Published online Apr 16, 2019. doi: 10.4253/wjge.v11.i4.281
Peer-review started: February 11, 2019
First decision: March 11, 2019
Revised: March 18, 2019
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: April 16, 2019
Processing time: 75 Days and 5.1 Hours
For palliation of malignant biliary obstruction (MBO), the gold-standard method of biliary drainage is endoscopic retrograde cholangiopancreatography (ERCP) with the placement of metallic stents. Endoscopic ultrasound (EUS)-guided drainage is an alternative that is typically reserved for cases of ERCP failure. Recently, however, there have been robust randomized clinical trials (RCTs) comparing EUS-guided drainage and ERCP as primary approaches to MBO.
To compare EUS guidance and ERCP in terms of their effectiveness and safety in palliative biliary drainage for MBO.
This was a systematic review and meta-analysis, in which we searched the MEDLINE, Excerpta Medica, and Cochrane Central Register of Controlled Trials databases. Only RCTs comparing EUS and ERCP for primary drainage of MBO were eligible. All of the studies selected provided data regarding the rates of technical and clinical success, as well as the duration of the procedure, adverse events, and stent patency. We assessed the risk of biases using the Jadad score and the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation criteria.
The database searches yielded 5920 records, from which we selected 3 RCTs involving a total of 222 patients (112 submitted to EUS and 110 submitted to ERCP). In the EUS and ERCP groups, the rate of technical success was 91.96% and 91.81%, respectively, with a risk difference (RD) of 0.00% (95%CI: -0.07, 0.07; P = 0.97; I2 = 0%). The clinical success was 84.81% and 85.53% in the EUS and ERCP groups, respectively, with an RD of −0.01% (95%CI: -0.12, 0.10; P = 0.90; I2 = 0%). The mean difference (MD) for the duration of the procedure was -0.12% (95%CI: -8.20, 7.97; P = 0.98; I2 = 84%). In the EUS and ERCP groups, there were 14 and 25 adverse events, respectively, with an RD of -0.06% (95%CI: -0.23, 0.12; P = 0.54; I2 = 77%). The MD for stent patency was 9.32% (95%CI: -4.53, 23.18; P = 0.19; I2 = 44%). The stent dysfunction rate was significantly lower in the EUS group (MD = -0.22%; 95CI:-0.35, -0.08; P = 0.001; I2 = 0%).
EUS represents an interesting alternative to ERCP for MBO drainage, demonstrating lower stent dysfunction rates compared with ERCP. Technical and clinical success, duration, adverse events and patency rates were similar.
Core tip: No consensus is available in the literature regarding whether endoscopic retrograde cholangiopancreatography or endoscopic ultrasound-guided biliary drainage is more beneficial to the patient. This is the first systematic review and meta-analysis comparing the two methods. We investigated these two techniques in terms of technical and clinical success, as well as duration of the procedure, adverse events, stent dysfunction and stent patency.
- Citation: Logiudice FP, Bernardo WM, Galetti F, Sagae VM, Matsubayashi CO, Madruga Neto AC, Brunaldi VO, de Moura DTH, Franzini T, Cheng S, Matuguma SE, de Moura EGH. Endoscopic ultrasound-guided vs endoscopic retrograde cholangiopancreatography biliary drainage for obstructed distal malignant biliary strictures: A systematic review and meta-analysis. World J Gastrointest Endosc 2019; 11(4): 281-291
- URL: https://www.wjgnet.com/1948-5190/full/v11/i4/281.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i4.281
Endoscopic retrograde cholangiopancreatography (ERCP) is currently the gold-standard method to address malignant biliary obstruction (MBO) of the distal common bile duct[1,2], the procedure consists in endoscopic guidewire access to the duodenal papilla, with further injection of contrast on the bile ducts and placement of an endoscopic stent in order to treat MBO, and there are data favoring the use of self-expanding metal stents over that of plastic stents[3]. However, ERCP is not free of complications, the most common being post-ERCP pancreatitis and cholangitis[4]. In addition, there is a non-negligible risk of failed biliary cannulation in ERCP due to dysfunctional biliary sphincter or anatomical alterations[5].
Percutaneous transhepatic biliary drainage (PTBD) and surgical bilioenteric anastomosis are traditional alternatives to ERCP, although both have their particular drawbacks. PTBD requires multiple interventions and carries an increased risk of cholangitis, bacteremia, and hemobilia[6], whereas bilioenteric anastomosis is associated with high morbidity and mortality[7].
Endoscopic ultrasound (EUS) has long been of paramount importance for the workup of patients with biliary obstruction[8-10]. Some recent reports have described EUS-guided drainage as an alternative in cases of ERCP failure[11-13]. The efficacy and safety profile of EUS-guided drainage have improved over time, as has the availability of specific accessories, allowing some authors to test EUS-guided biliary drainage, in comparison with ERCP, as a primary approach to biliary obstruction.
Transluminal EUS-guided biliary drainage consists of needle access to the biliary ducts by hepatogastric or choledocoduodenal puncture under EUS guidance. Then, a guidewire is inserted through the needle, followed by dilation of the fistula and stent placement.
Although there have been a number of randomized clinical trials (RCTs) comparing EUS-guided biliary drainage and ERCP[14-16], there have yet to be any systematic reviews or meta-analyses regarding the topic. Therefore, the aim of the present study was to summarize all available data comparing EUS and ERCP in terms of their effectiveness and safety in the primary drainage of MBO. To that end, we conducted a systematic review and meta-analysis, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology, of RCTs comparing EUS and ERCP in the primary drainage of distal MBO, assessing technical success, clinical success, cost-effectiveness, duration of the procedure, adverse events, mortality, stent patency, and stent dysfunction.
This study followed the PRISMA guidelines[17] and was registered in the International Prospective Register of Systematic Reviews database[18] (CRD42018108712). The study was approved by the local institutional review board.
Only RCTs were considered eligible, without barriers as to the language or year of publication. We included RCTs that had evaluated patients diagnosed with distal MBO and undergoing primary drainage of the biliary tract under EUS guidance or by ERCP. Studies evaluating patients with benign biliary obstruction were excluded, as were those evaluating EUS-guided biliary drainage after failure of another method and those including only patients undergoing primary EUS-guided drainage due to an anatomical alteration that precluded ERCP.
We searched the MEDLINE, Excerpta Medica, Cochrane Central Register of Controlled Trials, Latin-American and Caribbean Health Sciences Literature databases, as well as the gray literature, for RCTs published up to and including November 2018. We employed descriptors available from the United States National Library of Medicine Medical Subject Headings and, to a lesser degree, other related terms aiming at a more sensitive strategy. For Medline, our search strategy was as follows: [(ERCP OR Endoscopic Retrograde Cholangiopancreatograph*) OR (EUS OR endosonography OR Endoscopic Ultrasonograph* OR Echo Endoscop*)] AND (decompression OR drain*). For the other databases, the following search strategy was applied: (EUS OR Endoscopic Ultrasonography) AND (decompression OR drainage).
Two independent researchers assessed titles and abstracts for eligibility. Any disagreement was resolved by consensus with a third experienced researcher. The articles were included after an evaluation of the full-text based on the study criteria.
Data related to EUS-guided and ERCP biliary drainage were collected using a preformatted Excel workbook. The data collected included technical and clinical success rates, as well as the duration of the procedure, adverse events, stent patency, and stent dysfunction.
In our quantitative analysis, we used the absolute values, means, and standard deviations. If a study expressed outcomes using median and interquartile range, mathematical formulas were used for data conversion[19]. In case of the study fails to present means and standard deviations or median and interquartile range of the continuous variables of specific outcomes, rendering impossible to include the data for meta-analysis evaluation, the study in question was excluded from the outcome appraisal.
The biases of the RCTs were assessed with the Jadad scale[20], which allows critical appraisal regarding blinding, randomization, and information on losses to follow-up. Jadad score is applied to evaluate the methodological quality of RCTs, rating the study from zero (poor quality) to five points (rigorous). The evaluation criteria are “description of the study as randomized”, “employment of appropriated ran-domization method”, “description of the method of blinding”, “employment of appropriated blinding method” and “description of losses to follow-up” whereupon each present criteria grants one point.
The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria with the GRADEpro Guideline Development Tool software (McMaster University, 2015; Evidence Prime, Inc., Ontario, Canada)[21]. GRADE is an approach to rate the quality of evidence based on criteria guideline developed by the GRADE working group and involves the appraisal of risk of bias, inconsistency, indirectness, imprecision and publication bias. Evaluation of biases and quality of studies was performed under supervision of our statistic team.
For the dichotomous variables, we calculated the risk difference (RD) values, using the Mantel-Haenszel test, together with the corresponding 95% confidence intervals. For continuous variables, we calculated the mean difference values, also with the corresponding 95% confidence intervals, using the inverse variance test. The results were displayed with forest plots.
We assessed the heterogeneity among studies using the Higgins test (I2). If there was an I2 < 50%, we used a fixed-effect model, whereas we used funnel plot analysis if there was an I2 > 50%. If we detected an outlier article, we removed it from the analysis and kept the fixed-effect model. If we could not detect an outlier, we switched to the random-effect model analysis to ameliorate the impact of the high heterogeneity. All analyses were carried out with Review Manager software, version 5.3.5 (RevMan 5; Cochrane Collaboration, Oxford, United Kingdom).
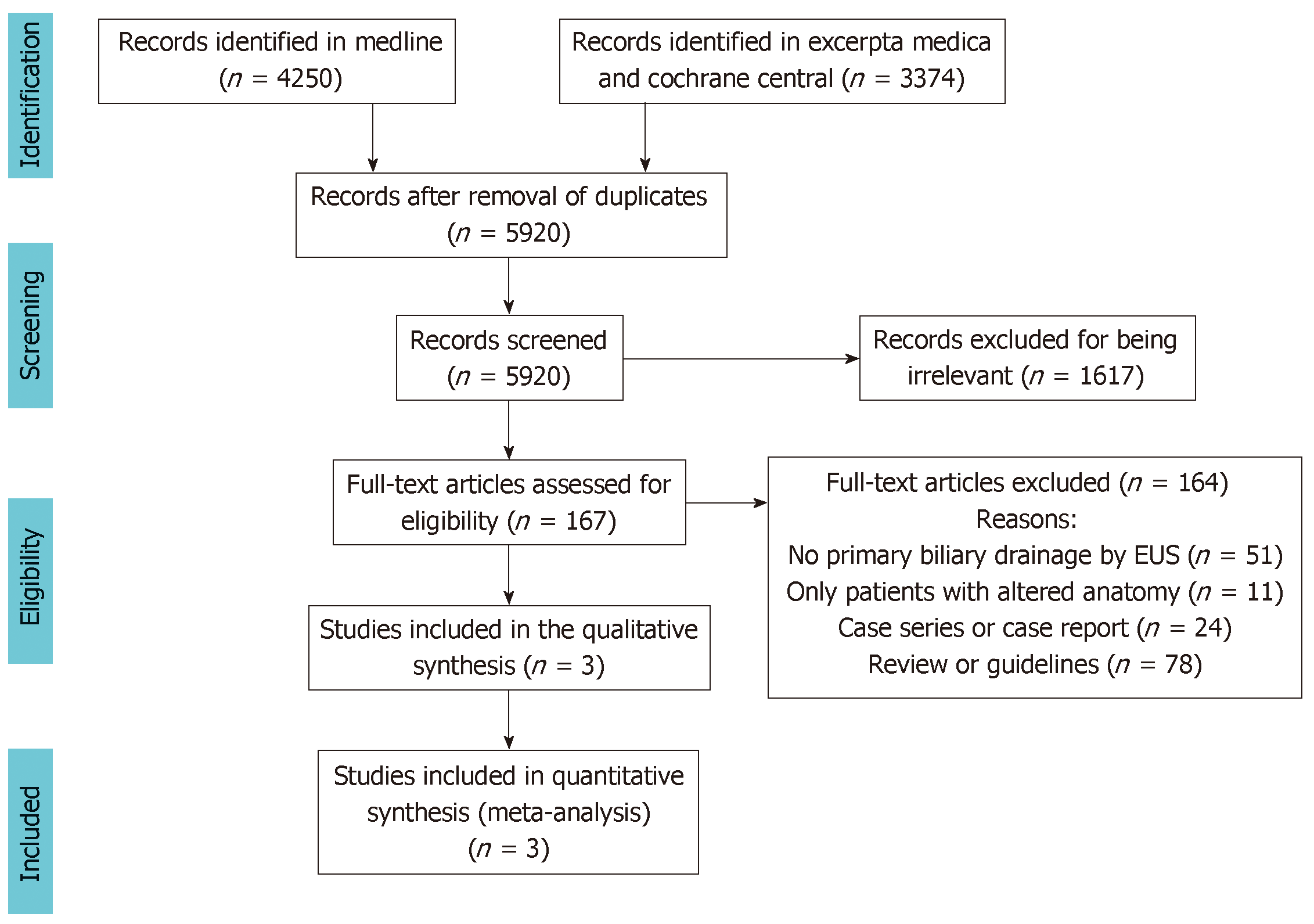
The database searches retrieved a total of 5920 studies, 164 of which were selected for full-text evaluation. Based on the study criteria, three RCTs were included in the qualitative analysis and meta-analysis (Figure 1).
The collective sample comprised 222 patients: 112 in the EUS group and 110 in the ERCP group. The mean age was similar between the two groups and among the samples of the RCTs included. The etiology of MBO in the studies selected is outlined in Table 1.
| Variable | Study | |||||
| Bang et al[16] | Paik et al[15] | Park et al[14] | ||||
| EUS | ERCP | EUS | ERCP | EUS | ERCP | |
| n | 33 | 34 | 64 | 61 | 15 | 15 |
| Age (yr), mean (SD) | 69.4 (12.6) | 69.2 (11.6) | 64.8 (12.5) | 68.4 (10.5) | 66.8 (8) | 65.4 (9.3) |
| Etiology of MBO | Pancreas (n = 33) | Pancreas (n = 31); pancreatic metastasis (n = 3) | Pancreas (n = 38); cholangiocarcinoma (n = 3); gallbladder (n = 4); papilla (n = 5); gastric (n = 4); duodenal (n = 2); other (n = 8) | Pancreas (n = 40); cholangiocarcinoma (n = 8); gallbladder (n = 4); papilla (n = 3); gastric (n = 2); duodenal (n = 1); hepatocellular carcinoma (n = 1); other (n = 2) | Pancreas (n = 14); cholangiocarcinoma (n = 1) | Pancreas (n = 13); metastatic lymph node (n = 2) |
On the Jadad scale (Table 2), all of the RCTs evaluated had a score of 3, which is the highest possible score for unblinded studies. According to the GRADE criteria for the quality of evidence, the evidence for technical success generated moderate certainty, the evidence for stent dysfunction generated low certainty, and the evidence for the remaining outcomes generated very low certainty (Table 3).
| Parameter | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence |
| No. of patients (studies) | ||||||
| Technical success | ||||||
| 222 (3 RCTs) | Not serious | Not serious | Not serious | Seriousa | None | Moderate |
| Clinical success | ||||||
| 155 (2 RCTs) | Seriousb | Not serious | Seriousc | Seriousa | None | Very low |
| Procedure duration | ||||||
| 222 (3 RCTs) | Not serious | Very seriousd | Seriouse | Seriousa | None | Very low |
| Adverse events | ||||||
| 222 (3 RCTs) | Not serious | Very seriousd | Not serious | Seriousa | None | Very low |
| Stent patency | ||||||
| 97 (2 RCTs) | Seriousb | Not serious | Seriouse | Seriousa | None | Very low |
| Stent dysfunction | ||||||
| 155 (2 RCTs) | Not serious | Not serious | Seriouse | Not serious | Strongly suspected | Low |
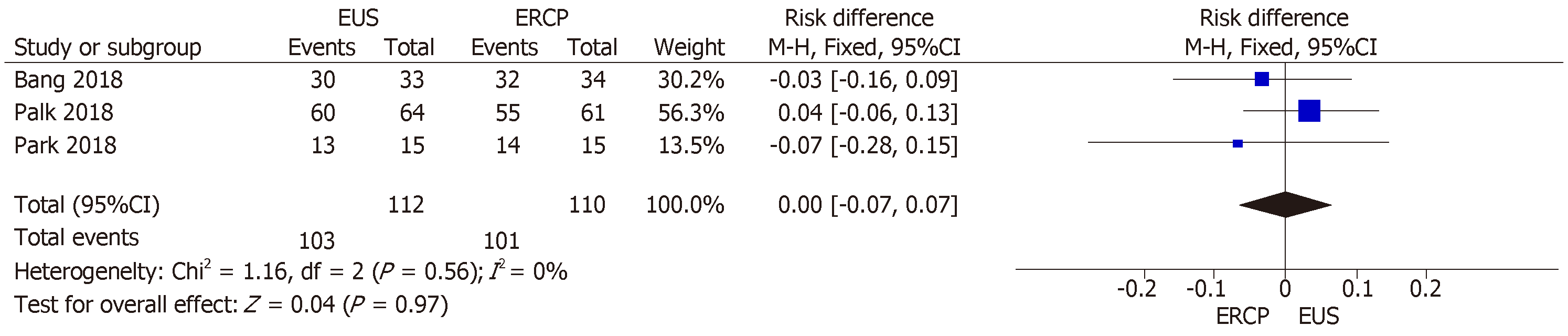
All three RCTs[14-16] reported technical success rates. The mean rate of technical success was 91.96% and 91.81% in the EUS and ERCP groups, respectively, with an RD of 0.00% (95%CI: −0.07, 0.07; P = 0.97), demonstrating no statistical difference between the two techniques (Figure 2).
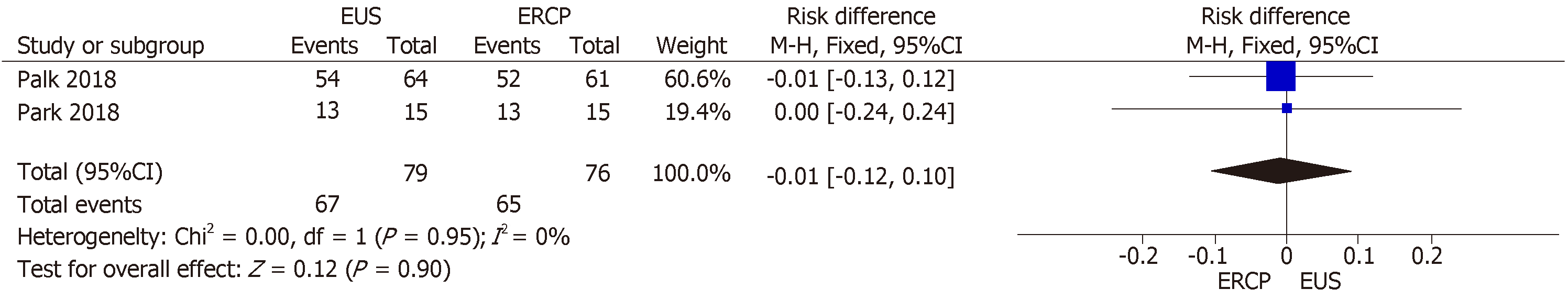
All three RCTs included data on clinical success[14-16]. However, Bang et al[16] included cross-over procedures in their final results, precluding the intention-to-treat analysis and thus excluding 67 patients. Therefore, the final collective sample in our analysis of clinical success comprised 155 patients: 79 in the EUS group and 76 in the ERCP group. The mean clinical success rate was 84.81% and 85.53% in the EUS and ERCP groups, respectively, with an RD of −0.01% (95%CI: −0.12, 0.10; P = 0.90), as shown in Figure 3.
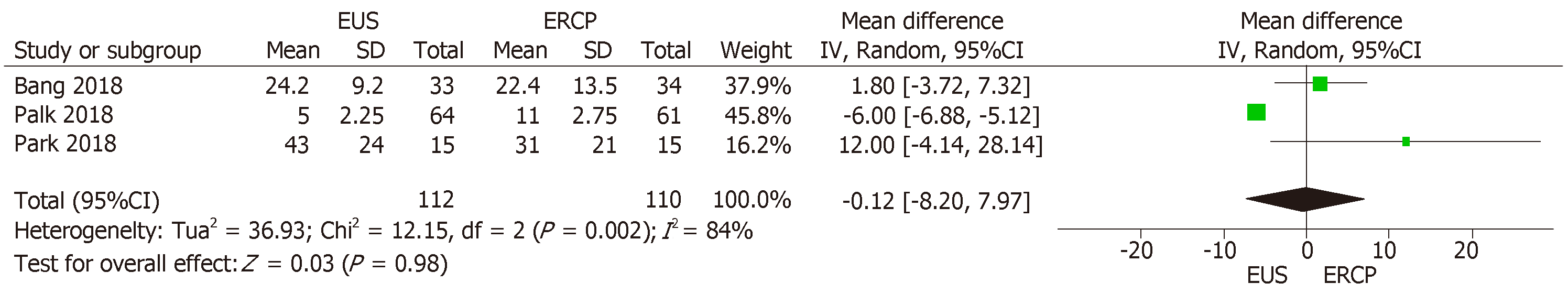
All three studies[14-16] reported the duration of the procedure in minutes. The mean time difference between EUS-guided and ERCP drainage was -0.12% (95%CI: -8.20, 7.97; P = 0.98), showing no statistical difference between the two groups (Figure 4). We found high heterogeneity among the studies (I2 = 84%). Because there were no outliers, we employed the random-effect model in our analysis.
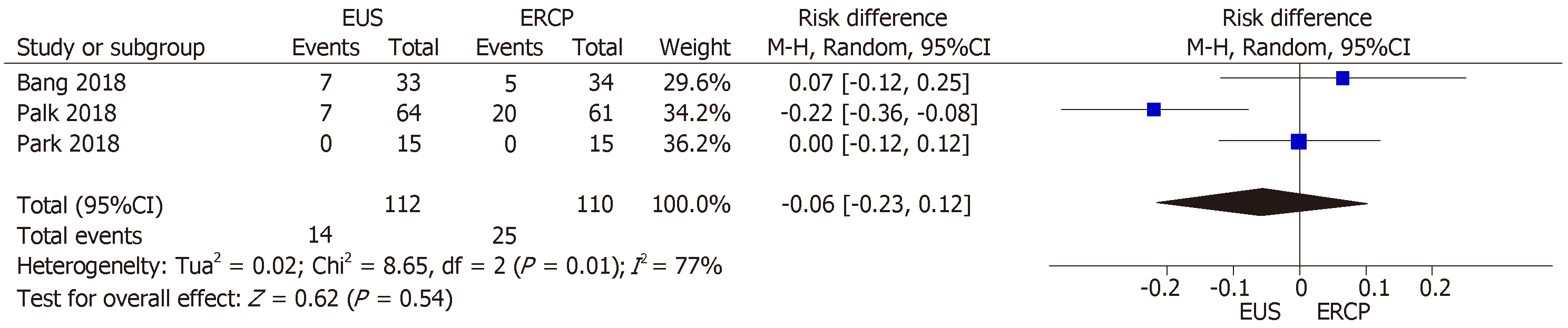
All three RCTs[14-16] described the adverse events reported. In the EUS group, 14 adverse events were reported: abdominal pain (n = 5); cholangitis (n = 4); pneumoperitoneum (n = 2); biliary peritonitis (n = 2); and cholecystitis (n = 1). In the ERCP group, there were 25 adverse events: pancreatitis (n = 10); cholangitis (n = 7); cholecystitis (n = 5); and abdominal pain (n = 3). No procedure-related mortality was reported in any of the studies.
Although we identified high heterogeneity (I2 = 77%), there were no outlier studies, and the random-effect model was therefore employed. The mean difference between the two techniques was −0.06% (95%CI: −0.23, 0.12; P = 0.54), indicating that there was no statistical difference (Figure 5).
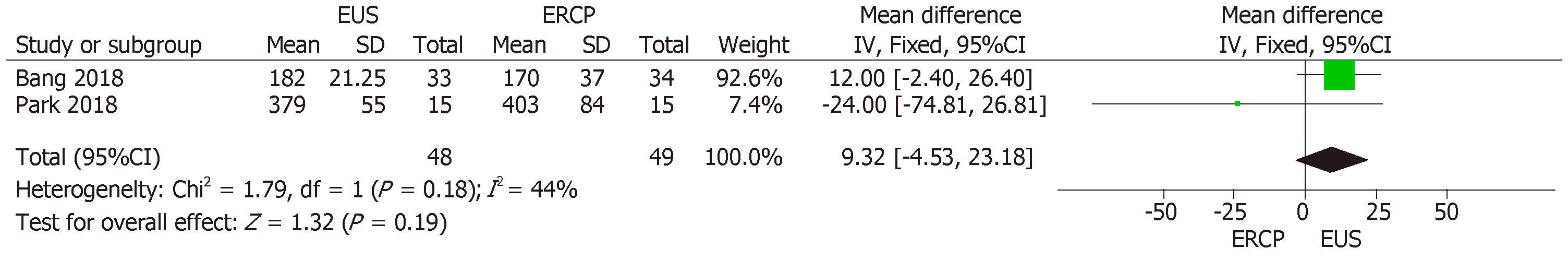
Although all three RCTs reported data on stent patency, Paik et al[15] did not detail standard deviation values, precluding the inclusion of that study in the analysis and thus excluding 125 patients. Therefore, the final collective sample in our analysis of stent patency comprised 97 patients: 48 in the EUS group and 49 in the ERCP group. The mean difference was 9.32% (95%CI: −4.53, 23.18; P = 0.19), demonstrating no significant difference between the two methods in terms of stent patency (Figure 6)
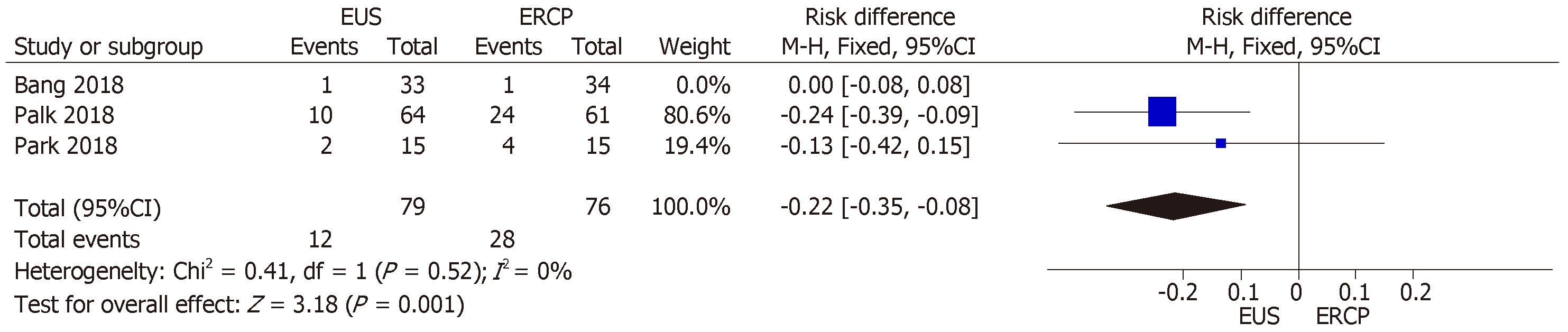
All three studies[14-16] provided data on stent dysfunction. We found high hete-rogeneity (I2 = 86%) among the studies, and the funnel plot analysis identified the Bang et al[16] study as an outlier. When we excluded that study from the analysis, the I2 value was 0%. Between the two remaining studies, there were 12 cases of stent dysfunction requiring intervention in the EUS group and 28 such cases in the ERCP group. The RD between the groups was −0.22% (95%CI: −0.35, −0.08; P = 0.001), thus favoring EUS-guidance over ERCP (Figure 7).
To our knowledge, this is the first systematic review and meta-analysis including only RCTs that compared EUS-guidance and ERCP as the primary approach to biliary drainage in cases of MBO. Our strict methodology, which included critical appraisal of biases, quality of evidence assessment, and a report prepared in accordance with the PRISMA guidelines[17], underscores the strength of our findings.
EUS-guided biliary drainage was first introduced as an alternative to be employed after ERCP failure[11–13]. Moole et al[22] recently published a systematic review and meta-analysis comparing PTBD and EUS-guided drainage as alternatives to be employed after failed ERCP, demonstrating that the latter was superior, as has been cor-roborated by other authors[23].
Because of improvements in the technique and accessories over time, some authors have reported EUS-guided biliary drainage as a first-line modality in patients presenting with factors predictive of difficult biliary access by ERCP (e.g., altered anatomy, duodenal obstruction, and previous duodenal stent)[24–28]. Okuno et al[27] published a prospective study of 20 patients undergoing EUS-guided hepa-ticogastrostomy with a 6-mm self-expanding metallic stent. The rates of technical success, clinical success, and adverse events were 100%, 95%, and 15%, respectively. In a recent multicenter cohort study[28], EUS-guided biliary drainage was compared with ERCP in patients with an indwelling duodenal stent. The authors identified a trend toward higher technical and clinical success rates in the EUS group and found no difference regarding adverse events. Finally, Nakai et al[29] published a retrospective study comparing primary and rescue EUS-guided biliary drainage in terms of the rates of technical success and adverse events, both of which the authors found to be similar between the two approaches.
In a recent retrospective study of patients with distal biliary obstruction, Kawabuto et al[30] demonstrated that EUS-guided choledochoduodenostomy was similar to transpapillary stenting in terms of the rates of clinical success and adverse events, although the duration of the procedure was shorter and there were no cases of pancreatitis among the patients submitted to the former. Therefore, the EUS-guided procedure was considered a plausible first-line method to address MBO. Subsequent RCTs comparing those techniques have shed light on the matter[14-16].
The availability of three high-quality RCTs allowed us to perform a consistent meta-analysis that will likely contribute to making daily practice more evidence based. Our analysis of technical success is extremely reliable because of the similar definitions employed and homogenous results among the three studies. However, the clinical success analysis lacked consistency because of indirectness due to different outcome definitions. In addition, Bang et al[16] included cross-over procedures in the data report, which impeded the intention-to-treat analysis. Therefore, caution should be taken in drawing conclusions based on the results of this analysis.
During our evaluation of the duration of procedures, we found high heterogeneity among the studies. Such true heterogeneity is likely attributable to the participation of endoscopists with different levels of expertise. In addition, various stents have been used in biliary drainage. Paik et al[15] employed insulated delivery systems to perform EUS-guided drainage, which probably shortened the duration of the procedure in their EUS group and promoted heterogeneity. Our analysis showed equivalence between the two methods regarding the duration of the procedure. It should be borne in mind that, whereas ERCP is a well-established technique, EUS-guided drainage is still in development, and its duration could therefore become shorter in the near future.
As to the safety of the procedure, our analysis showed similar rates of adverse events after EUS and ERCP. Although there was no difference between the two approaches regarding the overall rates, there was a substantial difference regarding the types of adverse events observed. In the ERCP group, the most common complication was pancreatitis, which was not reported in the EUS group. Conversely, pneumoperitoneum and biliary peritonitis were reported only in the EUS group, although none of patients required surgical intervention. Cholangitis was reported in both groups: 7 cases in the ERCP group and 4 in the EUS group.
The stent patency was equivalent for both methods, although the largest study[15] did not provide standard deviation values and was therefore excluded. That significantly reduced the size of the sample evaluated in the stent patency analysis.
Finally, the results of our analysis of the stent dysfunction rate favored EUS-guided drainage. That might be explained by the fact that this method allows a puncture far from the tumor rather than through it, thus avoiding tumor ingrowth or overgrowth. Although EUS-guided drainage can promote stent dysfunction due to food bolus impaction, that risk does not seem to outweigh its advantages. It should also be borne in mind that the employment of diverse stents for biliary drainage could be a confounding factor in the analysis of stent patency and dysfunction.
None of the RCTs evaluated in our systematic review described a cost-effectiveness analysis. Because the differences between ERCP and EUS-guided drainage are still slight, such information might create a tipping point to recommend one approach over the other. Future trials should address this knowledge gap.
Our study has some limitations. First, a lack of standard deviation data precluded the inclusion of the largest trial in the stent patency analysis, thus limiting our ability to draw conclusions regarding that aspect. Second, different definitions of clinical success resulted in a very low quality of evidence, also precluding any firm conclusions. Finally, the small number of RCTs included constitutes a major limitation. Future studies might therefore contradict our results. Nevertheless, to our knowledge, this is the first meta-analysis comparing ERCP and EUS-guided biliary drainage in MBO. Our findings could have significant clinical implications for the management of patients with MBO.
In patients with distal MBO, EUS-guided drainage shows rates of technical success, clinical success, adverse events, and stent patency similar to those of ERCP. The rates of stent dysfunction appear to be lower for stents placed under EUS guidance. Cost-effectiveness studies might solidify the role of EUS-guided drainage in the management of MBO.
Endoscopic retrograde cholangiopancreatography (ERCP) is currently the gold standard palliation approach for distal malignant biliary obstruction (MBO) but as endoscopic ultrasound (EUS)-guided techniques develop and became more commonly available question arises whether EUS-guided biliary drainage cloud be a first line method for treatment of distal MBO.
EUS-guided biliary drainage and ERCP are recognized endoscopic approaches for palliation of MBO. Our initial motivation was to compare EUS and ERCP techniques for primary drainage of distal MBO. By performing a systematic review and meta-analysis following a rigorous methodological approach we aimed to increase the available knowledge regarding endoscopic palliation of MBO.
To perform a systematic review and meta-analysis comparing EUS and ERCP as primary methods of biliary drainage in distal MBO regarding technical success, clinical success, duration of the procedure, adverse events, stent patency and stent dysfunction.
We conducted a systematic review and meta-analysis based on the PRISMA Statement and registered on PROSPERO international database. We searched the Medline, Excerpta Medica, and Cochrane Central Register of Controlled Trials databases. Only randomized clinical trials (RCTs) comparing EUS and ERCP for primary drainage of MBO were eligible. We assessed the risk of biases using the Jadad score and the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation criteria.
Three RCTs were included in the final analysis comprising a total of 222 patients (112 submitted to EUS and 110 submitted to ERCP). The stent dysfunction rate was significantly lower in the EUS group (MD = −0.22%; 95%CI: −0.35, −0.08; P = 0.001; I2 = 0%). There were no statistically significant difference regarding technical success, clinical success, duration of the procedure, adverse events and stent patency among the compared techniques.
In palliative drainage of distal MBO, EUS-guided and ERCP drainage presents similar rates of technical success, clinical success, adverse events, and stent patency. The rates of stent dysfunction appear to be lower for stents placed under EUS guidance.
We considered meaningful to stablish a present evaluation of both techniques and as the procedures continue to develop, further widespread and new technologies emerge, we encourage that additional RCT’s and meta-analisys are performed.Cost-effectiveness studies might solidify the role of EUS-guided drainage in the management of MBO.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Viswanath YKS, Ziogas DE S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 450] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Inamdar S, Slattery E, Bhalla R, Sejpal DV, Trindade AJ. Comparison of Adverse Events for Endoscopic vs Percutaneous Biliary Drainage in the Treatment of Malignant Biliary Tract Obstruction in an Inpatient National Cohort. JAMA Oncol. 2016;2:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Zorrón Pu L, de Moura EG, Bernardo WM, Baracat FI, Mendonça EQ, Kondo A, Luz GO, Furuya Júnior CK, Artifon EL. Endoscopic stenting for inoperable malignant biliary obstruction: A systematic review and meta-analysis. World J Gastroenterol. 2015;21:13374-13385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 4. | Dhir V, Itoi T, Khashab MA, Park DH, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Williams EJ, Ogollah R, Thomas P, Logan RF, Martin D, Wilkinson ML, Lombard M. What predicts failed cannulation and therapy at ERCP? Results of a large-scale multicenter analysis. Endoscopy. 2012;44:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Oh HC, Lee SK, Lee TY, Kwon S, Lee SS, Seo DW, Kim MH. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Bartlett EK, Wachtel H, Fraker DL, Vollmer CM, Drebin JA, Kelz RR, Karakousis GC, Roses RE. Surgical palliation for pancreatic malignancy: practice patterns and predictors of morbidity and mortality. J Gastrointest Surg. 2014;18:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Moura DTH, de Moura EGH, Matuguma SE, Dos Santos ME, Moura ETH, Baracat FI, Artifon E, Cheng S, Bernardo WM, Chacon D, Tanigawa R, Jukemura J. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open. 2018;6:E769-E777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 10. | De Moura DTH, Moura EGH, Bernardo WM, De Moura ETH, Baraca FI, Kondo A, Matuguma SE, Almeida Artifon EL. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc Ultrasound. 2018;7:10-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Park DH, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Wang K, Zhu J, Xing L, Wang Y, Jin Z, Li Z. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc. 2016;83:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 13. | Artifon EL, Takada J, Okawa L, Moura EG, Sakai P. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP. 2010;11:597-600. [PubMed] [DOI] [Full Text] |
| 14. | Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 15. | Paik WH, Lee TH, Park DH, Choi JH, Kim SO, Jang S, Kim DU, Shim JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018;113:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 16. | Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc. 2018;88:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 17. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15808] [Article Influence: 1580.8] [Reference Citation Analysis (1)] |
| 18. | PROSPERO. International prospective register of systematic reviews. Available from: https://www.crd.york.ac.uk/prospero/. |
| 19. | Kenney JF, Keepping ES. Standard Error of the Mean. 2nd edition. Princeton, NJ. 1951;. |
| 20. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12870] [Article Influence: 443.8] [Reference Citation Analysis (1)] |
| 21. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14823] [Article Influence: 871.9] [Reference Citation Analysis (0)] |
| 22. | Moole H, Bechtold ML, Forcione D, Puli SR. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore). 2017;96:e5154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Pu LZ, Singh R, Loong CK, de Moura EG. Malignant Biliary Obstruction: Evidence for Best Practice. Gastroenterol Res Pract. 2016;2016:3296801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Artifon EL, Okawa L, Takada J, Gupta K, Moura EG, Sakai P. EUS-guided choledochoantrostomy: an alternative for biliary drainage in unresectable pancreatic cancer with duodenal invasion. Gastrointest Endosc. 2011;73:1317-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Artifon EL, Ferreira F, Benevides G, Marcaccio FH, Otoch JP, Takada J, Carnevale FC, Mota AM, Moura E, Rasslan S, de Figueiredo LP, Sakai P. Extrahepatic anterograde covered self-expandable metallic stent placement across malignant biliary obstruction passed by endoscopic ultrasound guidance access: a challenging technique. Acta Gastroenterol Latinoam. 2012;42:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Artifon EL, Frazão MS, Wodak S, Carneiro FO, Takada J, Rabello C, Aparício D, de Moura EG, Sakai P, Otoch JP. Endoscopic ultrasound-guided choledochoduodenostomy and duodenal stenting in patients with unresectable periampullary cancer: one-step procedure by using linear echoendoscope. Scand J Gastroenterol. 2013;48:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Okuno N, Hara K, Mizuno N, Kuwahara T, Iwaya H, Ito A, Kuraoka N, Matsumoto S, Polmanee P, Niwa Y. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: A prospective clinical study. J Gastroenterol Hepatol. 2018;33:1413-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Yamao K, Kitano M, Takenaka M, Minaga K, Sakurai T, Watanabe T, Kayahara T, Yoshikawa T, Yamashita Y, Asada M, Okabe Y, Hanada K, Chiba Y, Kudo M. Outcomes of endoscopic biliary drainage in pancreatic cancer patients with an indwelling gastroduodenal stent: a multicenter cohort study in West Japan. Gastrointest Endosc. 2018;88:66-75.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 29. | Nakai Y, Isayama H, Yamamoto N, Matsubara S, Kogure H, Mizuno S, Hamada T, Takahara N, Uchino R, Akiyama D, Takagi K, Watanabe T, Umefune G, Ishigaki K, Tada M, Koike K. Indications for endoscopic ultrasonography (EUS)-guided biliary intervention: Does EUS always come after failed endoscopic retrograde cholangiopancreatography? Dig Endosc. 2017;29:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Kawakubo K, Kawakami H, Kuwatani M, Kubota Y, Kawahata S, Kubo K, Sakamoto N. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy. 2016;48:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |