Published online May 16, 2020. doi: 10.4253/wjge.v12.i5.138
Peer-review started: January 29, 2020
First decision: April 12, 2020
Revised: May 8, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: May 16, 2020
Processing time: 107 Days and 15.4 Hours
Colonoscopy screening for the detection and removal of colonic adenomas is central to efforts to reduce the morbidity and mortality of colorectal cancer. However, up to a third of adenomas may be missed at colonoscopy, and the majority of post-colonoscopy colorectal cancers are thought to arise from these. Adenomas have three-dimensional surface topographic features that differentiate them from adjacent normal mucosa. However, these topographic features are not enhanced by white light colonoscopy, and the endoscopist must infer these from two-dimensional cues. This may contribute to the number of missed lesions. A variety of optical imaging technologies have been developed commercially to enhance surface topography. However, existing techniques enhance surface topography indirectly, and in two dimensions, and the evidence does not wholly support their use in routine clinical practice. In this narrative review, co-authored by gastroenterologists and engineers, we summarise the evidence for the impact of established optical imaging technologies on adenoma detection rate, and review the development of photometric stereo (PS) for colonoscopy. PS is a machine vision technique able to capture a dense array of surface normals to render three-dimensional reconstructions of surface topography. This imaging technique has several potential clinical applications in colonoscopy, including adenoma detection, polyp classification, and facilitating polypectomy, an inherently three-dimensional task. However, the development of PS for colonoscopy is at an early stage. We consider the progress that has been made with PS to date and identify the obstacles that need to be overcome prior to clinical application.
Core tip: Dye-based chromoendoscopy has a stronger evidence base than existing virtual chromoendoscopy techniques for improving adenoma detection. However, it is inconvenient, and a novel approach is needed. Photometric stereo is a machine vision technique that captures surface normals. It has been applied successfully to colonic tissue and could be utilized in emerging computer-aided adenoma detection algorithms. However, the optimal method for processing specular reflections from colonic mucosa is unknown, and integration into commercial colonoscopy operating systems has not yet been attempted. Although photometric stereo could have a significant impact on colonoscopy in the future, that future remains distant.
- Citation: Shandro BM, Emrith K, Slabaugh G, Poullis A, Smith ML. Optical imaging technology in colonoscopy: Is there a role for photometric stereo? World J Gastrointest Endosc 2020; 12(5): 138-148
- URL: https://www.wjgnet.com/1948-5190/full/v12/i5/138.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i5.138
Colorectal cancer (CRC) is the third most common cancer in the world[1]. The detection and removal of colonic polyps during colonoscopy is central to efforts to reduce CRC mortality, through its earlier detection, and the detection and removal of its major precursor lesion, the adenoma.
Adenoma detection rate (ADR) has emerged as one of the most important measures of colonoscopy quality. It is used as a surrogate marker for post-colonoscopy CRC, which is the ultimate aim of colonoscopy screening. Post-colonoscopy CRC can arise from lesions missed at index colonoscopy or due to inadequately resected adenomas. A Dutch population study suggests that the majority of post-colonoscopy CRC is due to the former[2]. The miss rate for adenomas is estimated to be 22%-30%, and small (< 1 cm), flat, and proximal lesions are more likely to be missed[2-4].
Recent evidence has proven ADR to be an appropriate surrogate for post-colonoscopy CRC. Compared to patients examined by endoscopists with an ADR of 20% or greater, those examined by endoscopists with an ADR of less than 20% have a ten-fold increase in the hazard ratio of interval CRC and an absolute risk 0.12%[5]. Another study found that for every 1% increase in ADR, there is a 3% decrease in the risk of post-colonoscopy CRC[6].
ADR is determined by multiple service, patient, endoscopist and technical factors, which are displayed in Table 1, although the weight of evidence supporting the impact of each factor varies widely. There has also been a great deal of interest in harnessing new technologies to realize improvements in colonoscopy quality. In this paper we summarize the evidence for the impact of commercially available optical imaging technologies on ADR in the average-risk population, and review photometric stereo (PS), a machine vision technique with potential clinical applications in colonoscopy.
| Service factors | Patient factors | Endoscopist factors | Technical factors | Mechanical technologies |
| Allocated time per procedure[56] | Patient characteristics (age, medical history)[60] | Training[63] | Withdrawal time[65] | End-of-scope devices (cuffs, caps, rings)[72] |
| Morning vs afternoon list[57] | Indication for procedure[61] | Total experience[64] | Position change during withdrawal[66] | Third eye retroscope[73] |
| Enhanced patient instructions for bowel preparation[58] | Quality of bowel preparation[62] | Number of colonoscopies per year[64] | Re-examination of right colon[67] | |
| Setting (hospital based vs non-hospital based)[59] | Caecal intubation rate[59] | Retroflexion in right colon[67] | ||
| Specialty[59] | Rectal retroflexion[68] | |||
| Daily case load and fatigue[60] | Water-aided colonoscopy[69] | |||
| Antispasmodics[70] | ||||
| Second observer[71] |
Colonic adenomas have pronounced topographic features, such as elevations, recessions and pit patterns, which differentiate them from normal mucosa. In white light colonoscopy, the lighting illuminates the field of view and enhances coloration, but not topographic contrast. This may contribute to the number of missed lesions. Several optical imaging technologies have been introduced that enhance topographic contrast to facilitate the detection of adenomas.
High definition white light (HD-WL) colonoscopes and monitors produce higher resolution images and display more images per second than a standard definition white light (SD-WL) colonoscope. Although early studies did not report a significant improvement in ADR, more recent observational studies (4093 patients) demonstrated an increase in ADR of 4.5%-12.6% when HD-WL was compared to SD-WL colonoscopy[7-9]. However, patient characteristics and adjustment for potential confounders were not standardized across these studies. This might explain why the increase in ADR was more marked than that reported in a 2011 meta-analysis of five studies (4422 patients) comparing HD-WL to SD-WL colonoscopy, which showed a more modest 3.5% increase[10]. However, higher resolution images confer other benefits outwith ADR, and HD-WL colonoscopes are now in widespread use.
Chromoendoscopy is a technique where contrast dyes, such as indigo-carmine or methylene blue, are sprayed onto the colonic mucosa during the withdrawal of the colonoscope. The contrast dyes pool in recessions, thereby accentuating surface topography when viewed in HD- or SD-WL. A 2016 Cochrane review of seven randomized controlled trials (RCTs) (2727 patients) found that chromoendoscopy increased the odds of an average-risk patient having one or more neoplastic lesion detected by approximately 50% (pooled OR = 1.53, 95%CI: 1.31-1.79)[11]. However, some of the included trials compared chromoendoscopy to SD-WL colonoscopy, which has since been superseded by HD-WL in clinical practice, so the gains might now be smaller. A more recent large RCT (1065 patients) found a small increase in ADR when comparing chromoendoscopy to HD-WL colonoscopy, but this did not reach statistical significance (OR = 1.13, 95%CI: 0.87-1.48)[12].
In addition to dye-based chromoendoscopy, various optical imaging technologies have been developed commercially. These are commonly termed “virtual chromoendoscopy”, and include narrow band imaging (NBI), i-scan digital contrast (i-scan), flexible spectral imaging colour enhancement (FICE), blue light imaging (BLI), linked colour imaging (LCI) and autofluorescence imaging (AFI). Of these, NBI is the most established.
NBI uses red, green and blue light filters to enhance the superficial mucosa and vasculature. A 2012 Cochrane review of eight RCTs (3673 patients) found no difference in ADR between white light colonoscopy and NBI (RR = 0.94, 95%CI: 0.87-1.02)[13]. However, a 2019 meta-analysis of RCTs from which individual patient data was available (4491 patients) demonstrated a modest but statistically significant increase in ADR when NBI was compared to white light colonoscopy, but only when second generation NBI was used (OR = 1.28, 95%CI: 1.05-1.56) or bowel preparation was excellent (OR = 1.30, 95%CI: 1.05-1.56)[14].
The i-scan digital image processing system offers surface enhancement, contrast enhancement and tone enhancement. FICE uses a computed spectral estimation system to narrow the bandwidth of light in order to enhance the visibility of mucosal and vascular details. A 2014 meta-analysis of five RCTs (3032 patients) compared both i-scan and FICE to HD-WL colonoscopy and found no increase in ADR (RR = 1.09, 95%CI: 0.97-1.23)[15]. However, a recent large RCT (740 patients) comparing i-scan to HD-WL colonoscopy found a significant increase in ADR in the intention-to-treat analysis (47.2% vs 37.7%; P = 0.01)[16]. The observed increase in ADR was largely due to enhanced detection of small, flat, proximal adenomas, which are the lesions most likely to be missed at screening colonoscopy.
BLI and LCI are more recent techniques for virtual chromoendoscopy, based on narrow-band observation of mucosa illuminated by a laser light source. In a large RCT (963 patients) comparing BLI to white light colonoscopy, no increase in ADR was observed, although this was not the primary outcome measure[17]. One RCT (141 patients) comparing LCI to white light colonoscopy demonstrated a significantly increased per-patient ADR (37% vs 28%)[18].
In AFI a rotating filter produces short-wave light bursts that excite different tissue types. The excited tissues emit fluorescent light that is detected and reconstructed in two dimensions (2D). A 2015 meta-analysis of six RCTs (1199 patients) showed no significant difference in ADR between AFI and white light colonoscopy (OR = 1.01, 95%CI: 0.74-1.37)[19]. A subsequent RCT (802 patients) confirmed no increase in ADR using updated AFI instead of white light colonoscopy, but did demonstrate a significant increase in the detection of proximal flat lesions[20].
It is widely accepted that dye-based chromoendoscopy improves ADR. However, this has not been demonstrated consistently in RCTs, and no studies have demonstrated an increase in the detection of advanced neoplasia compared to white light colonoscopy. In addition, dye-based chromoendoscopy is cumbersome to perform, and any increase in ADR must be balanced against the financial and opportunity costs of the additional time required to perform each procedure. As a result, this technique is generally only recommended for high-risk populations, such as those with inflammatory bowel disease or hereditary polyposis syndromes[21]. Even in these populations, national bodies have drawn different conclusions from the same evidence base[21,22].
Virtual chromoendoscopy does have advantages over dye-based chromoendoscopy, not least the ease with which it can be performed. However, additional training and experience are required to interpret the enhanced images correctly[23], and the evidence that these technologies increase ADR, in a clinically meaningful and repeatable manner, is lacking.
It is clear that there is still a need for research into less established imaging technologies that have the potential to enhance surface topography during colonoscopy, and might thereby increase ADR. One such technology is PS.
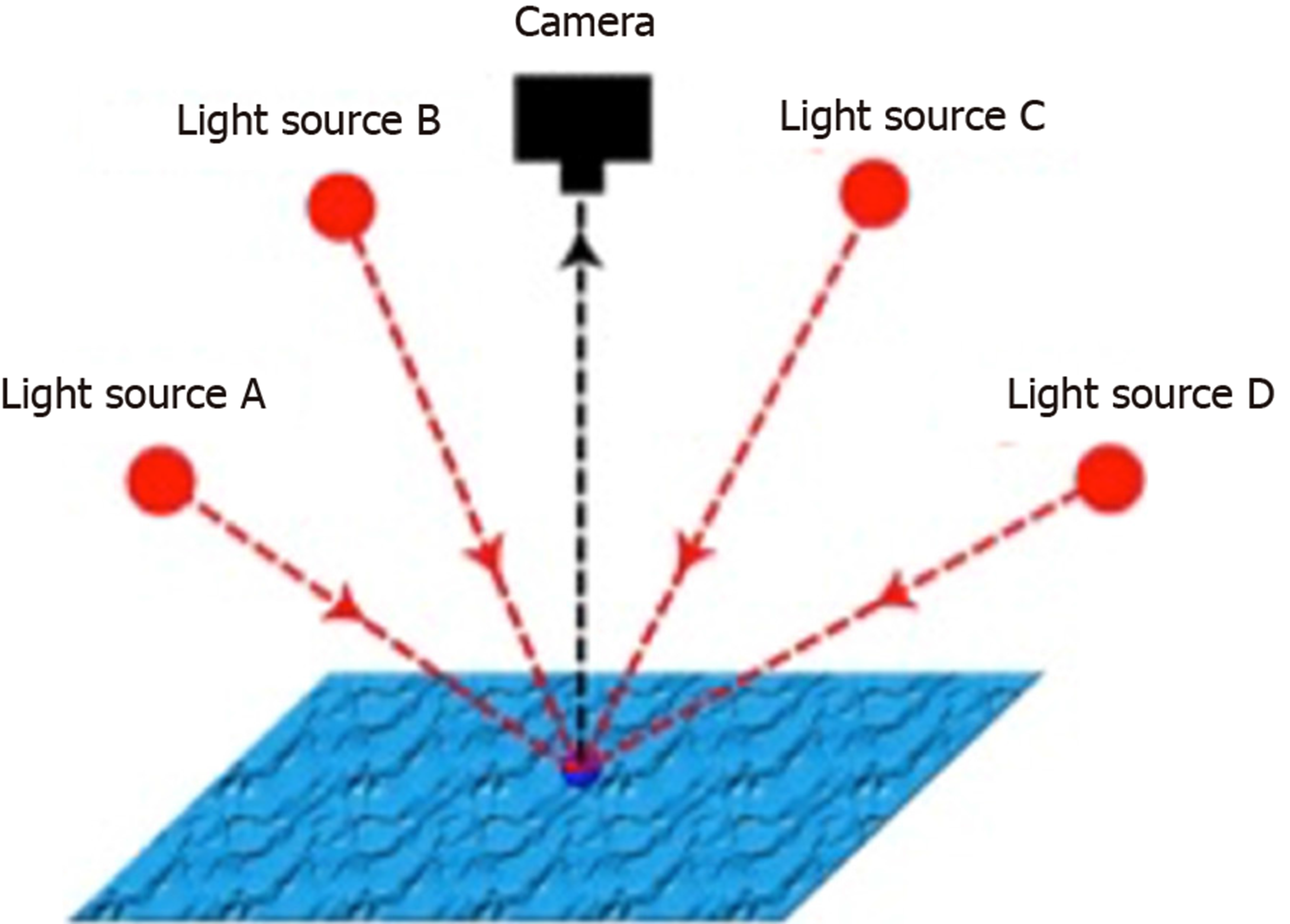
PS is a machine vision technique introduced by Woodham[24] in 1980. In PS, a series of images of an object are obtained from a single fixed viewpoint, with the object illuminated from multiple light sources orientated in different directions[24] (Figure 1). In addition to generating 2D colour images, the surface normals of the object can be estimated at each pixel location and the surface topography reconstructed in three dimensions (3D)[25] (Figure 2).
PS functions best in geometrically controlled situations, where the position of the camera and light sources relative to the object are known. Potential applications are wide-ranging, and PS has been demonstrated to provide accurate 3D surface topographic data for objects as diverse as particles and tablets in pharmaceutical manufacturing[26,27] through to nuclear reactors[28]. In plants, the application of PS for the in-field analysis of crops has been studied[29], in addition to early disease detection in asymptomatic plants, in combination with multispectral imaging[30].
In humans, PS has been used to perform contactless biometric identification using 3D handprints or finger knuckle patterns[31,32], 3D facial reconstruction and recognition[33,34], in vivo measurement of intravascular blood flow[35], ex and in vivo characterization of skin lesions[36,37], and 3D reconstruction of a phantom human tongue[38]. The latter has potential applications in traditional Chinese medicine, where visual inspection of the tongue surface is used to diagnose syndromes and diseases affecting distant organs[39].
Unlike commercially available virtual chromoendoscopy techniques, the study of PS in colonoscopy is at a nascent stage. A Boston-Madrid consortium has carried out a number of proof-of-concept studies using the PS technique. Firstly, they imaged a silicon phantom colon, using a bench top PS imager with cross-polarizers placed in front of the light sources and camera lens to reduce specular reflections at image acquisition[40]. They demonstrated accurate 3D reconstruction of the haustra and three 0.5-1 mm sessile elevations in the phantom colon[40].
Following on from this work, they imaged three human ex vivo gastrointestinal tissues using the same bench top PS imager: A colonic post-polypectomy site, a benign sessile colonic polyp, and a small bowel melanoma metastasis[41]. These specimens were wet, and therefore represent a better model for the reflective properties of colonic mucosa encountered in colonoscopy. Each pathological finding was identifiable in the 3D reconstructions[40]. However, these results were obtained under conditions dissimilar to those encountered during colonoscopy, particularly in terms of the distance between the camera, light sources and mucosa.
The consortium subsequently modified a commercially available gastroscope by adding four additional light sources orientated equally around the gastroscope tip, and synchronizing the additional light sources with the video signal[40]. The resulting system had a total diameter of 14 mm, similar to commercial colonoscopes. The software modifications enabled real-time white light imaging, and topographical reconstructions every four frames. Unfortunately, the dimensions of the gastroscope tip precluded the use of cross-polarizers. Using a non-specular 3D-printed phantom colon, they compared the images obtained using the modified gastroscope to those obtained using their previously described bench top PS imager. The elevations and depressions in the phantom colon were accurately reconstructed in 3D by both imaging systems[40].
The consortium then imaged three ex vivo porcine colons, which had been dissected and laid flat, using the modified PS gastroscope fixed above the tissue[42]. They carried out dye-based chromoendoscopy on the samples using indigo-carmine, and compared these images with images obtained by virtual chromoendoscopy by colour-equalisation, and with images obtained by virtual chromoendoscopy combined with PS. They detected statistically significant image improvement when virtual chromoendoscopy and PS were combined, compared to virtual chromoendoscopy alone[42]. However, it should be noted that this study compared still images, rather than real-time video, and the working distance was fixed. Both challenges need to be overcome prior to clinical application.
Finally, they evaluated the capability of PS to capture topographic data in the human rectum in vivo, using their modified gastroscope on eight human subjects[43]. The white light images obtained through the modified gastroscope were displayed in real-time, but the 3D topography could only be reconstructed in post-processing. When imaging obliquely to the mucosa, elevations from blood vessels and diminutive lesions were reconstructed appropriately, relative to qualitative inspection of the white light images. However when imaging perpendicular to the rectal mucosa, specular reflections caused insurmountable topographical artifacts[43].
The Boston-Madrid consortium has not published any new data since 2014, and is presumably no longer active. However, one researcher from the consortium has continued to study PS imaging. In 2019 conference proceedings they reported the successful imaging of a synthetic phantom colon using a multimodal system that combined white light, PS and speckle contrast flow imaging[44]; and described a deep learning method for depth estimation using computer-generated PS images[45].
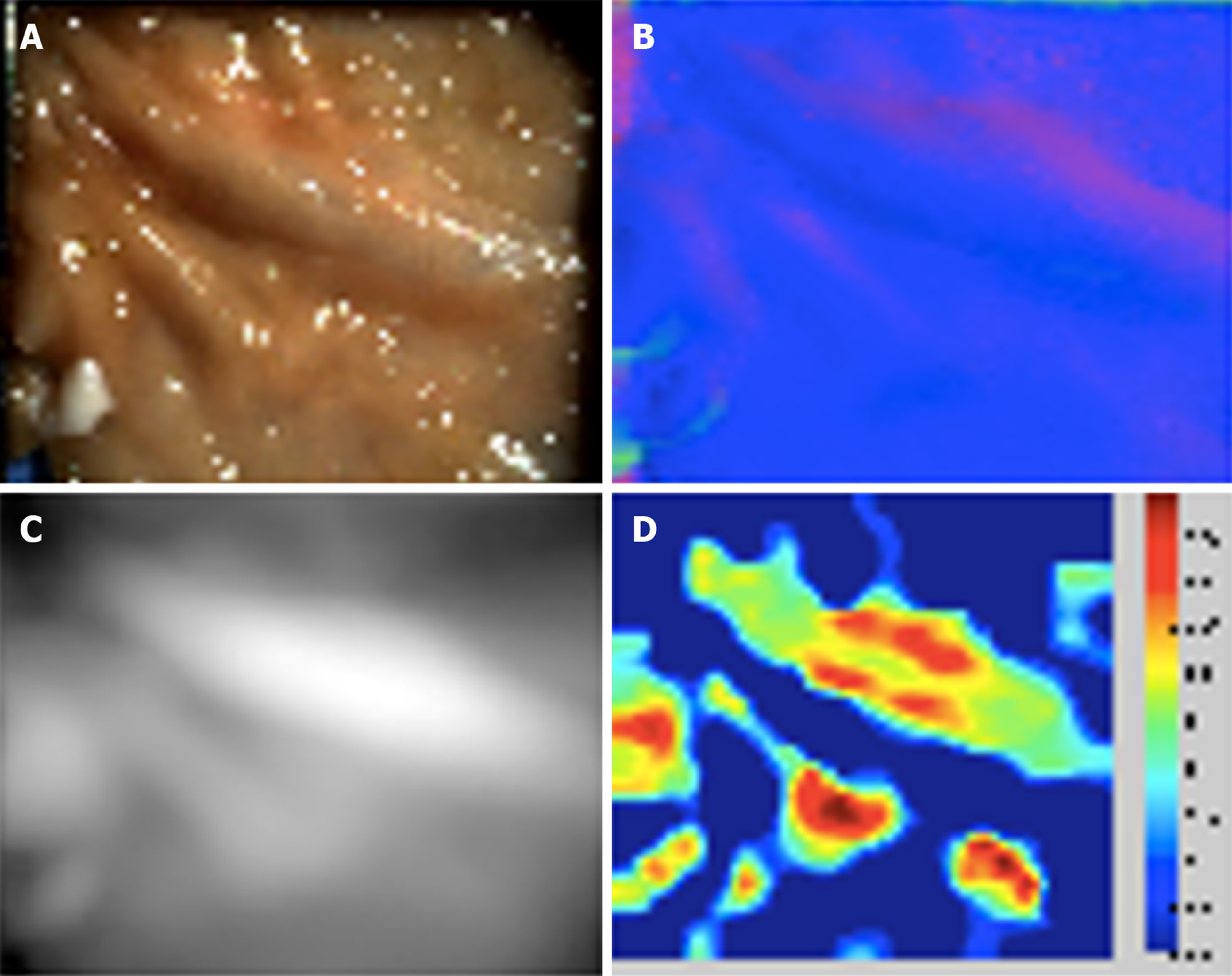
Outside of the Boston-Madrid consortium, very little has been published about PS in the gastrointestinal tract. Our group in the United Kingdom has applied PS to ex vivo porcine gut, using a handheld PS imaging system to capture topographic data in the porcine colon, duodenum, oesophagus, and gastro-oesophageal junction[25,46] (Figure 2). Phantom polyps were created by raising small areas of the mucosa by submucosal injection of saline solution. A least squares approximation method was used to adjust for specular reflections, and a 3D reconstruction generated. We demonstrated that the Shape Index differentiated locally spherical phantom polyps from the adjacent normal mucosa[25] (Figure 3). Such an approach could potentially be applied to the computer-aided detection of pedunculated or sessile, though not flat, polyps.
A South Korean group constructed a bench top multimodal endoscopic system that combined white light imaging, multispectral imaging and PS[47]. They tested its function using a 3D-printed polyp-mimicking phantom of the human colon and four ex vivo mouse colons that had been inoculated with human CRC cell lines. They demonstrated that the PS component could detect and reconstruct phantom sessile polyps with diameters as small as 0.5 mm[47]. In the mouse colons, the PS-derived 3D reconstructions demonstrated a polypoid surface distinct from the adjacent normal mucosa, the locations of which aligned with the spectral-classified tumour sites. Formal histological analysis of the multimodally-detected tumour sites demonstrated CRC, the margins of which correlated with the polypoid surface delineated in the PS-generated 3D image[47].
These early studies have demonstrated that PS can differentiate abnormal tissue from the surrounding normal mucosa, which has potential applications in colonoscopy.
The primary clinical application of PS would be to increase ADR by emphasising the surface topography of adenomas in the field of view – essentially as a novel method of virtual chromoendoscopy. The evidence generally supports dye-based chromoendoscopy as a technique to increase ADR, whilst that supporting existing virtual chromoendoscopy technologies is less compelling, at least in average risk populations. This may be because the origin of the enhanced surface definition in dye-based chromoendoscopy is the surface topography itself (i.e., the dye accumulating in pits and crevices in the mucosa), whereas the enhancement derived from commercial virtual chromoendoscopy is instead based on the optical properties of the mucosa. In this respect, PS has more in common with conventional chromoendoscopy than with established virtual chromoendoscopy techniques. However, PS has an advantage over dye-based chromoendoscopy in that it could be readily integrated into computer-aided adenoma detection systems.
When a polyp is detected, the type of lesion must be diagnosed to determine future CRC risk[48]. Optical diagnosis using existing optical imaging technologies can achieve acceptable sensitivities and specificities in expert hands[49]. However these results have not been replicated in routine clinical practice[50], and adequate training is not widely available. PS has been shown to differentiate between benign and malignant skin lesions[36,37], and could improve optical diagnosis by generating 3D data for interpretation by the endoscopist, given sufficient training, or by a computer-aided diagnostic algorithm. However, no published studies have applied PS to polyp diagnosis rather than detection.
With no proven methods of optical diagnosis in widespread use, most detected polyps are subsequently removed by polypectomy and sent for histological analysis. Polypectomy is a challenging task, particularly more advanced techniques such as endoscopic mucosal resection or endoscopic submucosal dissection. It is a procedure that is inherently performed in 3D, but the endoscopist must infer depth information from indirect cues from a 2D video monitor. In laparoscopy, 3D systems that provide binocular depth perception have been shown to reduce procedure time and error rates in experimental settings[51]. It is conceivable that 3D colonoscopy, such as could be rendered by PS in the future, could reduce procedure times and complications in polypectomy as well.
Only small proof-of-concept studies have been carried out to date, and there are multiple obstacles that must be overcome prior to clinical application. Firstly, PS assumes Lambertian reflectance, and the moist colonic mucosa is an innately non-Lambertian surface. This gives rise to specular reflections, which can cause artifactual distortion in the 3D reconstruction. This issue has been reported in all studies of PS in the gastrointestinal tract, and may be exaggerated when the camera lens is perpendicular to the mucosa[43].
A variety of post-processing approaches have been used to try to compensate for these specular reflections, including least squares approximation[25], exclusion of the reflections using spectral or directional cues[52], combining perspective projection and the Blinn-Phong reflectance model[53], and simultaneous mesh-based computation of surface normals and reflectance[54]. However, the optimal approach to take in the application of PS to colonoscopy is unknown.
In the multimodal imaging study by Kim et al[47], the total time for image acquisition and reconstruction was 9 s, during which the lesion and image plane had to be fixed. This acquisition time is impractical for colonoscopy, as the distance between the colonoscope tip and the colonic mucosa is constantly changing due to movement of the colonoscope tip and the colon itself. However, the majority of this processing time was attributable to the multispectral component, with the PS acquisition and 3D reconstruction taking approximately 1 s, which is more promising. In addition, PS imaging has previously been applied to fast-moving surfaces in other fields, such as quality control in manufacturing[55]. A similar technique could potentially be applied to colonoscopy.
In their in vivo study of human rectums, the Boston-Madrid consortium was able to display white light images in real-time using their PS-modified gastroscope, with the PS data extracted in post-processing[43]. With further advances in computer processing, it is anticipated that real-time PS topographic data could be made available to endoscopists in the future, either as a visual representation or via a computer-aided detection system. However, even when this is achieved, the most effective way to convey 3D information to the endoscopist remains unknown.
Finally, although the technology is relatively inexpensive and unsophisticated compared to some other approaches, the hardware has not yet been miniaturized for integration into commercial colonoscopes. However, colonoscope tips already house a camera and multiple light sources, and the Boston-Madrid consortium documented their conversion of a commercial gastroscope to obtain PS images using a bespoke end-of-scope device. With commercial input, integrating PS into the next generation of colonoscopes should not be an insurmountable task.
PS can derive accurate 3D surface topographic data from colon phantoms, animal models and human colonic tissue. However, research into the application of PS to colonoscopy is at a very early stage. In humans, PS imaging has only been performed on a single ex vivo colonic polyp and eight in vivo rectums to date. Several obstacles have been identified and incompletely resolved, particularly how to deal with specular reflections and an unfixed field of view. Furthermore, whilst miniaturization of the existing technology to permit integration into the next generation of commercial colonoscopes is certainly possible, it has yet to be attempted. Although PS imaging could have a significant impact on colonoscopy in the future, that future remains distant.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: British Society of Gastroenterology (0013758).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Manes G, Rawat K S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3280] [Article Influence: 410.0] [Reference Citation Analysis (3)] |
| 2. | le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 3. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 4. | Gavin DR, Valori RM, Anderson JT, Donnelly MT, Williams JG, Swarbrick ET. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut. 2013;62:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 5. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1462] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 6. | Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA, Wallace MB. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Jrebi NY, Hefty M, Jalouta T, Ogilvie J, Davis AT, Asgeirsson T, Luchtefeld M. High-definition colonoscopy increases adenoma detection rate. Surg Endosc. 2017;31:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Bond A, O'Toole P, Fisher G, Subramanian S, Haslam N, Probert C, Cox T, Sarkar S. New-Generation High-Definition Colonoscopes Increase Adenoma Detection when Screening a Moderate-Risk Population for Colorectal Cancer. Clin Colorectal Cancer. 2017;16:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Brown SR, Baraza W, Din S, Riley S. Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database Syst Rev. 2016;4:CD006439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Lesne A, Rouquette O, Touzet S, Petit-Laurent F, Tourlonias G, Pasquion A, Rivory J, Aguero Garcete G, Scanzi J, Chalumeau S, Chambon-Augoyard C, Moussata D, Leger-Nguyen F, Degeorges S, Chauvenet M, Fontanges T, Baubet S, Brulet P, Billioud C, Thimonier E, Stroeymeyt-Martin K, Hamel B, Graillot E, Cruiziat C, Scalone O, O'Brien M, Péré-Vergé D, Souquet JC, Phelip JM, Poincloux L, Poupon-Bourdy S, Denis A, Magaud L, Ponchon T, Pioche M. Adenoma detection with blue-water infusion colonoscopy: a randomized trial. Endoscopy. 2017;49:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Atkinson NSS, Ket S, Bassett P, Aponte D, De Aguiar S, Gupta N, Horimatsu T, Ikematsu H, Inoue T, Kaltenbach T, Leung WK, Matsuda T, Paggi S, Radaelli F, Rastogi A, Rex DK, Sabbagh LC, Saito Y, Sano Y, Saracco GM, Saunders BP, Senore C, Soetikno R, Vemulapalli KC, Jairath V, East JE. Narrow-Band Imaging for Detection of Neoplasia at Colonoscopy: A Meta-analysis of Data From Individual Patients in Randomized Controlled Trials. Gastroenterology. 2019;157:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Omata F, Ohde S, Deshpande GA, Kobayashi D, Masuda K, Fukui T. Image-enhanced, chromo, and cap-assisted colonoscopy for improving adenoma/neoplasia detection rate: a systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:222-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Kidambi TD, Terdiman JP, El-Nachef N, Singh A, Kattah MG, Lee JK. Effect of I-scan Electronic Chromoendoscopy on Detection of Adenomas During Colonoscopy. Clin Gastroenterol Hepatol. 2019;17:701-708.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Ikematsu H, Sakamoto T, Togashi K, Yoshida N, Hisabe T, Kiriyama S, Matsuda K, Hayashi Y, Matsuda T, Osera S, Kaneko K, Utano K, Naito Y, Ishihara H, Kato M, Yoshimura K, Ishikawa H, Yamamoto H, Saito Y. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc. 2017;86:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Min M, Deng P, Zhang W, Sun X, Liu Y, Nong B. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc. 2017;86:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Zhao ZY, Guan YG, Li BR, Shan YQ, Yan FH, Gao YJ, Wang H, Lou Z, Fu CG, Yu ED. Detection and miss rates of autofluorescence imaging of adenomatous and polypoid lesions during colonoscopy: a systematic review and meta-analysis. Endosc Int Open. 2015;3:E226-E235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Takeuchi Y, Sawaya M, Oka S, Tamai N, Kawamura T, Uraoka T, Ikematsu H, Moriyama T, Arao M, Ishikawa H, Ito Y, Matsuda T. Efficacy of autofluorescence imaging for flat neoplasm detection: a multicenter randomized controlled trial (A-FLAT trial). Gastrointest Endosc. 2019;89:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, Pellisé M, Antonelli G, Bustamante Balen M, Coron E, Cortas G, Iacucci M, Yuichi M, Longcroft-Wheaton G, Mouzyka S, Pilonis N, Puig I, van Hooft JE, Dekker E. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:1155-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (1)] |
| 22. | Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, Ilyas M, Kaur A, Lalloo F, Latchford A, Rutter MD, Tomlinson I, Thomas HJW, Hill J; Hereditary CRC guidelines eDelphi consensus group. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020;69:411-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 291] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 23. | ASGE Technology Committee. Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JT, Murad FM, Siddiqui UD, Wallace MB, Banerjee S. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Woodham RM. Photometric method for determining surface orientation from multiple images. Opt Eng. 1980;19:139-144. [DOI] [Full Text] |
| 25. | Emrith K, Slabaugh G, Poullis A, Groves C, Smith M. Photometric stereo reconstruction for surface analysis of mucosal tissue. In: Reyes-Aldasoro CC, Slabaugh G, editors. Proceedings of the 18th Conference on Medical Image Understanding and Analysis; 2014 July 9-11; London, UK. London: UoL, 2014. Available from: https://uwe-repository.worktribe.com/output/814742/photometric-stereo-reconstruction-for-surface-analysis-of-mucosal-tissue. |
| 26. | Burggraeve A, Sandler N, Heinämäki J, Räikkönen H, Remon JP, Vervaet C, De Beer T, Yliruusi J. Real-time image-based investigation of spheronization and drying phenomena using different pellet formulations. Eur J Pharm Sci. 2011;44:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Allesø M, Holm P, Carstensen JM, Holm R. Quantitative surface topography assessment of directly compressed and roller compacted tablet cores using photometric stereo image analysis. Eur J Pharm Sci. 2016;87:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Huang S, Xu K, Li M, Wu M. Improved Visual Inspection Through 3D Image Reconstruction of Defects Based on the Photometric Stereo Technique. Sensors (Basel). 2019;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Smith LN, Zhang W, Hansen MF, Hales IJ, Smith ML. Innovative 3D and 2D machine vision methods for analysis of plants and crops in the field. Comput Ind. 2018;97:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Veys C, Chatziavgerinos F, AlSuwaidi A, Hibbert J, Hansen M, Bernotas G, Smith M, Yin H, Rolfe S, Grieve B. Multispectral imaging for presymptomatic analysis of light leaf spot in oilseed rape. Plant Methods. 2019;15:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Hansen MF, Smith L, Smith M. Multispectral contactless 3D handprint acquisition for identification. In: Arabnia HR, de la Fuente D, Kozerenko EB, Olivas JA, Tinetti FG, editors. The Proceedings of the 2018 International Conference on Artificial Intelligence; 2018 July 30-Aug 2; Las Vegas, USA. Sterling: Stylus, 2018. Available from: https://uwe-repository.worktribe.com/output/919501. |
| 32. | Cheng KHM, Kumar A. Contactless Biometric Identification using 3D Finger Knuckle Patterns. IEEE Trans Pattern Anal Mach Intell. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Zafeiriou S, Atkinson GA, Hansen MF, Smith WA, Argyriou V, Petrou M, Smith ML, Smith LN. Face recognition and verification using photometric stereo: The photoface database and a comprehensive evaluation. IEEE T Inf Foren Sec. 2013;8:121-135. [DOI] [Full Text] |
| 34. | Roth J, Yiying Tong, Xiaoming Liu. Adaptive 3D Face Reconstruction from Unconstrained Photo Collections. IEEE Trans Pattern Anal Mach Intell. 2017;39:2127-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Mazdeyasna S, Huang C, Zhao M, Agochukwu NB, Bahrani AA, Wong L, Yu G. Noncontact speckle contrast diffuse correlation tomography of blood flow distributions in tissues with arbitrary geometries. J Biomed Opt. 2018;23:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Zhou Y, Smith M, Smith L, Warr R. Using 3D differential forms to characterize a pigmented lesion in vivo. Skin Res Technol. 2010;16:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Zhou Y, Smith M, Smith L, Farooq A, Warr R. Enhanced 3D curvature pattern and melanoma diagnosis. Comput Med Imaging Graph. 2011;35:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Cai Y, Zhang L, Sheng N, Wang L, Zhang X. Dynamic 3D reconstruction of tongue surface based on photometric stereo technique. In: Liu H, Kubota N, Zhu X, Dillmann R, Zhou D, editors. Lecture Notes in Computer Science 9244: Intelligent Robotics and Applications; 2015 Aug 24-27; Portsmouth, UK. Portsmouth: Springer, 2015. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Jung CJ, Jeon YJ, Kim JY, Kim KH. Review on the current trends in tongue diagnosis systems. Integr Med Res. 2012;1:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Parot V, Lim D, González G, Traverso G, Nishioka NS, Vakoc BJ, Durr NJ. Photometric stereo endoscopy. J Biomed Opt. 2013;18:076017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Durr NJ, Gonzalez G, Lim D, Traverso G, Nishioka NS, Vakoc BJ, Parot V. System for clinical photometric stereo endoscopy. In: Vo-Dinh T, Mahadevan-Jansen A, Grundfest WS, editors. Proceedings of SPIE 8935: Advanced Biomedical and Clinical Diagnostic Systems XII; 2014 Feb 4-6; San Francisco, USA. San Francisco: SPIE, 2014. [DOI] [Full Text] |
| 42. | González G, Parot V, Lo W, Vakoc BJ, Durr NJ. Feature space optimization for virtual chromoendoscopy augmented by topography. Med Image Comput Comput Assist Interv. 2014;17:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Durr NJ, Parot V, Traverso G, Puricelli WP, Vakoc BJ, Nishioka NS, Gonzalez G. Imaging colonic surface topography with photometric stereo endoscopy. Gastrointest Endosc. 2014;79:AB352. [DOI] [Full Text] |
| 44. | Bobrow TL, Durr NJ. An adaptive-coherence light source for hyperspectral, topographical, and flow-contrast imaging. In: Azar, FS, Intes X, Fang Q, editors. Proceedings of SPIE 10871: Multimodal Biomedical Imaging XIV; 2019 Feb 02-03; San Francisco, USA. Bellingham: SPIE, 2019. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Mahmood F, Borders D, Chen R, Sweer J, Tilley II, S, Nishioka NS, Stayman, JW, Durr NJ. Robust photometric stereo endoscopy via deep learning trained on synthetic data. In: Azar, FS, Intes X, Fang Q, editors. Proceedings of SPIE 10871: Multimodal Biomedical Imaging XIV; 2019 Feb 02-03; San Francisco, United States. Bellingham: SPIE, 2019. [DOI] [Full Text] |
| 46. | Poullis A, Groves C, Slabaugh G, Emrith K, Smith M. A novel photometric stereo imaging sensor for endoscopy imaging: proof of concept studies on a porcine model. Gut. 2014;63:A46-A47. [DOI] [Full Text] |
| 47. | Kim J, Al Faruque H, Kim S, Kim E, Hwang JY. Multimodal endoscopic system based on multispectral and photometric stereo imaging and analysis. Biomed Opt Express. 2019;10:2289-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A, Hazewinkel Y, Jover R, Kalager M, Loberg M, Pox C, Rembacken B, Lieberman D; European Society of Gastrointestinal Endoscopy. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 49. | ASGE Technology Committee. Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JT, Murad FM, Siddiqui UD, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1-502.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 50. | Rees CJ, Rajasekhar PT, Wilson A, Close H, Rutter MD, Saunders BP, East JE, Maier R, Moorghen M, Muhammad U, Hancock H, Jayaprakash A, MacDonald C, Ramadas A, Dhar A, Mason JM. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut. 2017;66:887-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 51. | Yim C, Lo CH, Lau MH, Fan R, Lai HM, Foo DC. Three-dimensional laparoscopy: is it as good as it looks? - a review of the literature. Ann Laparosc Endosc Surg. 2017;2:131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Barsky S, Petrou M. The 4-source photometric stereo technique for three-dimensional surfaces in the presence of highlights and shadows. IEEE Trans Pattern Anal Mach Intell. 2003;25:1239-1252. [DOI] [Full Text] |
| 53. | Khanian M, Boroujerdi AS, Breuß M. Photometric stereo for strong specular highlights. Comp. Visual Media. 2018;4:83-102. [DOI] [Full Text] |
| 54. | Xie W, Nie Y, Song Z, Wang CCL. Mesh-Based Computation for Solving Photometric Stereo With Near Point Lighting. IEEE Comput Graph Appl. 2019;39:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Smith ML, Smith LN. Dynamic Photometric Stereo - A New Technique for Moving Surface Analysis. Image Vision Compu. 2005;23:841-852. [DOI] [Full Text] |
| 56. | Whitson MJ, Bodian CA, Aisenberg J, Cohen LB. Is production pressure jeopardizing the quality of colonoscopy? A survey of U.S. endoscopists' practices and perceptions. Gastrointest Endosc. 2012;75:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Xu Y, Chen K, Xu L, Yuan X, Wu Y, Chen P. Diagnostic yield is not influenced by the timing of screening endoscopy: morning versus afternoon. Scand J Gastroenterol. 2018;53:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Guo X, Yang Z, Zhao L, Leung F, Luo H, Kang X, Li X, Jia H, Yang S, Tao Q, Pan Y, Guo X. Enhanced instructions improve the quality of bowel preparation for colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;85:90-97.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 60. | Wang H, Wang P, Liu X, Li L, Xiao X, Liu P, Zhang D, Li Y, Xu G, Tu M, Song Y. Factors predicting the colorectal adenoma detection rate in colonoscopic screening of a Chinese population: A prospective study. Medicine (Baltimore). 2019;98:e15103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Gimeno García AZ, González Y, Quintero E, Nicolás-Pérez D, Adrián Z, Romero R, Alarcón Fernández O, Hernández M, Carrillo M, Felipe V, Díaz J, Ramos L, Moreno M, Jiménez-Sosa A. Clinical validation of the European Panel on the Appropriateness of Gastrointestinal Endoscopy (EPAGE) II criteria in an open-access unit: a prospective study. Endoscopy. 2012;44:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 698] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 63. | Kaminski MF, Anderson J, Valori R, Kraszewska E, Rupinski M, Pachlewski J, Wronska E, Bretthauer M, Thomas-Gibson S, Kuipers EJ, Regula J. Leadership training to improve adenoma detection rate in screening colonoscopy: a randomised trial. Gut. 2016;65:616-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 64. | Rees CJ, Bevan R, Zimmermann-Fraedrich K, Rutter MD, Rex D, Dekker E, Ponchon T, Bretthauer M, Regula J, Saunders B, Hassan C, Bourke MJ, Rösch T. Expert opinions and scientific evidence for colonoscopy key performance indicators. Gut. 2016;65:2045-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 65. | Shaukat A, Rector TS, Church TR, Lederle FA, Kim AS, Rank JM, Allen JI. Longer Withdrawal Time Is Associated With a Reduced Incidence of Interval Cancer After Screening Colonoscopy. Gastroenterology. 2015;149:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 66. | East JE, Bassett P, Arebi N, Thomas-Gibson S, Guenther T, Saunders BP. Dynamic patient position changes during colonoscope withdrawal increase adenoma detection: a randomized, crossover trial. Gastrointest Endosc. 2011;73:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Kushnir VM, Oh YS, Hollander T, Chen CH, Sayuk GS, Davidson N, Mullady D, Murad FM, Sharabash NM, Ruettgers E, Dassopoulos T, Easler JJ, Gyawali CP, Edmundowicz SA, Early DS. Impact of retroflexion vs. second forward view examination of the right colon on adenoma detection: a comparison study. Am J Gastroenterol. 2015;110:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 68. | Hanson JM, Atkin WS, Cunliffe WJ, Browell DA, Griffith CD, Varma JS, Plusa SM. Rectal retroflexion: an essential part of lower gastrointestinal endoscopic examination. Dis Colon Rectum. 2001;44:1706-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Liu Y, Huang QK, Dong XL, Jin PP. Water exchange versus air insufflation for colonoscopy: A meta-analysis. Saudi J Gastroenterol. 2018;24:311-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Sanagapalli S, Agnihotri K, Leong R, Corte CJ. Antispasmodic drugs in colonoscopy: a review of their pharmacology, safety and efficacy in improving polyp detection and related outcomes. Therap Adv Gastroenterol. 2017;10:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Xu L, Zhang Y, Song H, Wang W, Zhang S, Ding X. Nurse Participation in Colonoscopy Observation versus the Colonoscopist Alone for Polyp and Adenoma Detection: A Meta-Analysis of Randomized, Controlled Trials. Gastroenterol Res Pract. 2016;2016:7631981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Jain D, Sandhu N, Singhal S. New Developments in Mechanical Enhancement of Colonoscopy: Cuffs, Caps and Rings. Digestion. 2016;93:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI, Dewit O, Ignjatovic A, Odstrcil E, East J, Deprez PH, Saunders BP, Kalloo AN, Creel B, Singh V, Lennon AM, Siersema PD; Third Eye Retroscope Randomized Clinical Evaluation [TERRACE] Study Group. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |