Published online Jan 16, 2019. doi: 10.4253/wjge.v11.i1.61
Peer-review started: October 30, 2018
First decision: November 29, 2018
Revised: December 5, 2018
Accepted: December 13, 2018
Article in press: December 13, 2018
Published online: January 16, 2019
Processing time: 80 Days and 19 Hours
Self-expandable metal stents (SEMSs) are frequently used in the setting of palliation for occluding, inoperable colorectal cancer (CRC). Among possible complications of SEMS positioning, re-obstruction is the most frequent. Its management is controversial, potentially involving secondary stent-in-stent placement, which has been poorly investigated. Moreover, the issue of secondary stent-in-stent re-obstruction and of more-than-two colonic stenting has never been assessed. We describe a case of tertiary SEMS-in-SEMS placement, and also discuss our practice based on available literature.
A 66-year-old male with occluding and metastatic CRC was initially treated by positioning of a SEMS, which had to be revised 6 mo later when a symptomatic intra-stent tumor ingrowth was treated by a SEMS-in-SEMS. We hereby describe an additional episode of intestinal occlusion due to recurrence of intra-stent tumor ingrowth. This patient, despite several negative prognostic factors (splenic flexure location of the tumor, carcinomatosis with ascites, subsequent chemotherapy that included bevacizumab and two previously positioned stents (1 SEMS and 1 SEMS-in-SEMS)) underwent successful management through the placement of a tertiary SEMS-in-SEMS, with immediate clinical benefit and no procedure-related adverse events after 150 d of post-procedural follow-up. This endoscopic management has permitted 27 mo of partial control of a metastatic disease without the need for chemotherapy discontinuation and, ultimately, a good quality of life until death.
Tertiary SEMS-in-SEMS is technically feasible, and appears to be a safe and effective option in the case of recurrent SEMS obstruction.
Core tip: Endoscopic positioning of self-expandable metal stents (SEMSs) has an established role in the palliation of obstructing metastatic colorectal cancers (CRCs). More controversial is the management of re-obstruction due to intrastent tumor ingrowth. In our case, a patient with obstructing, metastatic, carcinomatous CRC, primary palliated with SEMS placement, experienced two different episodes of intrastent tumor in-growth. This occurred along with a long-lasting history of partial efficacy of chemotherapy, including bevacizumab. Both these episodes were successfully treated through subsequent stent-in-stent placement, with immediate symptom relief, no procedure-related complications (notwithstanding different negative prognostic factors), no need for chemotherapy discontinuation and, ultimately, a good quality of life.
- Citation: Vanella G, Coluccio C, Di Giulio E, Assisi D, Lapenta R. Tertiary stent-in-stent for obstructing colorectal cancer: A case report and literature review. World J Gastrointest Endosc 2019; 11(1): 61-67
- URL: https://www.wjgnet.com/1948-5190/full/v11/i1/61.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i1.61
Colorectal cancer (CRC) is one of the most commonly encountered neoplasms, especially in Western countries, with an increasing incidence over the last years[1,2]. More than a quarter of CRC patients are diagnosed with stage IV disease, and about ten percent of CRC patients present with large bowel occlusion[3-5], involving a management regimen (endoscopic vs surgical) that is still debated[3,6-8]. The application of self-expandable metal stents (SEMSs) for CRC obstruction has been increasingly used over time in the setting of palliation of inoperable cases as an alternative to emergency surgery[9]. Surgical procedures are burdened by a high mortality rate in this setting[4,10,11], are not oncologically indicated in advanced disease, and require a time interval before undertaking chemotherapy[12]. This represents the only possibility of disease control for these patients. On the contrary, endoscopic procedures, despite being less invasive[13] and requiring shorter hospitalization times[11,14-16], suffer from a suboptimal technical success rate (particularly for tortuous colorectal flexures[17]) and from the possibility of these complications[6,10,17-22]: colonic perforation (10%), stent migration (9%) and re-obstruction (18%)[10]. The issue of complications is even more noteworthy when patients are candidates for chemotherapy with antiangiogenic agents (e.g., bevacizumab), a described risk factor for colonic perforation in the presence of a SEMS[23,24]. While some complications need to be treated by surgery, re-obstruction can be treated by stent-in-stent deployment[25]. Nevertheless, technical success and clinical efficacy are scarcely reported, yet are still lower than primary stenting[3,25]. Moreover, the issue of the stent-in-stent patency and the possible management of an additional intra-stent neoplastic ingrowth has never been assessed.
We describe the case of a man with occluding splenic flexure CRC that was treated by SEMS positioning due to metastatic, carcinomatous disease, with chemotherapy starting immediately after the first stent positioning. However, in two different occasions (6 mo and 22 mo after primary stenting), the patients experienced symptoms of a radiologically confirmed bowel occlusion due to endoscopically-diagnosed intra-stent tumor ingrowth. Nevertheless, systemic disease was substantially under control with chemotherapy. Despite the presence of negative prognostic factors (splenic-flexure location, carcinomatosis, bevacizumab subsequently added to chemotherapy regimen, previously positioned SEMS and SEMS-in-SEMS), both of the two episodes were successfully treated with positioning of additional stent-in-stents, which allowed for substantial chemotherapeutic continuity and lasting partial disease control of carcinomatous disease.
A 66-year-old man was diagnosed in March 2016 with occluding colonic cancer, immediately proximal to and partially involving the splenic flexure. Due to the presence of liver metastasis and peritoneal carcinomatosis with ascites, a 22 mm/6 cm through-the-scope (TTS) SEMS (WallflexTM, Boston Scientific) was placed through the stenosis, and chemotherapy with fluorouracil/folinic acid/irinotecan was immediately started. Six months later, an occluding intra-stent tumor ingrowth (with responsive systemic disease) was treated by the placement of a colonic stent-in-stent (22 mm/9 cm TTS SEMS; WallflexTM, Boston Scientific), and chemotherapy resumed 2 d after.
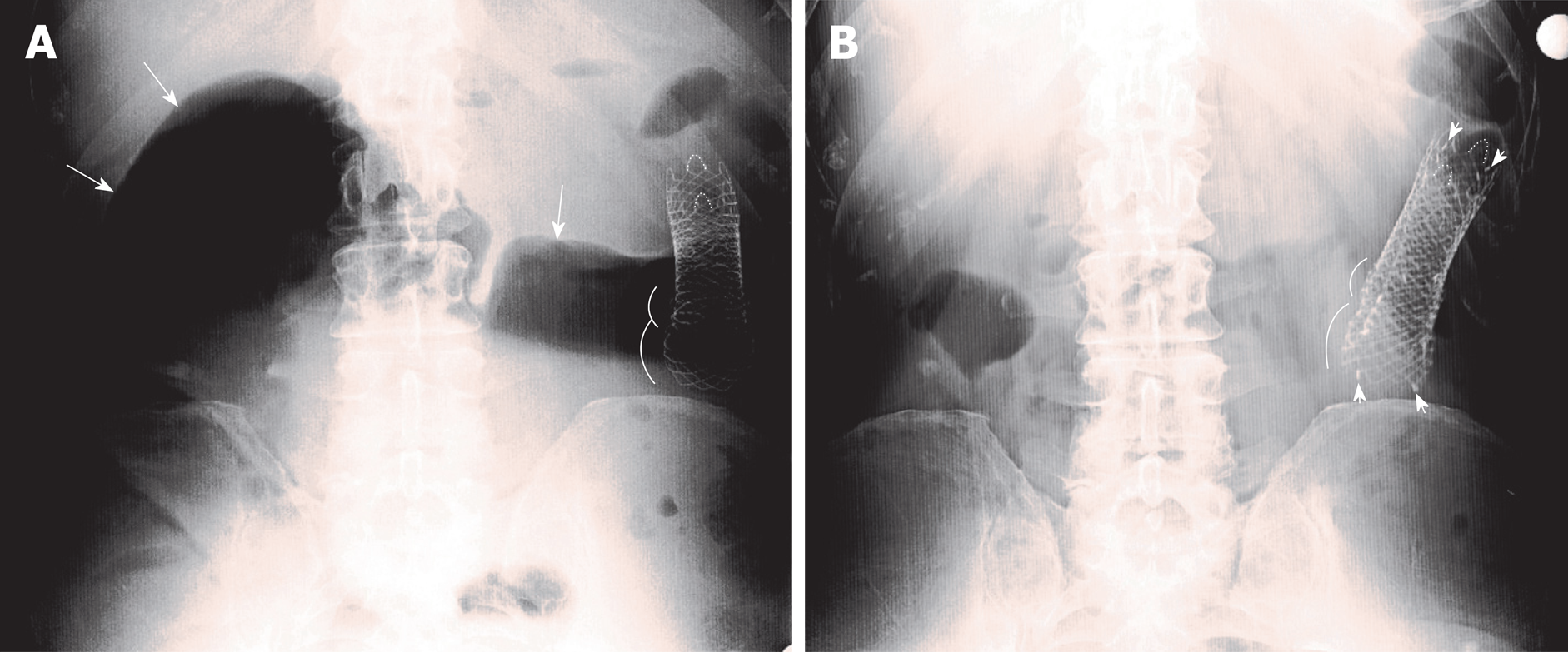
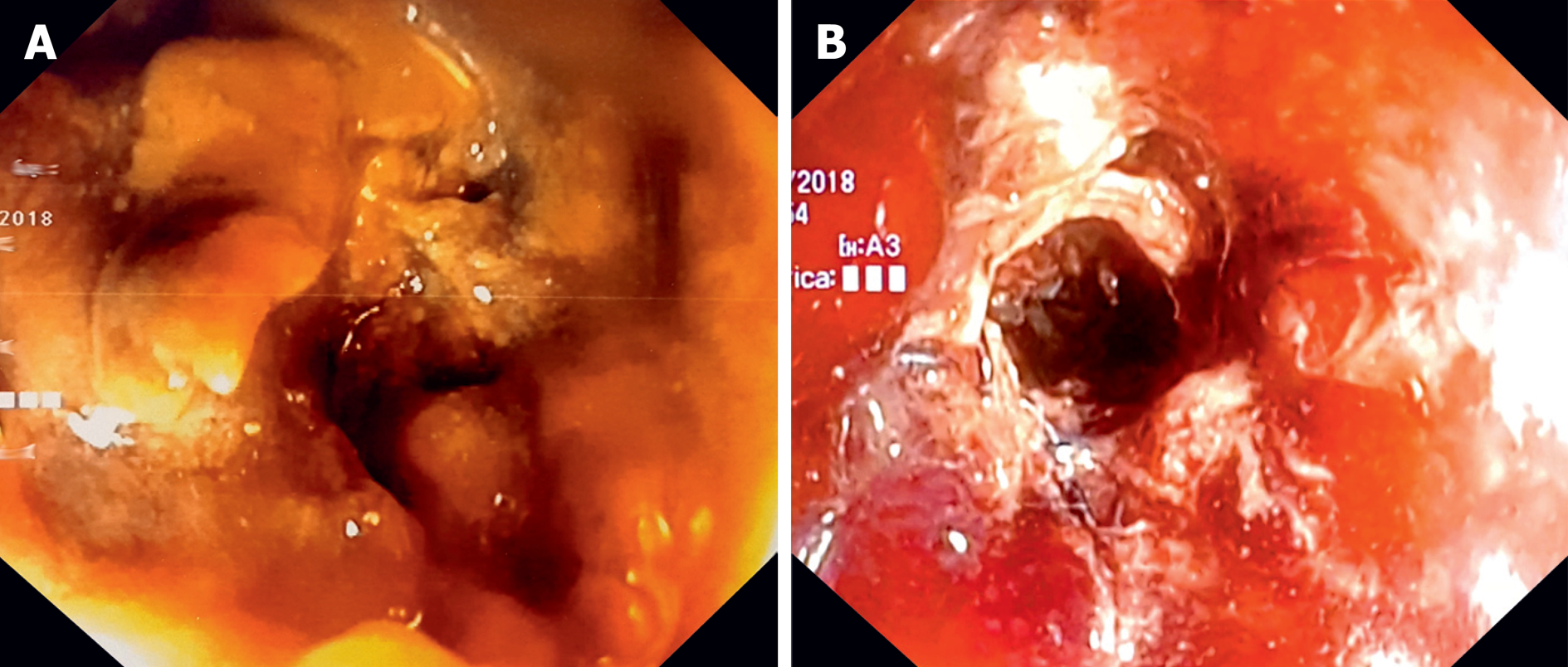
After more than 1 year of substantial disease stability (although bevacizumab was added to the chemotherapy regimen 18 mo after diagnosis), the patient experienced symptoms of intestinal occlusion in January 2018 (+16 mo from the second stent). Physical examination revealed abdominal distension, hyper-tympanism on percussion, and tinkling bowel sounds. An abdominal X-ray showed ileocolic dilation proximal to the correctly located previous stents (Figure 1A), and colonoscopy showed new intrastent tumor ingrowths (Figure 2A).
The conclusive diagnosis was bowel occlusion due to intrastent tumor ingrowth in a patient with previously positioned multiple SEMS for the palliation of an obstructing, metastatic, carcinomatous CRC with partial disease control under chemotherapy, including bevacizumab.
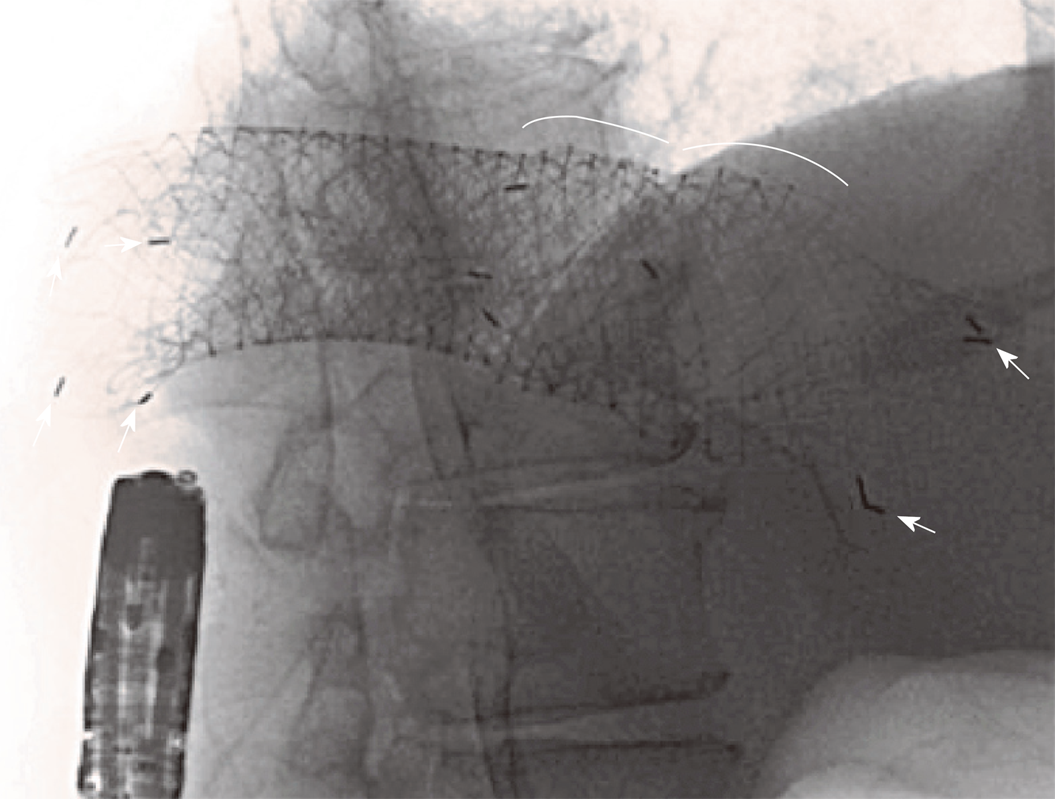
Considering the presence of carcinosis and ascites, as well as the patient’s willingness to avoid a stoma, an additional TTS 22 mm/8 cm SEMS (Hanarostent®, M.I.Tech) was successfully positioned within the two previous stents (Figure 3) using fluoroscopic guidance and deep sedation. This procedure was performed after multidisciplinary discussion and informed consent.
Despite that the stent opening appeared endoscopically not brilliant (Figure 2B), the patient experienced immediate relief of occlusive symptoms, the following X-ray showed no residual bowel dilation (Figure 1B) and chemotherapy was resumed 8 d after.
No SEMS-related adverse events occurred until June 2018 (+150 d from the last procedure and +27 mo from diagnosis), when the patient died due to systemic complications related to disease progression.
This 66-year-old patient with metastatic, carcinomatous, occluding CRC was successfully treated with multiple endoscopic procedures (1 SEMS and 2 SEMS-in-SEMS placements), without procedure-related complications and with clinical benefit, good quality of life and partial systemic disease control for more than 2 years.
Some aspects made us consider this case of interest for endoscopy, gastroenterology and oncology practitioners. Firstly, to our knowledge, the possibility of positioning a third stent-in-stent for recurrent intrastent tumor ingrowth has never been considered and described. Moreover, some presumptive technical difficulties and negative prognostic factors[26] did not interfere with the success and efficacy of the endoscopic palliation. For example, a significantly lower technical success of SEMS placement[27] and patency after SEMS placement[25] has been demonstrated in patients with carcinomatosis (83% vs 93% for technical success[27] and 118 d vs 361 d for SEMS patency[25]). Carcinomatosis and the proximal location of the obstruction were found to be independent predictors of technical failure[27]. Moreover, concomitant chemotherapy with bevacizumab has been preliminary associated with increased perforation risk when compared to either chemotherapy without bevacizumab or no chemotherapy[23,24,28-30]. However, in our case, neither the presence of peritoneal carcinomatosis with ascites, the splenic flexure location, nor subsequent chemotherapy that included bevacizumab affected the technical success or lasting clinical benefits of the procedures. In fact, the patient has remained asymptomatic, on a varied fiber-free diet and with an acceptable quality of life until death, which was due to disease progression.
In one study focusing on secondary stent-in-stent placement[25], the rate of technical success was not reported (patients with attempted but failed secondary SEMS placement were excluded). In this study, overall clinical success after secondary positioning was achieved in 75% of patients (which is lower than reported in the setting of primary stenting), and long-term clinical failure was reported in 52% of patients with initially successful secondary positioning. In addition, the presence of carcinomatosis was associated with reduced long-term clinical success. However, in our case, the presence of the two previously positioned SEMS did not hamper the successful positioning and clinical efficacy of the third stent-in-stent.
The same authors also compared, for the first time, the clinical outcomes of an endoscopic re-stenting vs palliative surgery after a first stent failure in 115 patients, which was due to malignant re-obstruction. They demonstrated that among patients undergoing secondary SEMS placement, the overall mortality rate and median duration of hospital stay were significantly lower than in the surgery group, where the median lumen patency was higher (7.9 mo vs 3.4 mo for the stent patency). Notably, no significant differences were registered in overall and progression-free survival between the two groups[31]. In this scenario, we report one case with an extraordinary stent patency of 480 d after secondary stenting and of 150 d after tertiary stenting.
All these data highlight the need for a cautious evaluation of the solution best suited for one specific patient with stent re-obstruction.
In this case, despite the presence of negative prognostic factors and technical difficulties, tertiary stenting was technically successful. The patient experienced immediate relief of symptoms, has not encountered any SEMS-related adverse events, and has maintained a good quality of life during the 150 d following the last stent positioning, without the need for chemotherapy discontinuation. Despite the fact that the evidence on multiple stenting is controversial, and that data on tertiary stent-in-stenting are lacking, this report suggests that the positioning of a third stent-in-stent in patients with recurrent intra-stent tumor in-growth should be considered and might be a reasonable and effective option. Further research is needed to confirm the safety and reproducibility of this approach compared with surgical options, not only in terms of technical feasibility, but also regarding quality of life and long-term outcomes.
SEMS positioning is an established treatment for the palliation of occluding unresectable CRCs. Even in the presence of negative prognostic factors, the feasibility of endoscopic palliation may be discussed in multidisciplinary tumor boards in facilities with high endoscopic expertise and prompt surgical back-up.
SEMS re-obstruction is the most frequent complication of the endoscopic palliation of occluding CRCs, and SEMS-in-SEMS placement has proven to be a valid option in this setting.
Even after secondary failure of SEMS-in-SEMS due to recurrent tumor ingrowth, a tertiary SEMS-in-SEMS placement is technically feasible and might be an option in referral centers.
The authors would like to thank the nursing staff of the Endoscopy Unit and the medical staff of Oncological Division of Regina Elena Cancer Institute, which has collaborated in retrieving clinical information during the procedure and during patient follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Iliescu EL, Sipos F S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Song H
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13166] [Article Influence: 1880.9] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55825] [Article Influence: 7975.0] [Reference Citation Analysis (132)] |
| 3. | van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A; European Society of Gastrointestinal Endoscopy (ESGE). Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc. 2014;80:747-761.e1-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Jullumstrø E, Wibe A, Lydersen S, Edna TH. Colon cancer incidence, presentation, treatment and outcomes over 25 years. Colorectal Dis. 2011;13:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Cheynel N, Cortet M, Lepage C, Benoit L, Faivre J, Bouvier AM. Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis Colon Rectum. 2007;50:1568-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg. 2014;207:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, Miyahima NT, Coronel Cordero MA, Visconti TAC, Ide E, Sakai P, de Moura EGH. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558-E567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Fugazza A, Galtieri PA, Repici A. Using stents in the management of malignant bowel obstruction: the current situation and future progress. Expert Rev Gastroenterol Hepatol. 2017;11:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Sagar J. Role of colonic stents in the management of colorectal cancers. World J Gastrointest Endosc. 2016;8:198-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Zhao XD, Cai BB, Cao RS, Shi RH. Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol. 2013;19:5565-5574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Vemulapalli R, Lara LF, Sreenarasimhaiah J, Harford WV, Siddiqui AA. A comparison of palliative stenting or emergent surgery for obstructing incurable colon cancer. Dig Dis Sci. 2010;55:1732-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Súarez J, Jiménez J, Vera R, Tarifa A, Balén E, Arrazubi V, Vila J, Lera JM. Stent or surgery for incurable obstructive colorectal cancer: an individualized decision. Int J Colorectal Dis. 2010;25:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Law WL, Choi HK, Chu KW. Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg. 2003;90:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Fiori E, Lamazza A, De Cesare A, Bononi M, Volpino P, Schillaci A, Cavallaro A, Cangemi V. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:265-268. [PubMed] |
| 15. | Carne PW, Frye JN, Robertson GM, Frizelle FA. Stents or open operation for palliation of colorectal cancer: a retrospective, cohort study of perioperative outcome and long-term survival. Dis Colon Rectum. 2004;47:1455-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Tomiki Y, Watanabe T, Ishibiki Y, Tanaka M, Suda S, Yamamoto T, Sakamoto K, Kamano T. Comparison of stent placement and colostomy as palliative treatment for inoperable malignant colorectal obstruction. Surg Endosc. 2004;18:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Liang TW, Sun Y, Wei YC, Yang DX. Palliative treatment of malignant colorectal obstruction caused by advanced malignancy: a self-expanding metallic stent or surgery? A system review and meta-analysis. Surg Today. 2014;44:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 19. | van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA; Dutch Colorectal Stent Group. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, Gontikakis M, Kontis M, Paraskevas I, Vassilobpoulos P, Paraskevas E. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost-effectiveness analysis. Surg Endosc. 2004;18:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Fernández-Esparrach G, Bordas JM, Giráldez MD, Ginès A, Pellisé M, Sendino O, Martínez-Pallí G, Castells A, Llach J. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Sousa M, Pinho R, Proença L, Silva J, Ponte A, Rodrigues J, Carvalho J. Predictors of Complications and Mortality in Patients with Self-Expanding Metallic Stents for the Palliation of Malignant Colonic Obstruction. GE Port J Gastroenterol. 2017;24:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Imbulgoda A, MacLean A, Heine J, Drolet S, Vickers MM. Colonic perforation with intraluminal stents and bevacizumab in advanced colorectal cancer: retrospective case series and literature review. Can J Surg. 2015;58:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | van Halsema EE, van Hooft JE, Small AJ, Baron TH, García-Cano J, Cheon JH, Lee MS, Kwon SH, Mucci-Hennekinne S, Fockens P, Dijkgraaf MG, Repici A. Perforation in colorectal stenting: a meta-analysis and a search for risk factors. Gastrointest Endosc. 2014;79:970-982.e7; quiz 983.e2, 983.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Yoon JY, Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Outcomes of secondary stent-in-stent self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2011;74:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Kuwai T, Yamaguchi T, Imagawa H, Yoshida S, Isayama H, Matsuzawa T, Yamada T, Saito S, Shimada M, Hirata N, Sasaki T, Koizumi K, Maetani I, Saida Y. Factors related to difficult self-expandable metallic stent placement for malignant colonic obstruction: A post-hoc analysis of a multicenter study across Japan. Dig Endosc. 2019;31:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Yoon JY, Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Clinical outcomes and risk factors for technical and clinical failures of self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2011;74:858-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Manes G, de Bellis M, Fuccio L, Repici A, Masci E, Ardizzone S, Mangiavillano B, Carlino A, Rossi GB, Occhipinti P, Cennamo V. Endoscopic palliation in patients with incurable malignant colorectal obstruction by means of self-expanding metal stent: analysis of results and predictors of outcomes in a large multicenter series. Arch Surg. 2011;146:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Cennamo V, Fuccio L, Mutri V, Minardi ME, Eusebi LH, Ceroni L, Laterza L, Ansaloni L, Pinna AD, Salfi N, Martoni AA, Bazzoli F. Does stent placement for advanced colon cancer increase the risk of perforation during bevacizumab-based therapy? Clin Gastroenterol Hepatol. 2009;7:1174-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Outcomes of secondary self-expandable metal stents versus surgery after delayed initial palliative stent failure in malignant colorectal obstruction. Digestion. 2013;88:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |