Published online Aug 16, 2019. doi: 10.4253/wjge.v11.i8.443
Peer-review started: May 14, 2019
First decision: June 3, 2019
Revised: June 16, 2019
Accepted: July 20, 2019
Article in press: July 3, 2019
Published online: August 16, 2019
Processing time: 96 Days and 8.6 Hours
The present armamentarium of endoscopic hemostatic therapy for non-variceal upper gastrointestinal hemorrhage includes injection, electrocautery and clips. There are newer endoscopic options such as hemostatic sprays, endoscopic suturing and modifications of current options including coagulation forceps and over-the-scope clips. Peptic hemorrhage is the most prevalent type of nonvariceal upper gastrointestinal hemorrhage and traditional endoscopic interventions have demonstrated significant hemostasis success. However, the hemostatic success rate is less for other entities such as Dieulafoy’s lesions and bleeding from malignant lesions. Novel innovations such as endoscopic submucosal dissection and peroral endoscopic myotomy has spawned a need for dependable hemostasis. Gastric antral vascular ectasias are associated with chronic gastrointestinal bleeding and usually treated by standard argon plasma coagulation (APC), but newer modalities such as radiofrequency ablation, banding, cryotherapy and hybrid APC have been utilized as well. We will opine on whether the newer hemostatic modalities have generated success when traditional modalities fail and should any of these modalities be routinely available in the endoscopic toolbox.
Core tip: New devices are available for hemostasis of non-variceal upper gastrointestinal hemorrhage that may supplement or supplant traditional modalities. These devices however have a varying track record in hemostasis with different learning curves, costs and detriments.
- Citation: Friedel D. Potential role of new technological innovations in nonvariceal hemorrhage. World J Gastrointest Endosc 2019; 11(8): 443-453
- URL: https://www.wjgnet.com/1948-5190/full/v11/i8/443.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i8.443
Non-variceal upper gastrointestinal hemorrhage is prevalent and associated with significant morbidity and mortality. The most common cause of non-variceal upper gastrointestinal hemorrhage (NVUGIH) is peptic hemorrhage but there is a broad range of other pathologies including Dieulafoy lesions, Mallory-Weiss tears, malignant lesions, vascular ectasias and iatrogenic causes. Prompt endoscopy for diagnosis and potential hemostasis usually results in a favorable outcome. However, refractory or recurrent bleeding can occur with standard medical management and possible endoscopic intervention in up to 13% of patients, often necessitating other interventions such as interventional radiology or surgery[1]. Novel endoscopic interventions such as endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM) have a particular penchant to potentially have a wide area for bleeding and impingement of adjacent vascular structures[2]. Gastric antral vascular ectasias (GAVE) can result in chronic and occasionally acute gastrointestinal blood loss and this entity is readily treated by argon plasma coagulation (APC) but newer modalities have also demonstrated efficacy[3]. We will discuss the experience to date with these new interventions and discuss whether they should be routinely available.
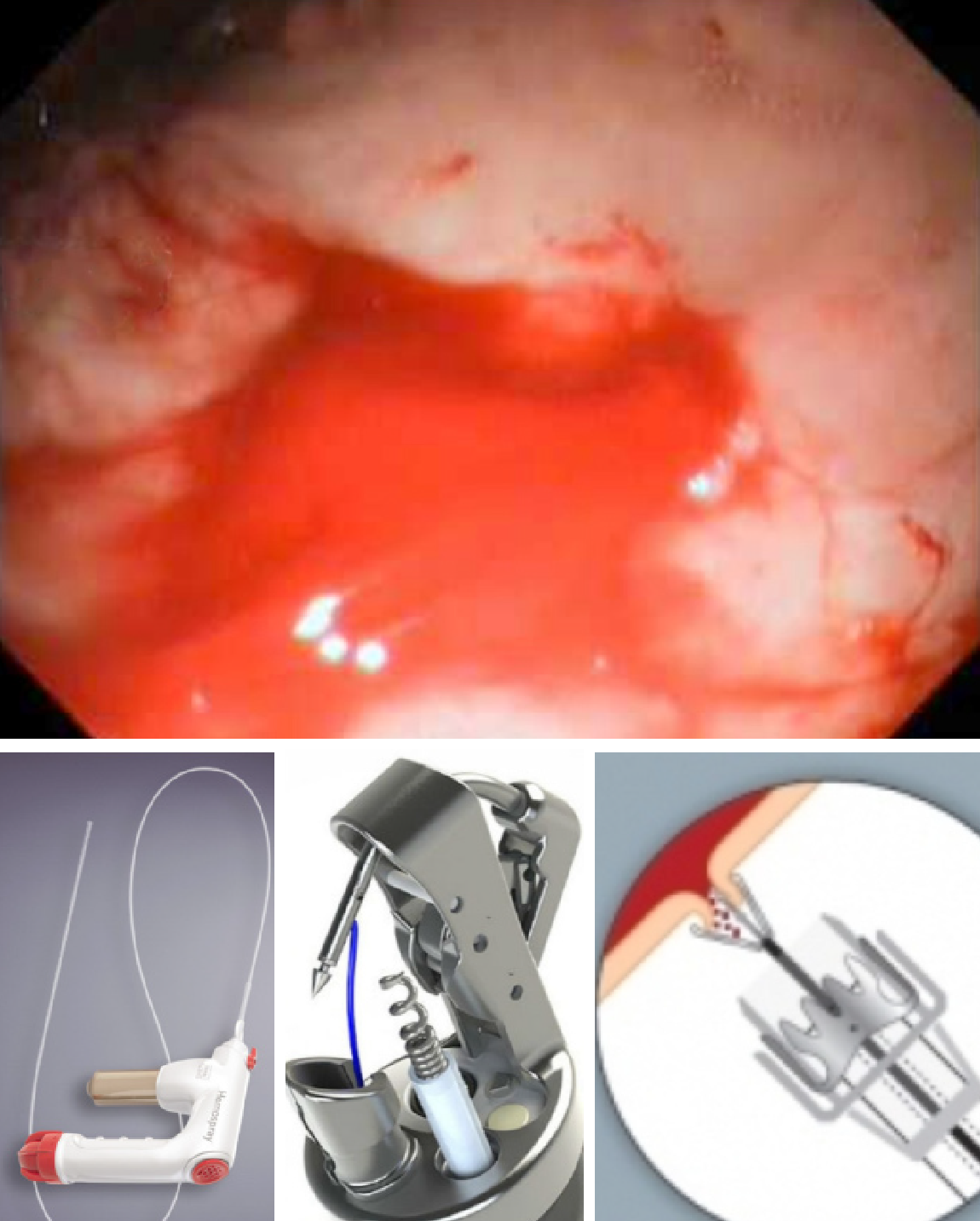
The over-the-scope clip (OTSC) (Ovesco Endoscopy, Tubingin Ger) has demonstrated efficacy in closing perforations and hemostasis[4]. This clip has proved itself capable in achieving hemostasis (> 90%) both as rescue and first line therapy for peptic and Dieulafoy’s lesions[5,6]. It is particularly useful for lesions with a large visible, fibrotic base and bleeding sites not easily treated by devices passed through the accessory channel[7]. Validating series have included Dieulafoy’s and Mallory-Weiss lesions but most are peptic lesions[5,6,8]. Though multiple clip placement has been described, good endoscopic visualization and precise placement of the clip is paramount as these clips are very difficult to remove. There is limited hemostatic experience for another OTSC-the Padlock Clip (Aponos Medical Kingston, NH)[9]. Overall, the experience of OTSC’s for NVUGIH has been impressive (Figure 1).
A recent multicenter series of 10 patients with refractory peptic hemorrhage were all successfully treated with the Apollo endoscopic suturing system (Apollo Endosurgery Austin Tx) and no rebleeding was noted[10]. This device has been useful in mini-mizing chronic blood loss from marginal and anastomosis ulcers[11]. Endoscopic suturing after endoscopic mucosal resection and ESD is an attractive option, but studies to date have not specifically addressed hemostasis[12].
Hemospray (Cook Medical, Winston-Salem, NC) is a nonabsorbable powder that becomes adhesive and cohesive when hydrated. Unlike cautery and clips, it does not treat the underlying bleeding lesion. Sixty-three patients compiled from a registry with NVUGIH (half ulcer-related) were treated with Hemospray[13]. Fifty-five were only treated with Hemospray and 8 were treated as a salvage intervention when traditional therapy failed. The monotherapy group had 85% primary hemostasis with 15% rebleed at 7 d. The salvage therapy group had 100% primary hemostasis and 25% rebleed at 7 d[13]. This and other work supported use in NVUGIH including peptic lesions, Mallory-Weiss tears and anastomosis ulcers. A small randomized comparison study of NVUGIH demonstrated therapeutic equivalency between clips and Hemospray when each was combined with epinephrine[14]. The topical hemostasis niche is likely to become crowded as several new products are being evaluated[15].
The literature contains a plethora of miscellaneous interventions reflecting the innovative vision of endoscopists. Endoscopic banding for ulcers has largely been abandoned but occasionally banding can be used for other lesions such as a Dieulafoy’s[16]. Detachable snares in concert with clips have been used for NVUGIH[17]. Metal stents have been used for esophageal NVUGIH and post-sphincterotomy bleeding[18,19]. Some centers tout the usefulness of EUS-guided therapy and vascular (doppler) probes to assess arteries[20,21].
Most operators performing ESD and potentially extraluminal procedures such as POEM desire a monopolar device with precise clasping and coaptive ability such as the Coagrasper (Olympus Endoscopy, Center Valley Pa)[22]. ESD defects are often not practically closed by clips and Hemospray, suturing and fibrin glue have been employed though none is standard. Polyglycolic shields adhered by fibrin glue have been proposed as method to minimize post-ESD bleeding but results regarding this are mixed[23,24].
Endoscopic banding appears comparable to APC-the current standard- in treating GAVE[25]. It may be di-fficult to band after APC due to fibrosis however. Radiofrequency ablation (RFA) has also been well validated for GAVE hemostasis[26]. Cryotherapy and hybrid APC had been evaluated[27,28]. Multiple other modalities have also been utilized for GAVE (Table 1).
| Over-the-scope clips | Hemospray | Endoscopic suturing | |
| Hemostatic efficacy | Very good | Moderate | Good |
| Ease of use | Good | Very Good | Fair |
| Cross utilization | Good | Poor | Very good |
| Cost | Moderate | High | Moderate |
The decision of which of the newer modalities to have available for endoscopic hemostasis depends on track record of hemostatic success, respective ease-of-use (largely related to prior experience and/or training), cross- utilization and cost. The OTSC’s fare quite well with these criteria in that the Ovesco clip has been well validated as a hemostatic instrument, only moderately challenging to use even with limited experience, utilized in high-volume units for perforation/fistula closure and relatively inexpensive. Hemospray also fares well in that it has a limited but positive record regarding hemostasis, and is exceptionally easy to use. It is moderately expensive and has no cross-utilization however. The Apollo suturing device is not expensive, but has a moderately steep learning curve and its use for ulcer hemostasis would likely be infrequent. RFA has a moderate record in GAVE treatment and easy to use but it is expensive and should only be available if it is also used for Barrett’s ablation. Endoscopic banding is cheap and variceal experience can be extrapolated to NVUGIH hemostasis. However, it has a sparse record in hemostasis. Experienced ESD operators will likely have a monopolar device which would be compatible with their cautery unit. The issue of tissue shields after ESD is intriguing, but it will likely be years before a formal recommendation could be made.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jonaitis L, Yakoot M S-Editor: Cui LJ L-Editor:AE-Editor:Zhou BX
| 1. | Han YJ, Cha JM, Park JH, Jeon JW, Shin HP, Joo KR, Lee JI. Successful Endoscopic Hemostasis Is a Protective Factor for Rebleeding and Mortality in Patients with Nonvariceal Upper Gastrointestinal Bleeding. Dig Dis Sci. 2016;61:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol. 2016;22:5927-5935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Hsu WH, Wang YK, Hsieh MS, Kuo FC, Wu MC, Shih HY, Wu IC, Yu FJ, Hu HM, Su YC, Wu DC. Insights into the management of gastric antral vascular ectasia (watermelon stomach). Therap Adv Gastroenterol. 2018;11:1756283X17747471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Skinner M, Gutierrez JP, Neumann H, Wilcox CM, Burski C, Mönkemüller K. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Manno M, Mangiafico S, Caruso A, Barbera C, Bertani H, Mirante VG, Pigò F, Amardeep K, Conigliaro R. First-line endoscopic treatment with OTSC in patients with high-risk non-variceal upper gastrointestinal bleeding: preliminary experience in 40 cases. Surg Endosc. 2016;30:2026-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Chan SM, Chiu PW, Teoh AY, Lau JY. Use of the Over-The-Scope Clip for treatment of refractory upper gastrointestinal bleeding: a case series. Endoscopy. 2014;46:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Nojkov B, Cappell MS. Gastrointestinal bleeding from Dieulafoy's lesion: Clinical presentation, endoscopic findings, and endoscopic therapy. World J Gastrointest Endosc. 2015;7:295-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Dinelli M, Omazzi B, Andreozzi P, Zucchini N, Redaelli A, Manes G. First clinical experiences with a novel endoscopic over-the-scope clip system. Endosc Int Open. 2017;5:E151-E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Agarwal A, Benias P, Brewer Gutierrez OI, Wong V, Hanada Y, Yang J, Villgran V, Kumbhari V, Kalloo A, Khashab MA, Chiu P, Ngamruengphong S. Endoscopic suturing for management of peptic ulcer-related upper gastrointestinal bleeding: a preliminary experience. Endosc Int Open. 2018;6:E1439-E1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Jirapinyo P, Watson RR, Thompson CC. Use of a novel endoscopic suturing device to treat recalcitrant marginal ulceration (with video). Gastrointest Endosc. 2012;76:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Kukreja K, Chennubhotla S, Bhandari B, Arora A, Singhal S. Closing the Gaps: Endoscopic Suturing for Large Submucosal and Full-Thickness Defects. Clin Endosc. 2018;51:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ghassemi KA, Jensen DM. Evolving techniques for gastrointestinal endoscopic hemostasis treatment. Expert Rev Gastroenterol Hepatol. 2016;10:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Baracat FI, de Moura DTH, Brunaldi VO, Tranquillini CV, Baracat R, Sakai P, de Moura EGH. Randomized controlled trial of hemostatic powder versus endoscopic clipping for non-variceal upper gastrointestinal bleeding. Surg Endosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Vitali F, Naegel A, Atreya R, Zopf S, Neufert C, Siebler J, Neurath MF, Rath T. Comparison of Hemospray® and Endoclot™ for the treatment of gastrointestinal bleeding. World J Gastroenterol. 2019;25:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 16. | Alis H, Oner OZ, Kalayci MU, Dolay K, Kapan S, Soylu A, Aygun E. Is endoscopic band ligation superior to injection therapy for Dieulafoy lesion? Surg Endosc. 2009;23:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lee JH, Kim BK, Seol DC, Byun SJ, Park KH, Sung IK, Park HS, Shim CS. Rescue endoscopic bleeding control for nonvariceal upper gastrointestinal hemorrhage using clipping and detachable snaring.. Endoscopy. 2013;45:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Zhou Y, Huo J, Wang X, Liu D. Covered self-expanding metal stents for the treatment of refractory esophageal nonvariceal bleeding: a case series. J Laparoendosc Adv Surg Tech A. 2014;24:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Shah JN, Marson F, Binmoeller KF. Temporary self-expandable metal stent placement for treatment of post-sphincterotomy bleeding. Gastrointest Endosc. 2010;72:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Jain D, Thosani N, Singhal S. Endoscopic ultrasound-assisted gastrointestinal hemostasis: an evolving technique. Therap Adv Gastroenterol. 2016;9:635-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Jensen DM, Ohning GV, Kovacs TO, Ghassemi KA, Jutabha R, Dulai GS, Machicado GA. Doppler endoscopic probe as a guide to risk stratification and definitive hemostasis of peptic ulcer bleeding. Gastrointest Endosc. 2016;83:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Tanaka S, Toyonaga T, Morita Y, Ishida T, Hoshi N, Grimes KL, Ohara Y, Yoshizaki T, Kawara F, Umegaki E, Azuma T. Efficacy of a new hemostatic forceps during gastric endoscopic submucosal dissection: A prospective randomized controlled trial. J Gastroenterol Hepatol. 2017;32:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Fischer JC, Parker PM, Shaw WW. Laser Doppler flowmeter measurements of skin perfusion changes associated with arterial and venous compromise in the cutaneous island flap. Microsurgery. 1985;6:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Kataoka Y, Tsuji Y, Hirasawa K, Takimoto K, Wada T, Mochizuki S, Ohata K, Sakaguchi Y, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Endoscopic tissue shielding to prevent bleeding after endoscopic submucosal dissection: a prospective multicenter randomized controlled trial. Endoscopy. 2019;51:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc. 2012;24:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | St Romain P, Boyd A, Zheng J, Chow SC, Burbridge R, Wild D. Radiofrequency ablation (RFA) vs. argon plasma coagulation (APC) for the management of gastric antral vascular ectasia (GAVE) in patients with and without cirrhosis: results from a retrospective analysis of a large cohort of patients treated at a single center. Endosc Int Open. 2018;6:E266-E270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Patel AA, Trindade AJ, Diehl DL, Khara HS, Lee TP, Lee C, Sethi A. Nitrous oxide cryotherapy ablation for refractory gastric antral vascular ectasia. United European Gastroenterol J. 2018;6:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. |
Hernández Mondragón OV, Lopez Valenzuela LA, Blancas Valencia JM, Espinosa Saavedra D, Blanco Velasco G. Safety and efficacy of Hybrid-APC for the treatment of refractory GAVE..
|