Published online May 16, 2019. doi: 10.4253/wjge.v11.i5.373
Peer-review started: March 25, 2019
First decision: April 11, 2019
Revised: May 11, 2019
Accepted: May 13, 2019
Article in press: May 14, 2019
Published online: May 16, 2019
Processing time: 55 Days and 0.7 Hours
Capsule endoscopy and balloon-assisted enteroscopy (BAE) enable visualization of rare small bowel conditions such as small intestinal malignant tumors. However, details of the endoscopic characteristics of small intestinal malignant tumors are still unknown.
To elucidate the endoscopic characteristics of small intestinal malignant tumors.
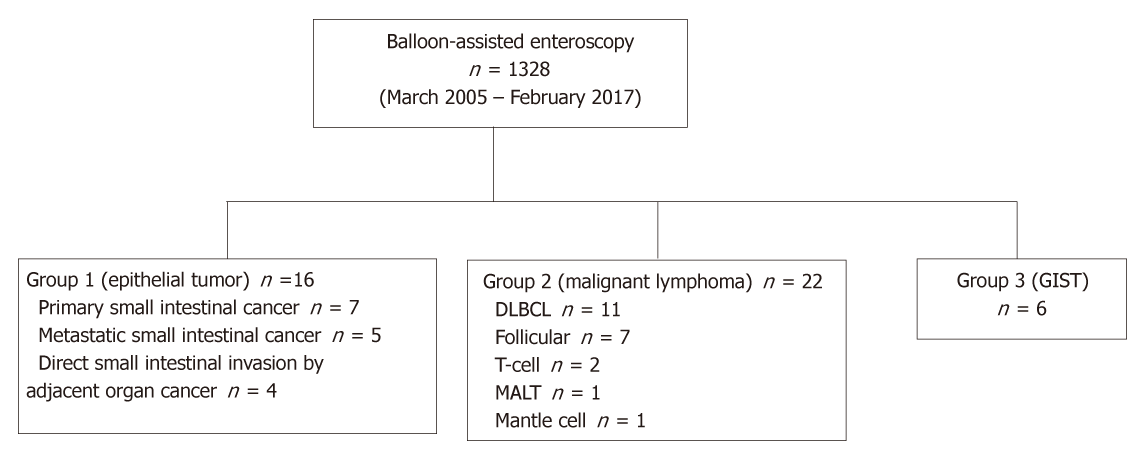
From March 2005 to February 2017, 1328 BAE procedures were performed at Keio University Hospital. Of these procedures, malignant tumors were classified into three groups, Group 1: epithelial tumors including primary small intestinal cancer, metastatic small intestinal cancer, and direct small intestinal invasion by an adjacent organ cancer; Group 2: small intestinal malignant lymphoma; and Group 3, small intestinal gastrointestinal stromal tumors. We systematically collected clinical and endoscopic data from patients’ medical records to determine the endoscopic characteristics for each group.
The number of patients in each group was 16 (Group 1), 22 (Group 2), and 6 (Group 3), and the percentage of solitary tumors was 100%, 45.5%, and 100%, respectively (P < 0.001). Patients’ clinical background parameters including age, symptoms, and laboratory data were not significantly different between the groups. Seventy-five percent of epithelial tumors (Group 1) were located in the upper small intestine (duodenum and ileum), and approximately 70% of gastrointestinal stromal tumors (Group 3) were located in the jejunum. Solitary protruding or mass-type tumors were not seen in malignant lymphoma (Group 2) (P < 0.001). Stenosis was seen more often in Group 1, (68.8%, 27.3%, and 0%; Group 1, 2, and 3, respectively; P = 0.004). Enlarged white villi inside and/or surrounding the tumor were seen in 12.5%, 54.5%, and 0% in Group 1, 2, and 3, respectively (P = 0.001).
The differential diagnosis of small intestinal malignant tumors could be tentatively made based on BAE findings.
Core Tip: The aim of this study was to elucidate the endoscopic characteristics of small intestinal malignant tumors. Balloon-assisted enteroscopy procedures at our institution were enrolled in the analysis. Malignant tumors were classified into three groups, Group 1: epithelial tumors; Group 2: small intestinal malignant lymphoma; and Group 3, small intestinal gastrointestinal stromal tumors. We collected data from patients’ medical records to determine the endoscopic characteristics for each group. Group 1 and Group 2 were observed as solitary tumors. Enlarged white villi inside and/or surrounding the tumor were seen in 12.5%, 54.5%, and 0% in Group 1, 2, and 3, respectively (P <0.001).
- Citation: Horie T, Hosoe N, Takabayashi K, Hayashi Y, Kamiya KJL, Miyanaga R, Mizuno S, Fukuhara K, Fukuhara S, Naganuma M, Shimoda M, Ogata H, Kanai T. Endoscopic characteristics of small intestinal malignant tumors observed by balloon-assisted enteroscopy. World J Gastrointest Endosc 2019; 11(5): 373-382
- URL: https://www.wjgnet.com/1948-5190/full/v11/i5/373.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i5.373
The small intestine is a long luminal organ that constitutes 75% of the length of gas-trointestinal tract and 90% of its mucosal surface area. Small intestinal cancer is relatively rare, accounting for less than 5% of gastrointestinal cancers[1] and with an incidence of 6.8 cases per million[2]. The gastrointestinal tract is a major organ affected by extranodal malignant lymphoma, accounting for 30%–40% of all extranodal lymphomas and 5%–20% of all non-Hodgkin lymphomas[3]. The most frequent primary gastrointestinal site of malignant lymphoma is the stomach (60%–70%), followed by the small intestine (20%–30%)[4]. Until the development of balloon-assisted enteroscopy (BAE) and video capsule endoscopy, small intestinal malignant tumors could not be observed endoscopically. Moreover, BAE enables direct observation of these small intestinal lesions, and also permits biopsy and endoscopic therapy, such as stent placement and endoscopic tattooing for subsequent surgical therapy[5,6]. A previous study reported the incidence of small intestinal tumors detected by BAE[6,7]; however, the endoscopic characteristics of small intestinal tumors have been reported only in a limited number of case reports[8-10], and the details of these characteristics are still unknown.
The aim of this study was to investigate the endoscopic characteristics of small intestinal malignant tumors observed by BAE.
This study was a retrospective medical record analysis and was approved by the ethics committee of Keio University Hospital (approval number, 20160431). Data was collected from patients’ medical records, and the endoscopy findings were collected using an endoscopy reporting system (Solemio ENDO®, Olympus, Tokyo, Japan). Patients who underwent BAE (EN450/T5 or EN450/P5; Fujifilm, Tokyo, Japan or SIF-Q260; Olympus, Tokyo, Japan) between March 2005 and February 2017 in Keio University Hospital were screened. Of the 1328 procedures, 44 small intestinal malignant tumors were seen endoscopically, and data for these tumors were included in the analysis. Benign small intestinal polyp and polyposis syndrome such as Peutz–Jeghers syndrome and familial adenomatous were excluded from the analysis. The included small intestinal malignant tumors were classified into three groups: Group 1: epithelial tumors including primary small intestinal cancer (ade-no-carcinoma), metastatic small intestinal cancer (adenocarcinoma), and direct small intestinal invasion by adjacent organ cancer (adenocarcinoma); Group 2: small intestinal malignant lymphoma; and Group 3: small intestinal gastrointestinal stromal tumors (GIST). Patients’ clinical background parameters included age, symptom, and laboratory data. To define the endoscopic characteristics for each group, endoscopic data such as tumor location, solitary or multiple lesions, type or form, presence of stenosis, presence of bleeding, and presence of white villi were systematically collected from patients’ medical charts and the endoscopy reporting system. Solitary and multiple lesions were confirmed by computed tomography and/or barium swallow. Stricture was defined as a stenosis through which we could not pass an enteroscope (EN450/T5 or SIF-Q260). Bleeding was defined as spontaneous bleeding before passing the enteroscope. We also focused on the endoscopic findings of white villi in malignant lymphoma. To determine the morphological and pathological characteristics of white villi in malignant lymphoma, we compared the pathological findings and endoscopic findings from each biopsy site.
Statistical analyses were performed using the Fisher’s exact test for percentages and one way ANOVA to assess differences in parameters showing a normal distribution. Non- normally distributed data were analyzed using the Kruskal–Wallis test. P-values < 0.05 were considered significant, and SPSS version 22 software (SPSS Inc., Tokyo, Japan) was used for all statistical analyses.
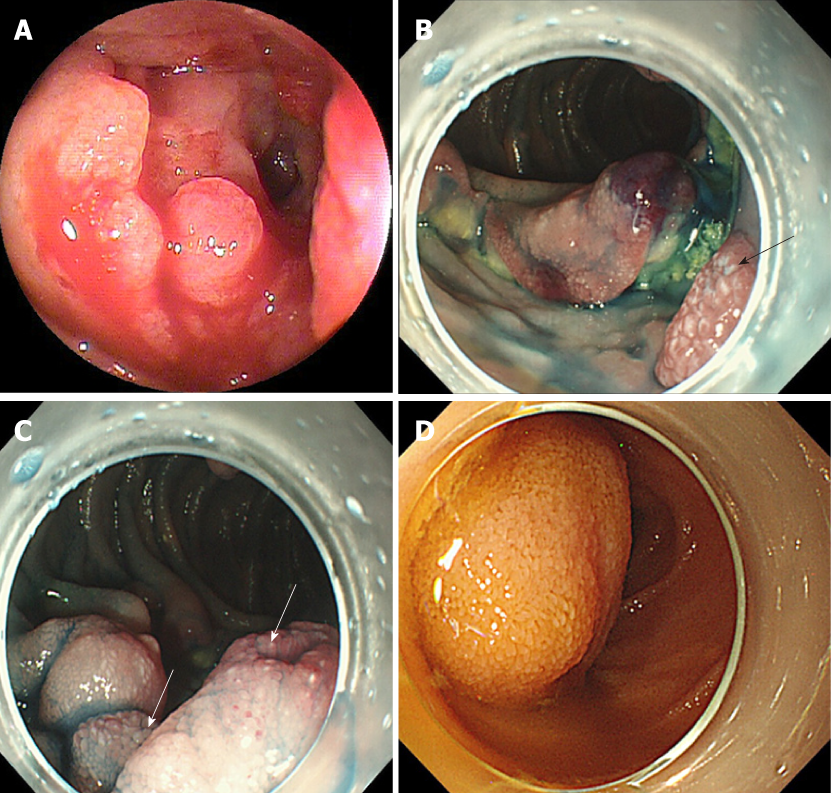
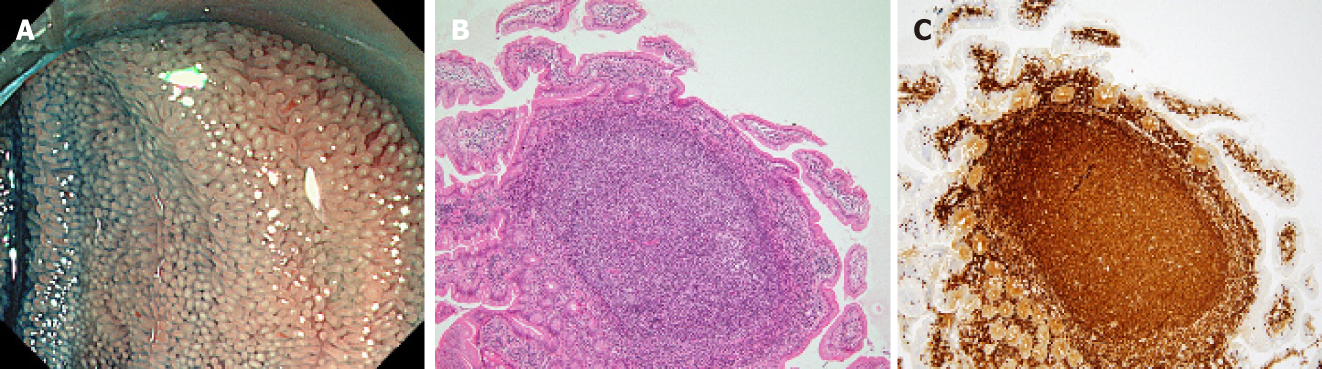
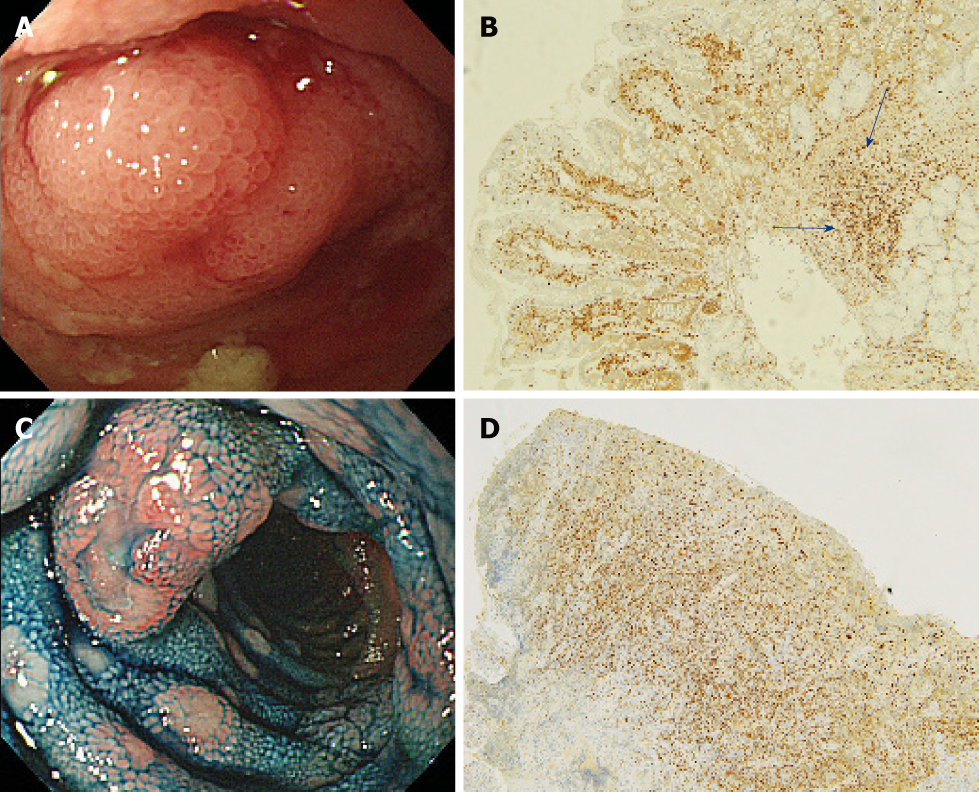
A flow diagram of patient enrollment is shown in Figure 1. In total, 1328 BAE procedures were performed from March 2005 to February 2017. Of these 1328 procedures, the number of patients in each group was 16 (Group 1), 22 (Group 2), and 6 (Group 3) (Figure 1). Table 1 shows the patients’ characteristics. We found no statistically-significant difference in age, symptoms (epigastric pain, melena, weight loss), and blood test results (white blood cell count, hemoglobin, lactate dehydrogenase) between the groups. The endoscopic characteristics of the small intestinal malignant tumors are shown in Table 2 and Figure 2. Seventy-five percent of epithelial tumors (Group 1) were located in the upper small intestine (duodenum and jejunum), and approximately 70% of GISTs were located in the jejunum. The percentage of solitary tumors was 100%, 45.5%, and 100% in Group 1, 2, and 3, respectively (P < 0.001). Solitary protruding or mass-type tumors were not seen in malignant lymphoma (Group 2) (P <0.001). Solitary infiltrative ulcerated type tumors were seen only in Group1 (P = 0.007) (Figure 2A). Multiple lesions with ulcerated surfaces or polyposis were seen only in Group 2, and stenosis was seen more frequently in Group 1, (68.8%, 27.3%, and 0%; Group 1, 2, and 3, respectively; P = 0.004). Although the difference was not statistically significant, Group1 tended to have more bleeding compared with Group 2 and 3. Enlarged white villi inside and/or surrounding the tumor were seen in 12.5%, 54.5%, and 0% in Group 1, 2 and 3, respectively (P < 0.001) (Figure 2B and C). We further investigated the pathological and morphological features of white villi in Group 2. Adequate biopsy samples were not obtained from four patients; therefore, we excluded data for these patients from the analysis (Table 3). Of the 22 Group 2 patients, enlarged white villi were seen in 12 patients. At the biopsy sites where most of the white villi were seen, lymphoma cells infiltrated into the villi with an intact epithelium; villi were filled with lymphoma cells (Table 3 and Figure 3). When the intact epithelium was ulcerated or lymphoma cells were present in the deep mucosa, white villi could not be seen (Table 3 and Figure 4).
| Group 1 (epithelial) | Group 2 (malignant lymphoma) | Group 3 (GIST) | P-value | |
| Age (mean ± SD) | 62.9 ± 13.7 | 67.7 ± 7.1 | 67.0 ± 11.9 | 0.47 |
| Symptom (%) | ||||
| Epigastric pain | 25.0 (4/16) | 27.3 (6/22) | 0 (0/6) | 0.36 |
| Melena | 25.0 (4/16) | 13.6 (3/22) | 16.7 (1/6) | 0.67 |
| Weight loss | 6.3 (1/16) | 4.5 (1/22) | 0 (0/16) | 0.82 |
| Other | 43.8 (7/16) | 54.5 (12/22) | 83.3 (5/6) | N/A |
| Blood test results (mean ± SD) | ||||
| WBC (/μL) | 7712.5 ± 3428.1 | 6536.4 ± 2858.5 | 4950.0 ± 1312.6 | 0.25 |
| Hb (g/dL) | 10.7 ± 2.7 | 11.6 ± 2.1 | 12.2 ± 2.4 | 0.63 |
| LDH (IU/L) | 201.0 ± 52.1 | 189.9 ± 49.1 | 175.0 ± 35.8 | 0.35 |
| Group 1 (Epithelial) (%) | Group 2 (Malignant lymphoma) (%) | Group 3 (GIST) (%) | P-value | |
| Tumor location | ||||
| Duodenum | 43.8 (7/16) | 18.2 (4/22) | 16.7 (1/6) | 0.18 |
| Jejunum | 31.3 (5/16) | 50.0 (11/22) | 66.7 (4/6) | 0.28 |
| Ileum | 25.0 (4/16) | 45.5 (10/22) | 16.7 (1/6) | 0.26 |
| Solitary lesion | 100.0 (16/16) | 45.5 (10/22) | 100.0 (6/6) | < 0.001 |
| Type or form | ||||
| Solitary | ||||
| Protruded or mass type | 31.3 (5/16) | 0 (0/22) | 66.7 (4/6) | < 0.001 |
| Ulcerated type (with raised margins) | 37.5 (6/16) | 40.9 (9/22) | 33.3 (2/6) | 0.94 |
| Infiltrative ulcerated type | 31.3 (5/16) | 0 (0/22) | 0 (0/6) | 0.007 |
| Multiple | ||||
| Multiple ulcers | 0 (0/16) | 22.7 (5/22) | 0 (0/6) | 0.06 |
| MLP | 0 (0/16) | 13.6 (3/22) | 0 (0/6) | 0.20 |
| Others | 0 (0/16) | 22.7 (5/22) | 0 (0/6) | N/A |
| Presence of stenosis | 68.8 (11/16) | 27.3 (6/22) | 0 (0/6) | 0.004 |
| Presence of bleeding | 43.8 (7/16) | 22.7 (5/22) | 16.7 (1/6) | 0.178 |
| White villi | 12.5 (2/16) | 54.5 (12/22) | 0 (0/6) | < 0.001 |
| White villi | P-value | ||
| Presence | Absence | ||
| Lymphoma cells infiltrating the villi with an intact epithelium | 91.7% (11/12) | 0 % (0/10) | < 0.001 |
| Lymphoma cells infiltrating the villi without an intact epithelium | 0% (0/12) | 50.0% (5/10) | 0.293 |
| Lymphoma cells present in the deep mucosa | 0% (0/12) | 20.0% (2/10) | 0.195 |
| Not assessed | 8.3% (1/12) | 30% (3/10) | N/A |
In the current study, we described the endoscopic characteristics of tumor location and morphology of small intestinal malignant tumors. Small intestinal malignant tumors were classified into three groups, and statistical analyses were performed between the groups. Patients’ clinical background parameters including age, symptoms, and laboratory data were not significantly different between the groups. First, we evaluated the endoscopic characteristics of tumor location and the number of tumors. Approximately three quarters of epithelial tumors were found in the duodenum or jejunum, and all were observed as solitary lesions (Table 2). Previous studies using BAE reported that primary small intestinal adenocarcinoma was located mainly in the duodenum or jejunum with a range of 77.8%–100.0%[6,7,11-14]. Primary small intestinal adenocarcinoma was reported as a solitary lesion in several studies[6,7,12-14], which was consistent with our results, whereas metastatic tumors were sometimes observed as multiple lesions[11,15]. In our classification, primary and metastatic tumors were classified into the same groups; however, it might be better to distinguish between metastatic and primary tumors. In the current study, malignant lymphoma lesions were located mainly in the jejunum and ileum, and approximately 60% were multiple lesions (Table 2), consistent with previous reports[11-14,16]. GISTs are reported mainly as solitary jejunal tumors[11-13,17,18]. Nakano et al[19] reported that 76% of patients with GIST had jejunal lesions, and that 3/25 patients had tumors in multiple sites (stomach and jejunum: 1; duodenum and jejunum: 1; and stomach, duodenum, and jejunum: 1). A particular type of GIST that is associated with neurofibromatosis type1 appears as multiple tumors[20-22]. However, as our results, most GISTs appeared as a solitary jejunal tumor, except for neurofibromatosis type1 associated type[20-22].
The endoscopic morphology of small intestinal tumors has not been systemically evaluated. In the current study, we evaluated the endoscopic morphology of small intestinal tumors. Epithelial tumors appeared as protruded or mass type, ulcerated type, or infiltrative ulcerated type, and 68.8% (11/16) were associated with stenosis. The infiltrative ulcerated type was typically recognized in epithelial tumors (Figure 2A). Malignant lymphoma appeared mainly as multiple lesions such as multiple ulcers or multiple lymphomatous polyposis (MLP), and stenosis was detected in 6/22 patients (27.3%). GIST was observed as the protruded or mass type [66.7% (4/6)], or the ulcerated type [33.3 % (2/6)] without stenosis. Chung et al[7] and Imaoka et al[12] reported that most small bowel adenocarcinomas appeared as the ulcerative form. Imaoka et al[12] and Almeida et al[23] reported that most epithelial small bowel tumors were associated with stenosis [70% (7/10) and 100% (3/3), respectively], similar to our findings. Previous reports[12,23,24] showed that malignant lymphoma occurred mainly as multiple lesions, such as multiple ulcers or MLP. MLP as multiple white nodules was observed in follicular lymphoma in previous studies[3,24-27]. Nakano et al[19] reported that the morphology of GISTs was classified into three groups: intraductal, extraductal, and mixed type. Lesions in 21 patients occurred as submucosal tumors and two occurred as diverticular transformation, in the 18 patients with ulceration. We saw no diverticular transformation in our study.
Of 22 patients with malignant lymphoma, enlarged white villi were seen in 12 patients (Table 2, Figure 3). The pathological and morphological features of the white villi biopsy sites showed that the lymphoma cells had infiltrated into the villi with an intact epithelium (Table 3 and Figure 3). In the current study, there were two types of malignant lymphoma that did not exhibit white villi. The first type showed the lymphoma cells sparsely infiltrated the villi, some lymphoma cells presented in the deep mucosa (Figure 4A and B). The second type showed the lymphoma cells infiltrated the mucosa without an intact epithelium (Figure 4C and D). Endoscopic findings of follicular lymphoma were described as “multiple polypoid lesions” and “multiple whitish small polyps” in a previous study[24]. These whitish polypoid lesions were seen as enlarged white villi using magnifying endoscopy[28-30]. Yamamoto et al[24] reported that each white enlarged villus was an enlarged neoplastic follicle consisting of lymphoma cells in the lamina propria, which was confirmed histologically. Another report showed that enlarged white duodenal villi were caused by infiltration of lymphoma cells into the villi, which formed lymphoid follicles[29]. From the pathological findings of our study, jejunal and/or ileal white villi in malignant lymphoma, even in other than the follicular type, are considered to consist of lymphoma cells in the lamina propria as with duodenal follicular lymphoma. Previous reports describe white villi of duodenal follicular lymphoma using esophagogastroduodenoscopy[24,28-30]. To our knowledge, ours is the first report of white villi in jejunal and ileal malignant lymphoma observed by BAE.
The endoscopic characteristics of the small intestinal tumors in our study are summarized in Table 4. Generally, epithelial small intestinal tumors appeared as solitary tumors with stenosis, small intestinal malignant lymphoma tumors as multiple tumors with white villi, and GISTs as solitary protruded lesions.
| Group 1 (Epithelial) | Group 2 (Malignant lymphoma) | Group 3 (GIST) | |
| Solitary tumor | 1 | 2 | 1 |
| Protruded or mass type | 2 | 3 | 1 |
| Infiltrative ulcerated type | 2 | 3 | 3 |
| Presence of stenosis | 1 | 2 | 3 |
| White villi | 3 | 2 | 3 |
Several limitations of this study should be addressed. First, this was a retrospective study; thus, many confounding factors could affect the results. Second, small intestinal tumors are very rare, and our sample size was relatively small. Future studies require higher numbers of patients to analyze larger datasets. However, there have been few studies to show the differences of endoscopic features among group1, 2 and 3. Furthermore, the importance of “white villi” could be emphasized in our study.
In conclusion, based on endoscopic findings during BAE, we were able to make tentative differential diagnoses of small intestinal malignant tumors.
Capsule endoscopy and balloon-assisted enteroscopy (BAE) enable visualization of rare small bowel conditions such as small intestinal malignant tumors.
The details of the endoscopic characteristics of small intestinal malignant tumors are still unknown.
The aim of this retrospective study was to elucidate the endoscopic characteristics of small intestinal malignant tumors.
This study was a retrospective medical record analysis. From March 2005 to February 2017, 1328 BAE procedures were performed at Keio University Hospital. Of these procedures, malignant tumors were classified into three groups, Group 1: epithelial tumors including primary small intestinal cancer, metastatic small intestinal cancer, and direct small intestinal invasion by an adjacent organ cancer; Group 2: small intestinal malignant lymphoma; and Group 3, small intestinal gastrointestinal stromal tumors. We systematically collected clinical and endoscopic data from patients’ medical records to determine the endoscopic characteristics for each group.
The number of patients in each group was 16 (Group 1), 22 (Group 2), and 6 (Group 3), and the percentage of solitary tumors was 100%, 45.5%, and 100%, respectively (P < 0.001). Solitary protruding or mass-type tumors were not seen in malignant lymphoma (Group 2) (P < 0.001). Stenosis was seen more often in Group 1, (68.8%, 27.3%, and 0%; Group 1, 2, and 3, respectively; P = 0.004). Enlarged white villi inside and/or surrounding the tumor were seen in 12.5%, 54.5%, and 0% in Group 1, 2, and 3, respectively (P = 0.001).
The differential diagnosis of small intestinal malignant tumors could be tentatively made based on BAE findings.
Future studies require higher numbers of patients to analyze larger datasets.
Conflict of interest statement: All authors disclose no financial conflicts of interest relevant to this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu CT, Wadhwa V S-Editor: Wang JL L-Editor: A E-Editor: Xing YX
| 1. | Neugut AI, Jacobson JS, Suh S, Mukherjee R, Arber N. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomarkers Prev. 1998;7:243-251. [PubMed] |
| 2. | Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19:58-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Matsumoto T. Gastrointestinal lymphoma: recent advances in diagnosis and treatment. Digestion. 2013;87:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003;97:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A, Ajibe H, Ido K, Sugano K. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 427] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Mitsui K, Tanaka S, Yamamoto H, Kobayashi T, Ehara A, Yano T, Goto H, Nakase H, Tanaka S, Matsui T, Iida M, Sugano K, Sakamoto C. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc. 2009;70:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Chung CS, Tai CM, Huang TY, Chang CW, Chen KC, Tseng CM, Wang HY, Chu CH, Wu JM, Chen Y, Wang HP. Small bowel tumors: A digestive endoscopy society of Taiwan (DEST) multicenter enteroscopy-based epidemiologic study. J Formos Med Assoc. 2018;117:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Kawano S, Miyashima Y, Miyabe Y, Kawai Y, Murata T, Uda M, Inokuchi T, Okada H. A case of small intestinal neuroendocrine carcinoma diagnosed using double-balloon endoscopy with long-term survival. Clin J Gastroenterol. 2018;11:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Li X, Gui Y, Shen F, Zhao CL, Yang Y, Han W. The application value of capsule endoscopy in diagnosing small intestinal carcinoma. J Cancer Res Ther. 2018;14:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ying X, Wang M, Verma V, Wang M, Ye S, Bi J, Zhou X, Han G, Zhen W. Metastatic spread of solid subtype lung adenocarcinoma to the small intestine with anemia and melena: A case report. Medicine (Baltimore). 2017;96:e7768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Honda W, Ohmiya N, Hirooka Y, Nakamura M, Miyahara R, Ohno E, Kawashima H, Itoh A, Watanabe O, Ando T, Goto H. Enteroscopic and radiologic diagnoses, treatment, and prognoses of small-bowel tumors. Gastrointest Endosc. 2012;76:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Imaoka H, Higaki N, Kumagi T, Miyaike J, Ohmoto M, Yamauchi K, Murakami T, Murakami H, Ikeda Y, Yokota T, Shibata N, Ninomiya T, Abe M, Hiasa Y, Matsuura B, Onji M, Umeda M, Horiike N. Characteristics of small bowel tumors detected by double balloon endoscopy. Dig Dis Sci. 2011;56:2366-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Chen WG, Shan GD, Zhang H, Li L, Yue M, Xiang Z, Cheng Y, Wu CJ, Fang Y, Chen LH. Double-balloon enteroscopy in small bowel tumors: a Chinese single-center study. World J Gastroenterol. 2013;19:3665-3671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Yamagami H, Oshitani N, Hosomi S, Suekane T, Kamata N, Sogawa M, Okazaki H, Watanabe K, Tominaga K, Watanabe T, Fujiwara Y, Arakawa T. Usefulness of double-balloon endoscopy in the diagnosis of malignant small-bowel tumors. Clin Gastroenterol Hepatol. 2008;6:1202-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Nishimura N, Mizuno M, Shimodate Y, Doi A, Mouri H, Matsueda K, Yamamoto H. The Role of Double-balloon Enteroscopy in the Diagnosis and Surgical Treatment of Metastatic Small Bowel Tumors. Intern Med. 2018;57:1209-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Lee BI, Choi H, Choi KY, Byeon JS, Jang HJ, Eun CS, Cheon JH, Shin SJ, Kim JO, Lee MS, Choi JH. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56:2920-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Zhou L, Liao Y, Wu J, Yang J, Zhang H, Wang X, Sun S. Small bowel gastrointestinal stromal tumor: a retrospective study of 32 cases at a single center and review of the literature. Ther Clin Risk Manag. 2018;14:1467-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Murino A, Nakamura M, Watanabe O, Yamamura T, Nagura A, Yoshimura T, Nakano A, Goto H, Hirooka Y. Effectiveness of Endoscopic Ultrasonography during Double Balloon Enteroscopy for characterization and management of small bowel submucosal tumours. Dig Liver Dis. 2016;48:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Nakano A, Nakamura M, Watanabe O, Yamamura T, Funasaka K, Ohno E, Kawashima H, Miyahara R, Goto H, Hirooka Y. Endoscopic Characteristics, Risk Grade, and Prognostic Prediction in Gastrointestinal Stromal Tumors of the Small Bowel. Digestion. 2017;95:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Salvi PF, Lorenzon L, Caterino S, Antolino L, Antonelli MS, Balducci G. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol. 2013;2013:398570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 22. | Hakozaki Y, Sameshima S, Tatsuoka T, Okuyama T, Yamagata Y, Noie T, Oya M, Fujii A, Ueda Y, Shimura C, Katagiri K. Rectal carcinoma and multiple gastrointestinal stromal tumors (GIST) of the small intestine in a patient with neurofibromatosis type 1: a case report. World J Surg Oncol. 2017;15:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Almeida N, Figueiredo P, Lopes S, Gouveia H, Leitão MC. Double-balloon enteroscopy and small bowel tumors: a South-European single-center experience. Dig Dis Sci. 2009;54:1520-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Yamamoto S, Nakase H, Yamashita K, Matsuura M, Takada M, Kawanami C, Chiba T. Gastrointestinal follicular lymphoma: review of the literature. J Gastroenterol. 2010;45:370-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Higuchi K, Komatsu K, Wakamatsu H, Kawasaki H, Murata M, Miyazaki K, Oikawa K, Ohwada M, Nanjo H, Otaka M, Watanabe S, Komatsu K. Small intestinal follicular lymphoma with multiple tumor formations diagnosed by double-balloon enteroscopy. Intern Med. 2007;46:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Nakamura M, Ohmiya N, Hirooka Y, Miyahara R, Ando T, Watanabe O, Itoh A, Kawashima H, Ohno E, Kinoshita T, Goto H. Endoscopic diagnosis of follicular lymphoma with small-bowel involvement using video capsule endoscopy and double-balloon endoscopy: a case series. Endoscopy. 2013;45:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Matsumoto T, Nakamura S, Esaki M, Yada S, Moriyama T, Yanai S, Hirahashi M, Yao T, Iida M. Double-balloon endoscopy depicts diminutive small bowel lesions in gastrointestinal lymphoma. Dig Dis Sci. 2010;55:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Iwamuro M, Kondo E, Otsuka F, Takata K, Yoshino T, Kawahara Y, Okada H. Detection of Minute Duodenal Follicular Lymphoma Lesions Using Magnifying Endoscopy. Acta Med Okayama. 2016;70:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Iwamuro M, Okada H, Takata K, Kawai Y, Kawano S, Nasu J, Kawahara Y, Tanaka T, Yoshino T, Yamamoto K. Magnified endoscopic features of duodenal follicular lymphoma and other whitish lesions. Acta Med Okayama. 2015;69:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Iwamuro M, Okuda M, Yumoto E, Suzuki S, Shirakawa A, Takata K, Yoshino T, Okada H, Yamamoto K. Magnifying endoscopy for intestinal follicular lymphoma is helpful for prompt diagnosis. Gut Liver. 2013;7:258-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |