid-19-asge-updates-for-members/gastroenterology-professional-society-guidance-on-endoscopic-procedures-during-the-covid-19-pandemic/. |
| 4. | Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellisè M, Arvanitakis M, Bhandari P, Bisschops R, Van Hooft JE, Kaminski MF, Triantafyllou K, Webster G, Pohl H, Dunkley I, Fehrke B, Gazic M, Gjergek T, Maasen S, Waagenes W, de Pater M, Ponchon T, Siersema PD, Messmann H, Dinis-Ribeiro M. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52:483-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 5. | Endoscopy activity and COVID-19: BSG and JAG guidance. [accessed 19 May 2020]. Available from: https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/. |
| 6. | Tse F, Borgaonkar M, Leontiadis GI. COVID-19: Advice from the Canadian Association of Gastroenterology for Endoscopy Facilities, as of March 16, 2020. J Can Assoc Gastroenterol. 2020;3:147-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | GESA. Updated Advice on Preventative Measures during Gastrointestinal (GI) Endoscopic Procedures during the COVID-19 Pandemic. [accessed 19 May 2020]. Available from: https://www.gesa.org.au/resources/covid-19/. |
| 8. | Chiu PWY, Ng SC, Inoue H, Reddy DN, Ling Hu E, Cho JY, Ho LK, Hewett DG, Chiu HM, Rerknimitr R, Wang HP, Ho SH, Seo DW, Goh KL, Tajiri H, Kitano S, Chan FKL. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut. 2020;69:991-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 9. | Irisawa A, Furuta T, Matsumoto T, Kawai T, Inaba T, Kanno A, Katanuma A, Kawahara Y, Matsuda K, Mizukami K, Otsuka T, Yasuda I, Tanaka S, Fujimoto K, Fukuda S, Iishi H, Igarashi Y, Inui K, Ueki T, Ogata H, Kato M, Shiotani A, Higuchi K, Fujita N, Murakami K, Yamamoto H, Ito T, Okazaki K, Kitagawa Y, Mine T, Tajiri H, Inoue H. Gastrointestinal endoscopy in the era of the acute pandemic of coronavirus disease 2019: Recommendations by Japan Gastroenterological Endoscopy Society (Issued on April 9th, 2020). Dig Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, Carrara S, Fugazza A, Di Leo M, Galtieri PA, Pellegatta G, Ferrara EC, Azzolini E, Lagioia M. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 11. | Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, Cabral-Prodigalidad P, Shergill A. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 12. | MOH Circular 73/2020 Revision of Suspect Case Definition for Coronavirus Disease 2019 (COVID-19). [accessed 27 May 2020]. Available from: https://www.ams.edu.sg/view-pdf.aspx?file=media%5C5316_fi_62.pdfofile=2.+MOH+SUSPECT+CASE+DEFINITION+FOR+CORONAVIRUS+DISEASE+54A-2020_1+9+March.pdf/. |
| 13. | Reprocessing Guideline Task Force. Petersen BT, Cohen J, Hambrick RD 3rd, Buttar N, Greenwald DA, Buscaglia JM, Collins J, Eisen G. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85:282-294.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Joint AGA/DHPA Guidance: Recommendations for Resumption of Elective Endoscopy during the COVID-19 Pandemic. [accessed 20 May 2020]. Available from: https://www.dhpassociation.org/2020/04/27/aga-dhpa-resume-endoscopy-covid19/. |
| 15. | Hennessy B, Vicari J, Bernstein B, Chapman F, Khaykis I, Littenberg G, Robbins D. Guidance for resuming GI endoscopy and practice operations after the COVID-19 pandemic. Gastrointest Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | BSG Guidance on recommencing GI Endoscopy in the deceleration early recovery phases of the COVID-19 pandemic. [accessed 20 May 2020]. Available from: https://www.bsg.org.uk/covid-19-advice/bsg-guidance-on-recommencing-gi-endoscopy-in-the-deceleration-early-recovery-phases-of-the-covid-19-pandemic/. |