Published online Jan 16, 2020. doi: 10.4253/wjge.v12.i1.42
Peer-review started: May 10, 2019
First decision: May 31, 2019
Revised: September 19, 2019
Accepted: November 6, 2019
Article in press: November 6, 2019
Published online: January 16, 2020
Processing time: 223 Days and 11.1 Hours
Esophagogastric leakage is one of the most severe postoperative complications. Partial disruption of the anastomosis, can be successfully treated with an endoscopic vacuum assisted closure (E-VAC). The advantage of that method of treatment is the ability to adjust a vacuum dressing individually to the size of the dehiscence and thus to reduce the risk of a secondary fistula or abscess. The authors present two patients with postoperative gastroesophageal leakage treated successfully with E-VAC.
Two male patients developed a potentially life threatening esophagogastric leakage. Patient A underwent resection of the distal half of the esophagus and upper part of the stomach due to Siewert type II adenocarcinoma of the gastroesophageal junction. Proximal resection of the stomach was performed in the patient B after massive bleeding from Mallory-Weiss tears. Both patients were treated successfully with an individually adapted E-VAC with concomitant correction of fluid and electrolyte disturbances, and treatment of sepsis with appropriate antibiotics.
Endoscopic vacuum closure is an effective alternative to endoscopic stenting or relaparotomy. Through individual approach it allows a more accurate assessment of healing.
Core tip: Postoperative esophagogastric leakage might be successfully treated with conservative measures either with well-established endoscopic stenting or endoscopic vacuum assisted closure with respect to the type and localization of leakage. The advantage of vacuum treatment is a continuous evacuation of septic discharge and an individual adjustment of the dressing depending on the size of the dehiscence. The application of an endoscopic vacuum dressing is relatively simple, cost-effective, easily accessible and in many cases, it avoids consecutive laparotomies.
- Citation: Cwaliński J, Hermann J, Kasprzyk M, Banasiewicz T. Endoscopic vacuum assisted closure of esophagogastric anastomosis dehiscence: A case report. World J Gastrointest Endosc 2020; 12(1): 42-48
- URL: https://www.wjgnet.com/1948-5190/full/v12/i1/42.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i1.42
Esophagogastric leakage remains the most severe and a life threatening complication of gastroesophageal junction (GEJ) resection. The reported leak rate following esophageal resection due to carcinoma varies from 5% to 17%[1,2]. The management depends on the clinical condition of a patient and a type of leakage, that might be secondary to dehiscence of less than 10% of the anastomosis circumference, disruption of 10%-50% of the circuit, or necrosis with anastomotic separation of more than 50% of the circumference[3,4]. The last type of leakage results usually in a catastrophic septic shock and requires urgent redo operation. The other types might be treated conservatively with correction of fluid and electrolyte disturbances, treatment of sepsis with appropriate antibiotics, percutaneous drainage, stent implantation or endoscopic vacuum assisted closure (E-VAC) placement[5,6]. Based on a limited experiences negative pressure drainage appears today a promising alternative to stenting[6,7]. The aim of the study is to show the possibilities of endoscopic vacuum therapy in the treatment of upper gastrointestinal tract leakage adapted individually to the size and location of a leakage.
Two male patients with postoperative esophagogastric leakage treated with individually adapted E-VAC.
Patient A, a 53-year-old male was admitted to the authors’ Surgery Clinic due to Siewert type II adenocarcinoma of GEJ. He underwent a radical resection of the distal half of the esophagus and upper part of the stomach through thoraco-phreno-laparotomy. Primary anastomosis of the esophageal remnant with gastric conduit was performed within the thoracic cavity to restore continuity of the digestive tract. The postoperative course was complicated with dehiscence of the esophagogastric anastomosis followed by leakage into the mediastinum limited by the gastric conduit, the lung, and the diaphragm but without entering the pleural cavity.
Patient B, a 23 year-old male was admitted to the Clinic from a county hospital with esophagogastric anastomosis dehiscence 4 d after urgent, proximal resection of the stomach due to massive bleeding from Mallory-Weiss tears which failed to respond to endoscopic treatment.
Patient A complained of dysphagia and anorexia and he lost weight in 6 mo, He was addicted to cigarettes and alcohol within 10 years before surgery. Gastroscopy revealed cancer of the gastro-oesophageal junction. The medical history of patient B was unencumbered and the esophageal rupture was a result of massive vomiting in the course of gastroenteritis.
Patient’s A general condition deteriorated on the fourth day after surgery. He developed dyspnoea and pain in the upper abdomen. The pulse increased to 110 bpm with permanent hypotension. There was also a purulent discharge through an abdominal drain located close to the hiatus followed by gastric content and saliva on the 6th d after surgery with a total volume up to 300 mL per day. Patient B after admission to the Clinic developed septic shock and underwent redo operation limited to drainage of the anastomotic region because of encapsulating peritonitis. He was admitted to the intensive care unit (ICU) for ventilator treatment, hemodialysis, and broad-spectrum antibiotic therapy. The abdominal drain left during the relaparotomy led gastric content and air.
Elevated inflammatory markers were characteristic of both patients. C-reactive protein at the moment of leak detection were 401 mg/L for patient A and 310 mg/L for patient B. Patient’s B procalcitonin level increased up to 20 ng/mL one day after relaparotomy. In turn, patient A manifested anemia on the fourth day after surgery and the level of white blood cells reached 40 G/L.
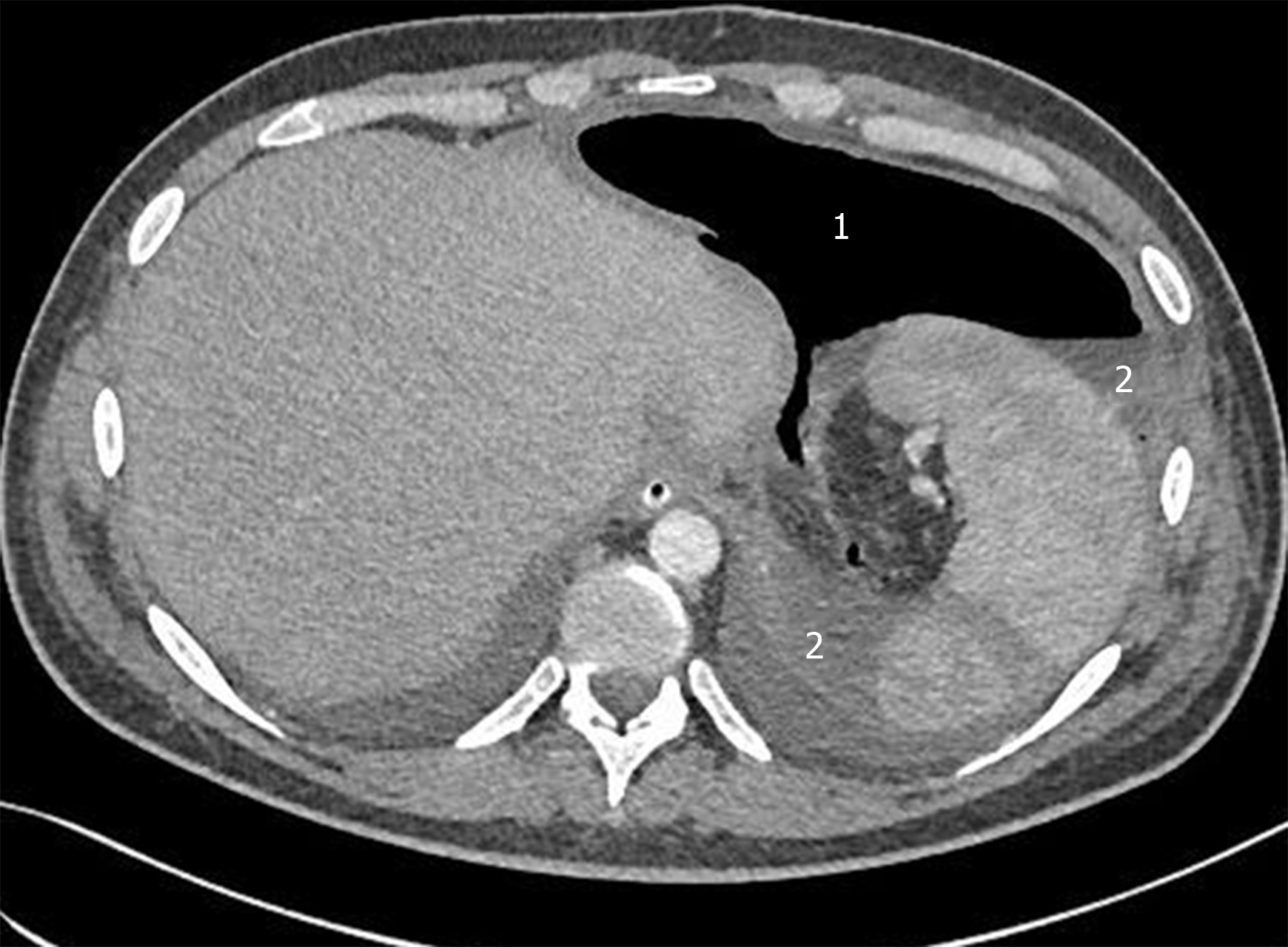
Patient A: Water-soluble contrast swallow showed the passage of contrast media beyond the gastrointestinal tract to the mediastinum (Figure 1). Endoscopic assessment revealed posterior disruption of esophagogastric anastomosis within 30% of the circumference (Figure 2A). Patient B: After relaparotomy and two-day stay at the ICU esophagogastroduodenoscopy was performed which revealed a small dehiscence of less than 10% of the anastomosis circumference in the form of a focal necrosis (Figure 2B). Computed tomography (CT) performed next day revealed a large amount of gas and fluid in the abdominal cavity (Figure 3).
Both patients showed postoperative gastroesophageal leakage classified as type II according Consensus for Defining and Reporting Complications After Esophagectomy.
In both cases after the decision to apply E-VAC, a hand-prepared drain consisting of a nasogastric catheter wrapped with a polyurethane foam (VivanoMed Foam, Paul Hartmann AG, Germany), was inserted into the lumen of the gastrointestinal tract (Figure 4).
A significant disruption of esophagogastric anastomosis diagnosed in patient A required the introduction of vacuum dressing into mediastinal cavity, preceded with aspiration of discharge for culture (Figure 2C). Throughout the treatment a stable negative pressure of 100 mmHg was maintained. E-VAC was changed four times in a fortnight and during each change either size of the foam was reduced and the catheter was gradually removed from the shrinking cavity with the aim to collapse it. Finally, E-VAC therapy was finished when the sinus reached a size of a small shallow esophageal diverticulum and when inflammatory markers levels normalized (Figure 2D). At the first session of vacuum therapy surgical treatment was combined with parenteral nutrition modified with immune modulatory amino acids such as L-arginine and L-glutamine, and medium chain triglycerides for hypoalbuminemia and lymphopenia. Next the realimentation was then continued via the enteral route through an enteric probe. Basal metabolic rate was evaluated according to European Society for Clinical Nutrition and Metabolism including anthropometric grade, and laboratory tests.
In the case of patient B, because of a very narrow range of dehiscence, a nasogastric catheter with a polyurethane foam was left in the lumen of the digestive tract to close to the anastomosis region (Figure 2E). Throughout the treatment a fluctuating negative pressure between 60- and 80-mmHg and changing every 5 min was applied to avoid damage to the mucosa. In addition, a nasoenteric tube was inserted for enteral nutrition. E-VAC was changed three times every third day. The patient’s general condition improved in 2 wk and he was finally discharged from the Clinic on oral diet.
Both patients were treated successfully with E-VAC of dehiscent anastomoses with concomitant correction of fluid and electrolyte disturbances, and treatment of sepsis with appropriate antibiotics. In both cases, a CT was performed at the end of the treatment to exclude a fluid reservoir (abscess, hematoma, intestinal fluid, etc.). A control gastroscopy performed in both patients after three months revealed complete healing of previous anastomotic disruption. In a case of Patient A, a small anastomotic stricture with shallow diverticulum was found (Figure 2F).
However, esophagogastric leakage after GEJ resection is one of the most severe complication with a relatively high mortality the management mainly depends on a patient’s general condition and the cause of leakage. According to Consensus for Defining and Reporting Complications After Esophagectomy reached by ECCG type II of anastomotic leak requires intervention but not surgical therapy[8]. This type of dehiscence might be treated successfully with conservative measures either with well-established endoscopic stenting or E-VAC with concomitant correction of fluid and electrolyte disturbances, and treatment of sepsis with appropriate antibiotics[4-6]. Although, stenting allows for prompt oral feeding, its migration occurs in over 25% of cases and it is not fully tight. In addition, late complications of stenting include difficulties with its retrieval, perforations, fistulas, and bleeding[9]. What is more, there was no communication between the collection of fluid and the pleural cavity in the presented case. Therefore, the anastomotic dehiscence which could have been sealed with a possible stent was the only approach for evacuation of septic discharge. Treatment of the leakage with E-VAC appears a significant alternative to stenting[7,9,10]. The advantage of vacuum treatment is a continuous evacuation of septic discharge, reduction of inflammatory edema, improvement of blood supply and lymphatic drainage. Negative pressure wound therapy (NPWT) result in shrinkage of a cavity and promotes formation of fresh granulation tissue[6,7]. The application of endoluminal closure is simple since the dressing consists of a polyurethane foam sutured to a regular nasogastric tube. That catheter equipped with a foam might be located either within a dehiscence for extended disruption of an anastomosis or close to it in the lumen of the gastrointestinal tract in a case of small defects. That tube is supposed to be connected then with every negative-pressure port. Immediate application of E-VAC just after diagnosis of esophagogastric leakage is of utmost importance[11]. The method is recommended today even to prevent esophagogastric leakage in jeopardized anastomoses[12]. E-VAC is today a widely used method of therapy for complications developing after operations within the upper part of the gastrointestinal tract with satisfactory early as well as long-term results and with good quality of life[13].
Our experience shows that E-VAC in the treatment of gastrointestinal anastomotic leakage is a valuable and effective method. In contrast to stenting, vacuum therapy provides constant access to the site of leakage, allowing the evaluation of healing and modification of surgical treatment. We hope endoscopic NPWT will be approved and becomes a valuable alternative in the treatment of surgical complications.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu B S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Dewar L, Gelfand G, Finley RJ, Evans K, Inculet R, Nelems B. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg. 1992;163:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Mishra PK, Shah H, Gupta N, Varshney V, Patil NS, Jain A, Saluja SS. Stapled versus hand-sewn cervical esophagogastric anastomosis in patients undergoing esophagectomy: A Retrospective Cohort Study. Ann Med Surg (Lond). 2016;5:118-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Persson S, Rouvelas I, Irino T, Lundell L. Outcomes following the main treatment options in patients with a leaking esophagus: a systematic literature review. Dis Esophagus. 2017;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, DʼJourno XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SY, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJ. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 843] [Article Influence: 84.3] [Reference Citation Analysis (1)] |
| 5. | Anderloni A, Genco C, Massidda M, Di Leo M, Fumagalli UR, Rosati R, Correale L, Maselli R, Ferrara EC, Jovani M, Repici A. Self-Expanding Metal Stents for the Treatment of Post-Surgical Esophageal Leaks: A Tertiary Referral Center Experience. Dig Surg. 2019;36:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Noh SM, Ahn JY, Lee JH, Jung HY, AlGhamdi Z, Kim HR, Kim YH. Endoscopic Vacuum-Assisted Closure Therapy in Patients with Anastomotic Leakage after Esophagectomy: A Single-Center Experience. Gastroenterol Res Pract. 2018;2018:1697968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 7. | Borejsza-Wysocki M, Szmyt K, Bobkiewicz A, Malinger S, Świrkowicz J, Hermann J, Drews M, Banasiewicz T. Endoscopic vacuum-assisted closure system (E-VAC): case report and review of the literature. Wideochir Inne Tech Maloinwazyjne. 2015;10:299-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Pera M, Low DE. Consensus for defining and reporting complications after esophagectomy: an important new step in place for using the same language. Cir Esp. 2015;93:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Hwang JJ, Jeong YS, Park YS, Yoon H, Shin CM, Kim N, Lee DH. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine (Baltimore). 2016;95:e3416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Aryaie AH, Singer JL, Fayezizadeh M, Lash J, Marks JM. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc. 2017;31:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp M, Klempnauer J, Manns MP, Schneider AS. Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc. 2010;71:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Neumann PA, Mennigen R, Palmes D, Senninger N, Vowinkel T, Laukoetter MG. Pre-emptive endoscopic vacuum therapy for treatment of anastomotic ischemia after esophageal resections. Endoscopy. 2017;49:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Dhayat SA, Schacht R, Mennigen R, Palmes D, Vogel T, Vowinkel T, Senninger N, Laukoetter MG. Long-Term Quality of Life Assessment After Successful Endoscopic Vacuum Therapy of Defects in the Upper Gastrointestinal Tract Quality of Life After EVT. J Gastrointest Surg. 2019;23:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |