Published online Oct 16, 2020. doi: 10.4253/wjge.v12.i10.341
Peer-review started: July 23, 2020
First decision: August 22, 2020
Revised: August 28, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 16, 2020
Processing time: 83 Days and 22.6 Hours
Acute gastrointestinal (GI) graft-vs-host disease (aGVHD) is the most complication of hematopoietic stem cell transplant (HSCT) in patients with hematologic malignancy. Limited data exists on endoscopic evaluation of GVHD in post-HSCT patients with differing GI symptoms. Further, the diagnostic value of gross endoscopic findings as well as the safety of endoscopy in this commonly thrombocytopenic and neutropenic patient population remains unclear.
To understand the diagnostic value of symptoms and gross endoscopic findings as well as safety of endoscopy in aGVHD patients.
We analyzed 195 endoscopies performed at City of Hope in patients who underwent allogeneic HSCT less than 100 d prior for hematologic malignancy and were subsequently evaluated for aGVHD via endoscopy. The yield, sensitivity, and specificity of diagnosing aGVHD were calculated for upper and lower endoscopy, various GI tract locations, and presenting symptoms.
Combined esophagogastroduodenoscopy (EGD) and flexible sigmoidoscopy (FS) demonstrated a greater diagnostic yield for aGVHD (83.1%) compared to EGD (66.7%) or FS (77.2%) alone with any presenting symptom. The upper and lower GI tract demonstrated similar yields regardless of whether patients presented with diarrhea (95.7% vs 99.1%) or nausea/vomiting (97.5% vs 96.8%). Normal-appearing mucosa was generally as specific (91.3%) as abnormal mucosa (58.7%-97.8%) for the presence of aGVHD. Adverse events such as bleeding (1.0%), infection (1.0%), and perforation (0.5%) only occurred in a small proportion of patients, with no significant differences in those with underlying thrombocytopenia (P = 1.000) and neutropenia (P = 0.425).
Combined EGD and FS with biopsies of normal and inflamed mucosa demonstrated the greatest diagnostic yield regardless of presenting symptom and appears to be safe in this population of patients.
Core Tip: We analyzed a retrospective cohort of 195 endoscopies performed in cancer patients who had a hematopoietic stem cell transplant less than 100 d prior to endoscopy and evaluated the diagnostic value of various endoscopic procedures, gross endoscopic findings, and presenting symptoms. Our findings show that combined esophagogastroduodenoscopy and flexible sigmoidoscopy with biopsies of normal and abnormal-appearing mucosa results in the greatest yield for diagnosing acute gastrointestinal graft-vs-host disease independent of symptoms. Additionally, we found no significant difference in adverse events in patients with and without thrombocytopenia and neutropenia.
- Citation: Rajan AV, Trieu H, Chu P, Lin J, Kidambi TD. Assessing the yield and safety of endoscopy in acute graft-vs-host disease after hematopoietic stem cell transplant. World J Gastrointest Endosc 2020; 12(10): 341-354
- URL: https://www.wjgnet.com/1948-5190/full/v12/i10/341.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i10.341
Graft-vs-host disease (GVHD) is the most common life-threatening complication of allogeneic hematopoietic stem cell transplant (HSCT) with the gastrointestinal (GI) tract commonly involved[1-4]. Classically, acute GVHD (aGVHD) occurs less than 100 d post-HSCT, while chronic GVHD (cGVHD) occurs after day 100[5]. Diagnosing aGVHD relies upon clinical findings and is confirmed with tissue biopsy. Endoscopy with esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy (FS), or combinations of these procedures are often performed to obtain GI tissue to confirm the diagnosis and assess the severity of GVHD histopathologically[6]. Histopathological evidence of GVHD is defined by the presence of apoptotic bodies in tissue specimens per updated 2014 NIH consensus guidelines[7,8].
Currently, there are limited primary data to guide endoscopic evaluation of aGVHD in the post-HSCT population. Previous studies examining clinical characteristics and endoscopic findings in aGVHD patients were limited to small case series in diverse patient populations (children and adults for a variety of indications)[9-22]. The largest studies focusing specifically on the population at risk for aGVHD included fewer than 175 endoscopic evaluations[9,10,14]. Based upon previous studies, FS with biopsy of the rectosigmoid colon is considered the standard evaluation for patients with symptoms localizing to the lower gut. The evidence supporting the use of EGD in the evaluation of GVHD in patients with upper GI symptoms is scarce. Further, the diagnostic value of gross endoscopic findings and presenting symptoms and their relationship to histopathological evidence of aGVHD remains unclear. To this end, the primary aim of our study was to characterize clinical symptoms and endoscopic findings in a large set of patients post-HSCT undergoing evaluation of aGVHD. A secondary aim was to understand which anatomical locations in the GI tract were most commonly involved by aGVHD and to assess whether presenting symptoms localized to specific portions of the GI tract histopathologically.
Additionally, there is inconsistent data on the safety of endoscopic evaluation in patients with thrombocytopenia and neutropenia[23-29], especially in those who have undergone HSCT. Intra and post-procedural bleeding are viewed as difficult to manage in thrombocytopenic patients given the perceived notion that it occurs diffusely rather than focally. In light of the higher perceived infectious and bleeding risks of performing endoscopy in this patient population, our final aim was to assess endoscopic safety.
A retrospective review was conducted of all endoscopic procedures performed at City of Hope (COH), an academic, tertiary care cancer center, between December 2017 and July 2019 to identify patients who had undergone allogenic HSCT with clinical suspicion for aGVHD. The institutional review board at COH approved this study. The endoscopic database included data from December 2017 onwards as part of a new electronic health record implemented at COH; endoscopic data prior to this did not interface with the new electronic health record and was not accessible for review.
Patients from the endoscopic database were included in the study if they: (1) Had a hematologic malignancy such as leukemia, lymphoma, or myelodysplastic or myeloproliferative syndromes; (2) Underwent allogeneic HSCT at COH; and (3) Developed symptoms prompting clinical suspicion for aGVHD leading to referral for endoscopic evaluation. Patients who underwent HSCT for immunodeficiencies, congenital metabolic defects, or hemoglobinopathies were excluded. Further, patients who did not have tissue biopsied during endoscopy or underwent HSCT greater than 100 d prior to endoscopy were excluded. If a single patient underwent multiple endoscopies at different times, each endoscopic evaluation was counted as a separate procedure.
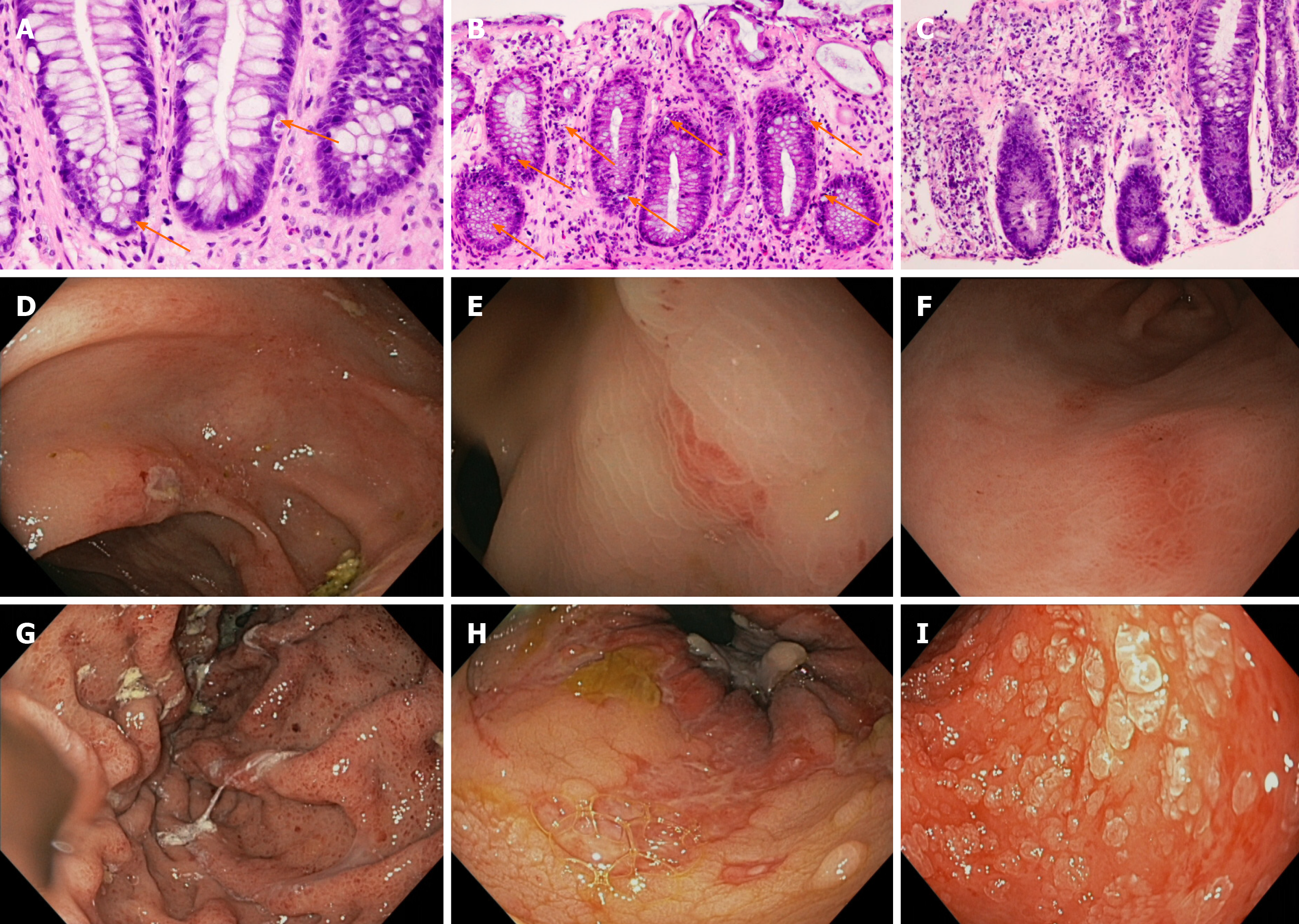
Two investigators (Rajan AV, Trieu H) reviewed all endoscopy and corresponding pathology reports, collected data on endoscopic findings as well as interventions performed, and reviewed the medical records. Pathology reports were also reviewed and pathological findings as well as their anatomical location were collected. All biopsy samples were sent for pathologic examination as part of routine clinical care and histology was evaluated by expert GI pathologists at COH. Histological grading of the severity of aGVHD was done on a scale ranging from mild, moderate, to severe as per standard of care at City of Hope Medical Center and in concordance with 2014 NIH Consensus Criteria[8]. Mild aGVHD was defined as rare or few apoptotic cells of individual crypts; moderate aGVHD was defined as apoptosis with crypt microabscesses and crypt cell flattening; and, severe aGVHD was defined as dropout of many crypts or flat mucosa with total denudation. In some instances, a single tissue sample was classified as a range of severities such as both mild and moderate. Illustrative histological images can be seen in Figure 1.
Demographic, clinical, and laboratory data from the electronic health records were collected. Hemoglobin count, platelet count, and absolute neutrophil count (ANC) were obtained from the last complete blood count (CBC) drawn prior to endoscopy and first CBC drawn post-endoscopy. Pre-procedure blood urea nitrogen and creatinine as well as pre and post-procedure international normalized ratio were obtained in a similar manner. Additionally, transfusion data was collected such as number of units of platelets, packed red blood cells (pRBC), and fresh frozen plasma/cryoprecipitate (FFP) transfused within 72 h prior to endoscopy and 72 h after. Thrombocytopenia was defined as a pre-procedure platelet count less than or equal to 50000 per microliter with no platelet transfusions or less than or equal to 75000 per microliter with one or more platelet transfusions. Neutropenia was defined as a pre-procedure ANC of less than 1000 cells per microliter.
Adverse events after endoscopy were defined as overt clinical GI bleeding, infection, luminal perforation, and/or death due to any cause within 1 wk. When an adverse event occurred, the medical record was reviewed for details related to endoscopic approaches for hemostasis and other interventions required to manage the adverse events.
Data were stored using the Research Electronic Data Capture (REDCap) 8.10.2 data management platform (Vanderbilt University, Nashville, Tennessee). Descriptive statistics were computed for demographic, clinical, endoscopic, and pathologic variables. Contingency tables were created to calculate the yield, sensitivity, and specificity of biopsies based on certain endoscopic findings and presenting symptoms for diagnosing aGVHD. Fisher’s exact test was performed to compare the incidence of post-endoscopic complications in the thrombocytopenic and neutropenic patients. All statistical analyses were performed using Stata/IC 15.1 (StataCorp, College Station, TX, United States). The statistical methods of this study were reviewed by Trieu H from the University of Southern California.
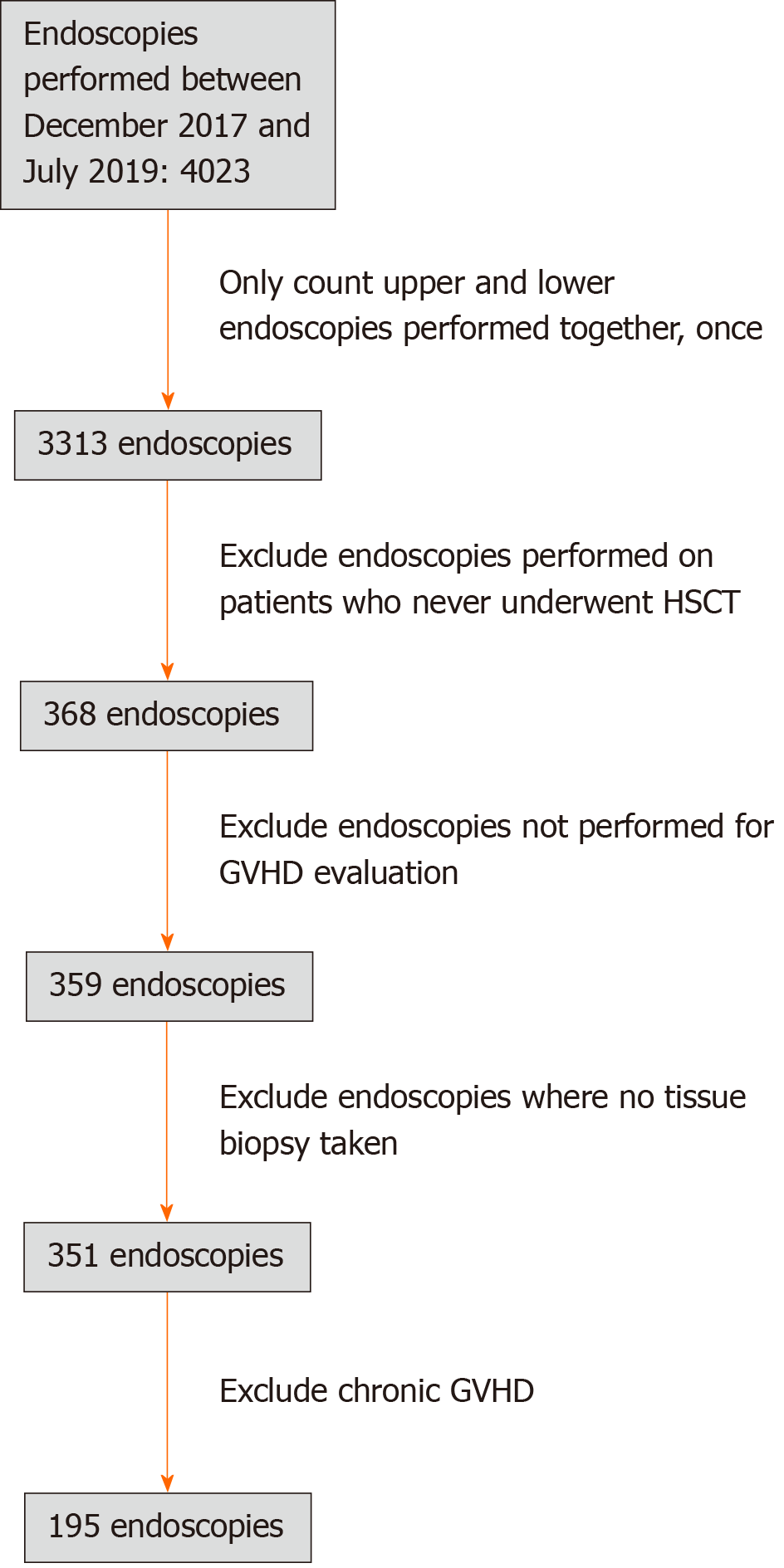
A total of 4023 endoscopies were performed at COH during the examined study period. As shown in Figure 2, 195 endoscopies met inclusion criteria and were included in the analysis. Females accounted for 51.8% of the patients, with a median age of 56 years (range 17-78) as shown in Table 1. Endoscopic evaluation for aGVHD occurred at a median of 27 d (range 9-98 days) following HSCT. The most common primary hematologic malignancy diagnoses were acute myelogenous leukemia in 41.0% and B-cell acute lymphoblastic leukemia in 17.4%. Acute GVHD was confirmed histologically in 76.4% of patients.
| Demographics and clinical characteristics | n = 195 |
| Age (yr), median (range) | 56 (17-78) |
| Female, n (%) | 101 (51.8) |
| Time since HSCT in days, median (range) | 27 (9-98) |
| Hematologic disorder, n (%)1 | |
| AML | 80 (41.0) |
| B-ALL | 34 (17.4) |
| MDS | 30 (15.4) |
| Myelofibrosis | 13 (6.7) |
| Presenting symptoms, n (%)1 | |
| Diarrhea | 144 (73.9) |
| Nausea/vomiting | 107 (54.9) |
| Abdominal pain | 50 (25.6) |
| Gross endoscopic findings, n (%)1 | |
| Edema/erythema | 108 (55.4) |
| Gastritis | 84 (43.1) |
| Ulcerations/erosions | 56 (28.7) |
| Colitis | 39 (20.0) |
| Esophagitis | 27 (13.9) |
| Duodenitis | 26 (13.3) |
| Normal | 25 (12.8) |
| Pathologic findings, n (%)1 | |
| Mild GVHD | 133 (68.2) |
| Chronic inflammation | 41 (21.0) |
| Moderate GVHD | 36 (18.5) |
| Ulceration/erosion | 25 (12.8) |
| Severe GVHD | 16 (8.2) |
| Location of pathologic findings, n (%)1 | |
| Sigmoid colon | 118 (60.5) |
| Stomach | 105 (53.9) |
| Rectum | 103 (52.8) |
| Duodenum | 94 (48.2) |
| Pre-procedure lab values, median (range) | |
| Hemoglobin (g/dL) | 9.0 (6.7-14.4) |
| Platelets (× 103/µL) | 80 (16-388) |
| ANC (× 103/µL) | 2.6 (0.0-23.0) |
| INR | 1.0 (0.9-1.7) |
| BUN | 14 (2-78) |
| Creatinine | 0.70 (0.28-3.32) |
| Pre-procedure transfusions, n (%) | |
| 1 or more units of platelets transfused | 78 (40.0) |
| 1 or more units of pRBCs transfused | 55 (28.2) |
| 1 or more units of FFP transfused | 3 (1.5) |
| Immunosuppressant use within 1-wk pre-procedure, n (%)1 | |
| Tacrolimus | 164 (84.1) |
| Sirolimus | 115 (59.0) |
| Mycophenolate | 70 (35.9) |
| Methylprednisone | 55 (28.2) |
| Hydrocortisone | 49 (25.1) |
Median pre-procedure hemoglobin count was 9.0 g/dL, platelet count 80 × 103/µL, and ANC 2.6 × 103/µL as shown in Table 2. The majority (greater than 90%) of pre-procedure hemoglobin and platelet counts were obtained after the last unit of product was transfused. Forty percent of patients required transfusions of 1 or more units of platelets, while 28.2% required 1 or more units of pRBCs, and only 1.5% required 1 or more units of FFP within 72 h prior to endoscopy.
| Endoscopic procedure | Confirmed GVHD in patients with any symptom, % (n) | Confirmed GVHD in patients presenting with diarrhea, % (n) | Confirmed GVHD in patients presenting with nausea/vomiting, % (n) | Confirmed GVHD in patients presenting with abdominal pain, % (n) |
| EGD (n = 45) | 66.7 (30/45) | 85.7 (6/7)1 | 70.6 (24/34) | 57.1 (8/14) |
| FS (n = 57) | 77.2 (44/57) | 76.8 (43/56) | 87.5 (7/8)2 | 76.9 (10/13) |
| Colonoscopy (n = 4) | 75.0 (3/4) | 75.0 (3/4) | - | 100.0 (1/1) |
| EGD + FS (n = 83) | 83.1 (69/83) | 84.9 (62/73) | 85.9 (55/64) | 90.5 (19/21) |
| EGD + colonoscopy (n = 6) | 50.0 (3/6) | 50.0 (2/4) | 0 (0/1) | 100.0 (1/1) |
Combined EGD and FS (43.0%) was the most common method of evaluation for aGVHD with fewer patients undergoing EGD (23.1%) or FS (29.2%) alone as shown in Table 2. Acute GVHD was confirmed histologically in 83.1% of patients who underwent combined EGD and FS and 77.2% of patients who underwent FS alone. When evaluating the diagnostic yield of endoscopic evaluation by presence of symptoms as shown in Table 2, combined EGD and FS provided the highest yield for diagnosing aGVHD in patients presenting with abdominal pain (90.5%). EGD alone and combined EGD and FS demonstrated comparably high yields in patients with diarrhea (85.7% and 84.9% respectively). Three of the six patients who had confirmed GVHD and had underwent EGD alone presented with both diarrhea and nausea/vomiting. FS alone and EGD with FS demonstrated similarly high yields in patients presenting with nausea/vomiting (87.5% and 85.9% respectively). Seven of the seven patients who had confirmed GVHD and had underwent FS alone presented with both nausea/vomiting and diarrhea.
The most common endoscopic findings were edema/erythema, gastritis, ulcerations/erosions, and colitis as shown in Table 1 and illustrated in Figure 1. Histopathologic examination revealed mild aGVHD in 68.2% of tissue specimens, chronic inflammation without histological evidence of aGVHD in 21.0%, moderate aGVHD in 18.5%, ulceration/erosion in 12.8%, and severe aGVHD in 8.2%.
We assessed the frequency with which endoscopic findings demonstrated histological evidence of aGVHD as well as the utility of endoscopic findings as markers for aGVHD anywhere in the GI tract, which is summarized in Table 3. Eighty-four percent of patients with biopsies of normal endoscopically appearing mucosa demonstrated histological features consistent with aGVHD, with all of these patients demonstrating mild aGVHD on pathology, less than 5% showing concurrent moderate aGVHD and none with severe aGVHD. The sensitivity of a normal endoscopic appearance for aGVHD anywhere in the GI tract was thus 14.1%. As shown in Table 3, the presence of general endoscopic abnormalities (i.e., ulceration, friability, blood clots) were more specific (58.7%-97.8%) than sensitive (2.7%-59.7%) for the presence of aGVHD anywhere along the GI tract on biopsy. Furthermore, the presence of esophagitis, colitis, gastritis, and duodenitis were particularly specific for aGVHD (94.3%, 93.0%, 88.9%, 78.6%, respectively) in biopsies obtained from the respective portions of the GI tract.
| Endoscopic finding | Patients with GVHD, % (n) | Sensitivity (%) | Specificity (%) | Patients with mild GVHD, % (n) | Patients with moderate GVHD, % (n) | Patients with severe GVHD, % (n) |
| Normal1 | 84.0 (21/25) | 14.1 | 91.3 | 100.0 (21/21) | 4.8 (1/21) | 0 (0/21) |
| General findings1 | ||||||
| Edema/erythema | 82.4 (89/108) | 59.7 | 58.7 | 88.8 (79/89) | 30.3 (27/89) | 12.4 (11/89) |
| Ulceration/erosion | 80.4 (45/56) | 30.2 | 76.1 | 77.8 (35/45) | 40.0 (18/45) | 26.7 (12/45) |
| Friability | 90.9 (10/11) | 6.7 | 97.8 | 80.0 (8/10) | 30.0 (3/10) | 20.0 (2/10) |
| Nodule | 80.0 (4/5) | 2.7 | 97.8 | 100.0 (4/4) | 50.0 (2/4) | 25.0 (1/4) |
| Specific findings2 | ||||||
| Gastritis | 84.5 (60/71) | 72.3 | 78.6 | 95.0 (57/60) | 16.7 (10/60) | 1.7 (1/60) |
| Duodenitis | 66.7 (14/21) | 16.1 | 88.9 | 78.6 (11/14) | 21.4 (3/14) | 21.4 (3/14) |
| Colitis | 97.1 (33/34) | 30.3 | 93.0 | 57.6 (19/33) | 42.4 (14/33) | 24.2 (8/33) |
| Esophagitis | 66.7 (14/21) | 70.0 | 94.3 | 100.0 (14/14) | 0 (0/14) | 0 (0/14) |
In this cohort, the most frequent indications for endoscopic evaluation were diarrhea (141/195, 72.3%), nausea/vomiting (94/195, 48.2%), and abdominal pain (42/195, 21.5%) as seen in Table 1. Further, 87.0% (60/69) of patients presenting with both diarrhea and nausea/vomiting had confirmed aGVHD on histopathology. We attempted to identify portions of the GI tract in which a biopsy would provide the greatest yield for diagnosing aGVHD in patients with each of the above presenting symptoms. To this end, we calculated the proportion of patients presenting with one of the above symptoms, a biopsy taken from a specific location, and histological evidence of aGVHD out of all patients presenting with one of the above symptoms, a biopsy taken from a specific location, and histological evidence of aGVHD in tissue taken from any location (Table 4).
| Presenting symptom and biopsy location1 | Histological evidence of GVHD in location, % (n) |
| Diarrhea (n = 144) | |
| Upper GI tract | 95.7 (67/70) |
| Esophagus | 51.9 (14/27) |
| Stomach | 82.1 (55/67) |
| Duodenum | 87.1 (61/70) |
| Lower GI tract | 99.1 (109/110) |
| Ileum | 100.0 (2/2) |
| Cecum | 100.0 (1/1) |
| Ascending colon | 100.0 (4/4) |
| Transverse colon | 75.0 (3/4) |
| Descending colon | 91.7 (11/12) |
| Sigmoid colon | 93.5 (101/108) |
| Rectum | 94.0 (94/100) |
| Nausea/vomiting (n = 107) | |
| Upper GI tract | 97.5 (77/79) |
| Esophagus | 51.6 (16/31) |
| Stomach | 84.6 (66/78) |
| Duodenum | 87.3 (69/79) |
| Lower GI tract | 96.8 (60/62) |
| Ileum | - |
| Cecum | - |
| Ascending colon | - |
| Transverse colon | - |
| Descending colon | 100.0 (6/6) |
| Sigmoid colon | 90.2 (55/61) |
| Rectum | 91.1 (51/56) |
| Abdominal pain (n = 50) | |
| Upper GI tract | 89.3 (25/28) |
| Esophagus | 66.7 (8/12) |
| Stomach | 89.3 (25/28) |
| Duodenum | 85.7 (24/28) |
| Lower GI tract | 96.8 (30/31) |
| Ileum | 100.0 (1/1) |
| Cecum | 100.0 (1/1) |
| Ascending colon | 100.0 (2/2) |
| Transverse colon | 100.0 (1/1) |
| Descending colon | 100.0 (4/4) |
| Sigmoid colon | 87.1 (27/31) |
| Rectum | 86.7 (26/30) |
In patients presenting with diarrhea, biopsying the lower GI tract demonstrated a slightly greater diagnostic yield compared to biopsying the upper tract (99.1% vs 95.7%). When considering specific locations within each tract, the ileum, cecum, and ascending colon all demonstrated 100% yield, however the number of patients who had tissue obtained from these locations were extremely low. Excluding these locations, the rectum and sigmoid colon demonstrated the greatest diagnostic yield. In patients presenting with nausea/vomiting, biopsy from either the upper or lower tract demonstrated similar yields (97.5% and 96.8%). Biopsying the descending colon resulted in 100% yield, however only 6 patients with nausea/vomiting had a biopsy obtained here. Excluding the descending colon, the sigmoid colon and rectum again demonstrated the greatest diagnostic yield. Biopsies from the lower GI tract demonstrated greater yield than biopsies of the upper tract (96.8% vs 89.3%) in patients with abdominal pain. Although biopsies taken from the ileum down to the descending colon demonstrated 100% yield, only 4 patients at most had biopsies taken from these locations. Excluding these areas, biopsies from the stomach and sigmoid colon demonstrated the diagnostic greatest yields (89.3% and 87.1%).
Death due to any cause within 1 wk of endoscopy occurred in 0% (0/195) of patients. Bleeding occurred in 1.0% (2/195), infection in 1.0% (2/195), and perforation in 0.5% (1/195). Both of the patients with bleeding required second look endoscopies to manage bleeding not resolved with supportive management and were successfully managed endoscopically.
Thrombocytopenia was identified in 67 patients. Adverse outcomes including death occurred in 1.5% (1/67) of patients in the thrombocytopenic group and 2.3% (3/128) of patients in the non-thrombocytopenic group with the bleeding occurring in 1.5% and 0.8% of patients, respectively. There was no significant difference in these adverse outcomes between the two groups (P = 1.000) (Table 5).
| Complication | Non-thrombocytopenic patients | Thrombocytopenic patients | Non-neutropenic patients1 | Neutropenic patients1 |
| Any-cause mortality within 1 wk of endoscopy, n (%) | 0/128 (0) | 0/67 (0) | 0/170 (0) | 0/25 (0) |
| Adverse outcomes excluding death, n (%) | 3/128 (2.3) | 1/67 (1.5) | 3/170 (1.8) | 1/25 (4.0) |
| Bleeding within 1 wk of endoscopy | 1/128 (0.8) | 1/67 (1.5) | 1/170 (0.6) | 1/25 (4.0) |
| Infection within 1 wk of endoscopy | 2/128 (1.6) | 0/67 (0) | 2/170 (1.2) | 0/25 (0) |
| Perforation within 1 wk of endoscopy | 1/128 (0.8) | 0/67 (0) | 1/170 (0.6) | 0/25 (0) |
| Adverse outcomes including death within 1 wk, n (%)2 | 3/128 (2.3) | 1/67 (1.5) | 3/170 (1.8) | 1/25 (4.0) |
Neutropenia was identified in 25 patients, all of whom were on broad spectrum antibiotics prior to endoscopy. Adverse outcomes including death occurred in 4.0% (1/25) of neutropenic patients and 1.8% (3/170) of non-neutropenic patients, with bleeding occurring in 4.0% and 0.6% of patients, respectively. No cases of infection occurred in the neutropenic patients. No significant difference in adverse outcomes was observed when comparing neutropenic to non-neutropenic patients (P = 0.425).
Intraprocedural bleeding occurred in 10 of 195 (5.1%) non-duplicate endoscopies which required hemostatic interventions such as hemoclips (9/195 or 4.6%), epinephrine injections (1/195 or 0.5%), or argon plasma coagulation (2/195 or 1.0%) during the index procedure. Two of these patients experienced recurrent post-procedure bleeding that was controlled during second look endoscopy. No patient with intraprocedural bleeding experienced other adverse outcomes (infection, perforation, or death) within one week of endoscopy.
We report the largest cross-sectional study to date on the management and safety of endoscopic evaluation of aGVHD in patients who have undergone HSCT. A number of prior studies have found that symptoms such as diarrhea often occur in the presence of aGVHD in the lower GI tract, warranting evaluation with FS, while upper GI symptoms such as nausea and vomiting warrant evaluation with EGD[6,14,16,18,19,22]. On the contrary, we found that combined EGD and FS with biopsies resulted in at least an 80% diagnostic yield in patients with any presenting symptom.
Interestingly, EGD alone in patients presenting with diarrhea and FS alone in patients presenting with nausea/vomiting demonstrated greater yields than combined EGD and FS. This finding could be due to the small number of patients presenting with diarrhea or nausea/vomiting who also underwent these modalities of endoscopic evaluation. Further, three of six patients with confirmed aGVHD who underwent EGD alone and all seven patients with confirmed GVHD who underwent FS alone presented with concurrent nausea/vomiting and diarrhea. Taken together, combined EGD and FS may be the most effective endoscopic approach to GVHD evaluation regardless of presenting symptoms.
To explore this concept further, we analyzed anatomical patterns of aGVHD localization for different presenting symptoms. Biopsies taken from either the upper or lower GI tracts demonstrated greater than 90% yield for histological evidence of aGVHD, with the lower GI tract demonstrating slightly greater yields across most presenting symptoms. When considering specific locations within the upper and lower tracts, the rectosigmoid colon demonstrated the greatest diagnostic yield across all symptoms except abdominal pain when excluding locations where fewer than seven patients had biopsies. Our findings are consistent with those of prior studies which recommend biopsies of the rectosigmoid colon for evaluation of lower GI aGVHD[14,16-18]. Thus, a biopsy approach targeted to the rectosigmoid colon may be ideal for patients who are not candidates for combined EGD with FS.
In addition, we hypothesized that macroscopic features observed on endoscopy may be suggestive of the presence and severity of aGVHD, acknowledging inconsistent findings in the literature[3,9,12,13,20]. We found that endoscopic evidence of inflammation was generally as specific as normal mucosa for the presence of aGVHD, however histological examination of inflamed mucosa more frequently revealed moderate to severe aGVHD. Normal appearing mucosa with aGVHD was typically mild with only one case of moderate grade findings. Our results thus confirm the clinical practice of performing biopsies of normal appearing mucosa (covering multiple segments of the GI tract) to evaluate for histological evidence of aGVHD. Abnormal mucosa should be biopsied to evaluate for actual histological grade of aGVHD.
The safety of endoscopic evaluation for aGVHD in HSCT patients continues to be an ongoing concern, given this population may be at increased risk for bleeding and infection. In our study, adverse events were relatively rare, complicating only 2.1% of all endoscopies. Further, none of the endoscopies that required hemostasis for intra-endoscopic bleeding had subsequent uncontrolled bleeding. Similarly low complication rates have been reported in the literature, including two studies of adult cancer patients with thrombocytopenia and neutropenia which found post-endoscopic complication rates of less than 5%[28,29]. Additionally, we found no significant differences in adverse events when comparing the thrombocytopenic and non-thrombocytopenic groups and the neutropenic and non-neutropenic groups. These findings suggest that endoscopic evaluation for aGVHD in this vulnerable population may be safe regardless of pre-procedure platelet and neutrophil count, challenging the need for thresholds set in place by endoscopy societies. Taken together with the findings of recent papers[28,29], a prospective, controlled study evaluating platelet and neutrophil thresholds for endoscopy should be conducted to potentially limit transfusions and aid in antibiotic and neutrophil-stimulation pharmacological stewardship efforts.
Our study had several limitations. Given the retrospective nature, there may have been confounding by indication accounting for the findings of high yield of FS alone or EGD alone across different symptoms. However, by review of the entire medical record to capture all symptoms, we limited the potential for this bias. Selection bias is also possible since all patients underwent endoscopic evaluation with biopsy, though this was the intent of our study. Our study lacked power for statistical comparisons given the low rate of adverse events – while this impaired our ability to perform multiple logistic regression and limited us to use of Fisher’s exact test, we believe the absolute incidence of these events have meaning and can be interpreted clinically and used as part of the risk/benefit calculations in post-HSCT patients referred for endoscopy. We believe that by reporting the largest endoscopic data in this patient population, our results add to the literature on the topic.
Determining the optimal endoscopic strategy for acute GVHD evaluation in patients with HSCT is challenging due to the inherent vulnerability of this population. Our findings suggest that combined EGD and FS with biopsy of the stomach and rectosigmoid colon results in the greatest diagnostic yield for most patients referred for evaluation of aGVHD, independent of symptoms. We confirm that biopsy of normal appearing mucosa is warranted and found that endoscopic evidence of severe inflammation is specific for more histologically severe GVHD. In resource limited settings, or in patients with high risk for sedation related complications, FS with rectosigmoid biopsies may be an appropriate approach given reasonable yield for detection of aGVHD. Our study also found no significant difference in adverse events between thrombocytopenic and neutropenic patients, confirming the safety of endoscopy in this patient population. Future, larger, controlled studies are needed to control for confounders and more accurately model the risk associated with endoscopy in the thrombocytopenic and neutropenic groups.
Gastrointestinal (GI) graft-vs-host disease (GVHD) is the most common complication of hematopoietic stem cell transplant (HSCT) and is often diagnosed via endoscopy with biopsy.
Limited data exists on optimal endoscopic strategy and safety for GVHD evaluation in cancer patients who have had HSCT.
To create a strategy of endoscopic approach based on symptoms, gross endoscopic findings, and biopsy location as well as understand the safety of endoscopy in acute GVHD (aGVHD) patients.
We analyzed 195 endoscopies performed at City of Hope in patients who underwent HSCT for hematological malignancy and were evaluated for aGVHD.
Evaluation using combined esophagogastroduodenoscopy (EGD) and flexible sigmoidoscopy (FS) demonstrated a greater diagnostic yield for aGVHD (83.1%) compared to EGD (66.7%) or FS (77.2%) alone in patients with any presenting symptom. Biopsies obtained from either the upper or lower GI tract, specifically the rectosigmoid colon, demonstrated comparably high yields in patients with diarrhea (95.7% vs 99.1%) or nausea/vomiting (97.5% vs 96.8%). Normal-appearing mucosa was generally as specific (91.3%) for the presence of aGVHD on biopsy as the presence of endoscopic abnormalities (58.7%-97.8%), however sensitivity was low. Adverse events occurred in a small proportion of patients, including bleeding (1.0%), infection (1.0%), and perforation (0.5%). There was no significant difference in occurrence of adverse events in thrombocytopenic compared to non-thrombocytopenic patients (P = 1.000) and neutropenic compared to non-neutropenic patients (P = 0.425).
Combined EGD and FS with biopsy of the stomach and rectosigmoid colon results in the greatest diagnostic yield for most patients referred for evaluation of aGVHD, independent of symptoms. Biopsy of normal appearing mucosa is warranted, and endoscopic evidence of severe inflammation is specific for more histologically severe GVHD. In resource limited settings, or in patients with high risk for sedation related complications, FS with rectosigmoid biopsies may be an appropriate approach given reasonable yield for detection of aGVHD. Our study also found no significant difference in adverse events between thrombocytopenic and neutropenic patients, confirming the safety of endoscopy in this patient population.
Future, larger, controlled studies are needed to control for confounders and more accurately model the risk associated with endoscopy in the thrombocytopenic and neutropenic groups.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Society of Gastrointestinal Endoscopy; and American College of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cerwenka H, Triantafyllou K S-Editor: Yan JP L-Editor: A P-Editor: Wang LL
| 1. | Naymagon S, Naymagon L, Wong SY, Ko HM, Renteria A, Levine J, Colombel JF, Ferrara J. Acute graft-versus-host disease of the gut: considerations for the gastroenterologist. Nat Rev Gastroenterol Hepatol. 2017;14:711-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 915] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 3. | Xu CF, Zhu LX, Xu XM, Chen WC, Wu DP. Endoscopic diagnosis of gastrointestinal graft-versus-host disease. World J Gastroenterol. 2008;14:2262-2267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Robak K, Zambonelli J, Bilinski J, Basak GW. Diarrhea after allogeneic stem cell transplantation: beyond graft-versus-host disease. Eur J Gastroenterol Hepatol. 2017;29:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 6. | Terdiman JP, Linker CA, Ries CA, Damon LE, Rugo HS, Ostroff JW. The role of endoscopic evaluation in patients with suspected intestinal graft-versus-host disease after allogeneic bone-marrow transplantation. Endoscopy. 1996;28:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, Cahn JY, Calderwood S, Gratwohl A, Socié G, Abecasis MM, Sobocinski KA, Zhang MJ, Horowitz MM. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 525] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, Kreft A, Longerich T, Morton T, Myerson D, Prieto VG, Rosenberg A, Treister N, Washington K, Ziemer M, Pavletic SZ, Lee SJ, Flowers ME, Schultz KR, Jagasia M, Martin PJ, Vogelsang GB, Kleiner DE. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21:589-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 9. | Nomura K, Iizuka T, Kaji D, Yamamoto H, Kuribayashi Y, Tanaka M, Furuhata T, Yamashita S, Kikuchi D, Matsui A, Mitani T, Ota Y, Taniguchi S, Hoteya S. Utility of Endoscopic Examination in the Diagnosis of Acute Graft-versus-Host Disease in the Lower Gastrointestinal Tract. Gastroenterol Res Pract. 2017;2017:2145986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kreisel W, Dahlberg M, Bertz H, Harder J, Potthoff K, Deibert P, Schmitt-Graeff A, Finke J. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant. 2012;47:430-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Oomori S, Takagi S, Kikuchi T, Utsunomiya K, Yokoyama H, Negoro K, Tohmiya Y, Aihara H, Yamada M, Takahashi S, Kameoka J, Kinouchi Y, Shimosegawa T. Significance of colonoscopy in patients with intestinal graft-versus-host disease after hematopoietic stem cell transplantation. Endoscopy. 2005;37:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Cruz-Correa M, Poonawala A, Abraham SC, Wu TT, Zahurak M, Vogelsang G, Kalloo AN, Lee LA. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy. 2002;34:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Mendelsohn R, Tang L, Ludwig E. Does Endoscopic Impression Correlate with Histologic Findings when Making the Diagnosis of Gastrointestinal Graft versus Host Disease? Am J Gastroenterol. 2009;104:S511. [DOI] [Full Text] |
| 14. | Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, Lee JH, Couriel D. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol. 2008;103:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Sung AD, Hassan S, Cardona DM, Wild D, Nichols KR, Mehdikhani H, Balmadrid B, Detweiler CJ, Shealy M, Cirrincione C, Li Z, Poleski M, Dalton TE, Siamakpour-Reihani S, Chao NJ, Sullivan KM. Late Gastrointestinal Complications of Allogeneic Hematopoietic Stem Cell Transplantation in Adults. Biol Blood Marrow Transplant. 2018;24:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Thompson B, Salzman D, Steinhauer J, Lazenby AJ, Wilcox CM. Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 2006;38:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Daniel F, Hassoun L, Husni M, Sharara A, Soweid A, Barada K, Haffar B, Massoud R, Shaib Y, Al-Hashash J, Bazarbachi A, El Cheikh J. Site specific diagnostic yield of endoscopic biopsies in Gastrointestinal Graft-versus-Host Disease: A tertiary care Center experience. Curr Res Transl Med. 2019;67:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Liu A, Meyer E, Johnston L, Brown J, Gerson LB. Prevalence of graft versus host disease and cytomegalovirus infection in patients post-haematopoietic cell transplantation presenting with gastrointestinal symptoms. Aliment Pharmacol Ther. 2013;38:955-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Cloutier J, Wall DA, Paulsen K, Bernstein CN. Upper Versus Lower Endoscopy in the Diagnosis of Graft-Versus-Host Disease. J Clin Gastroenterol. 2017;51:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Cheung DY, Kim JI, Kim SS, Sung HY, Cho SH, Park SH, Han JY, Kim JK, Lee JW, Min WS, Park GS, Kang CS. Endoscopic evaluation in gastrointestinal graft-versus-host disease: comparisons with histological findings. Dig Dis Sci. 2008;53:2947-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Altun R, Gökmen A, Tek İ, Soydan E, Kurt Yüksel M. Endoscopic evaluation of acute intestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Turk J Gastroenterol. 2016;27:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Scott AP, Tey SK, Butler J, Kennedy GA. Diagnostic Utility of Endoscopy and Biopsy in Suspected Acute Gastrointestinal Graft-versus-Host Disease after Hematopoietic Progenitor Cell Transplantation. Biol Blood Marrow Transplant. 2018;24:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | ASGE Standards of Practice Committee, Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (2)] |
| 24. | Van Os EC, Kamath PS, Gostout CJ, Heit JA. Gastroenterological procedures among patients with disorders of hemostasis: evaluation and management recommendations. Gastrointest Endosc. 1999;50:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Ross WA. Endoscopic Interventions in Patients with Thrombocytopenia. Gastroenterol Hepatol (N Y). 2015;11:115-117. [PubMed] |
| 26. | Tong MC, Tadros M, Vaziri H. Endoscopy in neutropenic and/or thrombocytopenic patients. World J Gastroenterol. 2015;21:13166-13176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Oh HJ, Park JM, Yoon SB, Lee HH, Lim CH, Kim JS, Cho YK, Lee BI, Cho YS, Choi MG. Bleeding After Endoscopic Procedures in Patients With Chronic Hematologic Thrombocytopenia. Dig Dis Sci. 2017;62:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Abu-Sbeih H, Ali FS, Coronel E, Chen HC, Wang X, Lum P, Shuttlesworth G, Bhutani MS, Raju GS, Lee JH, Stroehlein JR, Ross WA, Wang Y. Safety of endoscopy in cancer patients with thrombocytopenia and neutropenia. Gastrointest Endosc. 2019;89:937-949.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Krishna SG, Rao BB, Thirumurthi S, Lee JH, Ramireddy S, Guindani M, Ross WA. Safety of endoscopic interventions in patients with thrombocytopenia. Gastrointest Endosc. 2014;80:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |