Published online Jun 16, 2019. doi: 10.4253/wjge.v11.i6.403
Peer-review started: March 3, 2019
First decision: March 20, 2019
Revised: May 16, 2019
Accepted: May 23, 2019
Article in press: May 23, 2019
Published online: June 16, 2019
Processing time: 104 Days and 13.8 Hours
Endoscopic management of symptomatic pancreatic fluid collections (PFCs) using self-expandable metal stents (SEMS) placement has emerged as an innovative therapeutic approach with excellent efficacy, safety, and relatively few adverse outcomes. However, their use has not been studied in patients with cirrhosis. Cirrhotics tend to be considered less than optimal candidates due to concern for portal hypertension and coagulopathy related complications.
To compare the efficacy and safety of using SEMS for drainage of symptomatic PFCs in cirrhotic vs non-cirrhotic patients.
We conducted a retrospective comparative analysis of patients with symptomatic PFCs [pancreatic pseudocyst (PP) or walled-off necrosis (WON)] who underwent endoscopic ultrasound (EUS)-guided placement of fully covered self-expandable metals stents or lumen-apposing self-expandable metal stents. All patients were followed clinically until resolution of PFCs or death. Definition: (1) Technical success was defined as successful placement of SEMS; and (2) Clinical success was defined as complete resolution of the PFCs without additional interventions including interventional radiology or surgery. Number of procedures performed per patient, number of patients who achieved complete resolution of the PFCs without additional interventions and procedure related adverse events were recorded.
From January 2012 to December 2017, a total of 88 patients underwent EUS-guided drainage of symptomatic PFCs. Of these, 58 non cirrhotic patients underwent plastic stent insertion for management of PFC and 30 patients, 5 with cirrhosis and 25 without cirrhosis, underwent EUS-guided transmural drainage with SEMS, including 18 (60%) PP and 12 (40%) WON. Technical success was achieved in all 30 patients. Clinical success was achieved in 60% cirrhotic patients and 92% non-cirrhotics (P = 0.12). Procedure-related adverse events were 60% in cirrhotic and 28% non-cirrhotic (P = 0.62). Moreover, fatal adverse events were statistically more common in cirrhotics compared with non-cirrhotics (0 vs 40%; P = 0.023). Successful stent removal following resolution of the PFC, was 60% in cirrhotics and 80% in non-cirrhotics (P = 0.57). Post-procedure length of hospitalization was 18.6 ± 20.3 d in cirrhotics and 5.6 ± 13.7 d in non-cirrhotics (P = 0.084).
EUS-guided management of PFC using SEMS placement has a high technical and clinical success rate in non-cirrhotics. However, in cirrhotics caution must be exercised given the high morbidity and mortality as evidenced by our cohort, particularly for the endoscopic debridement of WONs. Larger, multicenter studies are warranted to further characterize the risk profile and outcomes in these patients.
Core tip: Endoscopic ultrasound-guided management of pancreatic fluid collections (PFCs) using self-expandable metal stents has a high technical and clinical success rate. However, their use in cirrhotics has not yet been studied. We conducted the first comparative study to assess the safety and outcomes of endoscopic management of symptomatic PFCs in cirrhotics vs non-cirrhotics. Despite a 100% technical success rate, clinical success was achieved in only 60% of cirrhotic patients with a procedure-related adverse event rate of 60%. Moreover, fatal adverse events were statistically more common in cirrhotics compared with non-cirrhotics (0% vs 40%; P = 0.023). Thus, given the high morbidity and mortality as evidenced by our cohort, caution must be exercised in this group. Larger, multicenter studies are warranted to further characterize the risk profile and outcomes in these patients.
- Citation: Laique S, Franco MC, Stevens T, Bhatt A, Vargo JJ, Chahal P. Clinical outcomes of endoscopic management of pancreatic fluid collections in cirrhotics vs non-cirrhotics: A comparative study. World J Gastrointest Endosc 2019; 11(6): 403-412
- URL: https://www.wjgnet.com/1948-5190/full/v11/i6/403.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i6.403
Pancreatic fluid collections (PFCs) develop due to damage to the pancreatic duct secondary to acute or chronic pancreatitis, iatrogenic causes (i.e., endoscopy or surgery) or trauma[1]. Most PFCs are asymptomatic and tend to resolve spontaneously over time[2]. However, some PFCs may rapidly enlarge and become infected, resulting in worsening abdominal pain, biliary obstruction with concomitant jaundice, sepsis, or gastric outlet obstruction, necessitating treatment[3]. Over the past decade, endoscopic ultrasound (EUS) guided drainage of symptomatic PFCs via placement of transmural stents has mostly replaced the more traditional approaches of surgery or percutaneous drainage[4,5]. This has mostly been due to its high success rate (87%-97%) coupled with low adverse event (6%-34%) and mortality (0%-1%) rates[6,7].
Even though EUS-guided transmural drainage of symptomatic PFC, using self-expandable metal stents (SEMS) has gone through much advancement in recent years, its use in cirrhotic patients has not yet been reported. These patients are less than optimal candidates given the underlying coagulopathy and hemodynamic alterations resulting in portal hypertension with its various complications – abdominal ascites, esophageal and splenic varices, and renal dysfunction, all of which place these patients at increased risk for procedure-related adverse events. In our study, we aim to compare the technical success rate and clinical outcomes of EUS guided drainage of symptomatic PFCs using SEMS in cirrhotics vs non-cirrhotics.
This was a retrospective study conducted at Cleveland Clinic, Cleveland, Ohio. The study was approved by the institutional review board. The endoscopy database at Cleveland Clinic was reviewed for patients who had undergone EUS-guided drainage of symptomatic PFCs [i.e., pancreatic pseudocyst (PP) and walled-off necrosis (WON)] between January 2012 and December 2017. Only patients with placement of SEMS [including both fully covered self-expandable metals stents (FCSEMS) and lumen-apposing self-expandable metal stents (LASEMS)] and 3-mo or longer follow-up were included in the study.
Technical success was defined as successful placement of SEMS; Clinical success was defined as complete resolution of the PFCs without additional interventions including interventional radiology or surgery; A PP was defined as an encapsulated collection of fluid with a well-defined inflammatory wall usually outside the pancreas with minimal or no necrosis seen more than four weeks after presentation (per the Revised Atlanta Classification)[1]; WON consisted of a mature, encapsulated collection of pancreatic and/or peripancreatic necrotic tissue contained within an enhancing wall of reactive tissue which is seen more than four weeks after presentation.
PFCs were characterized by magnetic resonance imaging (MRI) or computed topography (CT) in concordance with EUS findings. The indications for drainage of PFCs were as follows: (1) Gastric outlet obstruction; (2) Refractory abdominal pain; (3) Early satiety; (4) Rapid increase in size; and/or (5) Biliary obstruction/cholangitis; (6) Infection/ sepsis[8]. Patients with regional varices, suspected cystic neoplasms, coagulopathy [international normalized ratio (INR) > 1.5], thrombocytopenia (platelets < 50000/mm3) or imaging showing that the pseudocyst wall was not in close proximity (> 1 cm) to the EUS probe were all excluded from the study. Data were recorded from outpatient and hospital records to collect procedural details and the overall clinical course of the patient.
There were two different FCSEMS used in this study. Patient with PPs had a fully covered, biliary, 10 × 60 mm GORE® Viabil® stent (W.L. Gore and Associates, Flagstaff, AZ, United States) placed. While some patients with WON received a 18 × 60 mm fully covered through the scope (TTS) Taewoong (Taewoong-Medical Co., Ltd., South Korea) esophageal stent for endoscopic drainage/debridement of WON.
The LASEM evaluated in this study was a 10 mm, cautery enhanced, saddle-shaped, nitinol, braided, flexible stent fully covered with a silicon membrane (Xlumena Inc., Mountain View, California, United States; and AXIOS®, Boston Scientific, Marl-borough, MA, United States). The stent had bilateral double-walled anchoring flanges to hold the duodenal wall or stomach in direct apposition to the PFC wall. Two different lumen diameters stents were utilized-10 mm for management of PPs and the 15 mm for walled-off necrosis.
All patients underwent procedures performed by endoscopists with a large therapeutic endosonography experience (TS, AB, PC). All procedures were performed with general anesthesia assistance using the therapeutic linear array echoendoscope (Olympus Medical Systems; Center Valley, Pa, United States). Each patient received broad-spectrum antibiotics to decrease the risk of secondary infection. The optimum puncture site of the cyst (transduodenal or transgastric) was determined using EUS imaging and color doppler to exclude interposing vessels. The cyst was then punctured, under real-time imaging using a 19-gauge needle (Expect, Boston Scientific, Marlborough, MA, USA or EchoTip Access, Cook Medical, Winston Salem, NC, United States) and the cyst contents aspirated for visual inspection (e.g., viscosity, debris, pus). Under fluoroscopic guidance then a 0.035-inch guidewire was inserted through the needle and coiled into the cyst cavity. The needle was then withdrawn while leaving the guidewire in the cyst. A needle-knife was then used to create a path with dilation of the cystoenterostomy tract being done either with a 4 mm balloon or a 10 Fr cystotome with cautery, based on the preference of the endoscopist. After dilation, finally, a FCSEMS was placed under endoscopic and fluoroscopic views.
In patients who underwent placement of the LASEMS, the PFCs puncture was done with the tip of the delivery catheter. EUS guidance was used to deploy and then position the distal flange against the wall of the WON while the proximal flange was deployed under endoscopic guidance. The stent diameter was determined by the individual endoscopist. The larger 15-mm diameter stent was preferred for WON allowing for access to the cavity for future endoscopic necrosectomies and improved clearance of necrotic debris. The deployed stent lumen was then dilated, at the endoscopist’s discretion with a controlled radial expansion balloon (Boston Scientific) to allow for optimal stent luminal expansion.
In patients with WON, subsequent endoscopic necrosectomies were performed using an upper endoscope advanced through the LASEMS at the scheduling preference of the endoscopist until complete resolution of the necrotic cavity, con-firmed endoscopically and/or by cross-sectional imaging.
Immediate adverse events such as hypotension, respiratory distress, perforation, and bleeding were documented. Delayed adverse events (< 30 d after the procedure) were recorded by reviewing the electronic medical records for hospital admissions and ambulatory office visits.
All patients were followed-up with contrast-enhanced CT of the abdomen and pelvis at 4 to 8 wk after LASEMS placement. The stent was removed once the PFC had completely resolved without any residual fluid component left. Patients were followed at regular intervals in the ambulatory clinic, and repeat imaging was undertaken if there was a clinical concern for PFC recurrence.
The primary outcome of this study was to assess the technical and clinical success rates of PFC resolution using SEMS (FCSEMS or LASEMS) in cirrhotics compared to non-cirrhotic patients. Technical success was defined as successful endoscopic transmural placement of a removable SEMS[9,10]. The overall clinical success rate was defined as complete resolution of the PP or WON without the need for concomitant percutaneous or surgical drainage and resolution of the patient’s symptoms without the need for reintervention at three months after initial treatment[9].
Secondary outcomes evaluated included adverse events, PFC recurrence, number and type of reinterventions, successful stent removal after resolution and post-procedure length of hospitalization. Adverse events included procedure-related bleeding, infection, stent migration, or misdeployment. Reinterventions were defined as the need for repeat PFC drainage or debridement sessions due to persistent pseudocyst or necrosis or reintervention due to stent occlusion, cyst/necrotic cavity infection, or enlarging cyst size leading to symptoms.
Data are presented as mean ± SD or frequency (percent). A univariable analysis was performed to assess differences between cirrhotics and non-cirrhotics. Student’s t-tests were used to compare continuous variables and Fisher’s Exact tests were used for categorical factors. In addition, mean or percent differences between the groups and corresponding 95% confidence intervals are reported. Given the small sample size and low number of observed events, no multivariable analysis was performed. All analyses were performed using SAS (version 9.4, The SAS Institute, Cary, NC) and a P < 0.05 was considered statistically significant.
From January 2012 to December 2017, we identified 88 patients who underwent EUS-guided drainage of symptomatic PFCs; 58 patients (no cirrhotic in this 58 subset) received plastic stents for management of PFC and 30 patients (5 cirrhotics and 25 non-cirrhotics) had a SEMS placed with adequate (> 3 mo) follow up. All patients had PFCs arising in setting of acute pancreatitis. Table 1 presents a comparison of the clinical and PFCs’ characteristic of the non-cirrhotics and cirrhotic patients. Table 2 shows the demographic and clinical characteristics of the five patients with cirrhosis.
| Factor | Non-cirrhotic (n = 25) | Cirrhotic (n = 5) | Difference (95%CI) | P value |
| Age | 48.8 ± 15.8 | 57.8 ± 13.7 | 9.0 (-6.6, 24.6) | 0.25a |
| Gender | 0.62d | |||
| Male | 14 (56.0) | 4 (80.0) | 24.0 (-26.1, 71.6) | |
| Female | 11 (44.0) | 1 (20.0) | ||
| Anti-platelets | 4 (16.0) | 3 (60.0) | 44.0 (-6.3, 85.3) | 0.068d |
| Charlson co-morbidity index | 80.4 ± 29.4 | 50.4 ± 37.2 | -30.0 (-60.8, 0.73) | 0.055a |
| Type of PFC | 0.99d | |||
| WON | 10 (40.0) | 2 (40.0) | 0.00 (-52.2, 52.2) | |
| Pseudocyst | 15 (60.0) | 3 (60.0) | ||
| Location of PFC | 0.62d | |||
| Head | 9 (36.0) | 1 (20.0) | -16.0 (-64.4, 33.8) | |
| Body | 14 (56.0) | 3 (60.0) | 4.0 (-45.0, 52.4) | |
| Tail | 2 (8.0) | 1 (20.0) | 12.0 (-37.5, 60.1) | |
| Size of PFC (mm) | 122.9 ± 61.2 | 125.2 ± 68.4 | 2.3 (-60.2, 64.8) | 0.94a |
| Extension of fluid collection to paracolic gutter | 6 (24.0) | 1 (20.0) | -4.0 (-52.4, 45.0) | 0.99d |
| Pancreatic Duct on imaging | 0.99d | |||
| No leak | 18 (72.0) | 4 (80.0) | 8.0 (-41.3, 56.2) | |
| Leak | 3 (12.0) | 0 (0.0) | -12.0 (-60.1, 37.5) | |
| Complete disruption | 4 (16.0) | 1 (20.0) | 4.0 (-45.0, 52.4) | |
| Indication of collection drainage | 0.17d | |||
| Gastric outlet obstruction | 7 (28.0) | 2 (40.0) | 12.0 (-37.5, 60.1) | |
| Abdominal pain | 12 (48.0) | 1 (20.0) | -28.0 (-71.6, 22.2) | |
| Early satiety | 4 (16.0) | 0 (0.0) | -16.0 (-64.4, 33.8) | |
| Rapid increase in size | 2 (8.0) | 1 (20.0) | 12.0 (-37.5, 60.1) | |
| Jaundice | 0 (0.0) | 1 (20.0) | 20.0 (-29.9, 71.6) | |
| Etiology of pancreatitis | 0.24d | |||
| Gallstones | 10 (40.0) | 1 (20.0) | -20.0 (-71.6, 29.9) | |
| Alcohol | 8 (32.0) | 4 (80.0) | 48.0 (-2.2, 85.3) | |
| Other | 7 (28.0) | 0 (0.0) | -28.0 (-71.6, 22.2) | |
| Platelets | 248.7 ± 101.3 | 132.6 ± 57.5 | -116.1 (-214.5, -17.7) | 0.023a |
| Alkaline Phosphatase | 103.3 ± 57.3 | 790.8 ± 1461.4 | 687.5 (54.8, 1320.2) | 0.034a |
| ALT | 31.2 ± 36.4 | 100.6 ± 169.0 | 69.4 (-11.1, 149.9) | 0.088a |
| AST | 36.5 ± 50.9 | 173.6 ± 272.9 | 137.1 (10.0, 264.2) | 0.036a |
| Bilirubin | 0.61 ± 0.48 | 3.3 ± 3.1 | 2.7 (1.2, 4.1) | < 0.001a |
| INR | 1.1 ± 0.12 | 1.3 ± 0.30 | 0.24 (0.06, 0.42) | 0.012a |
| Factor | Cirrhotic (n = 5) |
| Statistics | |
| MELD score | 16.2 ± 7.9 |
| MELD (90 d/3 mo mortality) | 20.0 ± 19.7 |
| Child-Pugh Classification | |
| A | 1 (20.0) |
| B | 4 (80.0) |
| Esophageal varices | 3 (60.0) |
| Grade varices | |
| I | 3 (100.0) |
| Gastric varices | 1 (20.0) |
| PHG | 2 (40.0) |
| Previous UGI bleeding | 0 (0.0) |
| Chronic kidney disease | 2 (40.0) |
| Use of NSSB | 0 (0.0) |
Amongst the non-cirrhotic patients, 15 had PPs and 10 WON. Twenty-four patients underwent transgastric drainage. Successful insertion of a SEMS (9 HOT AXIOS™ and 16 FCSEMS) into the PFC cavity (technical success) was achieved in all 25 (100%) patients. Seven of the 10 patients with WON underwent direct endoscopic necro-sectomy.
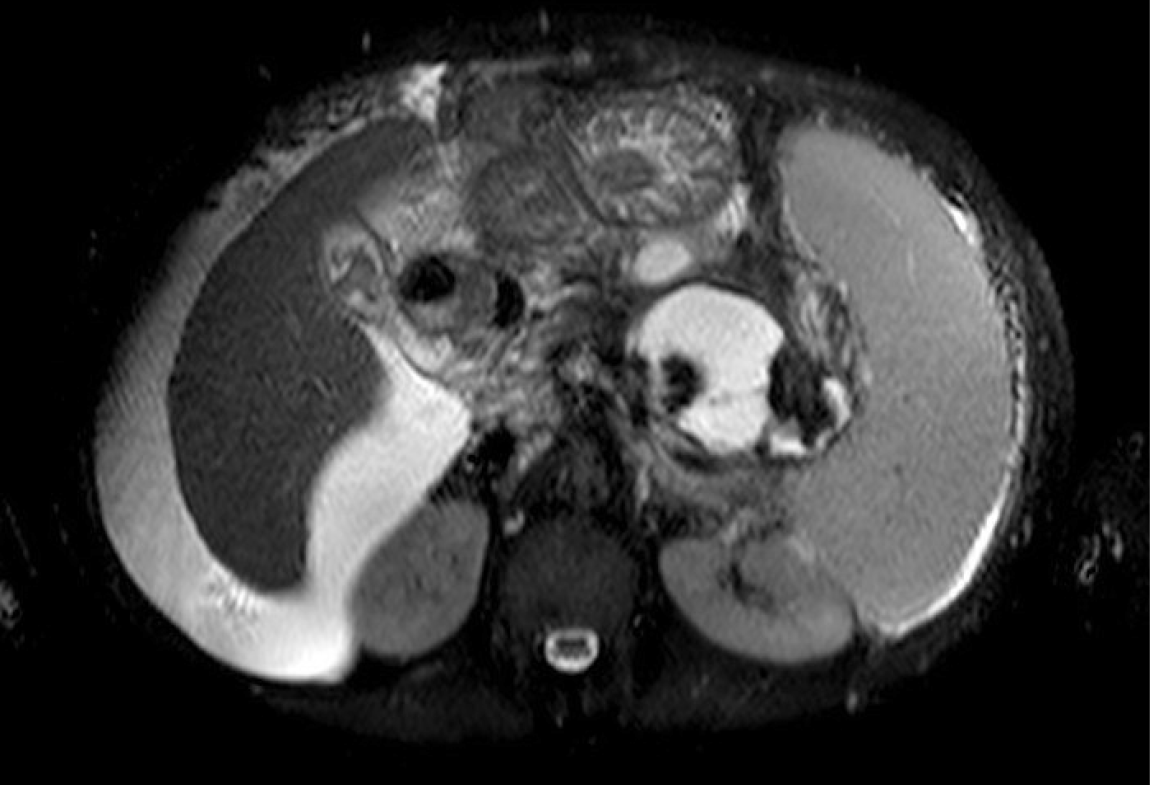
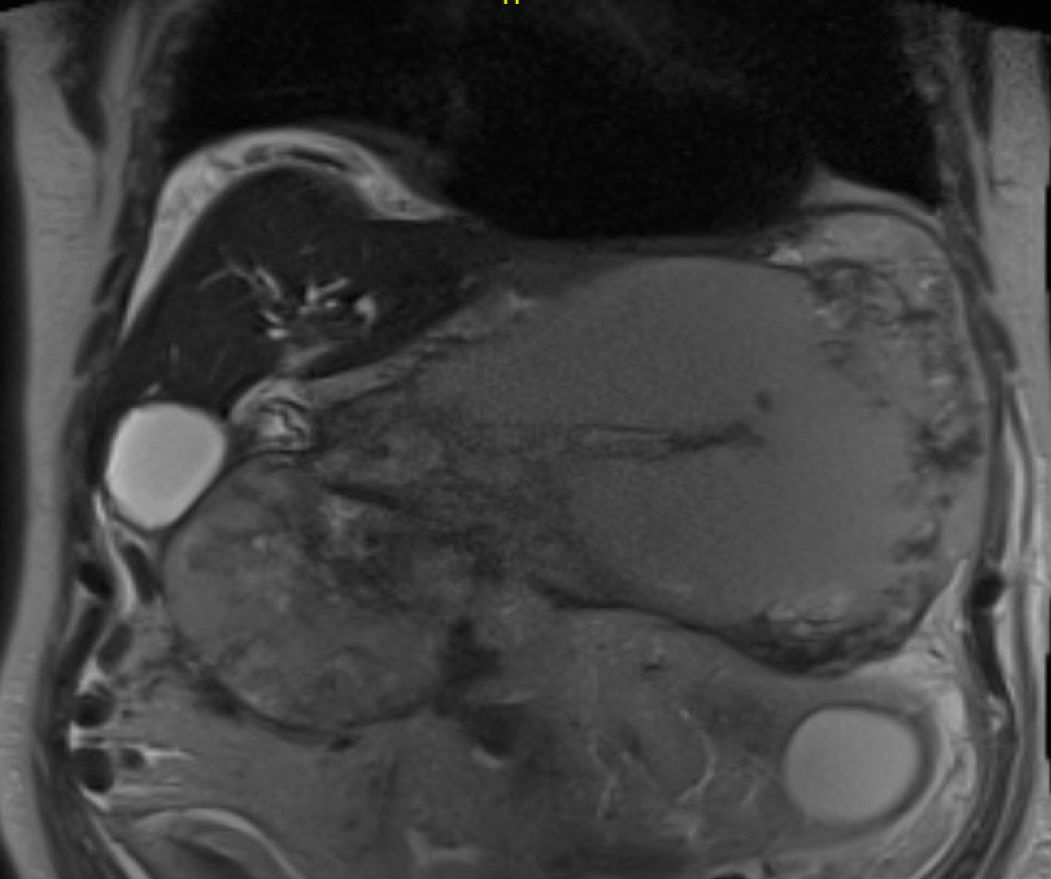
In the cirrhotic group, 3 patients had a PP and 2 WON (Figures 1 and 2). All patients underwent transgastric drainage with 4 patients having the HOT AXIOS™ inserted, and 1 patient with WON received a 18 mm × 6 cm FCSEMS. All procedures were technically successful. Procedural characteristics are summarized in Table 3.
| Factor | Non-cirrhotics | Cirrhotics | Statistics | P value |
| Stent type | 0.14d | |||
| Hot axios | 9 (36.0) | 4 (80.0) | 44.0 (-6.3, 85.3) | |
| FCSEMS | 16 (64.0) | 1 (20.0) | ||
| Drainage approach | 0.99d | |||
| Transgastric | 24 (96.0) | 5 (100.0) | 4.0 (-45.0, 52.4) | |
| Transduodenal | 1 (4.0) | 0 (0.0) | ||
| Tract dilation | 16 (64.0) | 1 (20.0) | -44.0 (-85.3, 6.3) | 0.14d |
| Technical Success | 25 (100.0) | 5 (100.0) | - | - |
| Endoscopic necrosectomy | 7 (28.0) | 2 (40.0) | 12.0 (-37.5, 60.1) | 0.62d |
| Hydrogen peroxide irrigation | 14 (56.0) | 2 (40.0) | -16.0 (-64.4, 33.8) | 0.64d |
| Need for concomitant percutaneous drainage | 1 (4.0) | 0 (0.0) | -4.0 (-52.4, 45.0) | 0.99d |
The procedures were technically successful in 100% patients. Clinical success was attained in 23 of the 25 (92%) non-cirrhotics and in 3 of the 5 (60%) cirrhotics (92% vs 60%; P = 0.12). Ten patients of the total sample experienced adverse events, with 7 of the 25 (28%) being non-cirrhotics and 3 of the 5 (60%) being cirrhotics (28% vs 60%; P = 0.62). Adverse events included bleeding, infection, and stent migration, and are detailed in Table 4. Two (40%) of the cirrhotic patients expired due to the ensuing complications, whereas, there were no fatalities in the non-cirrhotic group [CI: 40.0 (-10.3, 85.3); P = 0.023] (Table 4).
| Factor | Non-cirrhotics | Cirrhotics | Statistics | P value |
| Clinical success | 23 (92.0) | 3 (60.0) | -32.0 (-75.2, 18.3) | 0.12d |
| Successful stent removal following resolution | 20 (80.0) | 3 (60.0) | -20.0 (-71.6, 29.9) | 0.57d |
| Total number of endoscopic procedures prior to removal | 1.5 ± 0.94 | 2.0 ± 1.7 | 0.55 (-0.80, 1.9) | 0.41a |
| ERCP performed within 30 d post drainage | 5 (20.0) | 1 (20.0) | 0.00 (-52.2, 52.2) | 0.99d |
| Recurrence of PFC needing reintervention | 4 (16.0) | 0 (0.0) | -16.0 (-60.1, 37.5) | 0.99d |
| Need for surgery | 2 (8.0) | 0 (0.0) | -8.0 (-56.2, 41.3) | 0.99d |
| Any adverse events | 7 (28.0) | 3 (60.0) | 12.0 (-37.5, 60.1) | 0.62d |
| Bleeding | 1 (4.0) | 1 (20.0) | 16.0 (-33.8, 64.4) | 0.31d |
| Infection | 5 (20.0) | 2 (40.0) | 20.0 (-29.9, 71.6) | 0.57d |
| Stent migration | 1 (4.0) | 0 (0.0) | -4.0 (-52.4, 45.0) | 0.99d |
| Any severe AE | 2 (8.0) | 0 (0.0) | -8.0 (-56.2, 41.3) | 0.99d |
| Any fatal AE | 0 (0.0) | 2 (40.0) | 40.0 (-10.3, 85.3) | 0.023d |
| Any severe/fatal AE | 2 (8.0) | 2 (40.0) | 32.0 (-18.3, 75.2) | 0.12d |
| Length of post-procedure hospitalization (d) | 5.6 ± 13.7 | 18.6 ± 20.3 | 13.0 (-1.9, 27.9) | 0.084a |
| Follow-up (mo) | 14.1 ± 9.5 | 5.5 ± 5.1 | -8.6 (-17.6, 0.49) | 0.063a |
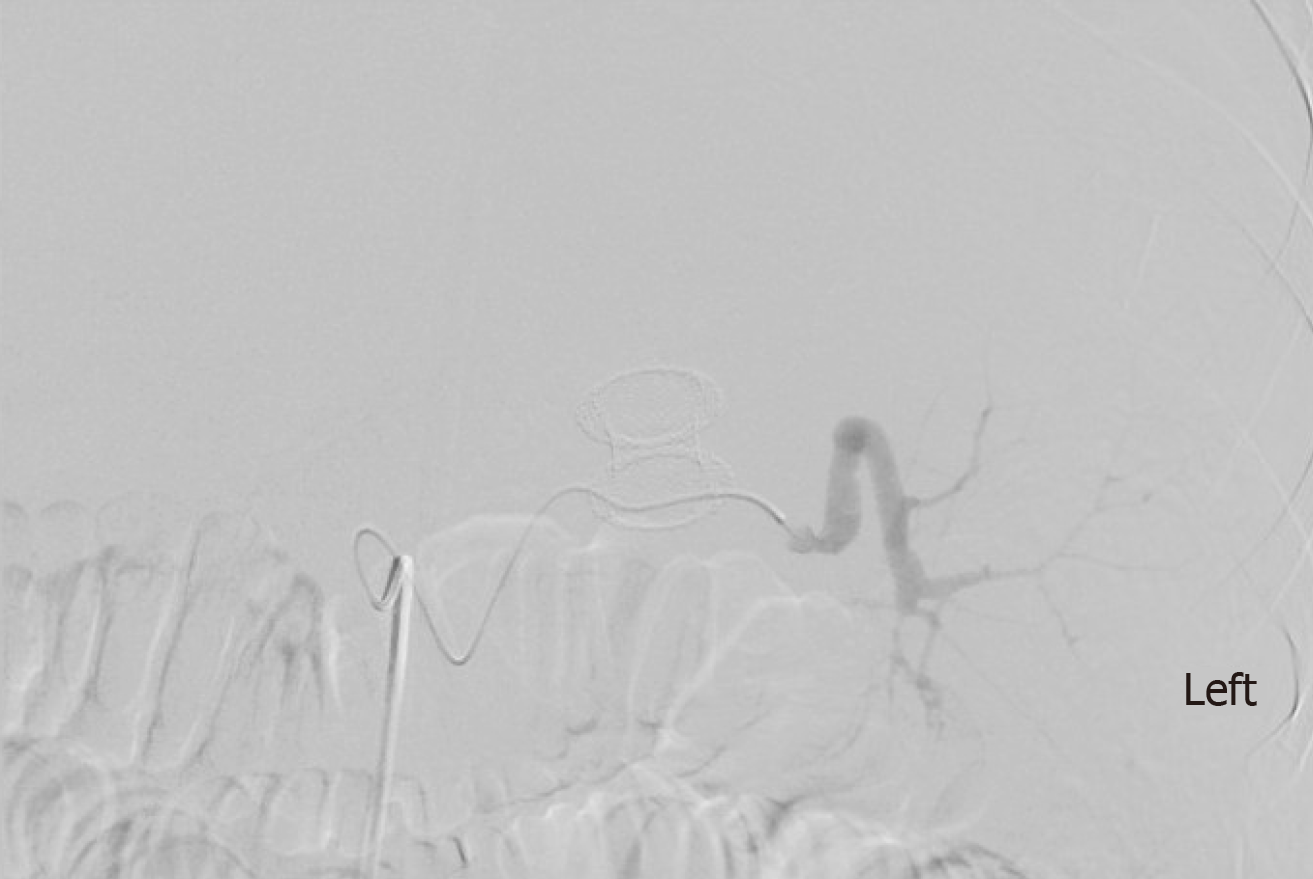
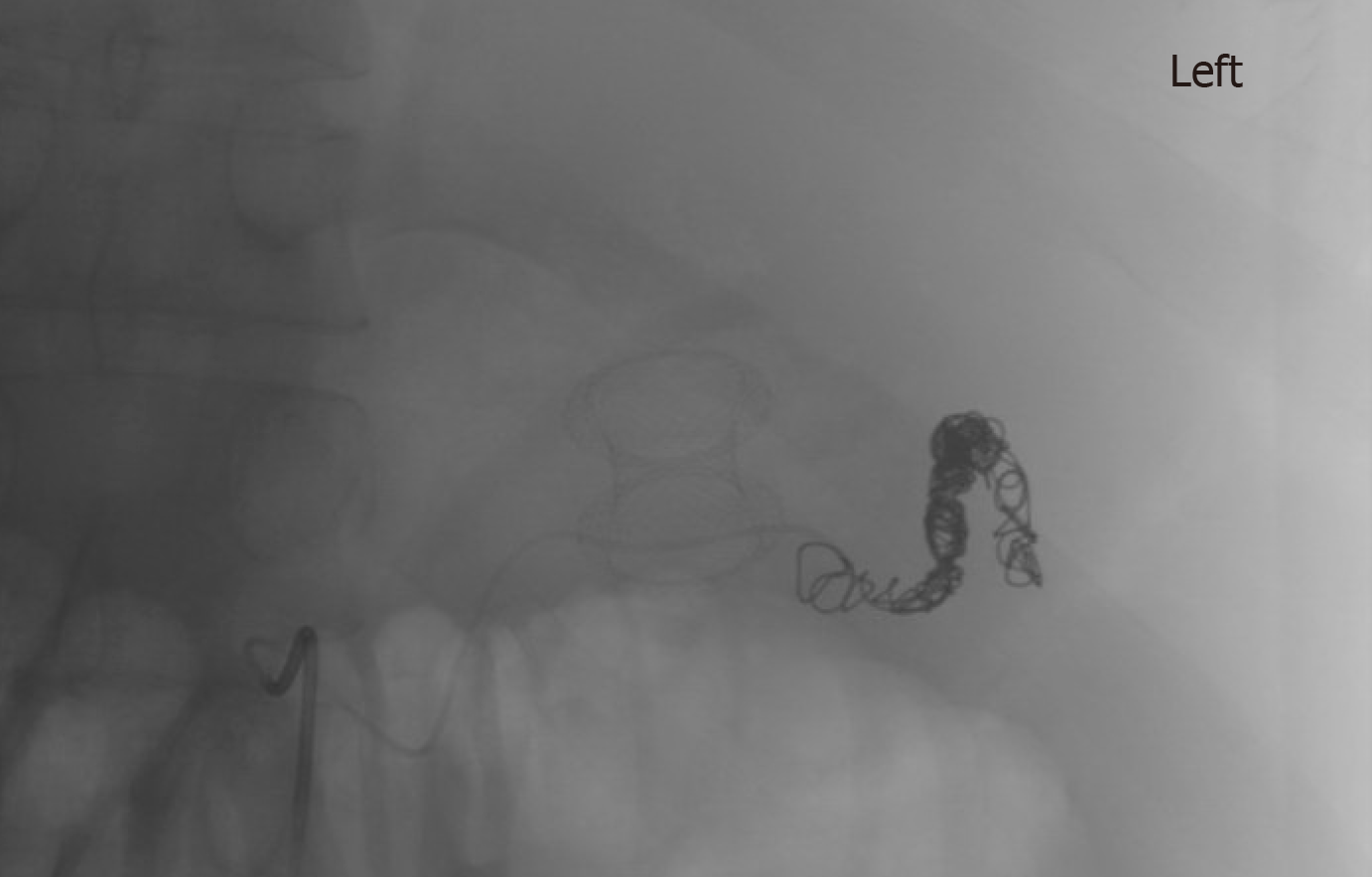
Amongst the non-cirrhotic patients who presented with adverse events, two had severe outcomes. One of these patients with a PP had a FCSEMS placed but had a splenic artery pseudoaneurysm rupture and subsequently developed severe intraabdominal infection requiring exploratory laparotomy, abdominal washout, and surgical cystograstomy due to the persistent collection. The other patient had a FCSEMS placed for a large WON and developed septic shock secondary to bilateral retroperitoneal extension of the WON requiring percutaneous drain placement and eventual laparoscopic retroperitoneal pancreatic debridement/necrosectomy. Amongst the cirrhotic patients with adverse events, two had a fatal adverse event. One of them (MELD: 17) received an AXIOS stent for management of a PP and developed severe upper gastrointestinal bleeding from rupture of a pseudoaneurysm of the main splenic artery after cystogastrostomy, which required urgent embolization (Figures 3 and 4). He ultimately developed severe sepsis and expired. The second patient (MELD: 28) with a 200 mm WON in the pancreatic head presented with cholangitis and gastric outlet obstruction, underwent AXIOS stent placement successfully. However, developed post-procedure hypovolemic shock due to massive PFC drainage necessitating intensive care unit admission. He subsequently underwent three endoscopic necrosectomies before he expired due to massive variceal bleeding with hypoxic respiratory failure.
Bleeding occurred in one patient in each group as detailed above. Infectious complications were seen in five non-cirrhotic and two cirrhotic patients. Recurrence of PFC needing reintervention happened in four non-cirrhotic patients only, three of them were managed endoscopically, with stent reposition or placement of a new stent, and two eventually required surgery.
Follow up data was available for cirrhotics for 5.5 ± 5.1 mo and 14.4 ± 9.5 mo for non-cirrhotics (P = 0.063). Post procedure hospitalization was longer in cirrhotics (18.6 ± 20.3 d vs 5.6 ± 13.7 d; P = 0.084). The number of endoscopic procedures performed before stent removal were cirrhotics 2.0 ± 1.7 vs 1.4 ± 0.93 in non-cirrhotics. Successful stent removal following resolution was lower in cirrhotics (60% vs 80%), however didn’t reach statistical significance [CI: -20.0 (-71.6, 29.9); P = 0.57].
The liver is the main organ involved in systemic metabolism. It plays an essential role in the immunological system by filtering the portal blood and clearing microbes, which may have invaded the bloodstream. In surgical patients, the liver also functions in the synthesis of plasma proteins, such as coagulation factors and albumin, and clearance of many drugs. Cirrhotic patients in whom these functions are impaired undergoing surgical procedures tend to be at a higher risk of adverse events, including infections, bleeding, major organ failure and anesthetic drugs side effects[11,12]. A study by Kim et al[13] reported a significantly higher incidence of 32.5% for postoperative adverse events and 10.2% mortality in cirrhotics compared to non-cirrhotics (all P < 0.001). In his study, even though the patients had undergone various surgical procedures; the Child-Pugh class, MELD score, and type of surgery were all independently associated with postoperative morbidity and mortality. EUS guided transmural drainage of symptomatic PFCs is considered a less invasive procedure compared to the traditional surgical approach. However, it still seems to pose cirrhotic patients to clinical decompensation, as evidenced by our cohort.
In our study, cirrhotic patients upon undergoing EUS guided transmural drainage of symptomatic PFCs using SEMS had poorer clinical outcomes when compared to non-cirrhotics. Despite a 100% technical success rate (endoscopist technique), clinically success was attained in only 60% cirrhotics, with two of the five cirrhotic patients having expired (P: 0.023) compared to 92% clinical success in non-cirrhotic and no fatalities. The rate of adverse events also tended to be higher in cirrhotic patients (60% cirrhotics vs 28% non-cirrhotics). In the cirrhotic group, there was also a trend toward lower successful stent removal following resolution of the PFC (60% vs 80%, P = 0.57) and longer post-procedure length of hospitalization (18.6 ± 20.3 d vs 5.6 ± 13.7 d; P = 0.084), supporting the higher post-procedure morbidity observed in these patients.
Our study is unique in many ways. This is the first study to report the use of SEMS for EUS guided transmural drainage of symptomatic PFCs in cirrhotics vs non-cirrhotics patients. Additionally, we assessed the efficacy, safety, and long-term clinical success of SEMS (FCSEMS and LASEMS) for PFCs (PPs and WONs) in these populations. There are several limitations to our study. Firstly, it was retrospective in nature with its inherent limitations (variable patient follow up, quality of cross-sectional imaging). Secondly, we had a small sample size which is tied to lower power. Additionally, the small number of cirrhotics did not allow us to accurately assess if distributional assumptions of t-tests were met. Lastly, we were unable to perform a multivariable analysis to adjust for possible confounders, which could affect some of the observed results, thus affecting thier reproducibility.
Our current literature review suggests that endoscopic management of PFCs using SEMS placement is an innovative therapeutic approach with excellent efficacy, safety, and relatively few adverse outcomes in non-cirrhotic patients. However, in cirrhotics caution must be exercised given the high morbidity and mortality as evidenced by our cohort, particularly for the endoscopic debridement of WONs. Larger, multicenter studies are warranted to further characterize the risk profile and outcomes in these patients.
Endoscopic ultrasound (EUS) guided drainage of symptomatic pancreatic fluid collections (PFCs), using self-expandable metal stents (SEMS) has a high technical and clinical success rate. However, their use in cirrhotics has not yet been studied. These patients are less than optimal surgical candidates given the underlying coagulopathy and portal hypertension related complications increasing their risk of adverse events.
Over the past decade, EUS guided drainage of symptomatic PFCs via placement of transmural stents has largely replaced the more traditional approaches of surgery or percutaneous drainage mainly been due to its high success rate (87%-97%) coupled with low adverse event (6%-34%) and mortality (0%-1%) rates. Thus, we wanted to study if this would be a viable option for cirrhotic patients.
Our study aimed to compare the technical success rate and clinical outcomes of EUS guided drainage of symptomatic PFCs using SEMS in cirrhotics vs non-cirrhotics.
We conducted a retrospective comparative analysis of patients with symptomatic PFCs [pancreatic pseudocyst (PP) or walled-off necrosis (WON)] who underwent EUS-guided placement of fully covered self-expandable metals stents (FCSEMS) or lumen-apposing self-expandable metal stents (LASEMS). All patients were followed clinically until resolution of PFCs or death. Definition: (1) Technical success was defined as successful placement of SEMS; and (2) Clinical success was defined as complete resolution of the PFCs without additional interventions including interventional radiology or surgery. Number of procedures performed per patient, number of patients who achieved complete resolution of the PFCs without additional interventions and procedure related adverse events were recorded.
From January 2012 to December 2017, a total of 88 patients underwent EUS-guided drainage of symptomatic PFCs. Of these, 58 non cirrhotic patients underwent plastic stent insertion for management of PFC and 30 patients, 5 with cirrhosis and 25 without cirrhosis, underwent EUS-guided transmural drainage with SEMS, including 18 (60%) PP and 12 (40%) WON. Technical success was achieved in all 30 patients. Clinical success was achieved in 60% cirrhotic patients and 92% non-cirrhotics (P = 0.12). Procedure-related adverse events were 60% in cirrhotic and 28% non-cirrhotic (P = 0.62). Moreover, fatal adverse events were statistically more common in cirrhotics compared with non-cirrhotics (0% vs 40%; P = 0.023). Successful stent removal following resolution was 60% in cirrhotics and 80% in non-cirrhotics (P = 0.57). Post-procedure length of hospitalization was 18.6 ± 20.3 d in cirrhotics and 5.6 ± 13.7 d in non-cirrhotics (P = 0.084).
Despite a 100% technical success rate (endoscopist technique), clinically success was attained in only 60% cirrhotics, with two of the five cirrhotic patients having expired (p: 0.023) compared to 92% clinical success in non-cirrhotic and no fatalities. The rate of adverse events also tended to be higher in the cirrhotic patients (60% cirrhotics vs 28% non-cirrhotics). Although the EUS guided transmural drainage of symptomatic PFCs is considered a less invasive procedure, when compared with the traditional surgical approach, it still seems to pose cirrhotic patients to clinical decompensation. Our study even though the first of its kind, was limited by its retrospective nature and small sample size and so these results must be interpreted as such.
In cirrhotic patients caution must be exercised when performing EUS guided drainage of symptomatic PFCs given the high morbidity and mortality as evidenced by our cohort, particularly for the endoscopic debridement of WONs. Larger, prospective, multicenter studies are warranted to further characterize the risk profile and outcomes in these patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Farhat S, Lei JJ, Liao KF, Neri V, Ramia JM, Yavuz A, Zhuge Y S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4338] [Article Influence: 361.5] [Reference Citation Analysis (45)] |
| 2. | Cheruvu CV, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl. 2003;85:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Yeo CJ, Bastidas JA, Lynch-Nyhan A, Fishman EK, Zinner MJ, Cameron JL. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet. 1990;170:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 4. | Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Jacobson BC, Baron TH, Adler DG, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ, Fanelli R, Wheeler-Harbaugh J, Faigel DO; American Society for Gastrointestinal Endoscopy. ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE, Taylor LJ, Munigala S, Bhat YM. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 2016;83:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 10. | Shah RJ, Shah JN, Waxman I, Kowalski TE, Sanchez-Yague A, Nieto J, Brauer BC, Gaidhane M, Kahaleh M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Frye JW, Perri RE. Perioperative risk assessment for patients with cirrhosis and liver disease. Expert Rev Gastroenterol Hepatol. 2009;3:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Macaron C, Hanouneh IA, Suman A, Lopez R, Johnston D, Carey WW. Safety of cardiac surgery for patients with cirrhosis and Child-Pugh scores less than 8. Clin Gastroenterol Hepatol. 2012;10:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Kim TH, Um SH, Yim SY, Seo YS, Yim HJ, Jeen YT, Lee HS, Chun HJ, Kim CD, Ahn H, Lee Y. The risk of perioperative adverse events in patients with chronic liver disease. Liver Int. 2015;35:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |