Published online Nov 16, 2020. doi: 10.4253/wjge.v12.i11.469
Peer-review started: April 20, 2020
First decision: June 15, 2020
Revised: June 24, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 16, 2020
Processing time: 210 Days and 1.2 Hours
Endoscopic retrograde cholangiopancreatography (ERCP) is the primary therapeutic procedure for the treatment of diseases affecting the biliary tree and pancreatic duct. Although the therapeutic success rate of ERCP is high, the procedure can cause complications, such as acute pancreatitis [post-ERCP pancreatitis (PEP)], bleeding and perforation.
To assess the efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) in preventing PEP during follow-up.
Databases such as MEDLINE, EMBASE and Cochrane Central Library were searched. Only randomized controlled trials (RCTs) comparing the efficacy of NSAIDs and placebo for the prevention of PEP were included. Outcomes evaluated included the incidence of PEP, severity of pancreatitis, route of administration, types, dose, and timing of administration of NSAIDs.
Twenty-six RCTs were considered eligible with a total of 8143 patients analyzed. Overall, 4020 patients used NSAIDs before ERCP and 4123 did not use NSAIDs (control group). Ultimately, 298 cases of post-ERCP acute pancreatitis were diagnosed in the NSAID group and 484 cases in the placebo group. The risk of PEP was lower in the NSAID group risk difference (RD): -0.04; 95% confidence interval (CI): -0.07 to - 0.03; number needed to treat (NNT), 25; P < 0.05. NSAID use effectively prevented mild pancreatitis compared to placebo use (2.5% vs 4.1%; 95%CI: -0.05 to -0.01; NNT, 33; P < 0.05), but information on moderate PEP and severe PEP could not be fully elucidated. Only rectal administration reduced the incidence of PEP with RD: -0.06; 95%CI: -0.08 to -0.04; NNT, 17; P < 0.05). Furthermore, only the use of diclofenac or indomethacin was effective in preventing PEP, at a dose of 100 mg, which must be administered before performing ERCP.
Rectal administration of diclofenac and indomethacin significantly reduced the risk of developing mild PEP. Additional RCTs are needed to compare the efficacy between NSAID routes of administration in preventing PEP.
Core Tip: The present systematic review and meta-analysis shows that the use of non-steroidal anti-inflammatory drugs reduced the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP). This review is the first to be carried out in Latin America with a large number of randomized controlled trials. The present study shows that rectal administration of diclofenac and indomethacin before endoscopic retrograde cholangiopancreatography can reduce the incidence of mild PEP in high, medium and low risk patients.
- Citation: Román Serrano JP, Jukemura J, Romanini SG, Guamán Aguilar PF, Castro JSL, Torres IT, Sanchez Pulla JA, Micelli Neto O, Taglieri E, Ardengh JC. Nonsteroidal anti-inflammatory drug effectivity in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. World J Gastrointest Endosc 2020; 12(11): 469-487
- URL: https://www.wjgnet.com/1948-5190/full/v12/i11/469.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i11.469
Endoscopic retrograde cholangiopancreatography (ERCP) is a useful tool in the treatment of biliopancreatic duct diseases with high technical and clinical success rates. The most common post-ERCP adverse events (AEs) are acute pancreatitis (AP), bleeding, perforation, and cholangitis[1]. AP is the most common, with an incidence between 3.5% and 9.7% and mortality ranging from 0.1% to 0.7%[2].
Mild AP is defined as the absence of organ failure and/or local and systemic complications, moderate AP as the presence of transient organ failure or local or systemic complications, and severe AP as the presence of persistent organ failure with or without complications. Persistent organ failure has a risk of mortality between 36% and 50% within the first phase[3]. Post-ERCP pancreatitis (PEP) is mild in 4%, moderate in 1.8% to 2.8%, and severe in 0.3% to 0.5%[4,5].
Risk factors associated with PEP are divided into patient- and procedure-related factors. Patient-related factors include sphincter of Oddi dysfunction (SOD), female gender, history of AP, and history of PEP, whereas procedure-related factors include difficult catheterization, passage of a guidewire in the main pancreatic duct (MPD) ≥ 1 time, and pancreatic injection ≥ 1 time[2]. The search for methods to prevent the occurrence of PEP is important to increase patient safety and reduce the incidence rate.
Studies have described preventive measures to avoid the occurrence of PEP, such as the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and pancreatic stent implantation. Theoretically, the use of NSAIDs that inhibit cyclooxygenase 2 (COX-2) improves the acute inflammatory effects of AP and reduces its systemic sequelae[6]. NSAIDs that inhibit phospholipase A2 (indomethacin and diclofenac) play a role in the early phase of the inflammatory cascade in AP. Research on the use of NSAIDs to prevent PEP started in the 1980s[7]. Randomized trials in animals have shown that indomethacin has a low mortality rate[7]. Its properties prevent papillary edema, at least theoretically decreasing the occurrence of PEP.
This systematic review and meta-analysis was performed to determine the effectiveness of NSAIDs in preventing PEP. The objective was to analyze the appropriate dose, route, time of administration, and the best NSAIDs to reduce the incidence of PEP.
This systematic review and meta-analysis was carried out in accordance with the recommendations of the Cochrane manual, following the items in the preferred reporting items for systematic reviews and meta-analyses (PRISMA)[8]. The review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database, under registration number 42016049582, and approved by the ethics committee of the Moriah Hospital, São Paulo, Brazil.
The eligibility criteria were organized according to the international standards for patient, intervention, comparison, and outcome. “Patient” (P) was those submitted to ERCP, “intervention” (I) was administration of different types of NSAIDs described in the literature, “comparison” (C) was the administration of placebo or other similar drugs to NSAIDs, and “outcome” (O) was the main outcome of PEP. The research was carried out using different databases or virtual libraries, among which were MEDLINE/PubMed, Embase, and central Cochrane library. The dates used were from the beginning of our study in July 2016 to December 2019.
The key words used in the MEDLINE research were ERCP, NSAIDs, pancreatitis, diclofenac, and indomethacin. For other databases, we used simpler terms, such as ERCP, pancreatitis, and NSAID. All types of studies that assessed the reduction in the incidence of PEP were researched. In this systematic review and meta-analysis, we included only randomized clinical trials (RCTs) that studied the incidence of PEP with the use of NSAIDs.
We excluded meta-analyses, prospective nonrandomized, retrospective studies, case series, pancreatic stent studies, NSAID vs NSAID, drugs that were not in the NSAID group, and abstracts and papers that were requested by the author without response. There was no restriction on the language and date of publication.
We included patients of any gender > 18 years old who underwent ERCP for the first time and with signed informed consent. We excluded those with previous sphincterotomy, periampullary tumor, signs of evident AP, chronic pancreatitis, allergies to NSAIDs, and active and healing gastric and duodenal ulcers.
The main outcome was the reduction in the overall incidence of PEP with the use of NSAIDs. We evaluated the reduction in incidence in relation to the severity of PEP (mild, moderate, and severe), types of NSAIDs (diclofenac, indomethacin, valdecoxib, ketoprofen, naproxen, and celecoxib), different routes of administration [rectal (R), oral (O), intramuscular (IM), and intravenous (IV)], and dose and time of administration (before, during, after, and before/after ERCP).
Two reviewers selected RCTs independently and by group analysis. Any disagreement was resolved by the reviewers and group members after consensus. The study selection process was described in the PRISMA flowchart[8]. This systematic review and meta-analysis was organized in relation to the critical assessment instruments according to the type of design of the JADAD scale[9]. Each study was classified according to the risk of bias, randomization, allocation, blinding, losses, prognostic factors, results, and patient number needed to treat (NNT).
Data were extracted based on the information on treatment intention. For all outcomes, risk difference (RD) was considered for analysis with a 95% confidence interval and statistical significance of P < 0.05. The difference between the outcomes of the analysis of each subgroup was calculated through RD together with dichotomous variables.
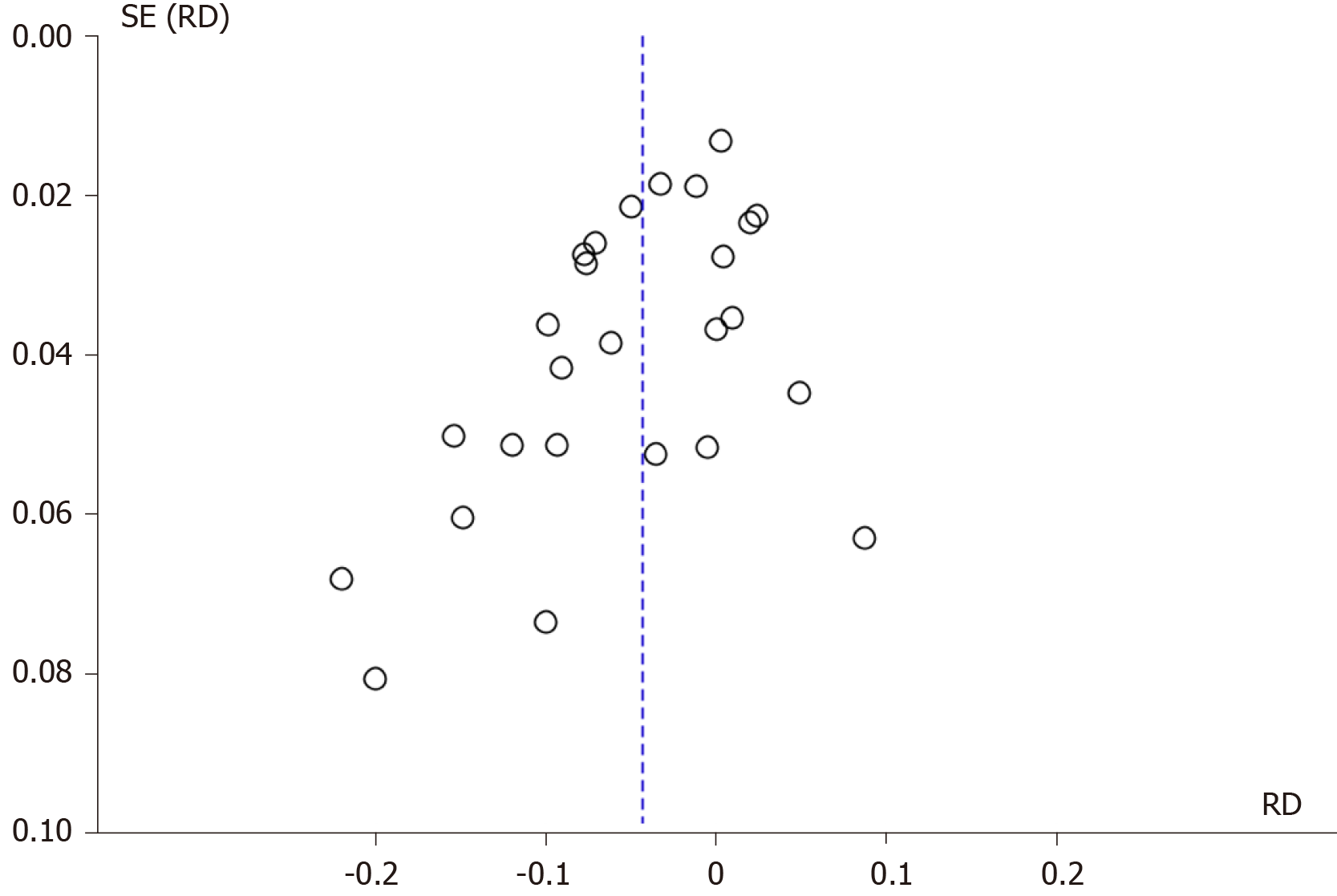
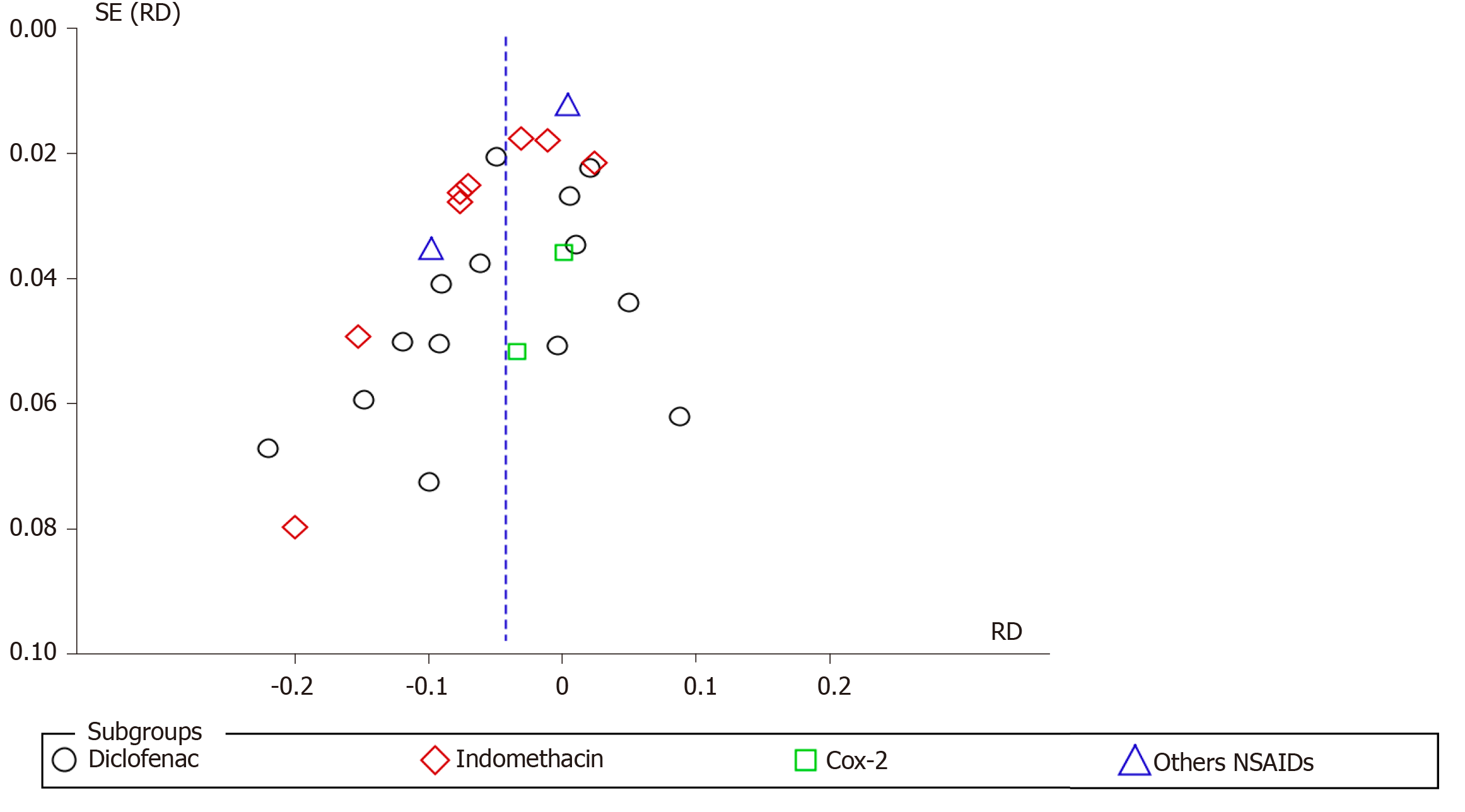
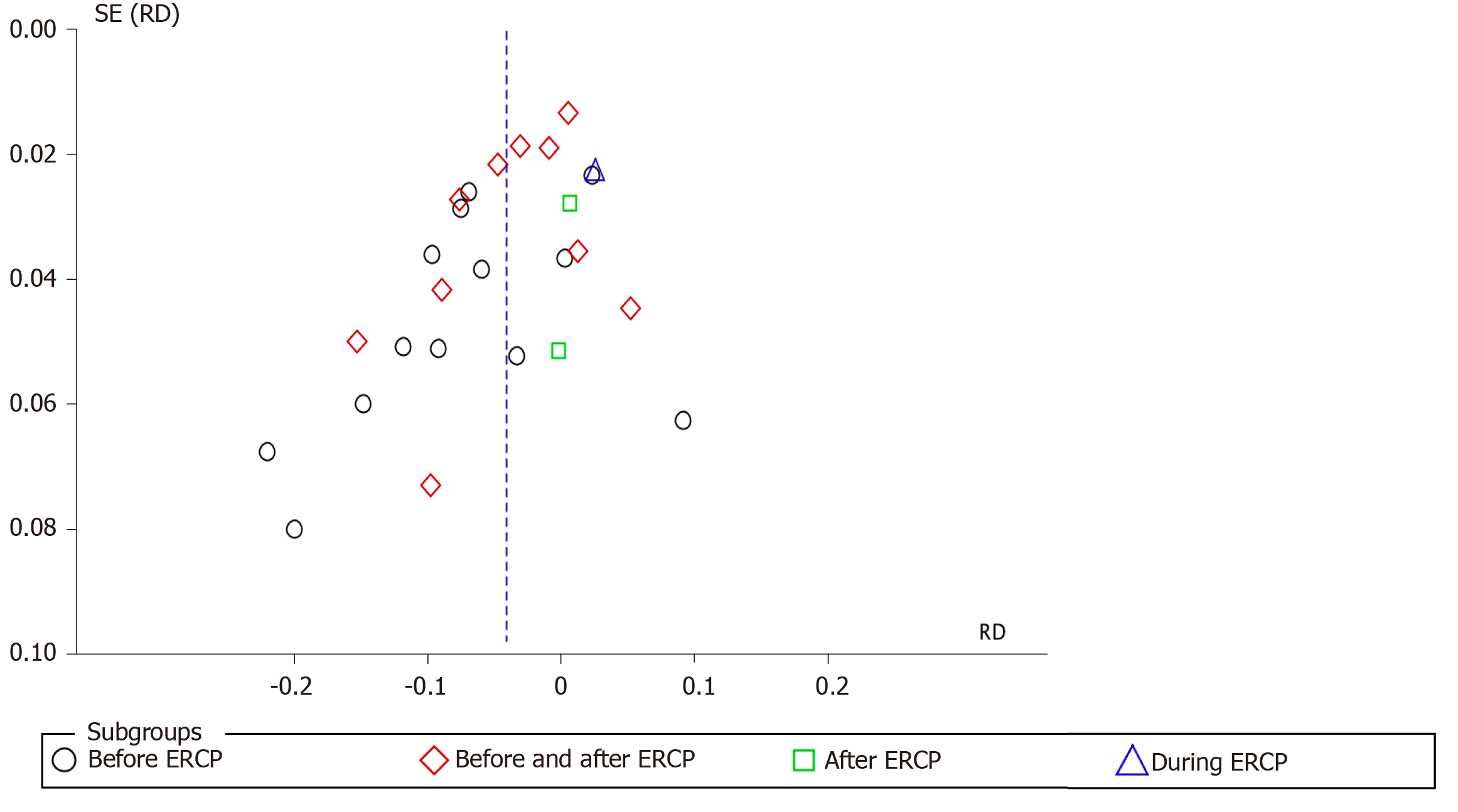
The analysis was performed with the statistical software RevMan 5.3 using the Mantel–Haenszel (MH) test with fixed effect (FE). Heterogeneity was considered by I2, with a cutoff of 50%. When a value ≥ 50% was found, sensitivity analysis was performed to try to identify a study with a higher probability of publication bias (“outlier”), through graphic expression of the “funnel plot” with the model or FE.
The sensitivity study aimed to identify the publication bias that justifies heterogeneity through the Egger funnel plot test. Once the publication biases were identified, which maintained heterogeneity ≥ 50%, it was decided to work with RD and randomized effect (RE) and work or interpret within the present systematic review and meta-analysis with a substantial or true heterogeneity.
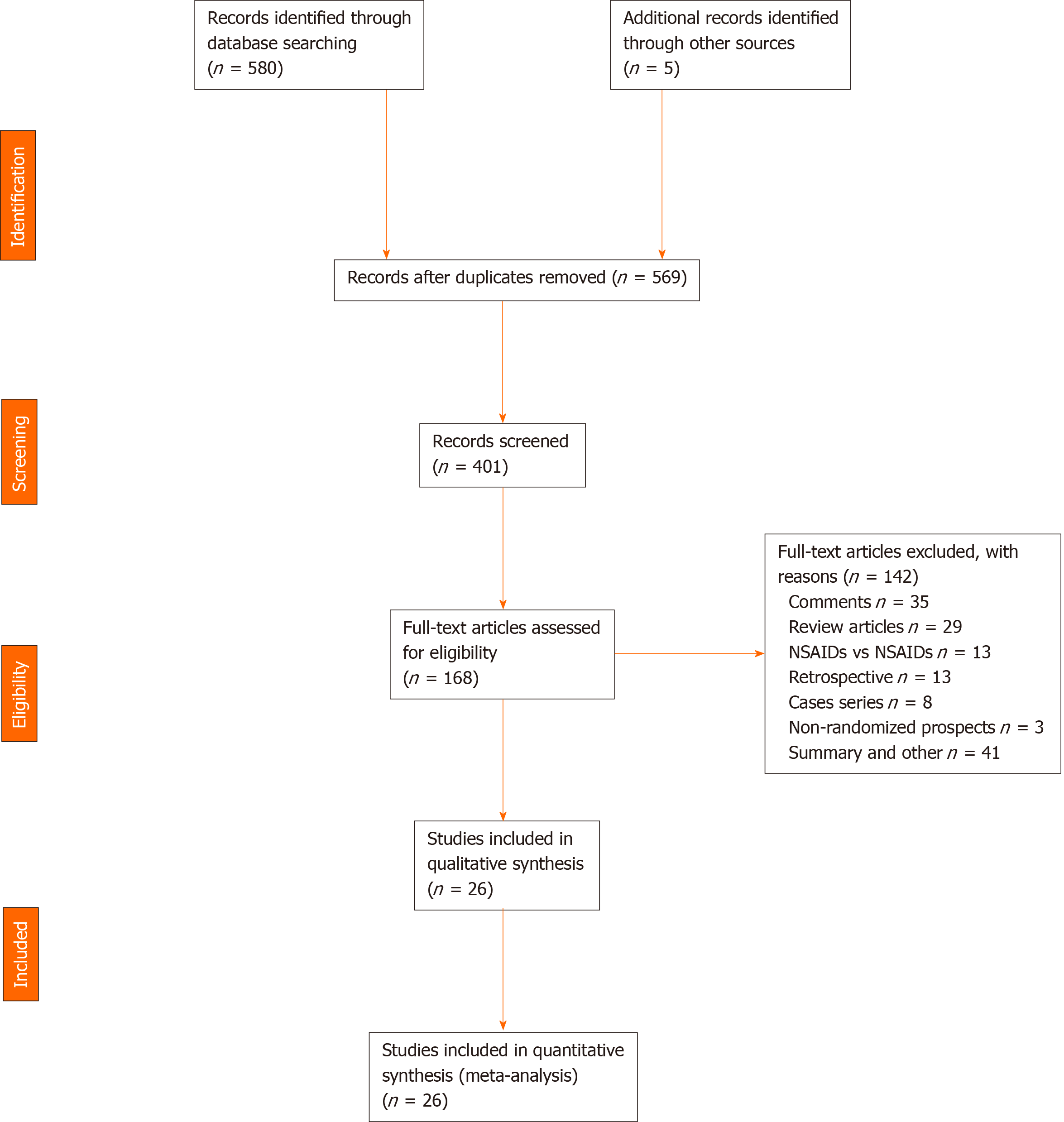
The evaluated articles are presented in the PRISMA flowchart and include 26 RCTs, 142 article were excluded (Figure 1). The 26 RCTs selected[6,7,10-33] were considered eligible and included a total of 8143 patients. The intervention group (NSAID) included 4020 patients and the comparison group (control) included 4123 patients (placebo and other substances).
We organized the studies after the consensus of two independent reviewers and after the group's consensus. Table 1 shows the included studies in alphabetical order, year, country of publication, route of administration, dose, and type of NSAIDs. Of the 26 RCTs, diclofenac was used in 12[10-21], indomethacin in 10[7,22-30], COX-2 inhibitors in 2[6,31], and other NSAIDs in 2[32,33]. Table 2 shows the included studies in alphabetical order, type of substance used (comparison) and number (n), and time of NSAID administration.
| Ref. | Year | Country | Route | Dose | NSAID type |
| Andrade et al[24], 2015 | 2015 | México | R | 100 mg | Indomethacin |
| Bhatia et al[6], 2011 | 2011 | India | IV | 20 mg | Valdecoxib |
| Cheon et al[10], 2007 | 2007 | United States | O | 50 mg | Diclofenac |
| Döbrönte et al[7], 2014 | 2014 | Hungary | R | 100 mg | Indomethacin |
| Elmunzer et al[25], 2012 | 2012 | United States | R | 100 mg | Indomethacin |
| Hauser et al[11], 2016 | 2016 | Croatia | R | 100 mg | Diclofenac |
| Ishiwatari et al[12], 2016 | 2016 | Japan | O | 100 mg | Diclofenac |
| Kato et al[31], 2017 | 2017 | Japan | O | 400 mg | Celecoxib |
| Kato et al[13], 2019 | 2019 | Japan | R | 25/50 mg | Diclofenac |
| Khoshbaten et al[14], 2008 | 2008 | Iran | R | 50 mg | Diclofenac |
| Leerhøy et al[15], 2016 | 2016 | Denmark | R | 100 mg | Diclofenac |
| Levenick et al[26], 2016 | 2016 | United States | R | 100 mg | Indomethacin |
| Li et al[27], 2019 | 2019 | China | R | 100 mg | Indomethacin |
| Lua et al[16], 2015 | 2015 | Malaysia | R | 100 mg | Diclofenac |
| Mansour et al[32], 2016 | 2016 | Iran | R | 500 mg | Naproxen |
| Masjedizadeh et al[26], 2017 | 2017 | Iran | R | 50 mg | Indomethacin |
| Montaño et al[23], 2007 | 2007 | México | R | 100 mg | Indomethacin |
| Hosseini et al[28], 2016 | 2016 | Iran | R | 100 mg | Indomethacin |
| Murray et al[17], 2003 | 2003 | Scotland | R | 100 mg | Diclofenac |
| Otsuka et al[18], 2012 | 2012 | Japan | R | 50 mg | Diclofenac |
| Park et al[21], 2014 | 2014 | South Korea | IM | 100 mg | Diclofenac |
| Patai et al[29], 2015 | 2015 | Hungary | R | 100 mg | Indomethacin |
| Quadros et al[33], 2016 | 2016 | Brazil | IV | 100 mg | Ketoprofen |
| Senol et al[19], 2009 | 2009 | United States | IV | 50 mg | Diclofenac |
| Sotoudehmanesh et al[30], 2007 | 2007 | Iran | R | 100 mg | Indomethacin |
| Uçar et al[20], 2016 | 2016 | Turkey | IM and IV | 75/100 mg | Diclofenac |
| Ref. | Comparison (n) | Administration time (after, before, and during) | n | Intervention |
| Andrade et al[24], 2015 | Glycerin (84) | Before ERCP | 166 | 82 |
| Bhatia et al[6], 2011 | Glyceryl trinitrate (127) | Before ERCP | 254 | 127 |
| Cheon et al[10], 2007 | Placebo SN (102) | Before and after ERCP | 207 | 105 |
| Döbrönte et al[7], 2014 | Placebo SN (318) | After ERCP | 665 | 347 |
| Elmunzer et al[25], 2012 | Placebo SN (307) | After ERCP | 602 | 295 |
| Hauser et al[11], 2016 | Ceftazidime (143) | Before ERCP | 272 | 129 |
| Ishiwatari et al[12]., 2016 | Placebo SN (214) | Before and after ERCP | 430 | 216 |
| Kato et al[31], 2017 | Saline solution (85) | Before ERCP | 170 | 85 |
| Kato et al[13]., 2019 | None (152) | Before ERCP | 303 | 151 |
| Khoshbaten et al[14], 2008 | Placebo SN (50) | Before ERCP | 100 | 50 |
| Leerhøy et al[15], 2016 | None (394) | After ERCP | 772 | 378 |
| Levenick et al[26], 2016 | Placebo SN (226) | During ERCP | 449 | 223 |
| Li et al[27], 2019 | Glycerin (50) | Before ERCP | 100 | 50 |
| Lua et al[16], 2015 | None (75) | After ERCP | 144 | 69 |
| Mansour et al[32], 2016 | Placebo SN (162) | Before ERCP | 324 | 162 |
| Masjedizadeh et al[26], 2017 | Placebo lactated Ringer’s solution (124) | Before ERCP | 186 | 62 |
| Montaño et al[23], 2007 | Glycerin (75) | Before ERCP | 150 | 75 |
| Hosseini et al[28], 2016 | Saline solution (205) | Before ERCP | 406 | 201 |
| Murray et al[17], 2003 | Placebo SN (110) | After ERCP | 220 | 110 |
| Otsuka et al[18], 2012 | Saline solution (53) | Before ERCP | 104 | 51 |
| Park et al[21], 2014 | Saline solution (170) | After ERCP | 343 | 173 |
| Patai et al[29], 2015 | Placebo SN (269) | Before ERCP | 539 | 270 |
| Quadros et al[33], 2016 | Saline solution (253) | After ERCP | 477 | 224 |
| Senol et al[19], 2009 | Placebo SN (40) | After ERCP | 80 | 40 |
| Sotoudehmanesh et al[30], 2007 | Placebo SN (245) | After ERCP | 490 | 245 |
| Uçar et al[20], 2016 | None (50) | Before ERCP | 150 | 100 |
| Total | - | - | 8103 | 4020 |
In assessing the risk of bias, all articles had adequate randomization, allocation, and blinding. The losses did not reach 20%. The JADAD score was above 3, which was satisfactory for inclusion in all studies. The description of each article is shown in Table 3. The time to diagnosis of PEP described in the RCTs ranged from 24 to 72 h and patients met at least two of Banks’ three diagnostic criteria: History of abdominal pain, nausea, or vomiting, increase in serum amylase, and images compatible with AP.
| Ref. | Randomization | Allocation | Blinding | Losses | Prognosis | AIT | JADAD |
| Andrade et al[24], 2015 | Yes | Yes | No | No | Homogeneous | Yes | 3 |
| Bhatia et al[6], 2011 | Yes | Yes | No | No | Homogeneous | No | 3 |
| Cheon et al[10], 2007 | Yes | Yes | Yes | Yes | Homogeneous | No | 5 |
| Döbrönte et al[7], 2014 | Yes | No | No | Yes | Homogeneous | No | 3 |
| Elmunzer et al[25], 2012 | Yes | Yes | Yes | No | Homogeneous | Yes | 5 |
| Hauser et al[11], 2016 | Yes | Yes | Yes | No | Homogeneous | Yes | 5 |
| Ishiwatari et al[12], 2016 | Yes | Yes | Yes | Yes | Homogeneous | No | 3 |
| Kato et al[31], 2017 | Yes | Yes | Yes | No | Homogeneous | Yes | 4 |
| Kato et al[13], 2019 | Yes | Yes | Yes | Yes | Homogeneous | No | 5 |
| Khoshbaten et al[14], 2008 | Yes | Yes | Yes | No | Homogeneous | No | 5 |
| Leerhøy et al[15], 2016 | Yes | No | No | No | Homogeneous | No | 3 |
| Levenick et al[26], 2016 | Yes | Yes | Yes | No | Homogeneous | Yes | 5 |
| Li et al[27], 2019 | Yes | Yes | Yes | Yes | Homogeneous | No | 5 |
| Lua et al[16], 2015 | Yes | Yes | No | Yes | Homogeneous | Yes | 3 |
| Mansour et al[32], 2016 | Yes | Yes | Yes | No | Homogeneous | Yes | 4 |
| Masjedizadeh et al[26], 2017 | Yes | No | Yes | No | Homogeneous | Yes | 4 |
| Montaño et al[23], 2007 | Yes | No | Yes | No | Homogeneous | No | 3 |
| Hosseini et al[28], 2016 | Yes | Yes | Yes | No | Homogeneous | No | 3 |
| Murray et al[17], 2003 | Yes | Yes | Yes | No | Homogeneous | No | 3 |
| Otsuka et al[18], 2012 | Yes | No | No | No | Homogeneous | Yes | 3 |
| Park et al[21], 2014 | Yes | Yes | Yes | No | Homogeneous | No | 3 |
| Patai et al[29], 2015 | Yes | Yes | Yes | Yes | Homogeneous | Yes | 5 |
| Quadros et al[33], 2016 | Yes | Yes | Yes | No | Homogeneous | Yes | 5 |
| Senol et al[19], 2009 | Yes | No | No | No | Homogeneous | No | 3 |
| Sotoudehmanesh et al[30], 2007 | Yes | Yes | Yes | No | Homogeneous | Yes | 4 |
| Uçar et al[20], 2016 | Yes | No | No | Yes | Homogeneous | No | 3 |
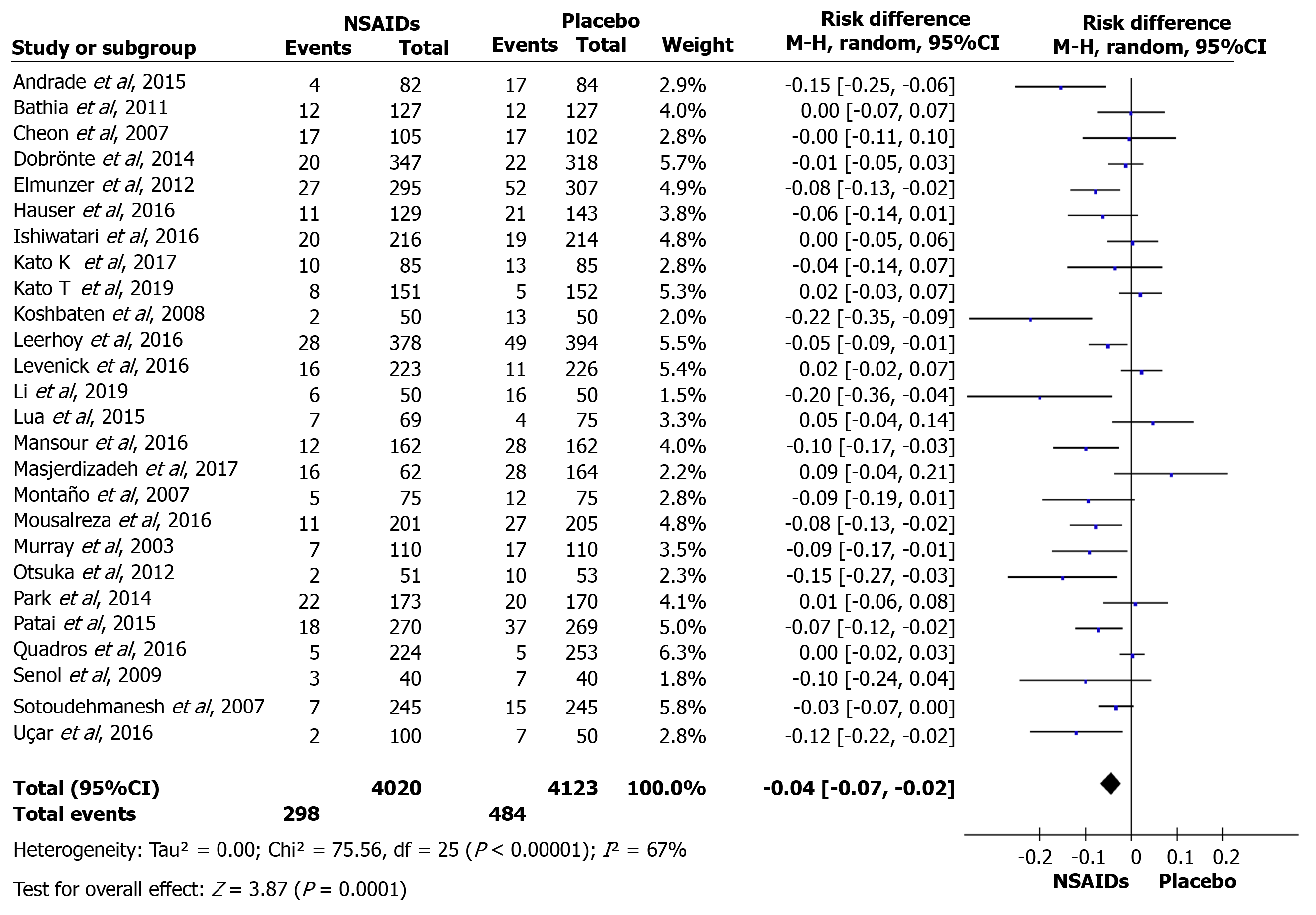
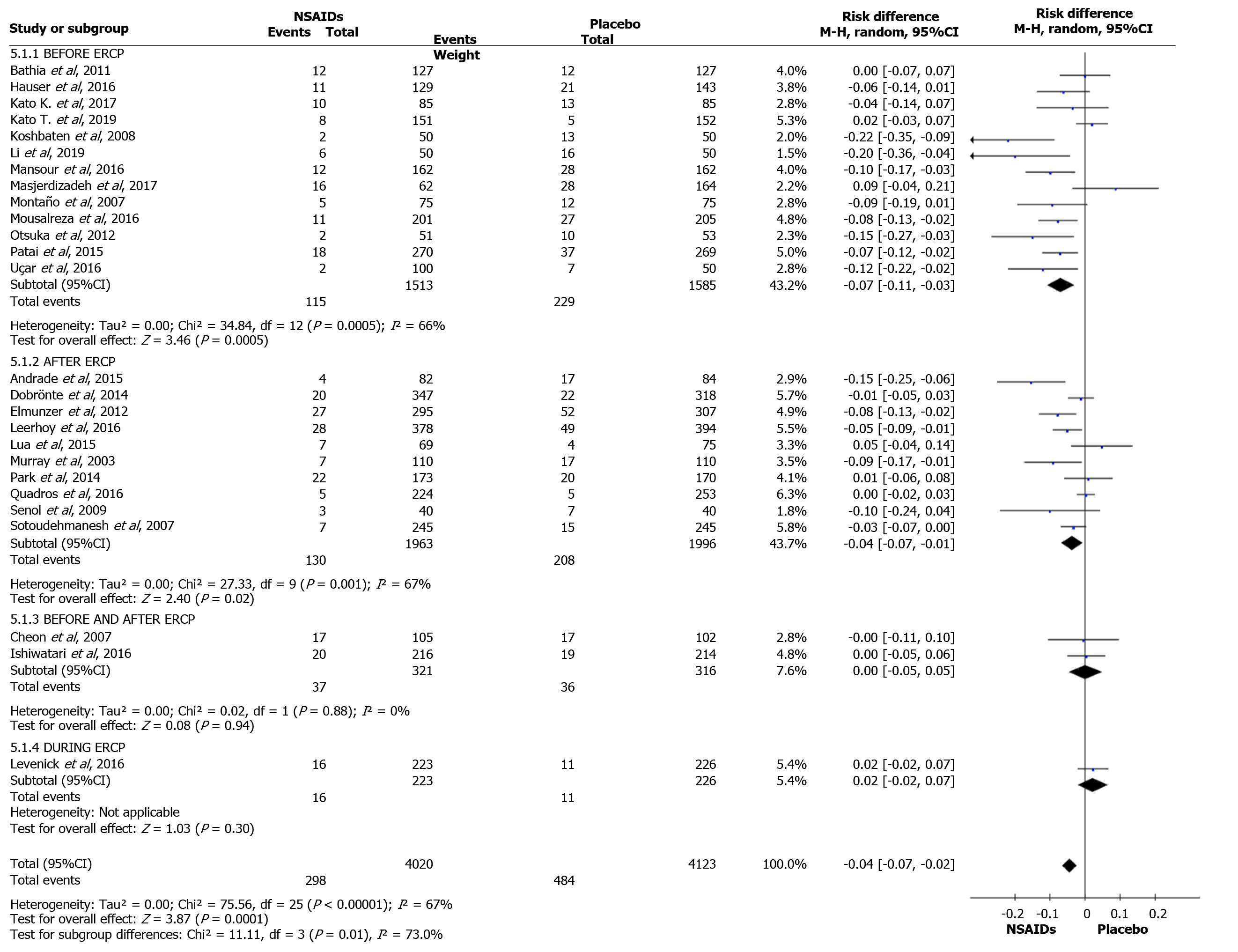
The overall incidence of PEP and a forest plot can be seen in Figure 2 and 3. In total there were 298 and 484 episodes of PEP in the intervention (4020) and comparison group (4123), respectively. RD was 95%CI -0.04 (-0.07, -0.03), P < 0.05, and NNT = 25.
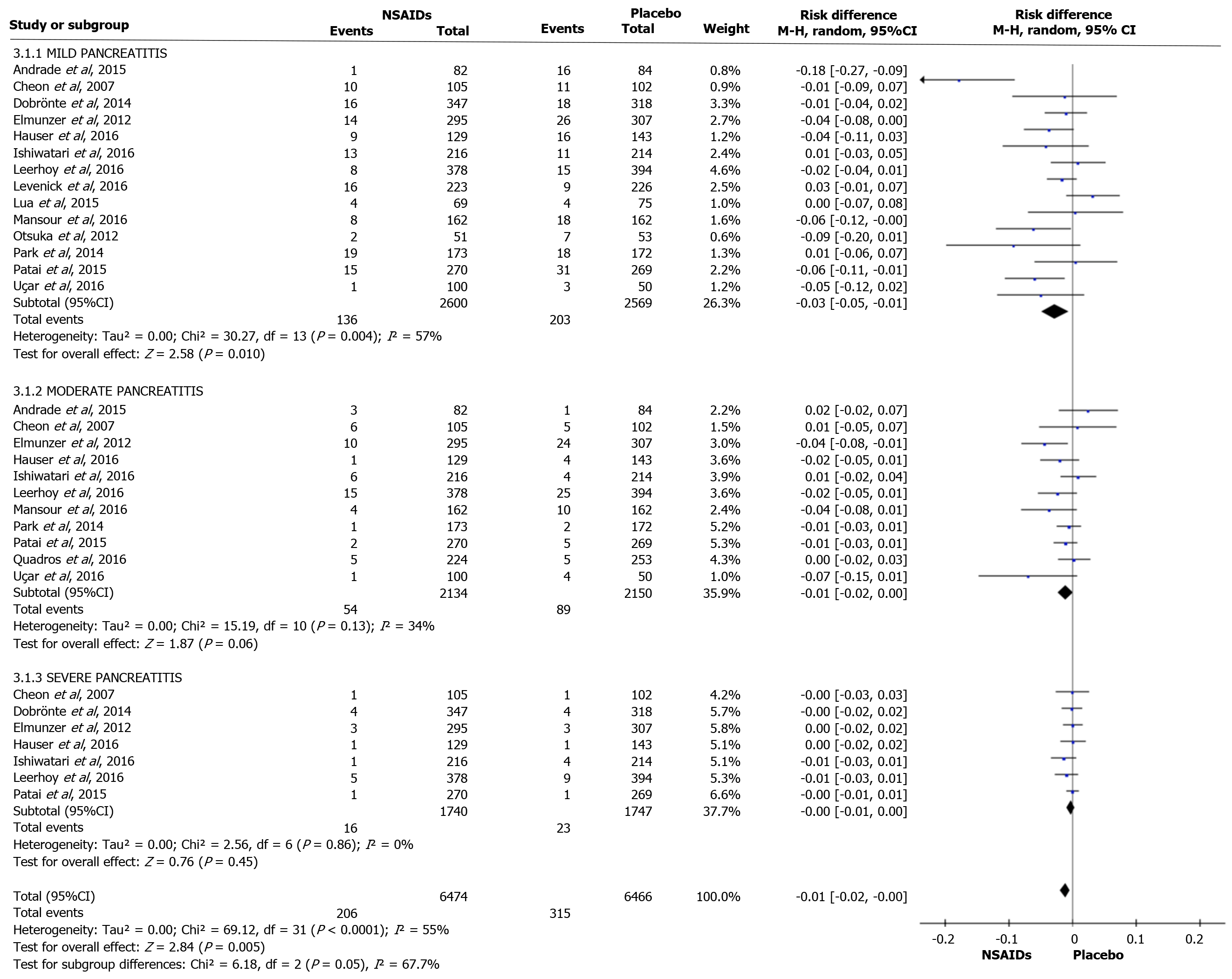
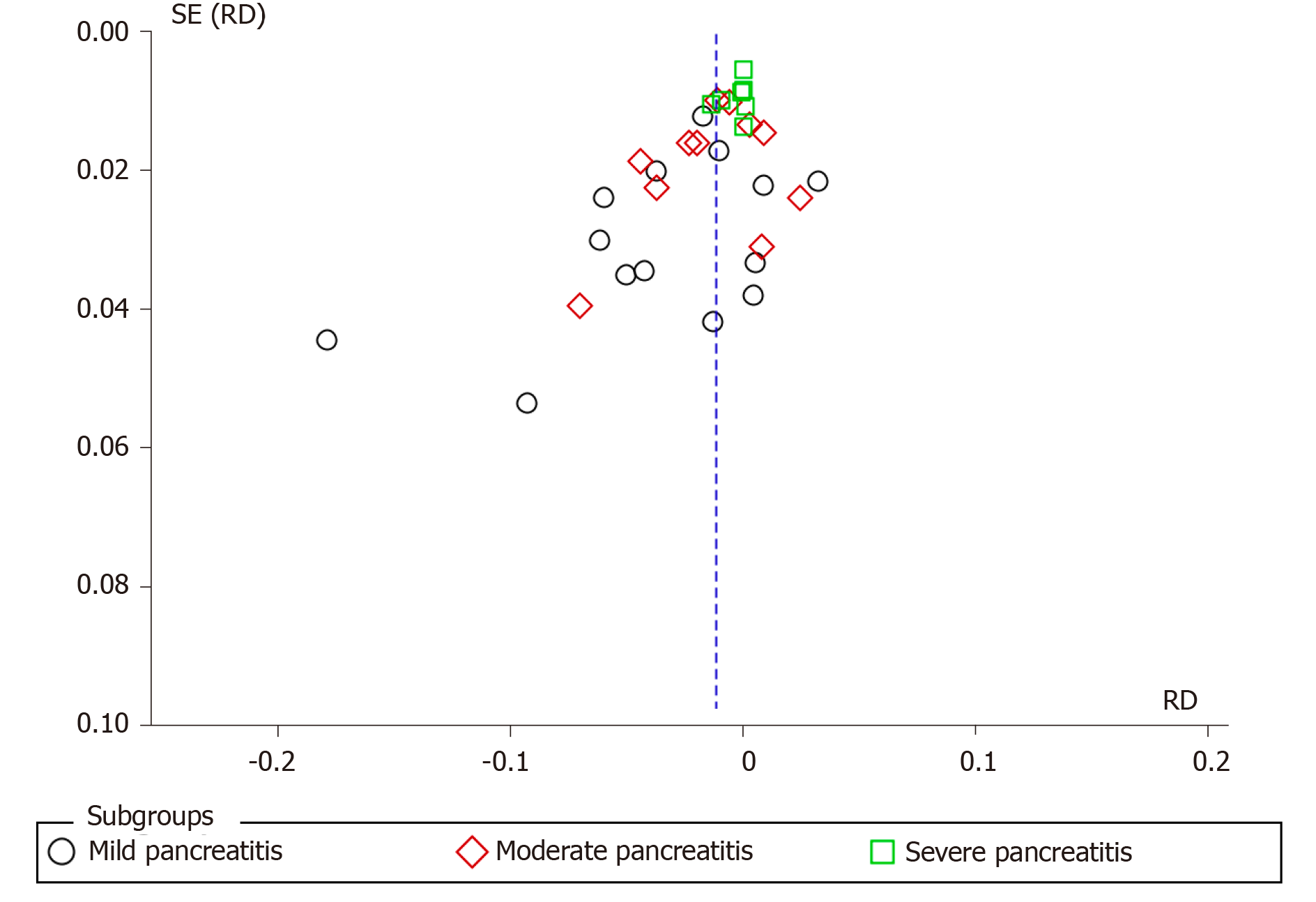
Fourteen articles evaluated the incidence rate of mild PEP. A total of 2600 and 2569 patients were allocated to the intervention and comparison groups, respectively. There were 136 and 203 episodes of mild AP in the intervention (2600) and comparison group (2569), respectively. RD was 95%CI 0.03 (-0.05, -0.01), P < 0.05, and NNT = 33. Eleven articles evaluated the incidence of moderate PEP. A total of 2134 and 2150 patients were allocated to the intervention and comparison groups, respectively. Moderate PEP was observed in 54 and 203 patients in the intervention and comparison group, respectively. RD was 95%CI -0.01 (-0.02, 0.00) and P > 0.05. Seven articles reported the incidence of severe PEP. A total of 1740 and 1747 patients were allocated to the intervention and comparison groups, respectively. Severe PEP was observed in 16 and 23 patients in the intervention and comparison group, respectively. RD was 95%CI -0.00 (-0.01, 0.00) and P > 0.05. The forest plot shows the severity of PEP (Figure 4 and 5).
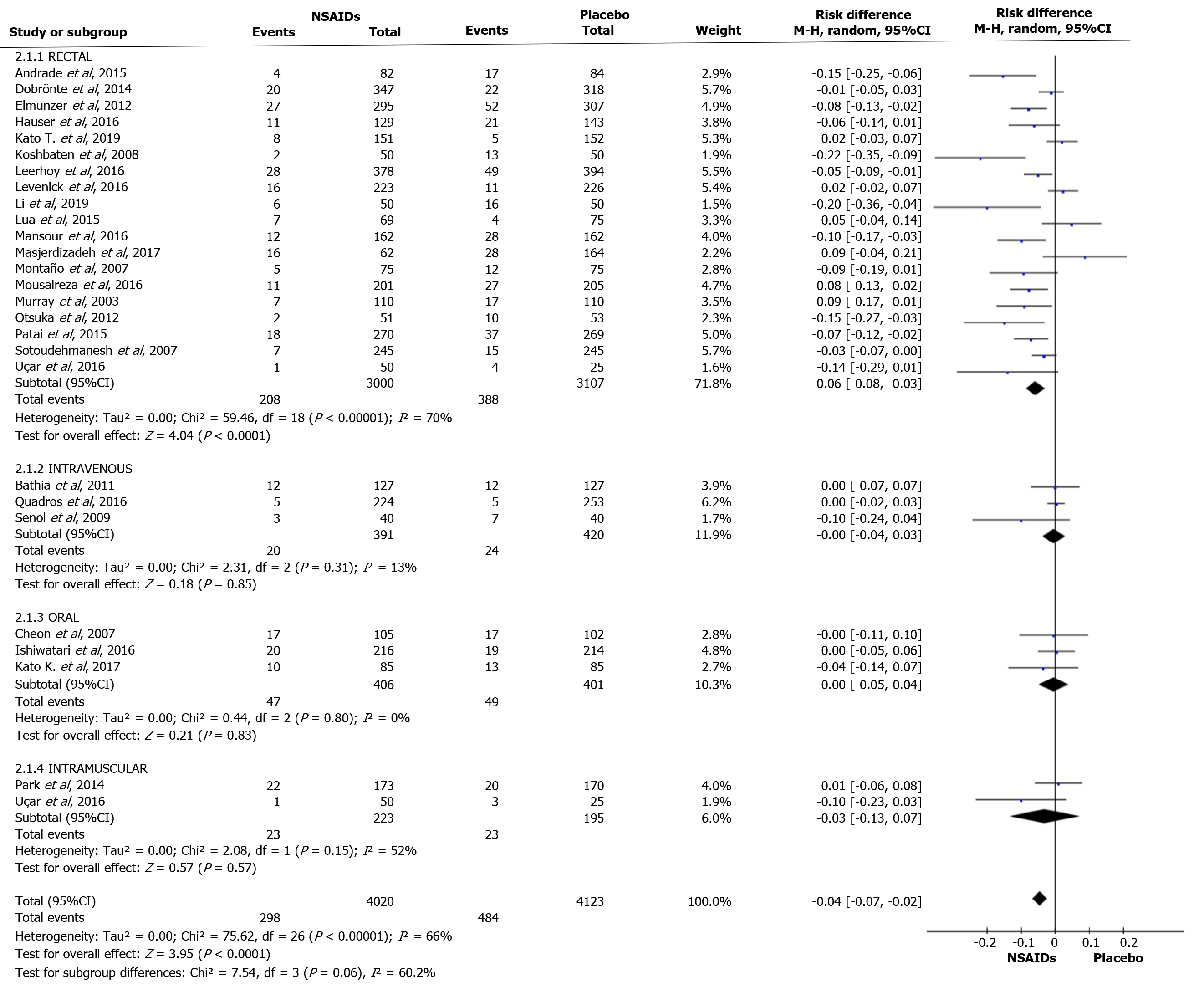
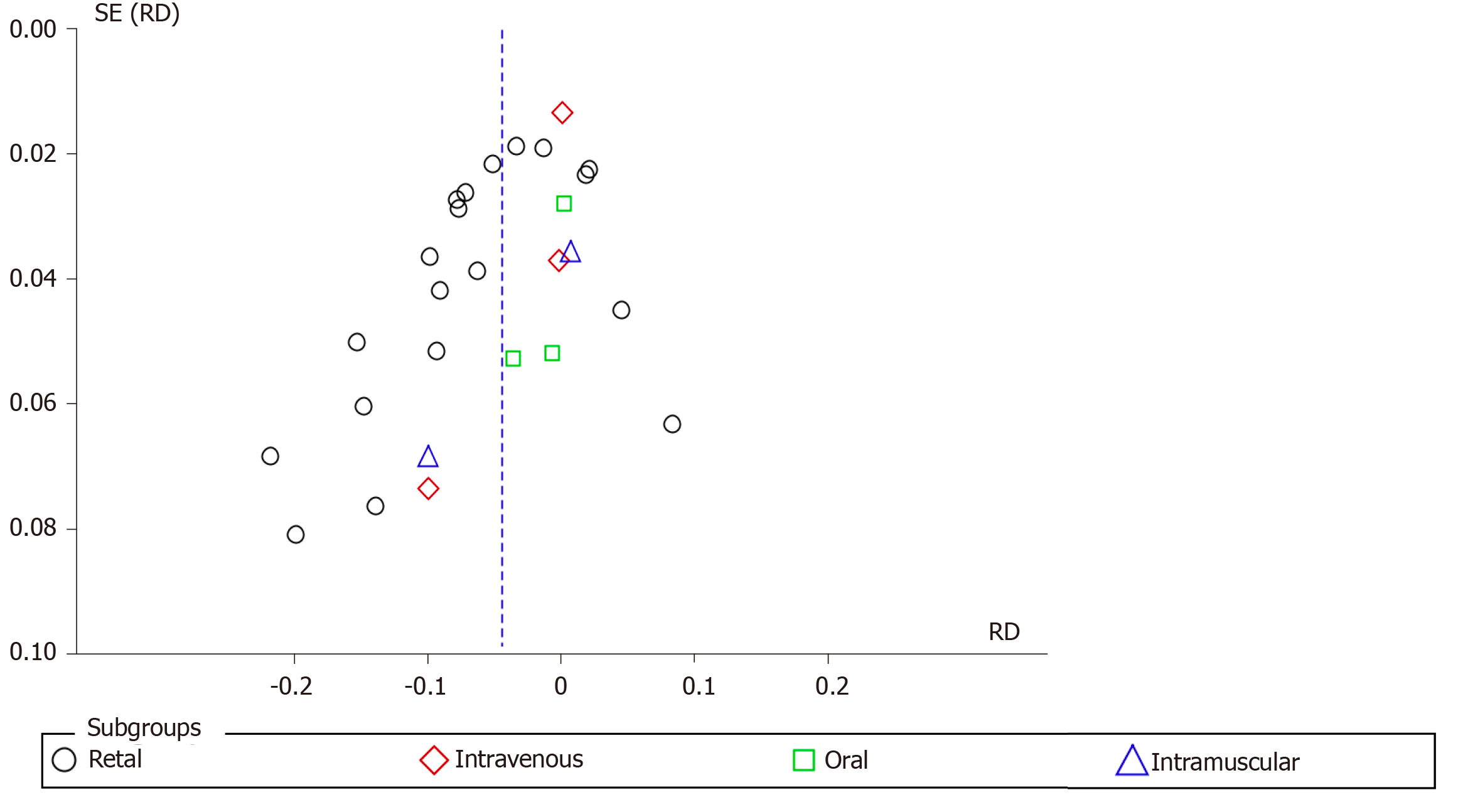
Nineteen articles described the rectal route for administering NSAIDs. A total of 3000 and 3017 patients were allocated to the intervention and comparison groups, respectively. PEP was observed in 208 and 388 patients in the intervention and comparison group, respectively. RD was 95%CI -0.06 (-0.08, -0.03), P < 0.05, and NNT = 17. In three articles, the IV route was described and the number of patients allocated to the intervention and comparison groups was 391 and 420 patients, respectively. PEP was observed in 20 and 24 patients in the intervention and comparison group, respectively. RD was 95%CI -0.00 (-0.04, 0.03) and P > 0.05. In three articles, the oral route of administration was described and the number of patients allocated to the intervention and comparison groups was 223 and 401 patients, respectively. PEP was observed in 47 and 49 patients in the intervention and comparison group, respectively. RD was 95%CI -0.00 (-0.05, 0.04) and P > 0.05. In two articles, the IM route was described, with 223 and 195 patients allocated to the intervention and comparison groups, respectively. PEP was observed in 23 and 23 patients in the intervention group and comparison group, respectively. RD was 95%CI -0.03 (-0.13, 0.07) and P > 0.05. The forest plot describes the different routes of administration (Figure 6 and 7).
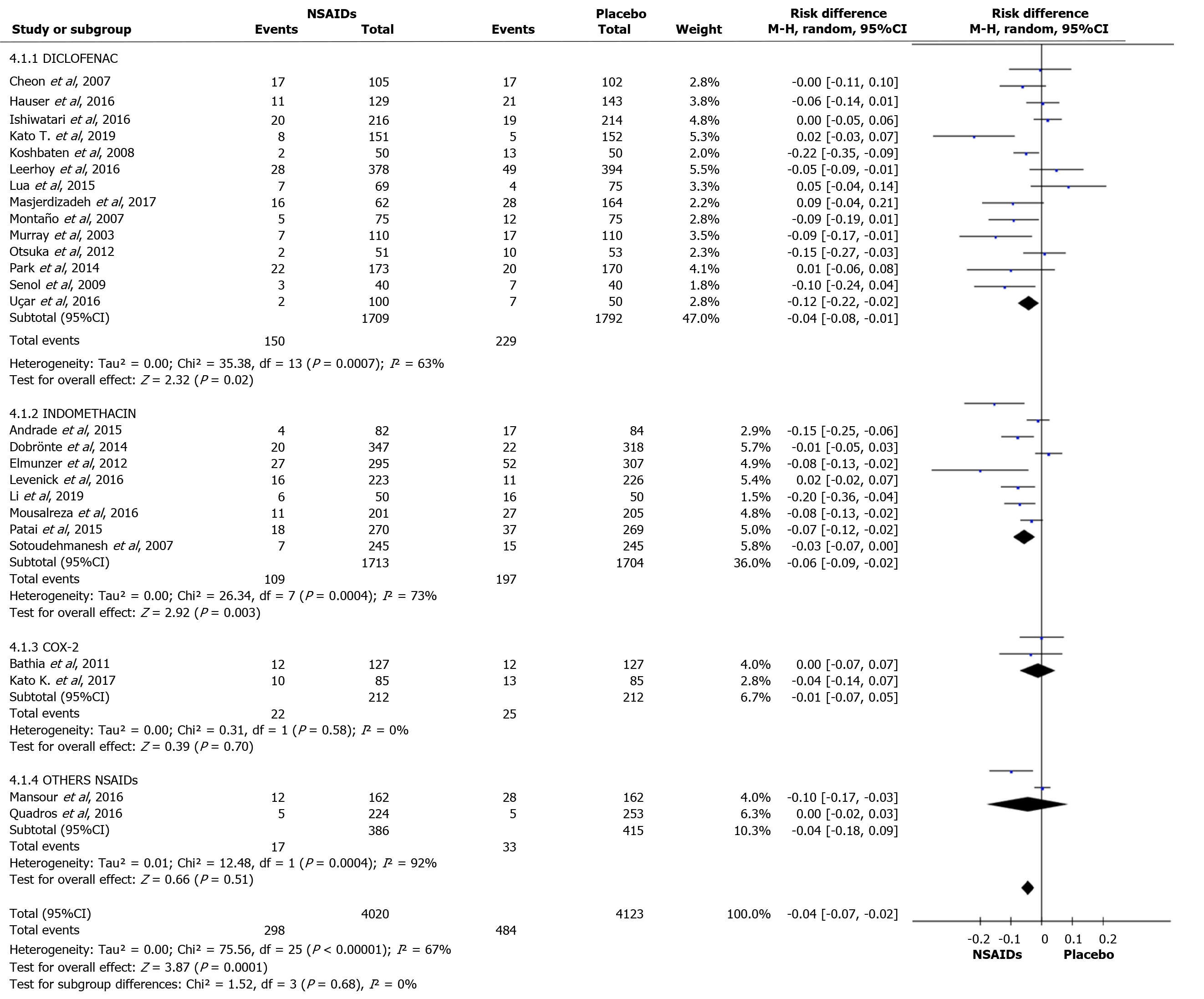
Diclofenac was used to prevent PEP in 15 articles. A total of 1709 and 1792 patients were allocated to the intervention and comparison groups, respectively. In the intervention and comparison group, PEP was observed in 150 and 229 patients, respectively. RD was 95%CI -0.04 (-0.08, -0.01), P < 0.05, and NNT = 25. Indomethacin was described in seven articles. A total of 1713 and 1704 patients were allocated to the intervention and comparison groups, respectively. In the intervention and comparison group, PEP was observed in 109 and 197 patients, respectively. RD was 95%CI -0.06 (-0.09, -0.02), P < 0.05, and NNT = 17. Two articles described the use of COX-2 inhibitors in the prevention of PEP. A total of 212 patients were allocated to the intervention and 212 to the comparison group. In the intervention and comparison groups, PEP was observed in 22 and 25 patients, respectively. RD was 95%CI -0.01 (-0.07, 0.05) and P > 0.05. Naproxen (1) and ketoprofen (1) have been described in the prevention of PEP. In the global analysis of both NSAIDs, 386 and 415 patients were allocated to the intervention and comparison group, respectively. In the intervention and comparison groups, 17 and 33 patients had PEP, respectively. RD was 95%CI -0.04 (-0.18, 0.09) and P > 0.05. Figure 8 and 9 shows the forest plot of the incidence of PEP using different types of NSAIDs.
Thirteen articles described the use of NSAIDs before ERCP to prevent PEP. A total of 1513 and 1585 patients were allocated to the intervention and comparison groups, respectively. PEP was observed in 115 and 229 patients in the intervention and comparison groups, respectively. RD was 95%CI -0.07 (-0.11, -0.03), P < 0. 05, and NNT = 14.
Ten articles described the use of NSAID after ERCP to prevent PEP. A total of 1963 and 1996 patients were allocated to the intervention and comparison groups, respectively. PEP was observed in 130 and 208 patients in the intervention and comparison groups, respectively. RD was 95%CI -0.04 (-0.07, -0.01), P < 0. 05, and NNT = 25. Two articles described the use of NSAID before and after ERCP to prevent PEP. A total of 321 and 316 patients were allocated to the intervention and comparison groups, respectively. PEP was observed in 37 and 36 patients in the intervention and comparison groups, respectively. RD was 95%CI 0.00 (-0.05, -0.05) and P > 0.05. Only one article described the use of NSAIDs during ERCP to prevent PEP. A total of 223 and 226 patients were allocated to the intervention and comparison groups, respectively. PEP was observed in 16 and 11 patients in the intervention and comparison groups, respectively. In this work, detailed statistical analysis was not possible. The forest plot in Figure 10 and 11 shows the incidence of PEP in relation to the timing of NSAID administration.
The use of NSAIDs and their impact on the prevention of PEP has been described in numerous RCTs. Although the number of RCTs is small and no convincing results have been presented, the major international societies of endoscopy and gastroenterology recommend its use in daily clinical practice, but make it clear that it is up to the endoscopist to decide whether or not to use it.
The European Society of Gastrointestinal Endoscopy (ESGE) recommends the use of diclofenac or indomethacin at a dose of 100 mg before ERCP in all patients whether they are at high, medium, or low risk for PEP and when there are no contraindications[2]. Japan Gastroenterological Endoscopy Society advocates a similar policy for the intrarectal administration of NSAIDs in all cases of ERCP when there are no contraindications[34]. The American Society for Gastrointestinal Endoscopy[35] recommends the administration of indomethacin in medium- and high-risk patients.
The Brazilian Society of Digestive Endoscopy does not define an effective method to prevent PEP. In Brazil, there are books dedicated to the subject that recommend the use of indomethacin as a method of preventing PEP[36]. A systematic Brazilian review showed a statistical significance with the use of indomethacin and diclofenac after analyzing 21 studies[37].
Unlike systematic reviews already published on NSAID use to reduce the risk of PEP, the current study included only RCTs, with a more robust methodology, in which an analysis was carried out in relation to the prevention of PEP and its incidence. This analysis according to the severity of AP episode, type of NSAID, dose, and time and route of administration showed a more detailed perception of important details, which contributed to a more robust conclusion.
The analysis of 26 RCTs showed a significant reduction in the risk of PEP with the use of NSAIDs in both high and low risk patients. However, this study revealed that AEs prevented by the use of NSAIDs mainly involved mild AP. This study showed the efficacy of rectal indomethacin (100 mg) or diclofenac (100 mg) before ERCP, with statistical significance and lower NNT compared to post-ERCP administration.
Due to the small number of RCTs published in the literature, it was not possible to identify whether another route of administration (oral, IV, and IM), another type of NSAID, another time of administration, and doses lower or greater than 100 mg are effective in preventing PEP. Thus, further large multicenter RCTs comparing other NSAIDs, other routes, and times and doses of administration are required to obtain robust conclusions. However, decisions on NSAIDs may be influenced by cost, as indomethacin is more expensive than diclofenac. A cost comparison of the types of NSAIDs to decrease the incidence of PEP should be conducted, in order to obtain more data on this issue. To our knowledge, this is the first meta-analysis on the prevention of PEP using NSAIDs, which includes all types of NSAIDs described in the literature, such as diclofenac, indomethacin, naproxen, valdecoxib, celecoxib, and ketoprofen.
COX-2 inhibitors, regardless of the initial trigger (the injured pancreatic acinar cell), quickly lead to a pro-inflammatory cascade with a short therapeutic intervention window for some types of interventions. COX enzymes play an important pro-inflammatory role in AP. The isoform of COX-2 is overexpressed in AP, while the expression of COX-1 remains constant. Pharmacological inhibition of COX-2 improves the severity of the acute effects on AP and its systemic and ischemic sequelae. COX-2 inhibitors may show some benefit in AP[6].
Diclofenac and indomethacin, by inhibiting phospholipase A2, play a role in the early phase of the inflammatory cascade in AP. Phospholipase A2 inhibition results in the suppression of several important classes of pro-inflammatory lipids (prostaglandins, leukotrienes, and platelet-activating factor). NSAIDs further inhibit neutrophil-endothelial cell binding. Of the NSAIDs studied in animals, indomethacin showed a lower mortality rate[7]. However, the effectiveness of other NSAIDs should be investigated.
It is important to emphasize that the results of this meta-analysis may have been influenced by heterogeneity > 50%, in relation to the weight of each RCT included in this study. When we refer to the weight of each study, we refer to the number of patients in each of them which was observed within the forest plot with a minimum weight of 1.5%[26] and a maximum weight of 6.3%[34]. These weights influence the time interpreted in the RevMan 5.3 software.
As mentioned by the ESGE, different demographic factors influence the development of PEP, such as patients with suspected SOD, females, previous AP, previous PEP, difficult cannulation, guidewire passage and MPD contrast, children, fine bile duct, absence of chronic pancreatitis, normal serum bilirubin, end-stage renal disease, previous sphincterotomy, pancreatic sphincterotomy, balloon sphincteroplasty, and failure to remove bile duct stones[37]. For these reasons, PEP prevention is important to increase patient safety.
This study emphasized how each RCT reached the diagnosis of AP, with each of the authors defining an episode of AP as the presence of abdominal pain 24 to 72 h after ERCP, increased pancreatic enzymes, and an image compatible with inflammatory alteration of the pancreatic gland (6.8, 11-34). The recent ESGE guideline suggests testing serum amylase and/or lipase 2 to 6 h after ERCP in patients with post-ERCP abdominal pain who should be discharged on the same day of ERCP. Patients with serum amylase and lipase values below 1.5 to 4 times the normal limit can be discharged without concern for PEP development[2]. Another limitation of the study was that not all RCTs stratified the severity of AP in order to be able to adequately interpret at what level of severity the use of NSAIDs may be most beneficial.
Of the 26 RCTs, 521 episodes of AP were assessed for severity. In 339, the AP episode was mild, representing 65% of stratified patients (339/521). Thus, our results demonstrated that the use of NSAIDs prevents the development of mild PEP. Finally, this systematic review focused solely and exclusively on PEP and its severity, but it is important to note that other AEs can occur post-ERCP which were not included in this review.
Thus, in relation to the subgroups examined, the rectal route adequately reduced the incidence of PEP. The use of NSAIDs was shown to be better in mild AP episodes. Both diclofenac and indomethacin were effective in preventing PEP. The best time to administer NSAIDs is before ERCP and the most appropriate dose that achieved the best results was 100 mg.
Other RCTs are needed to resolve some remaining doubts, such as: Would other NSAIDs be more effective? Would the IV route be better? Could smaller doses of more potent NSAIDs be more effective in preventing PEP?
It is concluded that rectal administration of 100 mg diclofenac or 100 mg indomethacin before ERCP prevents the occurrence of mild episodes of PEP.
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most widely performed therapeutic procedures for bile duct access. However, important complications can occur such as: Post-ERCP pancreatitis (PEP), bleeding, puncture and cholangitis. PEP is considered the main complication after the procedure. Large societies such as ASGE, European Society of Gastrointestinal Endoscopy and Japan Gastroenterological Endoscopy Society describe it as a very important complication and methods must be used to prevent and reduce this pathology. Various methods such as using non-steroidal anti-inflammatory drugs (NSAIDs), prostheses, somatostatin and others have been used, but NSAIDs showed a higher rate of effectiveness.
In many studies, NSAIDs have demonstrated good results, but there are also conflicting results. As there is still controversy as to whether the use of NSAIDs would help in reducing PEP, our group carried out the present study including all the randomized controlled trials (RCTs) described in the literature and the results showed that NSAIDs can help in the prevention of PEP.
Our main objective was to determine the effectiveness of NSAIDs vs “Placebo” as a method of choice or first-line therapy to reduce PEP, using the most recent RCTs. All NSAIDs mentioned in the literature, their route of administration and when they should be administered were investigated. In addition, we hope that this research will have important implications within the medical community.
We performed this meta-analysis according to the PRISMA guidelines. Virtual databases were searched up to December 2019 to identify RCTs without date or language restrictions. Following selection of the studies, they were organized according to the PICO criteria and the design followed the JADAD scale. Statistical analysis of the data was performed using RevMan 5.3 software. The main endpoint evaluated in this study was the reduction in the incidence of PEP. Subgroup analyses were also performed and included the severity of pancreatitis, route of administration, time of administration and the types of NSAIDs administered. The results were evaluated with the Higgins test method, using a risk difference with a random effect with a significance of P < 0.05, 95% confidence interval (CI) and interpreted as true heterogeneity.
Twenty-six high quality RCTs examining the use of NSAIDs vs Placebo for the reduction of PEP were included, involving a total of 8143 patients. 4020 patients used NSAIDs before ERCP and 4123 did not use NSAIDs (control group). A total of 298 cases of acute pancreatitis after ERCP were diagnosed in the NSAID group and 484 cases in the placebo group. The risk of PEP was lower (risk difference (RD)) in the NSAID group: -0.04; 95%CI: -0.07 to -0.02; number needed to treat (NNT), 25; P < 0.05. The use of NSAIDs effectively prevented mild pancreatitis compared to the use of placebo (2.5% vs 4.1%; 95%CI: -0.05 to -0.01; NNT, 33; P < 0.05), but data on moderate and severe PEP could not be fully elucidated. Only rectal administration reduced the incidence of PEP with the RD: -0.06 95%CI, -0.08 to -0.04; NNT, 17; P < 0.05.
In conclusion, the use of NSAIDs does reduce the incidence of PEP. In particular, NSAIDs reduce the incidence of mild acute pancreatitis. The most effective drugs were diclofenac and indomethacin. The best route of administration was rectal and the best time for NSAIDs administration was before ERCP.
It is hoped that these findings will help clinicians decide on the best treatment to prevent PEP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee CL S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Sajid MS, Khawaja AH, Sayegh M, Singh KK, Philipose Z. Systematic review and meta-analysis on the prophylactic role of non-steroidal anti-inflammatory drugs to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis. World J Gastrointest Endosc. 2015;7:1341–1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 499] [Article Influence: 99.8] [Reference Citation Analysis (1)] |
| 3. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4340] [Article Influence: 361.7] [Reference Citation Analysis (45)] |
| 4. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, Shaw MJ, Snady HW, Erickson RV, Moore JP, Roel JP. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 835] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 5. | Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, Van Dam J, Hughes M, Carr-Locke DL. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 6. | Bhatia V, Ahuja V, Acharya SK, Garg PK. A randomized controlled trial of valdecoxib and glyceryl trinitrate for the prevention of post-ERCP pancreatitis. J Clin Gastroenterol. 2011;45:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Döbrönte Z, Szepes Z, Izbéki F, Gervain J, Lakatos L, Pécsi G, Ihász M, Lakner L, Toldy E, Czakó L. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol. 2014;20:10151-10157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47187] [Article Influence: 2949.2] [Reference Citation Analysis (0)] |
| 9. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12887] [Article Influence: 444.4] [Reference Citation Analysis (1)] |
| 10. | Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, Schmidt S, Lazzell-Pannell L, Lehman GA. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc. 2007;66:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Hauser G, Blažević I, Salkić N, Poropat G, Giljača V, Bulić Z, Štimac D. Diclofenac sodium versus ceftazidime for preventing pancreatitis after endoscopic retrograde cholangiopancreatography: a prospective, randomized, controlled trial. Surg Endosc. 2017;31:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Ishiwatari H, Urata T, Yasuda I, Matsusaki S, Hisai H, Kawakami H, Ono M, Iwashita T, Doi S, Kawakubo K, Hayashi T, Sonoda T, Sakamoto N, Kato J. No Benefit of Oral Diclofenac on Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Dig Dis Sci. 2016;61:3292-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Katoh T, Kawashima K, Fukuba N, Masuda S, Kobatake H, Masaki K, Araki Y, Kawano K, Nishi K, Takenaka M, Ishihara S, Kinoshita Y. Low-dose rectal diclofenac does not prevent post-ERCP pancreatitis in low- or high-risk patients. J Gastroenterol Hepatol. 2020;35:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Khoshbaten M, Khorram H, Madad L, Ehsani Ardakani MJ, Farzin H, Zali MR. Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol. 2008;23:e11-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Leerhøy B, Nordholm-Carstensen A, Novovic S, Hansen MB, Jørgensen LN. Effect of body weight on fixed dose of diclofenac for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Scand J Gastroenterol. 2016;51:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Lua GW, Muthukaruppan R, Menon J. Can Rectal Diclofenac Prevent Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis? Dig Dis Sci. 2015;60:3118-3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, Noda T. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Senol A, Saritas U, Demirkan H. Efficacy of intramuscular diclofenac and fluid replacement in prevention of post-ERCP pancreatitis. World J Gastroenterol. 2009;15:3999-4004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Uçar R, Biyik M, Uçar E, Polat İ, Çifçi S, Ataseven H, Demir A. Rectal or intramuscular diclofenac reduces the incidence of pancreatitis afterendoscopic retrograde cholangiopancreatography. Turk J Med Sci. 2016;46:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Park SW, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Song SY. Intramuscular diclofenac for the prevention of post-ERCP pancreatitis: a randomized trial. Endoscopy. 2015;47:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Masjedizadeh A, Fathizadeh P, Aghamohamadi N. Comparative effectiveness of aggressive intravenous fluid resuscitation with lactated Ringer's solution and rectal indomethacin therapy in the prevention of pancreatitis after endoscopic retrograde cholangiopancreatography: a double blind randomised controlled clinical trial. Prz Gastroenterol. 2017;12:271-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Montaño Loza A, Rodríguez Lomelí X, García Correa JE, Dávalos Cobián C, Cervantes Guevara G, Medrano Muñoz F, Fuentes Orozco C, González Ojeda A. [Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes]. Rev Esp Enferm Dig. 2007;99:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Andrade-Dávila VF, Chávez-Tostado M, Dávalos-Cobián C, García-Correa J, Montaño-Loza A, Fuentes-Orozco C, Macías-Amezcua MD, García-Rentería J, Rendón-Félix J, Cortés-Lares JA, Ambriz-González G, Cortés-Flores AO, Alvarez-Villaseñor Adel S, González-Ojeda A. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, Waljee AK, Repaka A, Atkinson MR, Cote GA, Kwon RS, McHenry L, Piraka CR, Wamsteker EJ, Watkins JL, Korsnes SJ, Schmidt SE, Turner SM, Nicholson S, Fogel EL; U. S. Cooperative for Outcomes Research in Endoscopy (USCORE). A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 26. | Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, Hyder SM, Lacy BE, Bensen SP, Parr DD, Gardner TB. Rectal Indomethacin Does Not Prevent Post-ERCP Pancreatitis in Consecutive Patients. Gastroenterology. 2016;150:911-7; quiz e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 27. | Li L, Liu M, Zhang T, Jia Y, Zhang Y, Yuan H, Zhang G, He C. Indomethacin down-regulating HMGB1 and TNF-α to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. Scand J Gastroenterol. 2019;54:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Hosseini M, Shalchiantabrizi P, Yektaroudy K, Dadgarmoghaddam M, Salari M. Prophylactic Effect of Rectal Indomethacin Administration, with and without Intravenous Hydration, on Development of Endoscopic Retrograde Cholangiopancreatography Pancreatitis Episodes: A Randomized Clinical Trial. Arch Iran Med. 2016;19:538-543. [PubMed] |
| 29. | Patai Á, Solymosi N, Patai ÁV. Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. J Clin Gastroenterol. 2015;49:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Kato K, Shiba M, Kakiya Y, Maruyama H, Ominami M, Fukunaga S, Sugimori S, Nagami Y, Watanabe T, Tominaga K, Fujiwara Y. Celecoxib Oral Administration for Prevention of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Randomized Prospective Trial. Pancreas. 2017;46:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Mansour-Ghanaei F, Joukar F, Taherzadeh Z, Sokhanvar H, Hasandokht T. Suppository naproxen reduces incidence and severity of post-endoscopic retrograde cholangiopancreatography pancreatitis: Randomized controlled trial. World J Gastroenterol. 2016;22:5114-5121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | de Quadros Onófrio F, Lima JCP, Watte G, Lehmen RL, Oba D, Camargo G, Dos Santos CEO. Prophylaxis of pancreatitis with intravenous ketoprofen in a consecutive population of ERCP patients: a randomized double-blind placebo-controlled trial. Surg Endosc. 2017;31:2317-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, Itoi T, Sata N, Gabata T, Igarashi H, Kataoka K, Hirota M, Kadoya M, Kitamura N, Kimura Y, Kiriyama S, Shirai K, Hattori T, Takeda K, Takeyama Y, Hirota M, Sekimoto M, Shikata S, Arata S, Hirata K. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22:405-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 35. | ASGE Standards of Practice Committee, Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 536] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 36. | Sakai P, Ishioka S, Maluf F. Tratado de endoscopia digestiva digestiva diagnóstica e terapêutica. Vias biliares e páncreas. Segunda edição revisada e atualizada-Sao Paulo/Brazil: Editor Atheneu, 2015. |
| 37. | Serrano JPR, de Moura DTH, Bernardo WM, Ribeiro IB, Franzini TP, de Moura ETH, Brunaldi VO, Salesse MT, Sakai P, de Moura EGH. Nonsteroidal anti-inflammatory drugs versus placebo for post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E477-E486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |