Published online Nov 16, 2020. doi: 10.4253/wjge.v12.i11.493
Peer-review started: June 21, 2020
First decision: July 30, 2020
Revised: October 13, 2020
Accepted: October 23, 2020
Article in press: October 23, 2020
Published online: November 16, 2020
Processing time: 143 Days and 1.4 Hours
Pancreaticoduodenectomy is a technically demanding operation, with reported morbidity rates of approximately 40%–50%. A novel idea is to use endoscopic vacuum therapy (EVT) in a preemptive setting to prevent anastomotic leakage and pancreatic fistulas. In a recent case series, EVT was proven to be effective in preventing leaks in patients with anastomotic ischemia. There have been no previous reports on preemptive EVT after pancreaticoduodenectomy.
We describe the case of a 71-year-old woman with hypertension and diabetes who was admitted to the emergency room with jaundice, choluria, fecal acholia, abdominal pain, and fever. Admission examinations revealed leukocytosis and hyperbilirubinemia (total: 13 mg/dL; conjugated: 12.1 mg/dL). Abdominal ultrasound showed cholelithiasis and dilation of the common bile duct. Magnetic resonance imaging demonstrated a stenotic area, and a biopsy confirmed cholangiocarcinoma. Considering the high risk of leaks after pancreaticoduodenectomy, preemptive endoluminal vacuum therapy was performed. The system comprised a nasogastric tube, gauze, and an antimicrobial incise drape. The negative pressure was 125 mmHg, and no adverse events occurred. The patient was discharged on postoperative day 5 without any symptoms.
Preemptive endoluminal vacuum therapy may be a safe and feasible technique to reduce leaks after pancreaticoduodenectomy.
Core Tip: Leaks and fistulas represent a high cost burden to health systems worldwide, with high morbidity and mortality rates in affected patients. Preventing these transmural defects remains challenging. Despite the progress in surgical techniques, pancreaticoduodenectomy still has a high risk of adverse events, including leaks and pancreatic fistulas. Here, we present a feasible technique to reduce these complications of pancreaticoduodenectomy. To the best of our knowledge, this is the first report of preemptive endoluminal vacuum therapy after pancreaticoduodenectomy.
- Citation: de Medeiros FS, do Monte Junior ES, França RL, de Medeiros Neto HC, Santos JM, Almeida Júnior EA, da Silva Júnior SO, Tavares MHSMP, de Moura EGH. Preemptive endoluminal vacuum therapy after pancreaticoduodenectomy: A case report. World J Gastrointest Endosc 2020; 12(11): 493-499
- URL: https://www.wjgnet.com/1948-5190/full/v12/i11/493.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i11.493
Pancreaticoduodenectomy is a technically demanding operation, with reported morbidity rates of approximately 40%-50%[1,2]. Gastroparesis and bleeding are the most frequent complications, while pancreatic fistula, which can cause intra-abdominal abscess, sepsis, and occasionally death, is the most serious[3]. Patients may require another operation, representing a therapeutic challenge that directly impacts mortality, morbidity, and cost to health systems worldwide[4,5]. Several techniques, such as pancreatic duct occlusion, pancreatogastric anastomosis, Wirsung-jejunal duct-to-mucosa anastomosis, and drainage to the pancreatic duct, have been developed to prevent complications[6-8].
In recent years, endoscopic procedures have begun to fill the large gap between medical and surgical treatments aimed at avoiding fistulas and leaks[9-12]. There have been no previous reports of preemptive endoscopic vacuum therapy (EVT) after pancreaticoduodenectomy.
A 71-year-old woman was admitted to the emergency room with jaundice, choluria, fecal acholia, abdominal pain, and fever.
The patient had a 6-day history of worsening abdominal pain and fever 2 d before admission at the emergency unit.
She had a medical history of hypertension and type 2 diabetes mellitus, controlled with oral agents.
She had not undergone any prior abdominal surgery.
Physical examination revealed fever (38.5°C), jaundice, and tenderness in the upper abdomen.
Laboratory tests revealed elevated serum bilirubin levels (total: 13 mg/dL; conjugated: 12.1 mg/dL), leukocytosis (white blood cells: 16500/µL), and reduced serum albumin levels (2.1 g/dL). Carbohydrate antigen 19-9 and carcinoembryonic antigen levels were within normal limits.
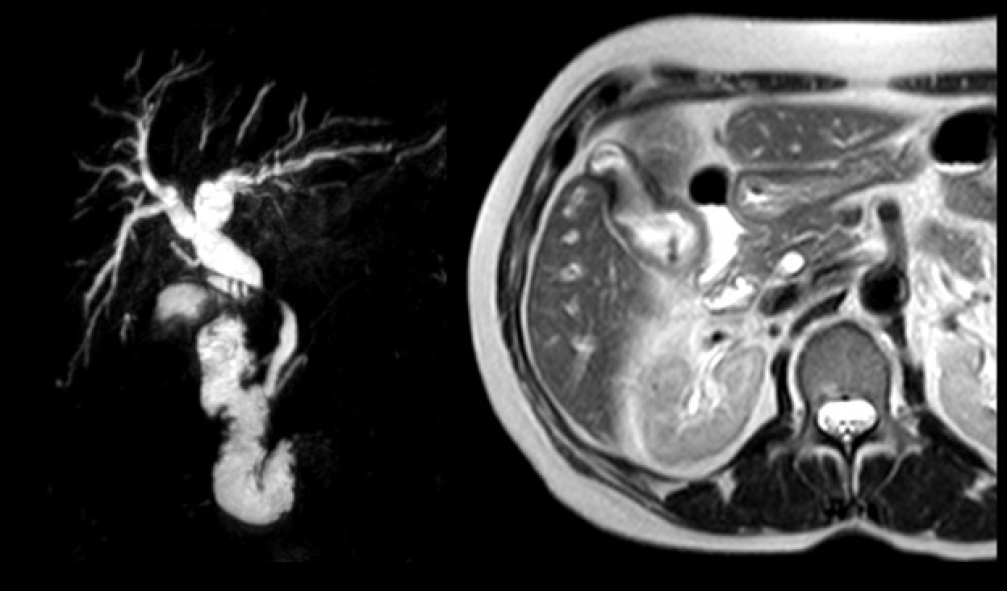
Abdominal ultrasonography showed cholelithiasis and intrahepatic and extrahepatic dilatations. Magnetic resonance imaging (MRI) demonstrated a circumferential tumor of the middle bile duct with upstream biliary dilatation, cholangitis, and cholelithiasis (Figure 1). The pancreas and caliber of the duct of Wirsung were normal. Endoscopic retrograde cholangiopancreatography (ERCP) was performed, although drainage was unsuccessful because the guidewire could not be passed across the lesion.
The patient was diagnosed with moderately differentiated biliary adenocarcinoma pT4pN0pM0 (stage group IIIB).
Considering the success of ERCP, we opted for percutaneous drainage. The patient had a history of weight loss, reduced serum albumin levels (2.1 g/dL), and lower oral food intake. Therefore, enteral nutrition was initiated through a nasoenteral feeding tube and high-protein oral supplementation.
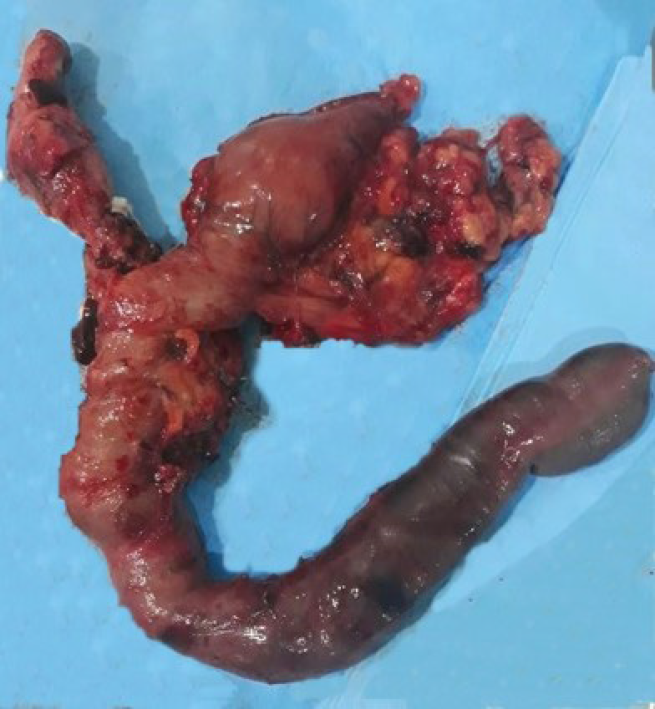
Pancreaticoduodenectomy (Figure 2) was performed on hospital day 14, after tumor staging and perioperative nutrition. A laparoscopic approach was attempted, although the procedure was converted to open pancreaticoduodenectomy because of bleeding from a branch of the portal vein. A harmonic power clamp was applied to a pancreatic section, showing a thin duct of Wirsung. End-to-side duct-to-mucosa pancreaticojejunostomy and choledochal-jejunal anastomosis were performed with Caprofyl. The surgical procedure lasted 10 h, requiring blood transfusion (4 units of red blood cells). The patient was admitted to the intensive care unit, where she remained for 1 d.
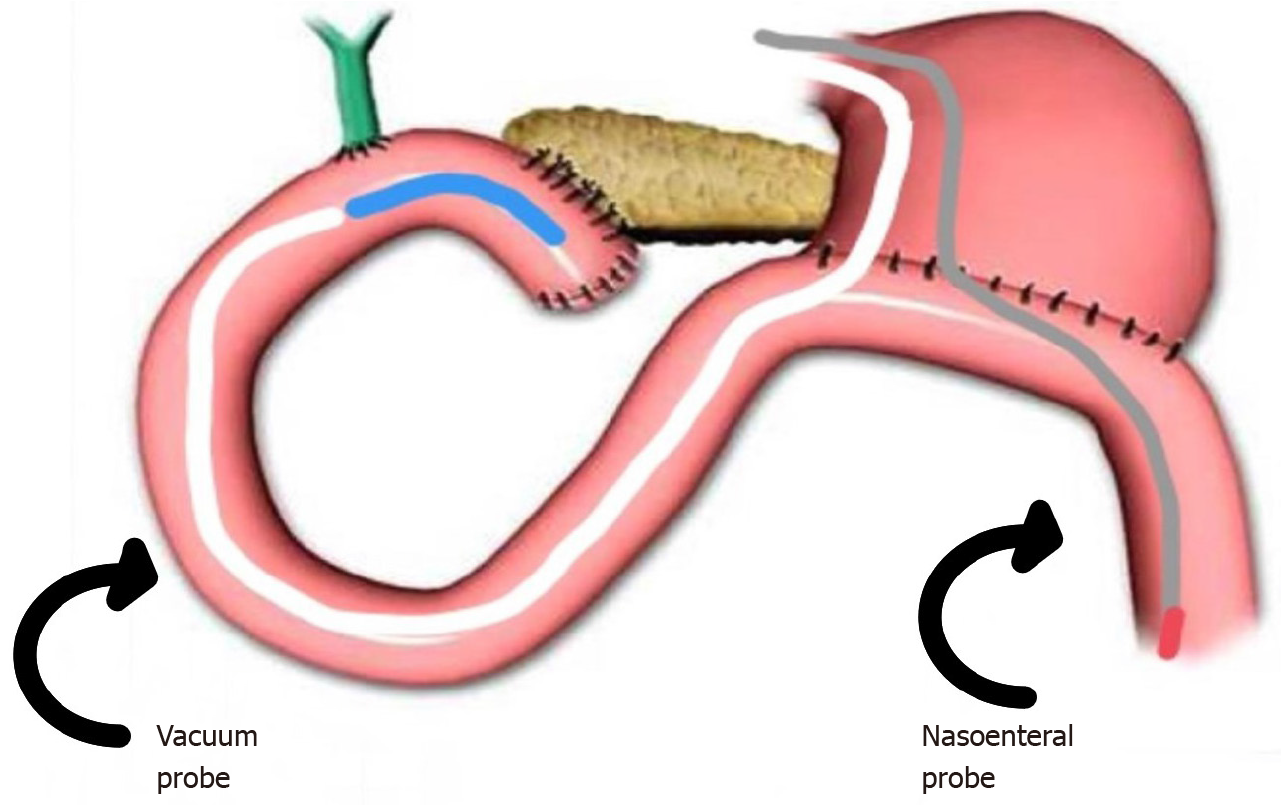
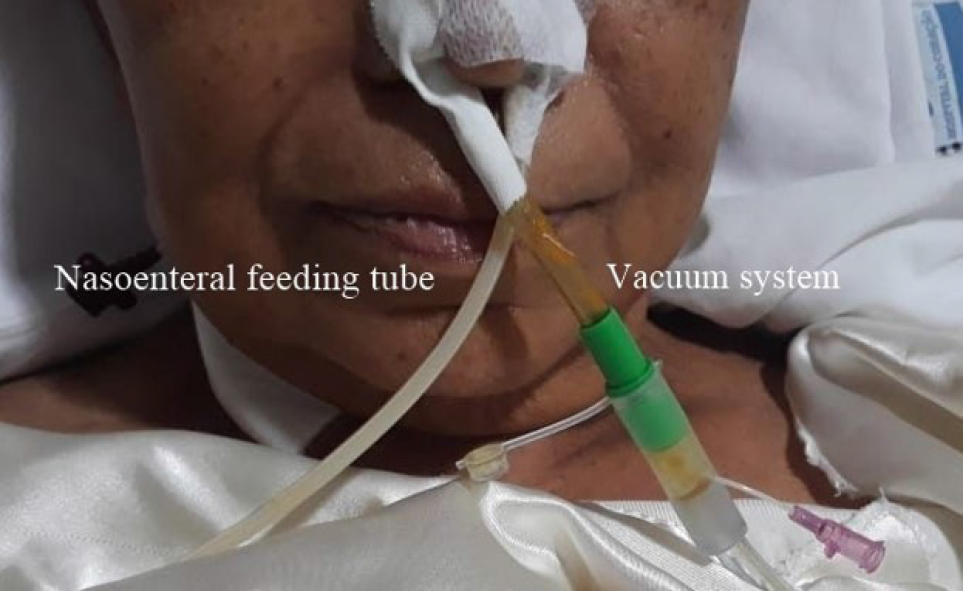
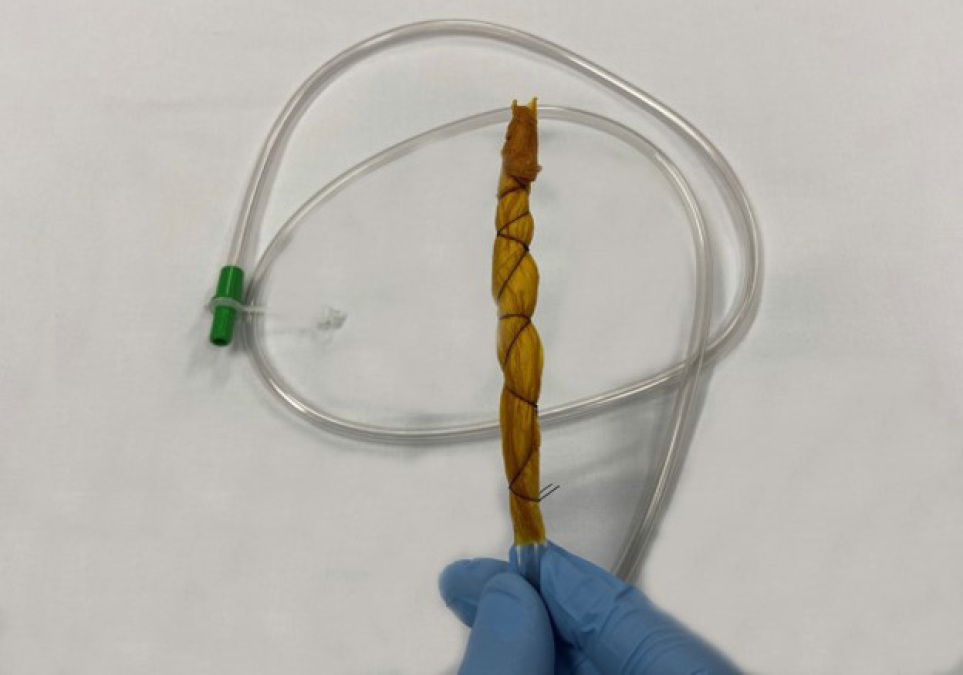
Preemptive endoluminal vacuum therapy was provided during surgery. The system implemented the Dr. Flaubert Sena technique[12] (Figure 3), using a nasogastric tube, antimicrobial incise drape, and gauze. The negative pressure was constant at 125 mmHg. The nasoenteral feeding tube was placed through the alimentary limb (Figure 4), and enteral feeding was initiated 12 h postoperatively. The endoscopic vacuum system was removed on postoperative day 3, without adverse events or symptoms.
The peripancreatic abdominal drain was removed on postoperative day 5, and the amylases were measured. The patient was discharged 5 d after pancreaticoduodenectomy.
At the time of drafting this manuscript, the patient was being followed up in the outpatient clinic and had developed no complications related to the procedure. The Oncology Group opted for no adjuvant therapy.
Pancreaticoduodenectomy is a technically demanding operation, with morbidity rates of approximately 40%–50%. The mortality rate is around 2.6%, and 37%–43% of deaths are directly linked to pancreatic fistulas[1,2,13,14]. However, the prevention of this complication remains challenging, being related to non-modifiable factors (patient’s age, comorbidities, pancreatic texture, and pancreatic duct size) and modifiable variables (anastomosis technique, somatostatin analogs, bleeding, and massive blood transfusions)[6,8,14]. These variables influence the development of leaks and pancreatic fistulas.
Although cavity drainage is unlikely to influence the rate of these adverse events, we proposed the use of an endoscopic vacuum system to prevent pancreatic fistulas[9,15,16]. The system is feasible and effective for the treatment of transmural defects[12]. Tools for building the system include a nasogastric tube (14 Fr), gauze, an antimicrobial incise drape, and a nylon suture. The first step is to make several holes in the nasogastric tube and cut the antimicrobial incise drape to match the size of the fenestrated portion. Subsequently, using an 18-G needle, several holes are made in the antimicrobial incise drape. The next step is wrapping the fenestrated portion of the nasogastric tube using gauze and then with an antimicrobial incise drape. Finally, a 2.0 nylon suture is used to fix the gauze and antimicrobial incise drape at the nasogastric tube (Figure 5). Polyvinyl alcohol foam and polyurethane foam are alternatives for making the system. However, gauze is just as safe and effective as these materials, and is less expensive.
Similar to the therapeutic vacuum, the endoscopic preemptive vacuum may also promote continuous exudate evacuation, reduction of inflammatory edema, improvement of blood supply, and lymphatic drainage. The negative pressure also promotes microdeformation and macrodeformation, leading to angiogenic factors that increase local healing.
Liu et al[17] proposed that the increased pressure in anastomosis associated with pancreatic enzymes (proteases and phosphatases) could lead to increased leakage in non-hermetic anastomoses due to self-digestion of the anastomosis, and a negative pressure could prevent this process. Therefore, we believe that the endoscopic vacuum system decreases pressure in the biliopancreatic limb and reduces contact between the anastomosis and pancreatic juice. Thus, the preemptive vacuum can prevent leaks and pancreatic fistulas.
In a systematic review of 60739 patients, Sergio Pedrazzoli[7] supported this theory, stating that for fluid flow from one lumen to another, there must be a pressure differential between the means to overcome gravity and peristaltic activity. However, at present, these pressures are not documented. Furthermore, the use of endoluminal drains would favor the removal of biliopancreatic secretions and could be used for the infusion of protease inhibitors.
With regard to complications, a recent systematic review with a meta-analysis published by do Monte Junior et al[18] demonstrated bleeding, stricture, and difficulty in removal as the main adverse events. Difficulty in removing the system only occurred in one patient. Considering that the preemptive vacuum therapy system remains in the anastomosis for no longer than 3 d, and the novel system has no sponge, the risk of those complications is small, although they may still exist. Neumann et al[19] performed preemptive vacuum therapy for the treatment of anastomotic ischemia after esophageal resection. Using a continuous suction of 125 mmHg, complete mucosal recovery was achieved in 75% of cases. Thus, ischemia caused by this regimen of negative pressure is improbable.
To diagnose pancreatic fistulas, we used the criteria of the International Pancreatic Fistula Study Group modified in 2016[20]. Our patient had several risk factors for the development of this complication, such as age, malignancy, fat-substituted pancreas, pancreatic duct size < 3 mm, intraoperative transfusion, and preoperative malnutrition. Nevertheless, there were no complications during her evolution[21]. An early oral diet was initiated on postoperative day 2 when peristaltic movements returned.
Various techniques are used to minimize the risk of anastomosis dehiscence, including the application of a fibrin sealant or a fibrin glue-coated collagen patch. The first advantage of preemptive vacuum therapy is that it allows enteral feeding while reducing the risk of leak and fistulas. Compared with the modified vacuum system, a preemptive vacuum is a feasible and cost-effective method for preventing those circumstances. Aside from this, recent studies demonstrated that fibrin sealant patches had no significant effect on the rate of postoperative pancreatic fistula[22,23].
Preemptive endoluminal vacuum therapy might be a safe and feasible technique to reduce leaks after pancreaticoduodenectomy.
The authors thank Dr. Yuri Raoni, Department of Radiology, Heart Hospital, for providing magnetic resonance imaging findings.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cwaliński J S-Editor: Liu M L-Editor: Webster JR P-Editor: Liu JH
| 1. | Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, Sugihara K, Mori M. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 2. | Søreide K, Labori KJ. Risk factors and preventive strategies for post-operative pancreatic fistula after pancreatic surgery: a comprehensive review. Scand J Gastroenterol. 2016;51:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Wang C, Qi R, Li H, Shi X. Comparison of Perioperative and Oncological Outcomes of Hybrid and Totally Laparoscopic Pancreatoduodenectomy. Med Sci Monit. 2020;26:e924190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Gubler C, Vetter D, Schmidt HM, Müller PC, Morell B, Raptis D, Gutschow CA. Preemptive endoluminal vacuum therapy to reduce anastomotic leakage after esophagectomy: a game-changing approach? Dis Esophagus. 2019;32:doy126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | D'Urso A, Komen N, Lefevre JH. Intraluminal flexible sheath for the protection of low anastomosis after anterior resection: results from a First-In-Human trial on 15 patients. Surg Endosc. 2020;34:5107-5116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Z'graggen K, Uhl W, Friess H, Büchler MW. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg. 2002;9:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 7. | Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017;96:e6858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 8. | Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2013;2013:CD008370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Borejsza-Wysocki M, Szmyt K, Bobkiewicz A, Malinger S, Świrkowicz J, Hermann J, Drews M, Banasiewicz T. Endoscopic vacuum-assisted closure system (E-VAC): case report and review of the literature. Wideochir Inne Tech Maloinwazyjne. 2015;10:299-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Noh SM, Ahn JY, Lee JH, Jung HY, AlGhamdi Z, Kim HR, Kim YH. Endoscopic Vacuum-Assisted Closure Therapy in Patients with Anastomotic Leakage after Esophagectomy: A Single-Center Experience. Gastroenterol Res Pract. 2018;2018:1697968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 11. | de Moura DTH, do Monte Junior ES, Hathorn KE, Ribeiro IB, de Medeiros FS, Thompson CC, de Moura EGH. The use of novel modified endoscopic vacuum therapies in the management of a transmural rectal wall defect. Endoscopy. 2020;epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | de Moura DTH, de Moura BFBH, Manfredi MA, Hathorn KE, Bazarbashi AN, Ribeiro IB, de Moura EGH, Thompson CC. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc. 2019;11:329-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (6)] |
| 13. | Ansari D, Andersson R. Pancreatic ductal adenocarcinoma: is a cure possible? Future Oncol. 2017;13:863-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Machado NO. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol. 2012;2012:602478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Diener MK, Tadjalli-Mehr K, Wente MN, Kieser M, Büchler MW, Seiler CM. Risk-benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbecks Arch Surg. 2011;396:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, Wakabayashi G, Kakeji Y, Mori M, Seto Y, Unno M. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci. 2017;24:243-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Liu QY, Zhang WZ, Xia HT, Leng JJ, Wan T, Liang B, Yang T, Dong JH. Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol. 2014;20:17491-17497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | do Monte Junior ES, de Moura DTH, Ribeiro IB, Hathorn KE, Farias GFA, Turiani CV, de Medeiros FS, Bernardo WM, de Moura EGH. Endoscopic vacuum therapy vs endoscopic stenting for upper gastrointestinal transmural defects: systematic review and meta-analysis. Digest Endosc. 2020 Epub ahead of print. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Neumann PA, Mennigen R, Palmes D, Senninger N, Vowinkel T, Laukoetter MG. Pre-emptive endoscopic vacuum therapy for treatment of anastomotic ischemia after esophageal resections. Endoscopy. 2017;49:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Pulvirenti A, Ramera M, Bassi C. Modifications in the International Study Group for Pancreatic Surgery (ISGPS) definition of postoperative pancreatic fistula. Transl Gastroenterol Hepatol. 2017;2:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Ogawa D, Hayashi H, Kitamura F, Uemura N, Miyata T, Okabe H, Imai K, Yamasita Y, Kubo S, Baba H. Multiple cholangiocarcinomas in the intrahepatic and extrahepatic biliary tree due to dichloromethane exposure: a case report. Surg Case Rep. 2020;6:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Kwon J, Shin SH, Lee S, Park G, Park Y, Lee SJ, Lee W, Song KB, Hwang DW, Kim SC, Lee JH. The Effect of Fibrinogen/Thrombin-Coated Collagen Patch (TachoSil®) Application in Pancreaticojejunostomy for Prevention of Pancreatic Fistula After Pancreaticoduodenectomy: A Randomized Clinical Trial. World J Surg. 2019;43:3128-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Park JS, Lee DH, Jang JY, Han Y, Yoon DS, Kim JK, Han HS, Yoon Y, Hwang D, Kang CM, Hwang HK, Lee WJ, Heo J, Chang YR, Kang MJ, Shin YC, Chang J, Kim H, Jung W, Kim SW. Use of TachoSil(®) patches to prevent pancreatic leaks after distal pancreatectomy: a prospective, multicenter, randomized controlled study. J Hepatobiliary Pancreat Sci. 2016;23:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |