Published online Nov 16, 2019. doi: 10.4253/wjge.v11.i11.523
Peer-review started: May 20, 2019
First decision: August 2, 2019
Revised: August 14, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 16, 2019
Processing time: 179 Days and 1.7 Hours
Hepatic cirrhosis is associated with greater adverse event rates following surgical procedures and is thought to have a higher risk of complications with interventional procedures in general. However, these same patients often require interventional gastrointestinal procedures such as endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS). While studies examining this scenario exist, the overall body of evidence for adverse event rates associated with ERCP/EUS procedures is more limited. We sought add to the literature by examining the incidence of adverse events after ERCP/EUS procedures in our safety-net hospital population with the hypothesis that severity of cirrhosis correlates with higher adverse event rates.
To examine whether increasing severity of cirrhosis is associated with greater incidence of adverse events after interventional ERCP/EUS procedures.
We performed a retrospective study of patients diagnosed with hepatic cirrhosis who underwent ERCP and/or EUS-guided fine needle aspirations/fine needle biopsies from January 1, 2016 to March 14, 2019 at our safety net hospital. We recorded Child-Pugh and Model for End-stage Liver Disease (MELD-Na) scores at time of procedure, interventions completed, and 30-day post-procedural adverse events. Statistical analyses were done to assess whether Child-Pugh class and MELD-Na score were associated with greater adverse event rates and whether advanced techniques (single-operator cholangioscopy, electrohydraulic lithotripsy/laser lithotripsy, or needle-knife techniques) were associated with higher complication rates.
77 procedures performed on 36 patients were included. The study population consisted primarily of middle-aged Hispanic males. 30-d procedure-related adverse events included gastrointestinal bleeding (7.8%), infection (6.5%), and bile leak (2%). The effect of Child-Pugh class C vs class A and B significantly predicted adverse events (β = 0.55, P < 0.01). MELD-Na scores also significantly predicted adverse events (β = 0.037, P < 0.01). Presence of advanced techniques was not associated with higher adverse events (P > 0.05). When MELD-Na scores were added as predictors with the effect of Child-Pugh class C, logistic regression showed MELD-Na scores were a significant predictor of adverse events (P < 0.01). The findings held after controlling for age, gender, ethnicity and repeat cases.
Increasing cirrhosis severity predicted adverse events while the presence of advanced techniques did not. MELD-Na score may be more useful in predicting adverse events than Child-Pugh class.
Core tip: We performed a retrospective review of all patients with hepatic cirrhosis who underwent interventional endoscopic retrograde cholangiopancreatography/ endoscopic ultrasound procedures over a 3 years span at our safety-net hospital and evaluated outcomes within a 30-d period. 77 cases were included. Both Model for End-stage Liver Disease (MELD-Na) score and Child-Pugh class C predicted adverse events (P < 0.01). When MELD-Na scores were added as predictors with the effect of Child-Pugh class C, only MELD-Na scores were a significant predictor of adverse events (P < 0.01). Our data demonstrates a correlation between cirrhosis severity and adverse events and suggests that MELD-Na score may be useful in assessing procedural risk.
- Citation: Yoo T, Epistola R, Epistola J, Ku L, Fleischman MW, Reicher S, Eysselein VE, Hou LA. Evaluating the risk of adverse events with interventional endoscopic retrograde cholangiopancreatography and endoscopic ultrasound procedures in cirrhotic patients. World J Gastrointest Endosc 2019; 11(11): 523-530
- URL: https://www.wjgnet.com/1948-5190/full/v11/i11/523.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i11.523
Liver cirrhosis is a major public health issue around the world, including in the United States where it affects over half a million people[1]. These individuals are just as likely, if not more so, as the general population to develop biliary or pancreatic tract diseases, many of which require interventional endoscopic retrograde cholangiopancreatography (ERCP) and/or endoscopic ultrasound (EUS) procedures[2,3]. The impaired hepatic function of cirrhotic patients cause systemic derangements which have been shown to increase the risk of morbidity and mortality in surgical or invasive procedures[4]. The presence of cirrhosis has been thought to increase the risk of post-procedural complications, including pancreatitis, bleeding, infection, and perforation[5-8]. Data examining the risks associated with cirrhotic patients undergoing ERCP/EUS procedures remains limited, though the available data suggests cirrhotic patients have a higher adverse event rate than the general population and that increasing severity of cirrhosis correlates with higher adverse event rates[5-8]. Given the relative dearth of information on the topic, we performed a retrospective review of cirrhotic patients who underwent interventional ERCP and/or EUS procedures at our safety net hospital to determine whether increasing severity of cirrhosis was associated with greater incidence of adverse outcomes with these procedures.
We performed a retrospective review of all patients diagnosed with hepatic cirrhosis who underwent inpatient or outpatient ERCP and/or interventional EUS [defined as EUS-guided liver biopsies or fine needle aspirations (FNA)/fine needle biopsies (FNB) of pancreatic and/or biliary masses/cysts] procedures from January 1, 2016 to March 14, 2019, at our tertiary referral safety net hospital in Southern California. EUS procedures that did not have an interventional component as detailed above were excluded. We recorded the age, sex, and ethnicity of the patients, etiology for cirrhosis, Child-Pugh and Model for End-stage Liver Disease (MELD-Na) scores at the time of procedure, indications for procedure, any interventions completed during the procedure, presence of advanced techniques (defined as single-operator cholangioscopy, laser or electrohydraulic/ electrohydraulic lithotripsy, or needle-knife techniques), and 30-day post-procedural adverse events (defined as evidence of infection, significant gastrointestinal (GI) bleeding, perforation, post-ERCP pancreatitis, and death). Statistical analysis was done to determine whether higher Child-Pugh scores and MELD-Na scores were associated with increased risk of adverse events (after controlling for repeat cases) and whether the presence of advanced techniques was associated with increased risk of adverse events.
All statistical analyses were performed using the RStudio software (Version 1.0.153). Chi-squared tests for independence were used to examine associations between categorical test variables (e.g., Child-Pugh scores, advanced procedures) and adverse events. Point-biserial correlation was used to examine the association between MELD-Na scores and adverse events. Multiple variable logistic regression was used in our prediction analyses to assess the associations between Child-Pugh class, advanced techniques, MELD-Na and adverse events, while controlling for age, gender, and repeat patients. Adverse events and advanced techniques were dummy coded in this analysis. Child-Pugh classes were effects coded using difference regression coding since they are ordinal categorical variables. A P value of less than 0.05 was considered statistically significant.
77 procedures (63 ERCP, 14 EUS-guided FNA/FNB/liver biopsies) performed on 36 patients were included. Of note, each procedure was treated as a unique case for the purposes of our study given that each procedure was an isolated event with differences in lab values and clinical status even among the same patient. The mean age of the study population was 58.7 years old (ranging from 21-83 years old) with males forming the majority of the group (58.4%). Individuals of Hispanic descent made up 56% of the cases. All 3 Child-Pugh classes were represented (31 Procedure with Child’s A, 34 procedures with Child’s B, 11 procedures with Child’s C, and 1 procedure with unreported Child-Pugh class). The mean MELD-Na score was 14 (range of 6-26). Cirrhosis was documented as being secondary to alcoholism (36.4%), viral hepatitis (24.7%), nonalcoholic steatohepatitis (18.2%), pyogenic cholangitis (16.9%), cryptogenic/unknown (14.3%), malignancy (9.1%), autoimmune hepatitis (3.9%), and primary biliary cirrhosis/primary sclerosing cholangitis (2%). A summary of patient demographics can be seen in Table 1.
| n = 77 | |
| Average age (yr) | 58.7 |
| Sex, n (%) | |
| Male | 45 (58.4) |
| Female | 32 (41.6) |
| Race, n (%) | |
| Hispanic | 56 (72.7) |
| Asian | 8 (10.4) |
| Caucasian | 4 (5.2) |
| African-American | 5 (6.5) |
| Not stated | 4 (5.2) |
| Average MELD-Na | 14 |
| Childs-Pugh Class, n (%) | |
| A | 31 (40.3) |
| B | 34 (44.2) |
| C | 11 (14.3) |
| Unreported | 1 (1.3) |
| Etiology for cirrhosis, n (%) | |
| Pyogenic cholangitis | 13 (16.9) |
| NASH | 14 (18.2) |
| Malignancy | 7 (9.1) |
| Alcoholism | 28 (36.4) |
| Hepatitis B virus | 10 (13) |
| Hepatitis C virus | 9 (11.7) |
| PBC/PSC | 2 (2.6) |
| Autoimmune hepatitis | 3 (3.9) |
| Cryptogenic/unknown | 11 (14.3) |
Documented indications for ERCP procedures included choledocholithiasis (n = 38, 49.4%), biliary stricture (n = 12, 15.6%), biliary leak (n = 11, 14.3%), cholelithiasis (n = 8, 10.4%), biliary obstruction secondary to mass (n = 8, 10.4%), cholangitis (n = 4, 5.2%), gallstone pancreatitis (n = 3, 3.9%), biliary obstruction due to stent-related issues (n = 1, 1.3%), and evaluation of common bile duct dilatation without choledocholithiasis or mass (n = 1, 1.3%). Indications for interventional EUS procedures included pancreatic or biliary mass biopsy (n = 12, 15.6%) and liver biopsy (n = 3, 3.9%). Interventions included stent placement/removal (n = 53, 68.8%), stone removal (n = 38, 49.3%), sphincterotomy (n = 22, 28.5%), FNA/FNB (n = 10, 13%), and liver biopsy (n = 3, 3.9%). Advanced techniques employed included single-operator cholangioscopy (n = 24, 31.2%), electrohydraulic lithotripsy (n = 13, 16.9%), needle-knife techniques (n = 6, 7.8%), and laser lithotripsy (n = 5, 6.5%). Of note, most cases were associated with multiple indications and interventions.
Thirty-day procedure-related adverse events included GI bleeding (n = 6, 7.8%), infection (n = 5, 6.5%), and bile leak (n = 1, 1.3%). No cases of post-ERCP pancreatitis were noted. 5 cases were associated with death within 30 d of the procedure date; however, none of the deaths were determined to be secondary to the procedure itself, with 4 of the deaths attributable to progression of previously known cancer and 1 case being associated with septic shock secondary to intraabdominal infection in the setting of cholangiocarcinoma. A summary of the procedures can be seen in Table 2.
| n = 77 | |
| Procedure type, n (%) | |
| EUS-guided FNA/FNB/liver biopsy | 14 (16.9) |
| ERCP | 63 (74) |
| Indication for procedure, n (%) | |
| Biliary stricture | 12 (15.6) |
| Biliary leak | 11 (14.3) |
| Choledocholithiasis | 38 (49.4) |
| Cholelithiasis | 8 (10.4) |
| Cholangitis | 4 (5.2) |
| Gallstone pancreatitis | 3 (3.9) |
| Obstruction secondary to mass | 8 (10.4) |
| Obstruction secondary to stent issue | 1 (1.3) |
| CBD dilatation w/o known mass | 1 (1.3) |
| Biopsy of mass | 12 (15.6) |
| Liver biopsy | 3 (3.9) |
| Interventions employed, n (%) | |
| Stone removal | 38 (49.3) |
| Stent placement/removal | 53 (68.8) |
| Sphincterotomy | 22 (28.5) |
| FNA/FNB | 10 (13) |
| Liver biopsy | 3 (3.9) |
| Spyglass cholangioscopy | 24 (31.2) |
| EHL | 13 (16.9) |
| Laser lithotripsy | 5 (6.5) |
| Needle-knife papillotomy | 6 (7.8) |
| Complications, n (%) | |
| Infection | 5 (6.5) |
| Gastrointestinal bleeding | 6 (7.8) |
| Bile leak | 1 (1.3) |
| Pancreatitis | 0 (0) |
Chi-square test of independence analyses indicated that Child-Pugh class was significantly associated with adverse events [χ2 (3, n = 76) 14.45, P < 0.01]. Adverse events were more likely to occur for Child C class patients than for Child A or B class patients. Presence of advanced techniques were not associated with adverse events [χ2 (2, n = 77) 0.02, P > 0.05]. MELD-Na scores were moderately positively correlated with adverse events occurring [r (74) = 0.49, P < 0.01].
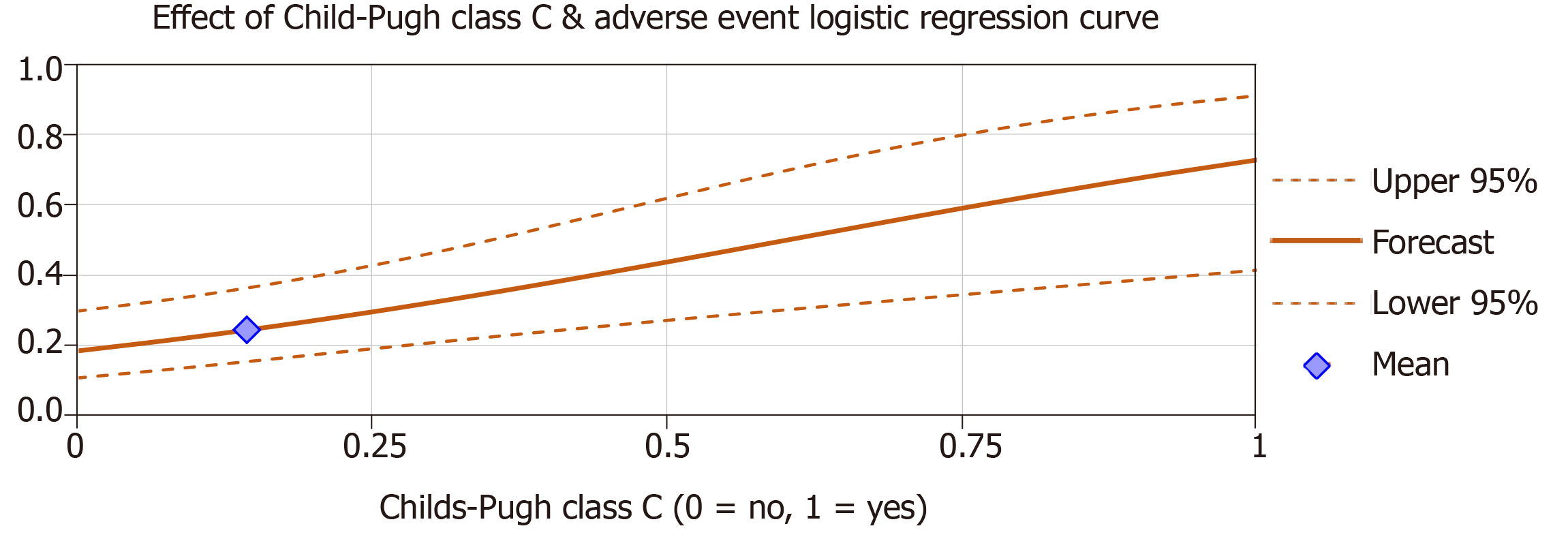
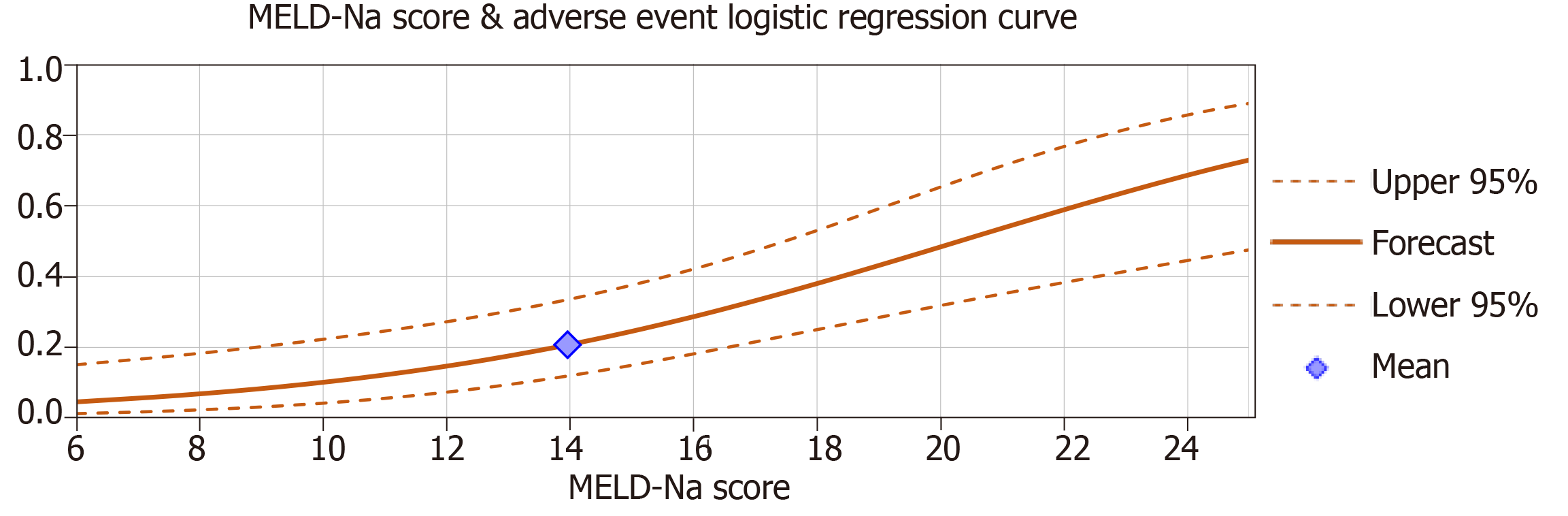
Multiple logistic regression analyses were used to examine if Child-Pugh class and MELD-Na scores significantly predicted adverse events. When analyzed in separate regression models, the effect of Child-Pugh class C vs class A and B significantly predicted the occurrence of adverse events (β = 0.55, P < 0.01) after controlling for age, gender and repeat cases (Figure 1). MELD-Na scores also significantly predicted adverse events (β = 0.037, P < 0.01) after controlling variables (Figure 2). The effect of Child-Pugh class B vs class A was not a significant predictor of adverse events (P > 0.05). Age, gender and repeat cases were also insignificant predictors in all regression models (P > 0.05).
When MELD-Na scores were added as predictors with the effect of Child-Pugh class C, logistic regression results indicated that only MELD-Na scores were a significant predictor of adverse events (β = 0.027, P < 0.01) after controlling for age, gender and repeat cases. The effect of Child-Pugh class C vs class A and B was insignificant (P > 0.05). MELD-Na scores and the effect of Child-Pugh class C were moderately positively correlated r (74) = 0.58, P < 0.01.
Our study examined all cirrhotic patients who underwent ERCP/interventional EUS procedures at our safety net hospital to evaluate the relationship between the severity of cirrhosis and rate of adverse events following these procedures. Analysis of our data noted a significantly increased risk of bleeding, infection, and bile leaks with both increasing MELD-Na score and higher Child-Pugh class even after controlling for age, gender and repeat cases. Interestingly, when Child-Pugh class and MELD-Na scores were combined in a logistical regression analysis, Child-Pugh class no longer became a significant predictor of adverse events while MELD-Na remained so. However, when performing the combined regression analysis separately with Child-Pugh class, the effect of Child-Pugh class C approached significance (P = 0.056). As such, the analyses suggest that MELD-Na score may be a more precise predictor of adverse outcomes with these procedures as it explains a unique portion of variance not attributable to Child-Pugh class. This finding adds to a growing body of research suggesting that only Child-Pugh class C is truly predictive of adverse events[5,8,9]. The implication that MELD-Na may be a more useful predictive tool is particularly notable as our review of the literature found that only a few studies evaluated MELD-Na score when examining outcomes of interventional GI procedures in patients with cirrhosis[6].
Our study also demonstrated a relatively high rate of post-procedural bleeding and infection, which occurred in 7.8% and 6.5% of cases respectively (all within ERCP procedures); these rates are notably higher than those seen among the general population (2% and 1.4%, respectively)[10]. Interestingly, our population did not experience any post-ERCP pancreatitis. These findings support those seen in previously published literature comparing ERCP outcomes between cirrhotics and non-cirrhotics and adds to the admittedly small data pool reporting safe outcomes with EUS-guided biopsies in cirrhotic individuals[5-8,11,12]. However, it is important to note that our study did not find an association between the use of advanced techniques and adverse events, suggesting that refinement of endoscopic techniques has improved safety of these procedures even in cirrhotic individuals.
The findings we present do have some limitations. Our study utilized a moderate sample size (n = 77 procedures), which restricts the interpretation of the Chi-square and logistic regression analyses. Additionally, many of our included patients underwent multiple procedures; 41 of our included procedures were repeat cases, with each unique patient undergoing an average of 2.14 procedures. The repeated cases further limited our interpretation of the statistical analyses. Finally, our study was performed at a single center and consisted primarily of middle aged Hispanic men from a lower socioeconomic class, affecting the generalizability of our results. However, our findings still held significance after correcting for age, gender, and repeat cases.
In conclusion, the findings presented above highlight the correlation between greater severity of cirrhosis and increasing risk of adverse events when performing interventional ERCP/EUS procedures. Our results support established literature yet offer intriguing implications regarding the use of MELD-Na score for assessing procedure risk and provides more data regarding safety of EUS-guided biopsies in cirrhotic patients. Further research is warranted in both these areas as the field continues to evolve.
Liver cirrhosis is a major health issue around the world and is associated with physiologic changes that have been shown to increase the risk of adverse events in surgical and other interventional procedures. Prior studies examining endoscopic retrograde cholangiopancreatography (ERCP) specifically have demonstrated increased risk of adverse outcomes with worsening cirrhosis severity within these individuals. We sought to evaluate the risk of adverse outcomes against cirrhosis severity with ERCP and interventional endoscopic ultrasound (EUS) procedures within our safety net hospital population as data on this population is lacking.
The study is designed to evaluate the risk of adverse events when performing ERCP and/or interventional EUS procedures on patients with liver cirrhosis. Knowing the adverse event risk against cirrhosis severity will help clinicians assess the risks/benefits of gastrointestinal (GI) procedures in cirrhotic individuals through the use of Child-Pugh Class or Model for End-stage Liver Disease (MELD-Na) score.
Our objective was to examine whether increasing severity of cirrhosis is associated with greater incidence of adverse events after interventional ERCP/EUS procedures in cirrhotic individuals within our safety net population.
We performed a retrospective study of patients diagnosed with hepatic cirrhosis who underwent ERCP and/or EUS-guided fine needle aspirations/fine needle biopsies over a 3 years span at our safety net hospital. Statistical analyses were done to assess whether Child-Pugh class and MELD-Na score were associated with greater adverse event rates and whether advanced techniques (single-operator cholangioscopy, electrohydraulic/laser lithotripsy, or needle-knife techniques) were associated with higher complication rates.
Our study included 77 procedures with the study population consisting primarily of middle-aged Hispanic men. 30-d procedure-related adverse events included GI bleeding (7.8%), infection (6.5%), and bile leak (2%). The effect of Child-Pugh class C vs class A and B significantly predicted adverse events (P < 0.01). MELD-Na scores also significantly predicted adverse events (P < 0.01). The presence of advanced techniques was not associated with higher adverse events (P > 0.05). When MELD-Na scores were added as predictors with the effect of Child-Pugh class C, logistic regression showed MELD-Na scores were a significant predictor of adverse events (P < 0.01). The findings held after controlling for age, gender, ethnicity and repeat cases. Our findings demonstrated that Child-Pugh class C and increasing MELD-Na scores were significant predictors of adverse events.
Increasing cirrhosis severity (as defined by MELD-Na score and Child-Pugh Class C) predicted adverse events while the presence of advanced techniques did not. The findings were largely in line with our initial hypothesis and the conclusions drawn by prior studies. Our research suggests that advanced techniques should not be withheld in cirrhotic individuals as they did not significantly contribute to increased adverse event rates. Additionally, our data implies that MELD-Na score may be more useful in predicting adverse events than Child-Pugh class.
The study confirms the results of prior studies and replicates those findings within a safety-net population, a population that has not been studied comprehensively in regards to this subject. Future research can consider comparing the usefulness of MELD-Na score vs Child-Pugh Class in assessing risk with ERCP/EUS procedures in patients with cirrhosis or consider replicating this study with a larger-scale safety net population.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lai JP S-Editor: Yan JP L-Editor: A E-Editor: Liu MY
| 1. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 504] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 2. | ASGE Standards of Practice Committee. Chathadi KV, Chandrasekhara V, Acosta RD, Decker GA, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fanelli RD, Fisher DA, Foley K, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The role of ERCP in benign diseases of the biliary tract. Gastrointest Endosc. 2015;81:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | ASGE Standards of Practice Committee. Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf RN, Shergill AK, Cash BD. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Adler DG, Haseeb A, Francis G, Kistler CA, Kaplan J, Ghumman SS, Laique SN, Munigala S, Taylor LJ, Cox K, Root B, Hayat U, Siddiqui A. Efficacy and safety of therapeutic ERCP in patients with cirrhosis: a large multicenter study. Gastrointest Endosc. 2016;83:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Leal C, Prado V, Colan J, Chavez-Rivera K, Sendino O, Blasi A, Roura P, Juanola A, Rodriguez de Miguel C, Pavesi M, Gomez C, Guarner C, Guarner-Argente C, Fernández J, Cardenas A. Adverse Events and Acute Chronic Liver Failure in Patients With Cirrhosis Undergoing Endoscopic Retrograde Cholangiopancreatography: A Multicenter Matched-Cohort Study. Am J Gastroenterol. 2019;114:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Navaneethan U, Njei B, Zhu X, Kommaraju K, Parsi MA, Varadarajulu S. Safety of ERCP in patients with liver cirrhosis: a national database study. Endosc Int Open. 2017;5:E303-E314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Li DM, Zhao J, Zhao Q, Qin H, Wang B, Li RX, Zhang M, Hu JF, Yang M. Safety and efficacy of endoscopic retrograde cholangiopancreatography for common bile duct stones in liver cirrhotic patients. J Huazhong Univ Sci Technolog Med Sci. 2014;34:612-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kim JY, Lee HS, Chung MJ, Park JY, Park SW, Song SY, Bang S. Bleeding Complications and Clinical Safety of Endoscopic Retrograde Cholangiopancreatography in Patients with Liver Cirrhosis. Yonsei Med J. 2019;60:440-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol. 2016;30:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Inamdar S, Berzin TM, Berkowitz J, Sejpal DV, Sawhney MS, Chutanni R, Pleskow DK, Trindade AJ. Decompensated cirrhosis may be a risk factor for adverse events in endoscopic retrograde cholangiopancreatography. Liver Int. 2016;36:1457-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Choudhary N, Bansal RK, Puri R, Singh RR, Nasa M, Shah V, Sarin H, Guleria M, Saigal S, Saraf N, Sud R, Soin AS. Impact and safety of endoscopic ultrasound guided fine needle aspiration on patients with cirrhosis and pyrexia of unknown origin in India. Endosc Int Open. 2016;4:E953-E956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |