Published online Feb 16, 2019. doi: 10.4253/wjge.v11.i2.95
Peer-review started: December 6, 2018
First decision: December 20, 2018
Revised: January 25, 2019
Accepted: February 13, 2019
Article in press: February 13, 2019
Published online: February 16, 2019
Processing time: 74 Days and 7.6 Hours
Malignant biliary strictures are usually linked to different types of tumors, mainly cholangiocarcinoma, pancreatic and hepatocellular carcinomas. Palliative measures are usually adopted in patients with nonresectable or borderline resectable biliary disease. Stent placement is a well-known and established treatment in patients with unresectable malignancy. Intraductal radiofrequency ablation (RFA) represents a procedure that involves the use of a biliary catheter device, via an endoscopic approach. Indications for biliary RFA described in literature are: Palliative treatment of malignant biliary strictures, avoiding stent occlusion, ablating ingrowth of blocked metal stents, prolonging stent patency, ablating residual adenomatous tissue after endoscopic ampullectomy. In this mini-review we addressed focus on technical success defined as deployment of the RF catheter, virtually succeeded in all patients included in the studies. About efficacy, three main outcome measures have been contemplated: Biliary decompression and stent patency, survival. Existing studies suggest a beneficial effect on survival and stent patency with RFA, but current impression is limited because most of studies have been performed using a retrospective design, on diminutive and dissimilar cohorts of patients.
Core tip: Intraductal radiofrequency ablation (RFA) represents a procedure that encompasses the use of a biliary catheter device, via an endoscopic approach, mainly endoscopic retrograde colangiopancreatography. Indications for biliary RFA described in literature are: Palliative treatment of malignant biliary strictures, avoiding stent occlusion, ablating ingrowth of blocked metal stents, prolonging stent patency, ablating residual adenomatous tissue after endoscopic ampullectomy. Existing studies suggest a favorable effect on survival and stent patency. Moreover, up-to-date feeling is that evidence supporting RFA is limited because most of the analyses have been achieved using a retrospective design, on diminutive and dissimilar cohorts of patients.
- Citation: Auriemma F, De Luca L, Bianchetti M, Repici A, Mangiavillano B. Radiofrequency and malignant biliary strictures: An update. World J Gastrointest Endosc 2019; 11(2): 95-102
- URL: https://www.wjgnet.com/1948-5190/full/v11/i2/95.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i2.95
The aim of this mini-review is to assess the utility of radiofrequency ablation (RFA) in malignant biliary obstruction (MBO). Malignant biliary strictures represent a diagnostic and therapeutic open question for biliary endoscopist. MBO is usually linked to different types of tumors, mainly cholangiocarcinoma, as well as pancreatic and hepatocellular carcinomas. Traditionally palliative measures have been adopted in patients with nonresectable or borderline resectable biliary disease. Stent placement is a well-established and widely accepted treatment in patients with unresectable malignancy[1,2], with a lower rate of adverse events such as procedural complications and post-stenting occlusion than surgical decompression[3]. The use of metal stents decreases the need for re-intervention and the occurrence of cholangitis compared to plastic or polyethylene stents[4]. However, stent patency is difficult to preserve due to neoplastic in- and over-growth, epithelial hyperplasia, and sludge deposition[5].
Efforts have been ongoing to develop different palliatives interventions to prolong patency of metallic biliary stents. Some of the interventions which have been studied include photodynamic therapy (PDT), intraductal radiotherapy and RFA[6-8].
RFA is a well-recognized percutaneous approach that has widely been used in the management of hepatocellular carcinoma and metastatic hepatic malignancy, with demonstrated effectiveness[9].
Even within the bile duct, RFA can be performed by specific endo-biliary probes that enable increased precision in the delivery of thermal energy in the biliary tree resulting in decreased epithelial hyperplasia and tumor ingrowth. Several studies have confirmed the safety and feasibility of these procedures for clinical use with promising results reported for the palliative treatment of malignant biliary strictures, preventing stent occlusion, ablating ingrowth of blocked metal stents, prolonging stent patency, ablating residual adenomatous tissue after endoscopic ampullectomy[10].
RFA creates an electrical passage through the body of monopolar probes, between an electrode and a grounding pad placed on the patient. Additionally, it may be generated by two interstitial electrodes with bipolar catheters, by using an alternating current. Resistance heats the surrounding tissues burning up to elevated temperature (50°C-100°C) and causing protein denaturation followed by cell desiccation and coagulative necrosis. The most contiguous areas to the electrode undergo to the highest current and heat shock due to reduced electrical conductivity of tissues. On the other hand, the parts of the tumor most distant are only burnt and necrosis is not determined because thermal conduction is not sufficiently high[11,12].
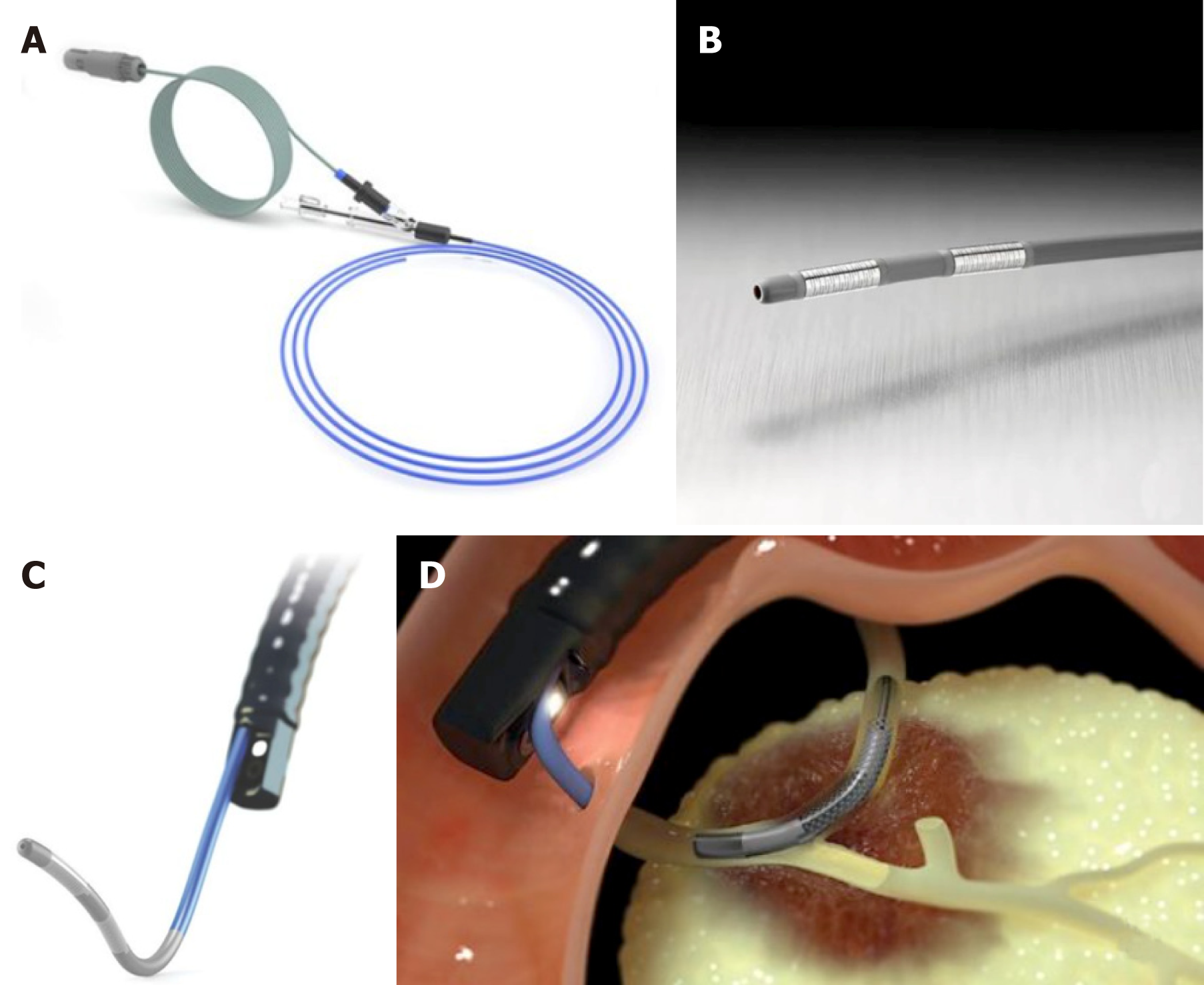
Intraductal RFA represents a procedure that encompasses the use of a biliary catheter device, via an endoscopic approach. For biliary RF, two devices are designed to be used over a guide wire during endoscopic retrograde colangiopancreatography (ERCP): Habib™ EndoHBP and ELRA™.
The RFA catheter Habib EndoHPB (EMcision Ltd, London, UK; Boston Scientific, Marlborough, Massachusetts, USA) is a disposable device properly designed for endoluminal delivery of RFA into the biliary system. It is an 8 Fr RFA probe, with bipolar conduction features. It is well-suited for large working channel of duodenoscopes, mostly over 0.035-inch guidewires. The catheter has two ring electrodes, 8 mm distant from each other. It provides local coagulative necrosis over a 2.5 cm length, in circular and ellipsoidal way (Figure 1). The highest energy accumulation is achieved between the electrodes, placed below and above the target. VIO 200D or 300D generator (Erbe Elktromedizin, Tubingen, Germany) are usually used, delivering high-frequency bipolar current. Generator setting mostly lies on: Power between 7 W-10 W, effect set at 8 for a duration of 30 s-90 s.
ELRA™ (EndoLuminal Radiofrequency Ablation, Taewoong Medical, South Korea) probe has been recently introduced. It allows strict control of temperature at the interface tissue-electrode. This probe has two sizes (18- and 33-mm length), with a diameter of 7 Fr. It contains four bipolar electrodes which provide linear ablation. There is no need for ground pads. The generator is VIVA (Taewoong Medical, South Korea) mostly set to two minutes interval, maximum temperature of 80°C and a power of 10 watts (Figure 2). In animal studies this represents the ideal setting to reduce the charring process, allowing more prolongated current stream and more effective tissue ablation[13,14].
To perform biliary RFA, biliary tract is cannulated as a standard ERCP procedure. Then a cholangiography is performed to distinctly visualize the location of the stricture and to define its extent and width. Though not crucial, a sphincterotomy is generally completed. In addition to this, dilation of the stricture, mostly by mean of a balloon, could be performed before RFA procedure. The probe is then inserted over the guidewire across the stricture.
RF energy is applied for the desired period, according to different RFA probe manufacturer’s indications. Before withdrawn the probe, a break period of about 60 s is necessary to prevent tissues from adhering to the electrodes. Usually multiple RF applications are completed during the same session. Generally it is preferred from the proximal verge of the target to the distal one, with tiny overlap in order to decrease the risk of complications, mainly perforation.
Once the probe has been removed, coagulated tissue debris are swiped by mean of balloon, and a plastic or metal stent is positioned to guarantee biliary drainage[15,16].
Over the last 8 years, more than 350 patients were reported in the literature to have been undergone endoscopic biliary RFA. Indications were mainly malignant strictures and occluded self-expanding metal stents (SEMS).
Nearly in all studies, malignant strictures accounted cholangiocarcinoma or pancreatic cancer, but also other malignant strictures have been considered, such gallbladder cancer, hepatic carcinoma and metastatic cancers as well.
In this mini-review we will focus on retrospective “largest” papers including more than 40 patients (including controls group) and all prospective and randomized controlled trial studies published on topic up to August 2018. Table 1 summarizes the main characteristics of the included studies (study design, population, intervention, RFA probe, outcomes, main findings)[17-25].
| Author (reference), Country, Year | Patients number | Study design | Intervention | Probe | Tumour type | Control group | Outcomes | Main findings |
| Steel et al[17], UK 2011 | 22 | Prospective | ERFA before SEMS | Habib EndoHPB | CC, PC | No | Technical and clinical success; adverse events | (1) 21/22 technical success; 18/21 stent patency at 90 d; and (2) 3 AE (1 pancreatitis, 2 cholecystitis) |
| Figueroa- Barojas et al[18], USA 2013 | 20 | Prospective | ERFA before stenting (metallic or plastic) | Habib EndoHPB | MBO | No | 30 d patency, stricture size; adverse events | (1) Significant increase of 3.5 mm CBD diameter after RFA; and (2) 2 AE (1 pancreatitis, 1 cholecystitis) |
| Dolak et al[19], Austria 2014 | 58 | Retrospective | Miscellaneous (ERFA before stenting, ERFA for blocked SEMS, percutaneous RFA) | Habib EndoHPB | MBO (mainly CC) | No | Patency, adverse events, mortality | (1) Median stent patency 170 d (95%CI 63-277): Metal vs plastic stenting (218 d vs 115 d, P = 0.051); and (2) 12 AE (1 partial liver infarction, 5 Cholangitis, 2 hemobilia, 2 cholangiosepsis, 1 hepatic coma, 1 left bundle branch block) |
| Sharaiha et al[20], USA 2014 | 66 | Retrospective | ERFA before stenting (26pts) vs stenting alone (40 pts) | Habib EndoHPB | CC, PC | Yes | Survival, stricture size; Adverse events | (1) ERFA independent predictor of survival [HR 0.29 (0.11-0.76), P = 0.012]; and (2) No differences in AE (2 RFA vs 3 no-RFA) |
| Strand et al[21], USA 2014 | 48 | Retrospective | ERFA (16 pts) vs PDT (32 pts) | Habib EndoHPB | CC | Yes | Survival; Adverse events | Similar survival; more stent occlusions in RFA group |
| Kallis et al[22], UK 2015 | 69 | Retrospective | ERFA before stenting (23 pts) vs stenting alone (46 pts) | Habib EndoHPB | PC | Yes | Survival, morbidity, and stent patency rates | Median survival in RFA group 226 d vs 123.5 d in controls (P < 0.01); SEMS patency equivalent |
| Sharaiha et al[23], USA 2015 | 69 | Retrospective (multicentric registry) | Miscellaneous (mainly ERFA before stenting) | Habib EndoHPB | MBO (mainly CC) | No | Survival; Adverse events | (1) Median survival 11.46 mo (6.2 mo-25 mo); and (2) AE 10 % (1 pancreatitis 2 cholecystitis, 1 hemobilia, 3 abdominal pain) |
| Laleman et al[24], Belgium 2017 | 18 | Prospective | ERFA before stenting | ELRA | CC, PC | No | Feasibility, safety, and biliary patency rate of a new RFA device | (1) Biliary patency 80% and 69% at 90 d and 180 d respectively; and (2) 6 AE (4 cholangitis, 2 pancreatitis) |
| Yang et al[25], China 2018 | 65 | RCT | ERFA before stenting (32 pts) vs stenting alone (33 pts) | Habib EndoHPB | CC | Yes | Overall survival, biliary patency; post-ERCP AE | (1) OS RFA + stent vs the stent-only (13.2 mo ± 0.6 mo vs 8.3 mo ± 0.5 mo, P < 0.001); Biliary patency RFA + stent longer than stent-only (6.8 mo vs 3.4 mo, P = 0.02); and (2) Similar AE [6.3% (2/32) vs 9.1% (3/33), P = 0.67] |
Technical success defined as deployment of the RF catheter was essentially succeeded in all patients. About efficacy, main outcome measures considered are: Biliary decompression and stent patency, survival. As for stent patency and biliary drainage different outcome measures have been considered: 30- or 90-d patency rate, median time patency. Moreover, in these studies different types of procedures have been grouped in the same series (RFA before stenting, RFA without stenting, RFA in occluded SEMS, combined endoscopic and/or percutaneous RFA), dissimilar stents have been used (metallic or plastic), different stenting replacement strategies have been adopted (on demanding, 3 mo scheduled ERCP). Despite this lack of homogeneity, the results of the included studies are quite similar, with 90-d patency ranging between 80%-86%, up to 69% ad 180-d[17,24]; median patency ranged between 170 d[19] and 200 d[25]. RFA + metallic stent placement outperformed RFA + plastic stent strategy, doubling median patency rate[19]. About survival, all but one study, in which similar results have been observed between RFA and PDT[21], showed very encouraging results in patients performing one or more RFA sessions. Overall survival ranged between 226 and 396 d[22,23,25], and RFA + stent outperformed stenting alone strategy in all study comparing them.
With regard to adverse events (AE), frequency ranged between 6.3% and 33.3%. Most of these concerns the bilio-pancreatic compartment: Acute pancreatitis, cholangitis, cholecystitis, and haemobilia. Only one study report two severe adverse events: One hepatic liver infarction and one hepatic coma[19].
Only two studies have specifically addressed biliary RFA in case of occluded metal stents. Kadayifci et al[26] matched endobiliary RFA to controls in which plastic stents were inserted across the stent. The study group included 25 patients treated with RFA using a Habib™ endoprobe inside the SEMS. The control group involved 25 patients treated only with placement of a plastic stent into an occluded SEMS. Biliary drainage was restored in all patients. Stent patency was evaluated at 90 d, reaching 56% and 24% in the RFA and control groups, respectively. In addition to this, stent patency was significantly longer in the RFA group compared to the control group (119.5 d vs 65.3 d, P = 0.03). 30-d mortality rate and 3- and 6-mo survival rates did not significantly differ between the RFA group and controls (P > 0.05).
The other study, recently published, is a feasibility prospective case series of 7 patients treated with novel temperature-controlled RFA probe ELRA™ (Taewoong, South Korea)[14]. Nine procedures were performed. Seventy percent of patients (5/7) required additional procedures and stent placement to guarantee optimal drainage. There were no procedure-related complications.
Ampullary adenomas are usually treated by endoscopic papillectomy. Nevertheless, ampullary adenomatous residuals spreading into the distal common bile duct or Wirsung represent a tricky condition.
Intraductal adenoma typically has been considered a contraindication to endoscopic management. Surgical treatment represents the gold standard in this condition. Conversely, a pancreaticoduodenectomy or a Whipple procedure are associated with high morbidity and mortality.
Firstly Valente et al[27] published a small series of three patients in which rescue endoscopic RFA for ampullary neoplasms with intraductal extension has been performed. They presented a long follow-up concluding that this approach may represent a safe alternative in patients refusing or not suitable for surgery. It could represent a long-term, palliative strategy in high risk patients.
A retrospective study evaluated the feasibility, safety, and efficacy in 14 patients with adenoma extension into the common bile duct and pancreatic duct. These patients underwent one RFA session (range, 1-5 sessions). At a median follow-up period of sixteen months after RFA, complete intraductal ablation was obtained in about 92% of patients. Adverse events occurred in 43% of cases, mainly represented by ductal strictures and a retro-duodenal abscess[28]. Suarez et al[29] published another small case series of 4 patients showing similar results, with 3 patients succeeding complete ablation of the intraductal adenoma and no adverse events noted during the short follow-up period.
Finally, Camus et al[30] published in 2018 the results of a prospective and open-label multicenter study including 20 patients with pathological confirmed endobiliary adenoma remnant undergoing intraductal RFA. Residual neoplasia was evident in 15% and 30% of patients at 6 mo and 12 mo, respectively, achieving seventy percent possibility of dysplasia eradication at 12 mo after a single session of RFA. At least one adverse event (no one severe) occurred in 40% patients during 12 mo follow-up.
Although small in number, in these studies RFA seems to be a reasonably safe and effective approach for the treatment of residual ampullary adenomas with endobiliary extension.
RFA is an additional treatment recently impemented to the advanced bilio-pancreatic endoscopy. In the field of unresectable neoplasia and MBO, in which treatment options are very restricted, great potential has been addressed to this procedure. Available studies suggest a beneficial effect on survival and stent patency with RFA, but current suggestion is limited because most of studies have been performed using a retrospective design, on diminutive and dissimilar cohorts of patients. As for complication, safety seems to be tolerable, though serious adverse events have been reported. Only few prospective studies and one randomized controlled trial are available and confirm and enhance these two main aspects: Increased survival and reduced rates of adverse events. Further efforts are needed to increase the degree of evidence and to comply with additional therapeutic indications such as occluded SEMS or adenomatous post-ampullectomy residuals.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gao BL, Guo XZ, Liu T, Rodrigo L S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
| 1. | Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82:256-267.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Kahaleh M, Tokar J, Conaway MR, Brock A, Le T, Adams RB, Yeaton P. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc. 2005;61:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc. 2012;76:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 5. | Loew BJ, Howell DA, Sanders MK, Desilets DJ, Kortan PP, May GR, Shah RJ, Chen YK, Parsons WG, Hawes RH, Cotton PB, Slivka AA, Ahmad J, Lehman GA, Sherman S, Neuhaus H, Schumacher BM. Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc. 2009;70:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Smith I, Kahaleh M. Biliary Tumor Ablation with Photodynamic Therapy and Radiofrequency Ablation. Gastrointest Endosc Clin N Am. 2015;25:793-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kahaleh M, Mishra R, Shami VM, Northup PG, Berg CL, Bashlor P, Jones P, Ellen K, Weiss GR, Brenin CM, Kurth BE, Rich TA, Adams RB, Yeaton P. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Deodato F, Clemente G, Mattiucci GC, Macchia G, Costamagna G, Giuliante F, Smaniotto D, Luzi S, Valentini V, Mutignani M, Nuzzo G, Cellini N, Morganti AG. Chemoradiation and brachytherapy in biliary tract carcinoma: long-term results. Int J Radiat Oncol Biol Phys. 2006;64:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Liu M, Huang GL, Xu M, Pan FS, Lu MD, Zheng KG, Kuang M, Xie XY. Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia. 2017;1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Alvarez-Sánchez MV, Napoléon B. Review of endoscopic radiofrequency in biliopancreatic tumours with emphasis on clinical benefits, controversies and safety. World J Gastroenterol. 2016;22:8257-8270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Goldberg SN, Gazelle GS. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48:359-367. [PubMed] |
| 12. | Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 13. | Cho JH, Lee KH, Kim JM, Kim YJ, Lee DH, Jeong S. Safety and Efficacy of a novel endobiliary radiofrequency ablation catheter (ELRA®) in a swine model. Gastrointest Endosc. 2015;81:AB350. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Nayar MK, Oppong KW, Bekkali NLH, Leeds JS. Novel temperature-controlled RFA probe for treatment of blocked metal biliary stents in patients with pancreaticobiliary cancers: initial experience. Endosc Int Open. 2018;6:E513-E517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Rustagi T, Jamidar PA. Intraductal radiofrequency ablation for management of malignant biliary obstruction. Dig Dis Sci. 2014;59:2635-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Mensah ET, Martin J, Topazian M. Radiofrequency ablation for biliary malignancies. Curr Opin Gastroenterol. 2016;32:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C, Wolkersdörfer G, Madl C, Berr F, Trauner M, Püspök A; Austrian Biliary RFA Study Group. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci. 2014;59:3099-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Kallis Y, Phillips N, Steel A, Kaltsidis H, Vlavianos P, Habib N, Westaby D. Analysis of Endoscopic Radiofrequency Ablation of Biliary Malignant Strictures in Pancreatic Cancer Suggests Potential Survival Benefit. Dig Dis Sci. 2015;60:3449-3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Sharaiha RZ, Sethi A, Weaver KR, Gonda TA, Shah RJ, Fukami N, Kedia P, Kumta NA, Clavo CM, Saunders MD, Cerecedo-Rodriguez J, Barojas PF, Widmer JL, Gaidhane M, Brugge WR, Kahaleh M. Impact of Radiofrequency Ablation on Malignant Biliary Strictures: Results of a Collaborative Registry. Dig Dis Sci. 2015;60:2164-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Laleman W, van der Merwe S, Verbeke L, Vanbeckevoort D, Aerts R, Prenen H, Van Cutsem E, Verslype C. A new intraductal radiofrequency ablation device for inoperable biliopancreatic tumors complicated by obstructive jaundice: the IGNITE-1 study. Endoscopy. 2017;49:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Kadayifci A, Atar M, Forcione DG, Casey BW, Kelsey PB, Brugge WR. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy. 2016;48:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Valente R, Urban O, Del Chiaro M, Capurso G, Blomberg J, Löhr JM, Arnelo U. ERCP-directed radiofrequency ablation of ampullary adenomas: a knife-sparing alternative in patients unfit for surgery. Endoscopy. 2015;47 Suppl 1 UCTN:E515-E516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Rustagi T, Irani S, Reddy DN, Abu Dayyeh BK, Baron TH, Gostout CJ, Levy MJ, Martin J, Petersen BT, Ross A, Topazian MD. Radiofrequency ablation for intraductal extension of ampullary neoplasms. Gastrointest Endosc. 2017;86:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Suarez AL, Coté GA, Elmunzer BJ. Adjunctive radiofrequency ablation for the endoscopic treatment of ampullary lesions with intraductal extension (with video). Endosc Int Open. 2016;4:E748-E751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Camus M, Napoléon B, Vienne A, Le Rhun M, Leblanc S, Barret M, Chaussade S, Robin F, Kaddour N, Prat F. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: a multicenter prospective study. Gastrointest Endosc. 2018;88:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |