Published online Jan 16, 2020. doi: 10.4253/wjge.v12.i1.1
Peer-review started: April 4, 2019
First decision: August 2, 2019
Revised: August 28, 2019
Accepted: October 19, 2019
Article in press: October 19, 2019
Published online: January 16, 2020
Processing time: 262 Days and 5.1 Hours
Upper gastrointestinal bleeding is defined as the bleeding originating from the esophagus to the ligament of Treitz and further classified into variceal and non-variceal gastrointestinal bleeding. Non-variceal upper gastrointestinal bleeding remains a common clinical problem globally. It is associated with high mortality, morbidity, and cost of the health care system. Despite the continuous improvement of therapeutic endoscopy, the 30-d readmission rate secondary to rebleeding and associated mortality is an ongoing issue. Available Food and Drug Administration approved traditional or conventional therapeutic endoscopic modalities includes epinephrine injection, argon plasma coagulation, heater probe, and placement of through the scope clip, which can be used alone or in combination to decrease the risk of rebleeding. Recently, more attention has been paid to the novel advanced endoscopic devices for primary treatment of the bleeding lesion and as a secondary measure when conventional therapies fail to achieve hemostasis. This review highlights emerging endoscopic modalities used in the management of non-variceal upper gastrointestinal related bleeding such as over-the-scope clip, Coagrasper, hemostatic sprays, radiofrequency ablation, cryotherapy, endoscopic suturing devices, and endoscopic ultrasound-guided angiotherapy. In this review article, we will also discuss the technical aspects of the common procedures, outcomes in terms of safety and efficacy, and their advantages and limitations in the setting of non-variceal upper gastrointestinal bleeding.
Core tip: In the last two decades, there has been drastic decline in the mortality and morbidity caused non-variceal upper gastrointestinal bleeding due to significant progress in the therapeutic endoscopy. The use of devices such as over the scope clips system, Coagrasper, hemospray and endoscopic suturing has tremendously evolved and expanded to achieve hemostasis as a primary method or when conventional therapeutic devices such as heater probe, hemoclips or epinephrine injection fails to control bleeding.
- Citation: Naseer M, Lambert K, Hamed A, Ali E. Endoscopic advances in the management of non-variceal upper gastrointestinal bleeding: A review. World J Gastrointest Endosc 2020; 12(1): 1-16
- URL: https://www.wjgnet.com/1948-5190/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i1.1
Gastrointestinal bleeding is a medical emergency that results in substantial morbidity, mortality, and health care cost[1,2]. It can present as a massive life-threatening hemorrhage, or a slow chronic bleed. Upper gastrointestinal bleeding (UGIB) is defined as any gastrointestinal bleeding that originates above the ligament of Treitz[3,4]. UGIB can be further classified as non-variceal UGIB (NVUGIB) and variceal UGIB (VUGIB). The common causes of NVUGIB are listed in Table 1. The incidence and mortality associated with NVUGIB have been decreasing due to the advancements in the prevention and management of NVUGIB[5-7]. Yet, it remains a common clinical problem with an annual incidence of about 90-108 per 100000 and mortality of 3% to 14%[8,9].
| Etiologies of non-variceal upper gastrointestinal bleeding | |
| Ulcer/ inflammation | Peptic ulcer disease |
| Erosive esophagitis, gastritis or duodenitis | |
| Anastomotic ulcers (post gastric bypass) | |
| Vascular lesions | Gastric antral vascular ectasia |
| Dieulafoy’s lesion | |
| Angiodysplasia/ Arteriovenous malformation | |
| Aorto-enteric fistula | |
| Congestive gastropathy | Portal hypertensive gastropathy |
| Malignant lesions | Gastrointestinal stromal tumors (GIST) |
| Non-GIST (e.g., Lipoma, schwannoma) | |
| Gastric and esophageal cancer | |
| Metastatic lesions in the upper GI tract | |
| Post procedural | Endoscopic mucosal and submucosal dissection |
| Post sphincterotomy | |
| Others | Mallory Weis tear |
| Cameron ulcers | |
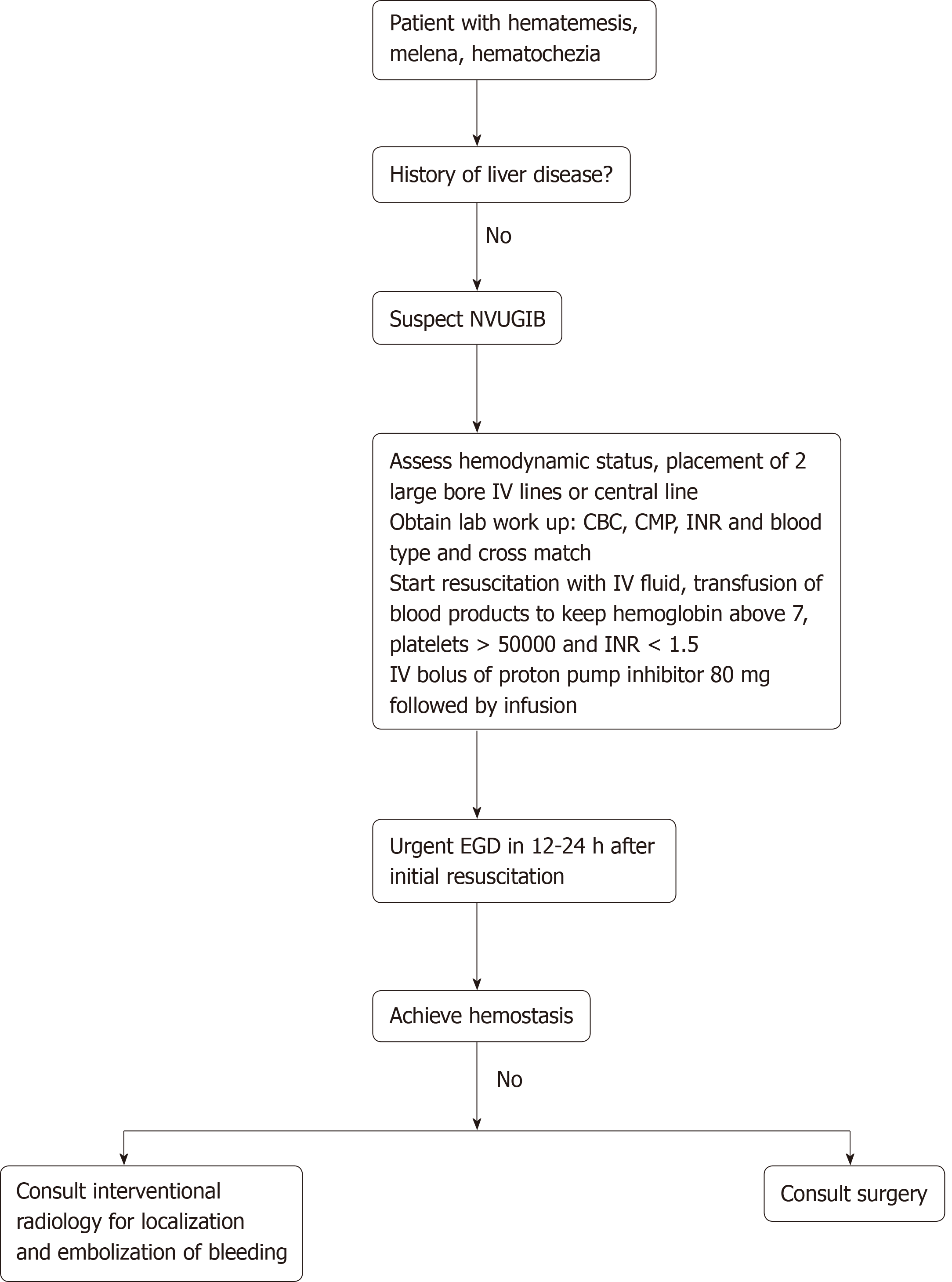
The initial approach to the patient presenting with acute NVUGIB is highlighted in Figure 1. Early endoscopic intervention within 24 h of presentation dramatically improves patient outcomes, and there was no difference observed compared to those who underwent endoscopic intervention < 12 h after presentation[10,11]. When an endoscopic approach is performed, it is crucial to have a standardized method of diagnosing the cause of bleeding, evaluating the stigmata of recent hemorrhage (i.e., active bleeding, a visible blood vessel, presence of clots, or red or black spots covering the ulcer lesion), and classifying gastric ulcers according to the Forrest classification[12,13]. Endoscopy is important in revealing the etiology of NVUGIB.
The development and widespread use of endoscopy has been a major contributor to the reduced need for surgery and morbidity associated with NVUGIB[14]. Endoscopic management is classified as injection, thermal, and mechanical methods. Amongst the traditional methods, injection of epinephrine is the most common and widely used modality because of its feasibility to perform and requires less coordination between endoscopist and assistant. However, epinephrine alone is less effective than combination with thermal or mechanical and other monotherapies such as clips, probes and electrocoagulation[15,16]. According to the Cochrane review, combination treatment has been associated with significant reduced risk of rebleeding, surgery and mortality in peptic ulcers with active bleeding or high-risk stigmata such as adherent clot[17]. Through the scope endoclips or hemoclips are found to be effective and safe hemostatic mechanical devices when applied precisely as mono or combine therapy. Clip grasp the vessel in the submucosa, seal the defect in the target blood vessel with or without approximation of the sides of the lesion. Furthermore, the tissue damage is minimal with clips and ulcer healing process is not hampered[18,19]. Introduced in clinical practice in 1990s, over the years, clips are evolved in terms of functionality (such as precision, tensile strength, rotatability, overshoot and strength of closure), physical characteristics and cost[20,21]. Recently published study by Wang et al[22] compared the functionality of the five different types of hemostatic clips. According to the study findings, Resolution 360 (Boston Scientific, Marlborough, Mass) was the fastest rotating clip when operated by the physicians. Instinct (Cook Medical, Bloomington, Ind) was found more mechanically stronger and performed better for compression of thick, fibrous tissue and crated ulcers. Overshoot and whipping (defined as > 30° and > 1 half revolution respectively) tends to happen when clips are rotated multiply in same direction. For both overshoot and whip, the SureClip 16 mm performed well when compared with other types of through the scope clips[22].
Despite the continued improvements, traditional therapies sometimes lack effectiveness at primary control or prevention of rebleeding, which are reported to be as high as 10%-24%[23-25]. Posterior duodenal wall ulcers or ulcers higher up the lesser curvature, actively bleeding lesions during endoscopy, ulcers larger than 2 cm in diameter, or with bleeding vessel > 2 mm are some of the major predictors of rebleeding[26]. For the past decade, there is much interest in developing and studying endoscopic methods to effectively achieve hemostasis and overcome the limitation of the traditional endoscopic methods. Tables 2 and 3 summarize emerging endoscopic modalities for the management of NVUGIB and their pros and cons. In this article, we will review the advanced endoscopic modalities currently available for the management of NVUGIB.
| Emerging endoscopic modalities | |
| Injection | Endoscopic ultrasound guided angiotherapy |
| Thermal therapies | Coagulation grasper, radiofrequency ablation, cryotherapy |
| Mechanical | Over the scope clip system, endoscopic suturing, flexible linear stapler (experimental) |
| Topical | Hemospray, endoclot, pure-Stat, ankaferd blood stopper, oxidized cellulose |
| Emerging endoscopic treatment | Pros | Cons |
| Over the scope clips | 1 Simple to use | 1 Difficult to close hard, chronic, and severely fibrotic lesions with OTSC |
| 2 Special endoscopic skills are not required to implant the clip | 2 Time consuming especially in the emergency situations (after identifying the bleeding source, the scope must be removed to mount OTSC system on the scope and reintroduce to deploy clips | |
| 3 Effective for the ulcers larger than 2 cm in diameter, or with bleeding vessel > 2 mm | ||
| Endoscopic suturing | 1 Technically more feasible and efficacious for larger, deep, and fibrotic ulcers | 1 Double channel endoscope and expert endoscopic skills are required to operate endoscopic suturing device |
| Endoscopic band ligation (EVL) | 1 Associated with the reduction of treatment sessions, control of bleeding and need for transfusion | Few cases of Hyperplastic gastric polyps |
| 2 EVL is safe, technically straightforward, and highly effective in this patient with complete eradication of GAVE | ||
| Coagrasper | 1 One of the safest and most efficacious hemostasis modalities due to large surface area of the forceps and anti-slip jaw design provides mechanical tamponade effect to the surrounding tissue | 1 Coagulation may be incomplete because of electrical leakage if the lesion submerged in water or lesion with large tissue volume or surface area |
| 2 The risk of perforation is extremely low because coagrasper works at a lower voltage as compared to other thermal treatments coagulates tissues without any carbonization and does not extend to deeper tissue | 2 Because the devices used for soft coagulation, including disposable hemostatic forceps, are relatively expensive, the method may be appropriate only for centers that perform ESD frequently | |
| 3 The forceps can be used to treat multiple bleeding sites proving to be cost-effective | 3 Few cases of aspiration pneumonia reported | |
| Radiofrequency ablation | 1 Feasible and safe in ablating GAVE lesions | 1 Endoscopic skills are required to perform RFA |
| 2 Able to deliver high energy captive coagulation of superficial mucosa including blood vessels | 2 Exact apposition of the gastric antral mucosa with electrode is required to allow effective delivery of the electric energy which means the endoscope may have to be removed, the electrode rotated, and reintroduced multiple times The newer through-the-scope internally rotatable ablating catheter may sidestep this disadvantage but has smaller surface area | |
| 3 Wider surface area coverage of mucosa owing to the various electrode sizes | ||
| 4 Contact technique with uniform zone of energy distribution and penetration such that deeper ectatic submucosal vascular channels are coagulated | ||
| Endoscopic ultrasound guided angiotherapy | 1 EUS-guided therapy of nonvariceal bleeding has been shown to be feasible and safe for peptic ulcer disease, Dieulafoy's lesions, bleeding tumors, and pseudoaneurysms due to the ability to directly visualize and target the bleeding vessel with a specific therapy and subsequently confirm hemostasis with real-time Doppler ultrasound are significant advantages of EUS-guided therapy | 1 Endoscopic skills are required to perform endoscopic ultrasound |
| 2 EUS guided angiotherapy more resource intensive than other routine hemostasis endoscopic procedures | ||
| Topical therapies, i.e., Hemospray and Endoclot | Easy to use, safe and effective Cost effective. Can be used for malignant GI hemorrhage | 1 Theoretically possible side effects of Hemospray include embolization, intestinal obstruction, and allergic reaction to the powder |
| 2 If hemostasis fails, there is the disadvantage that the powder attached to the mucous membrane may limit the use of other hemostatic modalities | ||
| 3 Hemospray works only on active bleeding |
It has been proved that mechanical hemostatic methods are more effective in achieving hemostasis than injections or thermal modalities alone. The Over-The-Scope Clip (OTSC, Ovesco Endoscopy GmbH, TÜbingen, Germany) is a Food and Drug Administration approved novel endoscopic clipping device[27]. The use of OTSC system was first reported in 2007 by Kirschniak et al[28] for gastrointestinal tissue approximation. Since then, the device has been widely used to control gastrointestinal bleeding, particularly caused by large and fibrotic ulcers at anatomic locations that are difficult to treat with through-the-scope (TTS) clips or at risk of perforation[29]. Other uses include the closure of perforation and fistula[30].
The OTSC® System Set consists of an applicator cap with a mounted over the scope clip, thread, thread retriever, and a hand wheel for clip release. The clip is made up of a super elastic nitinol alloy, which is delivered by means of an applicator cap and released by tightening the thread with the hand wheel. OTSC caps are available in 3 diameters (11, 12, and 14 mm) and 2 working depths (3 and 6 mm). There are three versions of OTSC versions available currently (atraumatic, traumatic and more gastric wall closure clip)[31]. Due to its unique design and elastic properties, the nitinol clip closes itself and secures the therapeutic effect by exerting constant circumferential compression force enough to stop bleeding from large size tissue defects and blood vessels[32].
Over the scope clip system has been established to be safe and effective as a first line and in the rescue management of non-variceal gastrointestinal bleeding. The first case series comprises a total of 9 patients (7 patients with GI bleeding) and was first published in 2009 by Repici et al[33] from Italy. It was followed by several other retrospective analyses of single and multi-center experience with OTSC to achieve hemostasis (Table 4)[33-43]. Four retrospective studies with large sample sizes (n = 67-93) were published between 2016-2018. The primary outcomes of these studies were the technical success to control bleeding and rebleeding rates. Most of the studies reported success rates between 78 % to 100 % with the rebleeding risk of < 1%. However, rebleeding was seen approximately 26% patients in a retrospective analysis conducted by Brandler et al[43]. In this study, the authors attributed high rebleeding rates and failure of OTSC to the history of coronary artery disease. Lamberts et al[42] reported rebleeding rates of 26%. Their data suggest that first line endoscopic treatment of the ulcer with OTSC has higher success and low rebleeding rates as compared to its use as second-line treatment. Also, they found OTSC as the less preferable treatment for diffusely bleeding polypoid lesions and vascular malformations.
| Authors and year of publication | Study design | Study participants | Sample size | Duration | Outcomes of the study | Success rate |
| Repici et al[33], 2009 | Retrospective | Mean age, 70 yr, gender (M/F): 5/2 | 7 | Unknown | Success rates with the first endoscopic therapy | Success rates with the first endoscopic therapy |
| Kirschniak et al[34], 2011 | Retrospective | Mean age, 68 yr, gender (M/F): 18/9 | 27 | 2006-2010 | 1 Success rates with the first endoscopic therapy | Primary hemostasis was achieved in all cases (100%) Rebleeding was observed in 2 cases |
| 2 Rebleeding episodes | ||||||
| Albert et al[35], 2011 | Retrospective | Mean age, 62 yr, gender (M/F): 5/2 | 7 | Unknown | 1 Success rates with the first endoscopic therapy | Primary success rate was observed in 100% |
| 2 Rebleeding episodes | ||||||
| Skinner et al[36], 2014 | Retrospective | Mean age, 59 yr, gender (M/F): 8/5 | 12 | 2012-2013 | 1 Success rates with the first endoscopic therapy | Hemostasis was achieved in all patients. Rebleeding occurred in two patients 1 d and 7 d after OTSC placement |
| 2 Rebleeding episodes | ||||||
| Nishiyama et al[37], 2013 | Retrospective | Mean age, 77 yr, gender (M/F): 5/4 | 9 | 2011-2012 | Success rates with the first endoscopic therapy | Primary success rate was observed in 77.8% |
| Manta et al[39], 2013 | Retrospective | Mean age, 64 yr, gender (M/F): 14/16 | 30 | 2011-2012 | 1 Success rates with the first endoscopic therapy | Primary hemostasis was achieved in 29 of 30 cases (97%) Rebleeding was observed in two cases (one duodenal bulb and one gastric ulcer) |
| 2 Rebleeding episodes | ||||||
| Manno et al[38], 2016 | Retrospective | Mean age, 69 yr, gender (M/F): 33/7 | 40 | 2013-2014 | 1 Success rates with the first endoscopic therapy | Technical success and primary haemostasis were achieved in all patients (100%). No re-bleeding need for surgical or radiological embolization treatment or other complications were observed during the follow-up period of 30 d |
| 2 Rebleeding episodes | ||||||
| Richter-Schrag et al[40], 2016 | Retrospective | Mean age, 72 yr, gender (M/F): 58/35 | 93 | 2012-2016 | 1 Success rates with the first endoscopic therapy | Primary hemostasis and clinical success of bleeding lesions (without rebleeding) was achieved in 88/100 (88%) and 78/100 (78%), respectively |
| 2 Rebleeding episodes | ||||||
| Wedi et al[41], 2016 | Retrospective | Mean age, 71 yr, gender (M/F): 50/34 | 84 | 2009-2012 | Success rates with the first endoscopic therapy | Success rate 35/41 (85.36%) |
| Lamberts et al[42], 2017 | Retrospective | Mean age, 71.7 yr, gender (M/F): 55/20 | 75 | February 2011 and June 2014 | 1 Success rates with the first endoscopic therapy | Application of the OTSC resulted in immediate hemostasis (primary success rate) in all 75 patients. However, in 34.7 % a rebleeding episode was noted that could be treated by further endoscopic interventions. Only 3 patients had to be sent to the operating room because of failure of endoscopic therapy. In the rebleeding group the use of antiplatelet therapies was higher (73.1% vs 48.9%) |
| 2 Rebleeding episodes | ||||||
| Brandler et al[43], 2018 | Retrospective | Mean age, 71 yr, gender (M/F): 38/29 | 67 | 2011-2015 | OTSC safety and efficacy in GI bleeding | OTSC success rate of 81.3% |
| Schmidt et al[44], 2018 | Prospective, randomized, controlled multicenter trial | Mean age: 77 yr, gender (M/F): 37/29 | 67 | March 2013 to September 2016 | 1 Persistent bleeding despite endoscopic therapy according to the protocol or | Persistent bleeding after per-protocol hemostasis was observed in 14 patients (42.4%) in the standard therapy group and 2 patients (6.0%) in the OTSC group (P < 0.001) Recurrent bleeding within 7 d occurred in 5 patients (16.1%) in the standard therapy group vs 3 patients (9.1%) in the OTSC group (P = 0.468) |
| 2 Recurrent bleeding within 7 d after initial successful endoscopic therapy |
Only one prospective randomized control multicenter trial compared OTSC with standard treatment (TTS clips or thermal therapy plus injection with diluted adrenaline) of severe recurrent UGIB was published by Schmidt et al[44]. According to the study, results demonstrated significant differences noted in the persistent bleeding rates between treatment (6.0%) and control group (42.4%) and rebleeding at 30 d. However, the rebleeding rates at day 7 were not significantly different between groups. A recently published study analyzed 1517 cases treated with OTSC in 30 published studies over a 9-year period. The overall success rate of OTSC to control hemorrhage was found to be 85% with the complication risk of about 1.7%. Procedural accidents for e.g. deviation of the over the scope clip system itself or deviation of the clip from fibrotic tissue, intraluminal stenosis, and perforation of the thin duodenal wall with the bear claw were a few of the reported complication in these studies[45].
One of the major advantages of the OTSC system is it’s simple to use and does not require special endoscopic skills to implant the clip[46]. However, it is difficult to close hard, chronic, and severely fibrotic lesions with OTSC. Another limitation is the application of the clip in the emergency situations because after identifying the bleeding source, the scope must be removed to mount OTSC system on the scope (just like variceal band ligator) and reintroduced to deploy clips[47].
An endoscopic suturing device to perform minimally invasive endoscopic interventions was first proposed by Kalloo et al[48] more than a decade ago. Since its development, the endoscopic suturing device (Overstitch TM, Apollo Endosurgery, Austin, TX, United States) has continuously evolved and been established to be successfully used in a variety of endoscopic procedures including gastrointestinal fistula closure, perforations, leaks, endoscopic revision of gastro-jejunal bypass after bariatric surgery, and endoscopic submucosal dissection (ESD)[49-51].
The endoscopic suturing device is introduced into the stomach through an over tube. The Overstitch system attaches proximally and distally to the double-channel endoscope, which is comprised of a cap-based suturing curved arm (to operate the tissue helix for atraumatic tissue manipulation), anchor exchange catheter (pass suture), and handle (to be mounted on the shaft of the endoscope to control suturing process). The suturing process begins at one of the edges of the ulcer using a curved needle. The curved needle then closes and grabbed by the anchor exchange and detached from the driver. The endoscope, with Overstitch, then moves proximally towards the other edge of the ulcer. This process repeats until the two edges of the ulcer are pulled together. Once the edges of the ulcer approximate each other, the 2 ‘O’ polypropylene suture, placed by the cinching device, is tightened and secured[52].
Endoscopic suturing is found to be a promising modality in the management of NVUGIB in several case reports and case series due to its excellent ability to close large mucosal defects after conventional methods fail to achieve hemostasis. Recently, Agarwal et al[53], published a case series of 10 patients and demonstrated the endoscopic suturing device was used successfully to control bleeding related to large recurrent peptic ulcers. Mean suturing time was reported to be 13.4 ± 5.6 (range 3.5-20) min. No early or delayed procedural related complications were reported[53].
The endoscopic suturing device has several advantages over OTSC and hemospray (HS). Although these devices have high success rates in controlling NVUGIB, the endoscopic suturing device is technically more feasible and efficacious for larger, deep, and fibrotic ulcers. However, bleeding from small, shallow, and non-fibrotic ulcers can be more efficiently controlled with OTSC placement[54]. HS, on the other hand, can be utilized as the temporizing measure to control bleeding, as published data suggested that its rebleeding rate is up to 29 % to 38 %[55]. Limitations of the endoscopic suturing device include the need for a double channel endoscope and expert endoscopic skills. The use of endoscopic suturing should be avoided if there is a suspicion of malignant ulcer[56].
Endoscopic band ligation (EBL) was initially developed for esophageal and hemorrhoidal ligation; however, it can be also used in the management of upper gastrointestinal vascular lesions, such as nodular gastric antral vascular ectasia (GAVE)[57]. Studies have demonstrated that EBL may be superior to argon plasma coagulation and endoscopic thermal therapy regarding the reduction of treatment sessions, control of bleeding and need for transfusion, proving to be a promising efficacious alternative modality[58,59]. However, further prospective studies are warranted with larger sample sizes, longer follow-up interval, and examination of cost-effectiveness and procedural time.
Coagrasper (Olympus Corp., Tokyo, Japan) or hemostatic forceps is a combination of a thermal and mechanical hemostasis device that delivers targeted monopolar coagulation at the precise site of bleeding[60]. It was initially developed to prevent and treat gastrointestinal bleeding associated with minimally invasive endoscopic procedures, such as EMRs, ESD, and resection of small gastric tumors[61]. Three sizes of the coagrasper are available with different jaw widths to allow effective hemostasis.
Coagrasper has several advantages over conventional heater probe thermal coagulation and hemoclips. Due to these unique properties, it is one of the safest and most efficacious hemostasis modalities[62]. The large surface area of the forceps and anti-slip jaw design provides mechanical tamponade effect to the surrounding tissue making it a highly efficacious hemostasis method. In addition, the risk of perforation is extremely low because coagrasper works at a lower voltage as compared to other thermal treatments coagulates tissues without any carbonization and does not extend to deeper tissue. The forceps can be used to treat multiple bleeding sites proving to be cost-effective[63].
A recent randomized prospective trial by Toka B and co-authors compared the efficacy of hemostatic forceps (n = 56) with hemoclip (n = 56) for NVUGIB[64]. The study reported an initial success rate in more than 98% of patients treated with coagrasper as compared to 80% in the hemoclip group. Rebleeding rates were lower in coagrasper group without adverse events. The shorter length of hospitalization and duration of endoscopic procedure in patients treated with coagrasper were reported. Another randomized controlled trial comparing efficacy of soft mode coagulation and heater probe thermocoagulation for peptic ulcer bleeding was published in 2015[65]. Significant differences were observed in achieving primary hemostasis in treatments groups with coagrasper (96%) and heater probe (67%). No reports of rebleeding and adverse events were observed in the coagrasper group. In contrast, perforation occurred in 2 patients treated with a heater probe, which were managed conservatively.
Radiofrequency ablation (RFA) was primarily used for the treatment of Barrett’s esophagus; however, it is an emerging endoscopic treatment for GAVE[66]. RFA can be performed by either using focal catheter (Barrx™ HALO90 and HALOULTRA) or Barrx TTS RFA catheter.
In a prospective open-label single center study, Raza and colleagues demonstrated 100% technical success with the HALO system and 67% clinical success in 9 patients after an 11-mo follow-up interval[67]. Further studies confirmed similar results of technical and clinical success with improved post-procedural hemoglobin without major adverse events observed[67,68]. Despite the promising results, the studies do not present a randomized design and have a short follow-up interval. A multicenter open-label retrospective case series demonstrated a significant increase in hemoglobin post-procedural with the HALO system, as well as a reduction of blood transfusions needed in 24 patients[68]. There are limited studies examining the use of RFA in other gastrointestinal related bleeds.
Cryotherapy has been proposed as a useful hemostasis modality by inducing cell necrosis through localized freezing of the large surface area of tissue[69]. Cho and colleagues demonstrated 50% of patients achieving complete response, while the other half achieved a partial response of GAVE related bleeding[70]. There was a reduction of blood transfusions required post-procedural, and an increase in hemoglobin was observed. There were no immediate complications observed. However, this was a small single-study pilot study with a short follow-up period. The number of treatment sessions and the type of cryogen need to be determined.
Endoscopic laser coagulation is another non-contact modality thermal method of hemostasis. An Nd: YAG laser is applied through the channel of an endoscope with the tip positioned 5 to 10 mm from the ulcer and the beam directed at the site of bleeding. Although ND: YAG laser therapy has been shown to be effective, it is not routinely used in the management of NVUGIB[55]. This is due to the technical constraints of the technique, the large size of laser delivery unit, requirement of special electrical and water supplies, and least cost-effective as compared to other modalities[71].
Hemostatic spray (Cook Medical, Winston-Salem, NC, United States), also known as HS or TC-325, is an absorptive inorganic powder that coalesces and adheres to the bleeding site forming a mechanical barrier[72]. It is not absorbed or metabolized by the gastrointestinal tract, limiting systemic toxicity, and sloughs off once hemostasis is achieved allowing for re-application if necessary[72]. HS does not require direct contact with the bleeding vessel and can, therefore, cover a larger surface area. In addition, it may promote platelet aggregation, activate the clotting cascade, as well as promote tissue formation[72]. HS has been evaluated as a monotherapy modality, such as in the management of a bulbar ulcer related bleed, as well as with other conventional therapy and as a rescue therapy[73]. In addition, it has been studied in malignancy related bleeding and use after therapeutic endoscopic interventions (Table 5).
| Study | Type of study | Sample size | Bleeding source | Modality | Outcomes | Results |
| Leblanc et al[72], 2013 | Case series, single arm (July 2011-March 2012) | 17 patients | Procedural (12/17) and malignancy related bleeding (5/17) | Monotherapy or rescue therapy | Immediate hemostasis, recurrent bleeding and mortality at 7 and 30 d, and related adverse events | Immediate hemostasis achieved in 100% patient in both groups; 2 patients with recurrent bleeding with 1 of 2 with treatment failure. No adverse events. No related complications |
| Sakai et al[73], 2016 | Case report | 1 patient | Ulcer related bleeding | Monotherapy | Immediate hemostasis | Immediate hemostasis achieved. No recurrent bleeding. No adverse events |
| Chen et al[55], 2015 | Retrospectiv single center study; (July 2011-July 2013) | 60 patients | 21 for nonmalignant nonvariceal upper gastrointestinal bleeding, 19 for malignant upper gastrointestinal bleeding, 11 for lower gastrointestinal bleeding, and 16 for intra-procedural bleeding | Monotherapy | Immediate hemostasis and early rebleeding (≤ 72 h) | Immediate hemostasis achieved in 66 cases including upper and lower (98.5 %), with 6 cases (9.5 %) of early rebleeding |
| Arena et al[74], 2017 | Retrospective cohort study; (January 2014-December 2015) | A total of 15 patients, 8 males, mean age 74 yr ± 7.7 | Malignancy related bleeding | Monotherapy | Immediate hemostasis, bleeding recurrence, adverse events, clinical outcome at 1 and 6 mo | Immediate hemostasis achieved in 93% (14/15). 3 (21%) patients with recurrent bleeding. 12/14 (80%) with good clinical outcome at 30 d and 50% (6/12) at 6 mo. No related adverse events |
| Pittayanon et al[75], 2018 | Retrospective study; (2011-2016) | 99 patients (70.5% were male, age 65 ± 14 yr | Malignancy related bleeding | Monotherapy and adjuvant therapy | Immediate hemostasis, early (≤ 3 d) and late (> 3 d) recurrent bleeding | Immediate hemostasis was 97.7%, with recurrent bleeding in 15% (early) and 17% (delayed). Six-month survival was 53.4% |
| Baracat et al[76], 2017 | Case report | 1 patient | Post-sphincterotomy bleeding | Rescue therapy | Hemostasis | Immediate hemostasis achieved |
| González et al[77], 2016 | Case report | 1 patient | Post-sclerotherapy bleeding | Monotherapy | Hemostasis | Immediate hemostasis achieved |
| Sung et al[78], 2011 | Prospective single-arm | 20 patients (18 men, 2 women; mean age 60.2 yr) | Peptic ulcer bleeding (Forrest score Ia or Ib) | Monotherapy | Immediate hemostasis (max of 2 applications allowed), bleeding recurrence post-operatively, after 72 h endoscopically, and after 30 d via phone; mortality, need for surgery, and complications | Immediate hemostasis in 95 % (19 / 20) of patients; (1/20) with a pseudoaneurysm requiring arterial embolization. Bleeding recurred in 2 patients ≤ 72 h (hemoglobin drop); neither had active bleeding at the 72-h endoscopy. No mortality, adverse events, or procedural-related complications at 30-d |
| Sinha et al[79], 2016 | Retrospective single center | 20 patients (median age of 75 yr; 50% men) | Peptic ulcer related bleeding (forrest 1a and 1b) | Adjuvant therapy to adrenaline, or to adrenaline with clips or a thermal device | Immediate hemostasis, 7 and 30-d rebleeding; all-cause and GI-related 30-d mortality | Initial hemostasis was attained in 95% with an overall rebleeding rate (RBR) at 7 d of 16%. No difference between the 7 and 30-d RBR. Hemospray + adrenaline = 100% initial hemostasis and 25% 7-d RBR. Hemospray as third agent = 92% initial hemostasis and 9% RBR. All-cause mortality was 15% with 1 GI-related death (3%) |
| Haddara et al[80], 2016 | Prospective registry; (published 2016) | 202 patients | Ulcer related bleeding in 75 patients, malignancy related bleed in 61 patients, procedural related bleed in 35 patients, and other in 31 patients | Monotherapy or rescue therapy | Feasibility, efficacy, re-bleeding rate at day 8 and 30 | Application of hemospray was found to be very easy or easy in 31.7 % and 55.4 %, respectively. Immediate hemostasis achieved in 96.5 %. Re-bleeding rate at day 8 and 30 were 26.7 % and 33.5 %, respectively |
| Yau et al[81], 2014 | Retrospective (February 2012-July 2013) | 19 patients (mean age 67.6 yr) | Peptic ulcers in 12 (63.2%) patients, Dieulafoy lesions in 2 (10.5%), mucosal erosion in 1 (5.3%), angiodysplastic lesion in 1 (5.3%), ampullectomy site in 1 (5.3%), polypectomy site in 1 (5.3%), and an unidentified lesion in 1 (5.3%) | Monotherapy, adjuvant therapy, and rescue therapy | Immediate hemostasis, recurrent bleeding at 7- and 30 d, mortality at 7 and 30 d (related to GIB), and adverse events (related to Hemospray) | Hemostasis in 14 of 15 (93.3%) patients; Rebleeding within 7 d in 7/18 (38.9%) patients. Potential adverse events in 2 (10.5%) patients (visceral perforation and splenic infarct). Mortality in 5 (26.3%) patients with 1 with hemoperitoneum |
| Smith et al[82], 2014 | Multicenter registry (June 2011-September 2011) | 63 patients (44 men; median age 65) | 30 patients with ulcer related bleeding | Monotherapy or rescue therapy | Immediate hemostasis | 47/55 (85%) patients in monotherapy group achieved immediate hemostasis |
| Sulz et al[83], 2014 | Case series; (published in 2014) | 16 patients | NVUGIB, unidentified | Monotherapy or rescue therapy | Immediate hemostasis | Immediate hemostasis of 93.75% (15/16) |
Several case series described the effect of HS on malignancy related bleeding. Chen et al[55] described 100% (5/5) of patients attaining immediate hemostasis with one recurrence of bleeding in a patient with severe metastatic disease complicated by disseminated intravascular coagulation[55,73]. As studied by Leblanc et al[72], 100% (5/5) of patients achieved immediate hemostasis (absent bleeding > 5 min after application) with one of two patients (esophageal tumor and stent placement) considered a treatment failure (not achieving immediate hemostasis or with recurrent bleeding despite 2 separate applications)[72]. Furthermore, Arena et al[74] demonstrated 93% achieving immediate hemostasis with a rebleeding rate (drop in hemoglobin > 2 g/dL) of 20%. Lastly, in a retrospective study, immediate hemostasis was achieved in 97.7% patients with recurrent bleeding of 15% (classified as early, < 3 d) and 17% (classified as delayed, > 3 d)[75]. No adverse events or procedural complications were observed in either study. Although bleeding may recur, HS appears to be effective for NVUGIB related to malignancies. The rate of recurrent bleeding and mortality have also been studied.
HS use in post-procedural related bleeds has also been studied. Leblanc and colleagues studied its efficacy after endoscopic intervention (5 patients after esophageal endoscopic mucosal resection, 4 after duodenal endoscopic mucosal resection, 2 after ampullary resection, and 1 after biliary sphincterotomy)[72]. Immediate hemostasis was achieved in 100% of patients whether used initially alone or as rescue therapy (after epinephrine injection and hemostatic clip placement)[72]. Further proving that HS is an appropriate and efficacious post-procedural hemostatic modality, two case reports highlighted immediate hemostasis achieved in post-sphincterotomy and post-sclerotherapy related bleeding[76,77].
HS can be used as adjunct and rescue therapy[78-81]. Per Sinha, it was used as an adjunct therapy to adrenaline in 40% of patients. Hemostasis was achieved in 95% of patients with an overall rebleeding rate of 16% at 7 d suggesting it should be considered as an adjunct therapy. Per Yau, HS was used as rescue therapy in 84.2% of patients with an overall hemostasis rate of 93.3%, however with a rebleeding rate of 38.9%[81]. Anticoagulant and antiplatelet use, coagulopathy, and thrombocytopenia likely contributed to the significant rebleeding rate[81].
To provide additional data on the efficacy of HS, there is a multicenter registry, by Smith and colleagues, which includes 63 patients[82]. Immediate hemostasis is defined as the absence of bleeding at the completion of the procedure, while rebleeding was defined as clinical manifestations of gastrointestinal bleeding and a reduction in hemoglobin by 2 g/dL. 10 of the 63 patients were treated for post-procedural bleeding. As a monotherapy use, 85% (47/55) achieved immediate hemostasis, while 100% achieved immediate hemostasis with HS used as adjunct therapy[82]. The efficacy of HS, whether as monotherapy, adjunct therapy, or rescue therapy, appears promising in the management of NVUGIB[83]. However, further, larger prospective studies are warranted to confirm.
Endoclot is an absorbable polysaccharide powder that has been proposed as a useful hemostatic agent. It has been shown to have similar rates of immediate hemostasis achieved and rebleeding compared to standard conventional therapy[84]. Examining endoclot as a primary monotherapy, Kim et al[85] studied its use in 12 patients with malignancy-related bleeding. 11 of the 12 patients had advanced gastric cancer. Immediate hemostasis was achieved, regardless of the tumor location and size, or previous use of antiplatelet medications, in all patients with a rebleeding rate in 2 patients (16%) at three and five days after treatment. There were no procedural related adverse events, nor all-cause mortality at 30 d after the procedure[85]. Although the sample size was small and limited to forrest 1b classification of bleeding, as well as the type of malignancy-related bleed, it appeared to be an efficacious modality.
To further evaluate its efficacy as a rescue therapy, Beg et al[86] studied the use of endoclot in 21 patients with various gastrointestinal bleeding lesions. Immediate hemostasis was achieved in all patients. The 30-d rebleeding rate was 4.8% and the mortality rate was 19.0%, however, without a statistically significant difference compared to the dual or triple endoscopic therapy group (P = 0.51 and P = 0.31, respectively). Only one death was attributed to the UGI bleed in a patient with a malignant related bleed and significant comorbidities[86].
Endoscopic ultrasound (EUS)–guided angiotherapy with doppler monitoring of the vascular response is a promising modality for the management of bleeding lesions that are inaccessible or refractory to standard endoscopic and interventional radiologic techniques[87]. EUS can detect vascular lesions in the gastrointestinal tract that are not visually apparent at endoscopy and target lesions for fine-needle injection of therapeutic agents[88]. Despite most reports on EUS-guided angiotherapy pertain to varices, the technique has also been described for the management of NVUGIB lesions. Although the feasibility and apparent safety of EUS-guided angiotherapy has been demonstrated, the use of EUS as an interventional tool in the managing NVUGIH has remained limited to a few centers worldwide. This is because of the lack of endosonographer training expertise and limited availability of EUS in the acute care setting.
In conclusion, NVUGIB continues to be a persistent challenge despite advancements in the both pharmacologic and endoscopic techniques. Several new modalities, as well as, modifications to traditional therapeutic modalities have clearly shown promise in improving outcomes whether used as monotherapy, adjuvant therapy, or rescue therapy for the management of NVUGIB. Due to the numerous NVUGIB etiologies, the indications, efficacy, and safety of the emerging endoscopic techniques continue to be defined. Additional studies are warranted to further define the role of these modalities into the treatment algorithm of NVUGIB and to determine the optimal treatment modality for specific NVUGIB pathology.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Madalinski M, Quach DT S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Alzoubaidi D, Lovat LB, Haidry R. Management of non-variceal upper gastrointestinal bleeding: where are we in 2018? Frontline Gastroenterol. 2019;10:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Luo PJ, Lin XH, Lin CC, Luo JC, Hu HY, Ting PH, Hou MC. Risk factors for upper gastrointestinal bleeding among aspirin users: An old issue with new findings from a population-based cohort study. J Formos Med Assoc. 2019;118:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 3. | Gralnek IM, Neeman Z, Strate LL. Acute Lower Gastrointestinal Bleeding. N Engl J Med. 2017;376:e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Khamaysi I, Gralnek IM. Acute upper gastrointestinal bleeding (UGIB) - initial evaluation and management. Best Pract Res Clin Gastroenterol. 2013;27:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Marmo R, Koch M, Cipolletta L, Capurso L, Pera A, Bianco MA, Rocca R, Dezi A, Fasoli R, Brunati S, Lorenzini I, Germani U, Di Matteo G, Giorgio P, Imperiali G, Minoli G, Barberani F, Boschetto S, Martorano M, Gatto G, Amuso M, Pastorelli A, Torre ES, Triossi O, Buzzi A, Cestari R, Della Casa D, Proietti M, Tanzilli A, Aragona G, Giangregorio F, Allegretta L, Tronci S, Michetti P, Romagnoli P, Nucci A, Rogai F, Piubello W, Tebaldi M, Bonfante F, Casadei A, Cortini C, Chiozzini G, Girardi L, Leoci C, Bagnalasta G, Segato S, Chianese G, Salvagnini M, Rotondano G. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol. 2008;103:1639-47; quiz 1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190-5; quiz 1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 8. | Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018;63:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 9. | Jairath V, Martel M, Logan RF, Barkun AN. Why do mortality rates for nonvariceal upper gastrointestinal bleeding differ around the world? A systematic review of cohort studies. Can J Gastroenterol. 2012;26:537-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Vergara M, Bennett C, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high-risk bleeding ulcers. Cochrane Database Syst Rev. 2014;CD005584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Fujishiro M, Iguchi M, Kakushima N, Kato M, Sakata Y, Hoteya S, Kataoka M, Shimaoka S, Yahagi N, Fujimoto K. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc. 2016;28:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Samuel R, Bilal M, Tayyem O, Guturu P. Evaluation and management of Non-variceal upper gastrointestinal bleeding. Dis Mon. 2018;64:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Troland D, Stanley A. Endotherapy of Peptic Ulcer Bleeding. Gastrointest Endosc Clin N Am. 2018;28:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-60; quiz 361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (1)] |
| 15. | Tsoi KK, Chiu PW, Chan FK, Ching JY, Lau JY, Sung JJ. The risk of peptic ulcer bleeding mortality in relation to hospital admission on holidays: a cohort study on 8,222 cases of peptic ulcer bleeding. Am J Gastroenterol. 2012;107:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Camus M, Jensen DM, Kovacs TO, Jensen ME, Markovic D, Gornbein J. Independent risk factors of 30-day outcomes in 1264 patients with peptic ulcer bleeding in the USA: large ulcers do worse. Aliment Pharmacol Ther. 2016;43:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 496] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 18. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P; International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 19. | Lai YC, Yang SS, Wu CH, Chen TK. Endoscopic hemoclip treatment for bleeding peptic ulcer. World J Gastroenterol. 2000;6:53-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kovacs TO, Jensen DM. Endoscopic therapy for severe ulcer bleeding. Gastrointest Endosc Clin N Am. 2011;21:681-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Gevers AM, De Goede E, Simoens M, Hiele M, Rutgeerts P. A randomized trial comparing injection therapy with hemoclip and with injection combined with hemoclip for bleeding ulcers. Gastrointest Endosc. 2002;55:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Wang TJ, Aihara H, Thompson AC, Schulman AR, Thompson CC, Ryou M. Choosing the right through-the-scope clip: a rigorous comparison of rotatability, whip, open/close precision, and closure strength (with videos). Gastrointest Endosc. 2019;89:77-86.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (36)] |
| 23. | Sarin N, Monga N, Adams PC. Time to endoscopy and outcomes in upper gastrointestinal bleeding. Can J Gastroenterol. 2009;23:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Laursen SB. Treatment and prognosis in peptic ulcer bleeding. Dan Med J. 2014;61:B4797. [PubMed] |
| 25. | Maggio D, Barkun AN, Martel M, Elouali S, Gralnek IM; Reason Investigators. Predictors of early rebleeding after endoscopic therapy in patients with nonvariceal upper gastrointestinal bleeding secondary to high-risk lesions. Can J Gastroenterol. 2013;27:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | García-Iglesias P, Villoria A, Suarez D, Brullet E, Gallach M, Feu F, Gisbert JP, Barkun A, Calvet X. Meta-analysis: predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment Pharmacol Ther. 2011;34:888-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Angsuwatcharakon P, Prueksapanich P, Kongkam P, Rattanachu-Ek T, Sottisuporn J, Rerknimitr R. Efficacy of the Ovesco Clip for Closure of Endoscope Related Perforations. Diagn Ther Endosc. 2016;2016:9371878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Kirschniak A, Traub F, Kueper MA, Stüker D, Königsrainer A, Kratt T. Endoscopic treatment of gastric perforation caused by acute necrotizing pancreatitis using over-the-scope clips: a case report. Endoscopy. 2007;39:1100-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Cahyadi O, Caca K, Schmidt A. Over-the-scope clip is an effective therapy for postbanding ulcer bleeding after initially successful transjugular intrahepatic portosystemic shunt therapy. Endoscopy. 2017;49:E258-E259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Samarasena J, Chen CL, Chin M, Chang K, Lee J. Successful closure of a cryotherapy-induced bleeding jejunal perforation with the over-the-scope clip system. Gastrointest Endosc. 2017;85:451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Haito-Chavez Y, Law JK, Kratt T, Arezzo A, Verra M, Morino M, Sharaiha RZ, Poley JW, Kahaleh M, Thompson CC, Ryan MB, Choksi N, Elmunzer BJ, Gosain S, Goldberg EM, Modayil RJ, Stavropoulos SN, Schembre DB, DiMaio CJ, Chandrasekhara V, Hasan MK, Varadarajulu S, Hawes R, Gomez V, Woodward TA, Rubel-Cohen S, Fluxa F, Vleggaar FP, Akshintala VS, Raju GS, Khashab MA. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc. 2014;80:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 33. | Repici A, Arezzo A, De Caro G, Morino M, Pagano N, Rando G, Romeo F, Del Conte G, Danese S, Malesci A. Clinical experience with a new endoscopic over-the-scope clip system for use in the GI tract. Dig Liver Dis. 2009;41:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Albert JG, Friedrich-Rust M, Woeste G, Strey C, Bechstein WO, Zeuzem S, Sarrazin C. Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest Endosc. 2011;74:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Skinner M, Gutierrez JP, Neumann H, Wilcox CM, Burski C, Mönkemüller K. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752-2760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Manno M, Mangiafico S, Caruso A, Barbera C, Bertani H, Mirante VG, Pigò F, Amardeep K, Conigliaro R. First-line endoscopic treatment with OTSC in patients with high-risk non-variceal upper gastrointestinal bleeding: preliminary experience in 40 cases. Surg Endosc. 2016;30:2026-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Manta R, Galloro G, Mangiavillano B, Conigliaro R, Pasquale L, Arezzo A, Masci E, Bassotti G, Frazzoni M. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endosc. 2013;27:3162-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Richter-Schrag HJ, Glatz T, Walker C, Fischer A, Thimme R. First-line endoscopic treatment with over-the-scope clips significantly improves the primary failure and rebleeding rates in high-risk gastrointestinal bleeding: A single-center experience with 100 cases. World J Gastroenterol. 2016;22:9162-9171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Wedi E, Gonzalez S, Menke D, Kruse E, Matthes K, Hochberger J. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Lamberts R, Koch A, Binner C, Zachäus M, Knigge I, Bernhardt M, Halm U. Use of over-the-scope clips (OTSC) for hemostasis in gastrointestinal bleeding in patients under antithrombotic therapy. Endosc Int Open. 2017;5:E324-E330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Brandler J, Baruah A, Zeb M, Mehfooz A, Pophali P, Wong Kee Song L, AbuDayyeh B, Gostout C, Mara K, Dierkhising R, Buttar N. Efficacy of Over-the-Scope Clips in Management of High-Risk Gastrointestinal Bleeding. Clin Gastroenterol Hepatol. 2018;16:690-696.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Schmidt A, Gölder S, Goetz M, Meining A, Lau J, von Delius S, Escher M, Hoffmann A, Wiest R, Messmann H, Kratt T, Walter B, Bettinger D, Caca K. Over-the-Scope Clips Are More Effective Than Standard Endoscopic Therapy for Patients With Recurrent Bleeding of Peptic Ulcers. Gastroenterology. 2018;155:674-686.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 45. | Mercky P, Gonzalez JM, Aimore Bonin E, Emungania O, Brunet J, Grimaud JC, Barthet M. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc. 2015;27:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Kobara H, Mori H, Nishiyama N, Fujihara S, Okano K, Suzuki Y, Masaki T. Over-the-scope clip system: A review of 1517 cases over 9 years. J Gastroenterol Hepatol. 2019;34:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 47. | Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc. 2012;76:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 48. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 902] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 49. | Mori H, Rahman A, Kobara H, Morishita A, Masaki T. The Development of Endoscopic Suturing Devices: Challenges in the Treatment of Iatrogenic Perforation and Bleeding. Intern Med. 2016;55:3075-3076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Barola S, Magnuson T, Schweitzer M, Chen YI, Ngamruengphong S, Khashab MA, Kumbhari V. Endoscopic Suturing for Massively Bleeding Marginal Ulcer 10 days Post Roux-en-Y Gastric Bypass. Obes Surg. 2017;27:1394-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Fujihara S, Mori H, Kobara H, Nishiyama N, Kobayashi M, Rafiq K, Masaki T. The efficacy and safety of prophylactic closure for a large mucosal defect after colorectal endoscopic submucosal dissection. Oncol Rep. 2013;30:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Chiu PW, Chan FK, Lau JY. Endoscopic Suturing for Ulcer Exclusion in Patients With Massively Bleeding Large Gastric Ulcer. Gastroenterology. 2015;149:29-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Agarwal A, Benias P, Brewer Gutierrez OI, Wong V, Hanada Y, Yang J, Villgran V, Kumbhari V, Kalloo A, Khashab MA, Chiu P, Ngamruengphong S. Endoscopic suturing for management of peptic ulcer-related upper gastrointestinal bleeding: a preliminary experience. Endosc Int Open. 2018;6:E1439-E1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Barola S, Fayad L, Hill C, Magnuson T, Schweitzer M, Singh V, Chen YI, Ngamruengphong S, Khashab MA, Kalloo AN, Kumbhari V. Endoscopic Management of Recalcitrant Marginal Ulcers by Covering the Ulcer Bed. Obes Surg. 2018;28:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Chen YI, Barkun A, Nolan S. Hemostatic powder TC-325 in the management of upper and lower gastrointestinal bleeding: a two-year experience at a single institution. Endoscopy. 2015;47:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Mori H, Kobara H, Kazi R, Fujihara S, Nishiyama N, Masaki T. Balloon-armed mechanical counter traction and double-armed bar suturing systems for pure endoscopic full-thickness resection. Gastroenterology. 2014;147:278-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Wells CD, Harrison ME, Gurudu SR, Crowell MD, Byrne TJ, Depetris G, Sharma VK. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 58. | Elhendawy M, Mosaad S, Alkhalawany W, Abo-Ali L, Enaba M, Elsaka A, Elfert AA. Randomized controlled study of endoscopic band ligation and argon plasma coagulation in the treatment of gastric antral and fundal vascular ectasia. United European Gastroenterol J. 2016;4:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 60. | Arima S, Sakata Y, Ogata S, Tominaga N, Tsuruoka N, Mannen K, Shiraishi R, Shimoda R, Tsunada S, Sakata H, Iwakiri R, Fujimoto K. Evaluation of hemostasis with soft coagulation using endoscopic hemostatic forceps in comparison with metallic hemoclips for bleeding gastric ulcers: a prospective, randomized trial. J Gastroenterol. 2010;45:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Fujishiro M, Abe N, Endo M, Kawahara Y, Shimoda R, Nagata S, Homma K, Morita Y, Uedo N. Retrospective multicenter study concerning electrocautery forceps with soft coagulation for nonmalignant gastroduodenal ulcer bleeding in Japan. Dig Endosc. 2010;22 Suppl 1:S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Nagata S, Kimura S, Ogoshi H, Hidaka T. Endoscopic hemostasis of gastric ulcer bleeding by hemostatic forceps coagulation. Dig Endosc. 2010;22 Suppl 1:S22-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Tanaka S, Toyonaga T, Morita Y, Ishida T, Hoshi N, Grimes KL, Ohara Y, Yoshizaki T, Kawara F, Umegaki E, Azuma T. Efficacy of a new hemostatic forceps during gastric endoscopic submucosal dissection: A prospective randomized controlled trial. J Gastroenterol Hepatol. 2017;32:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Toka B, Eminler AT, Karacaer C, Uslan MI, Koksal AS, Parlak E. Comparison of monopolar hemostatic forceps with soft coagulation versus hemoclip for peptic ulcer bleeding: a randomized trial (with video). Gastrointest Endosc. 2019;89:792-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Nunoue T, Takenaka R, Hori K, Okazaki N, Hamada K, Baba Y, Yamasaki Y, Kono Y, Seki H, Inokuchi T, Takemoto K, Taira A, Tsugeno H, Fujiki S, Kawahara Y, Okada H. A Randomized Trial of Monopolar Soft-mode Coagulation Versus Heater Probe Thermocoagulation for Peptic Ulcer Bleeding. J Clin Gastroenterol. 2015;49:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Maida M, Camilleri S, Manganaro M, Garufi S, Scarpulla G. Radiofrequency Ablation for Treatment of Refractory Gastric Antral Vascular Ectasia: A Systematic Review of the Literature. Gastroenterol Res Pract. 2017;2017:5609647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Raza N, Diehl DL. Radiofrequency ablation of treatment-refractory gastric antral vascular ectasia (GAVE). Surg Laparosc Endosc Percutan Tech. 2015;25:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Dray X, Repici A, Gonzalez P, Fristrup C, Lecleire S, Kantsevoy S, Wengrower D, Elbe P, Camus M, Carlino A, Pérez-Roldán F, Adar T, Marteau P. Radiofrequency ablation for the treatment of gastric antral vascular ectasia. Endoscopy. 2014;46:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Patel AA, Trindade AJ, Diehl DL, Khara HS, Lee TP, Lee C, Sethi A. Nitrous oxide cryotherapy ablation for refractory gastric antral vascular ectasia. United European Gastroenterol J. 2018;6:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Cho S, Zanati S, Yong E, Cirocco M, Kandel G, Kortan P, May G, Marcon N. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Macrì A, Saladino E, Versaci A, Basile A, Lamberto S, De Francesco F, Familiari L, Famulari C. Massive bleeding from a Dieulafoy's lesion of the duodenum successfully treated with "adjuvant" transarterial embolization and endoscopic laser coagulation. Acta Chir Belg. 2010;110:208-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Leblanc S, Vienne A, Dhooge M, Coriat R, Chaussade S, Prat F. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions (with videos). Gastrointest Endosc. 2013;78:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Sakai CM, Duarte RB, Baracat FI, Baracat R, de Moura EGH. Endoscopic treatment of upper-GI ulcer bleeding with hemostatic powder spray. VideoGIE. 2016;2:12-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Arena M, Masci E, Eusebi LH, Iabichino G, Mangiavillano B, Viaggi P, Morandi E, Fanti L, Granata A, Traina M, Testoni PA, Opocher E, Luigiano C. Hemospray for treatment of acute bleeding due to upper gastrointestinal tumours. Dig Liver Dis. 2017;49:514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Pittayanon R, Rerknimitr R, Barkun A. Prognostic factors affecting outcomes in patients with malignant GI bleeding treated with a novel endoscopically delivered hemostatic powder. Gastrointest Endosc. 2018;87:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Baracat FI, Tranquillini CV, Brunaldi VO, Baracat R, de Moura EGH. Hemostatic powder: a new ally in the management of postsphincterotomy bleeding. VideoGIE. 2017;2:303-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | González Ortiz B, Tapia Monge DM, Reyes Cerecedo A, Hernández Mondragón O. [Use of Hemospray® in post-sclerotherapy bleeding]. Bol Med Hosp Infant Mex. 2016;73:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 78. | Sung JJ, Luo D, Wu JC, Ching JY, Chan FK, Lau JY, Mack S, Ducharme R, Okolo P, Canto M, Kalloo A, Giday SA. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011;43:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 79. | Sinha R, Lockman KA, Church NI, Plevris JN, Hayes PC. The use of hemostatic spray as an adjunct to conventional hemostatic measures in high-risk nonvariceal upper GI bleeding (with video). Gastrointest Endosc. 2016;84:900-906.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Haddara S, Jacques J, Lecleire S, Branche J, Leblanc S, Le Baleur Y, Privat J, Heyries L, Bichard P, Granval P, Chaput U, Koch S, Levy J, Godart B, Charachon A, Bourgaux JF, Metivier-Cesbron E, Chabrun E, Quentin V, Perrot B, Vanbiervliet G, Coron E. A novel hemostatic powder for upper gastrointestinal bleeding: a multicenter study (the "GRAPHE" registry). Endoscopy. 2016;48:1084-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Yau AH, Ou G, Galorport C, Amar J, Bressler B, Donnellan F, Ko HH, Lam E, Enns RA. Safety and efficacy of Hemospray® in upper gastrointestinal bleeding. Can J Gastroenterol Hepatol. 2014;28:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 82. | Smith LA, Stanley AJ, Bergman JJ, Kiesslich R, Hoffman A, Tjwa ET, Kuipers EJ, von Holstein CS, Oberg S, Brullet E, Schmidt PN, Iqbal T, Mangiavillano B, Masci E, Prat F, Morris AJ. Hemospray application in nonvariceal upper gastrointestinal bleeding: results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol. 2014;48:e89-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 83. | Sulz MC, Frei R, Meyenberger C, Bauerfeind P, Semadeni GM, Gubler C. Routine use of Hemospray for gastrointestinal bleeding: prospective two-center experience in Switzerland. Endoscopy. 2014;46:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 84. | Park JC, Kim YJ, Kim EH, Lee J, Yang HS, Kim EH, Hahn KY, Shin SK, Lee SK, Lee YC. Effectiveness of the polysaccharide hemostatic powder in non-variceal upper gastrointestinal bleeding: Using propensity score matching. J Gastroenterol Hepatol. 2018;33:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Kim YJ, Park JC, Kim EH, Shin SK, Lee SK, Lee YC. Hemostatic powder application for control of acute upper gastrointestinal bleeding in patients with gastric malignancy. Endosc Int Open. 2018;6:E700-E705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Beg S, Al-Bakir I, Bhuva M, Patel J, Fullard M, Leahy A. Early clinical experience of the safety and efficacy of EndoClot in the management of non-variceal upper gastrointestinal bleeding. Endosc Int Open. 2015;3:E605-E609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Anastasiou J, Berzin TM. Endoscopic Ultrasound-Guided Vascular Interventions: From Diagnosis to Treatment. Saudi J Med Med Sci. 2018;6:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Satyavada S, Davitkov P, Akbar Ali M, Cooper G, Wong RCK, Chak A. Endoscopic Doppler Probe in the Diagnosis and Management of Upper Gastrointestinal Hemorrhage. ACG Case Rep J. 2018;5:e68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |