Published online Sep 16, 2018. doi: 10.4253/wjge.v10.i9.156
Peer-review started: March 23, 2018
First decision: April 26, 2018
Revised: June 17, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: September 16, 2018
Processing time: 178 Days and 20.6 Hours
The diagnostic and treatment guidelines of superficial non-ampullary duodenal tumors have not been standardized due to their low prevalence. Previous reports suggested that a superficial adenocarcinoma (SAC) should be treated via local resection because of its low risk of lymph node metastasis, whereas a high-grade adenoma (HGA) should be resected because of its high risk of progression to adenocarcinoma. Therefore, pretreatment diagnosis of SAC or HGA is important to determine the appropriate treatment strategy. There are certain endoscopic features known to be associated with SAC or HGA, and current practice prioritizes the endoscopic and biopsy diagnosis of these conditions. Surgical treatment of these duodenal lesions is often related to high risk of morbidity, and therefore endoscopic resection has become increasingly common in recent years. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are the commonly performed endoscopic resection methods. EMR is preferred due to its lower risk of adverse events; however, it has a higher risk of recurrence than ESD. Recently, a new and safer endoscopic procedure that reduces adverse events from EMR or ESD has been reported.
Core tip: Although superficial non-ampullary duodenal tumors are rare, they can progress to cancer and metastasize, and therefore early diagnosis and treatment of these duodenal tumors is essential. Pretreatment diagnosis for high-grade adenoma or superficial adenocarcinoma helps to determine the appropriate treatment strategy. Endoscopic resection has been adopted as an effective and minimally invasive treatment for these duodenal lesions; however, even though endoscopic mucosal resection has a lower risk of adverse events, it has a higher risk of recurrence than endoscopic submucosal dissection. Recently, a new and safer endoscopic procedure that reduces adverse events of endoscopic resection has been reported.
- Citation: Esaki M, Suzuki S, Ikehara H, Kusano C, Gotoda T. Endoscopic diagnosis and treatment of superficial non-ampullary duodenal tumors. World J Gastrointest Endosc 2018; 10(9): 156-164
- URL: https://www.wjgnet.com/1948-5190/full/v10/i9/156.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i9.156
The incidence of duodenal polyps has been reported as 1.0%-4.6% in patients undergoing an upper endoscopy[1-4]. Non-ampullary duodenal cancer is extremely rare, accounting for only 0.5% of all malignancies in the gastrointestinal tract[5]. Superficial non-ampullary duodenal tumors (SNADETs) are defined as lesions that are limited to the mucosa or submucosa, including adenoma and adenocarcinoma. According to a European study, the prevalence of duodenal villous adenoma was 0.1%-0.4% in patients undergoing a diagnostic or screening endoscopy[1,6]. Both duodenal adenomas in familial adenomatous polyposis (FAP) and sporadic non-ampullary adenomas have the potential to progress to carcinomas based on the adenoma-carcinoma sequence theory, similar to colonic adenomas[7-13]. In addition, superficial non-ampullary carcinomas occur de novo. Detection and treatment of SNADETs at an early stage is essential for good prognosis because of the poor prognosis of advanced duodenal carcinomas[14,15]. Conventionally, these lesions were removed surgically, but this procedure was associated with a high rate of morbidity and mortality[16-18]. Recently, endoscopic resections (ER) have been conducted for neoplasms in other organs including the esophagus, stomach, and colon, and ER appears to be an ideal treatment alternative to surgical resection for patients with SNADETs. However, ER for SNADETs is related to a high rate of adverse events, including delayed bleeding and perforation[19,20]. Standard diagnosis and treatment have not been established due to the low prevalence of SNADETs; therefore, this study provided the current evidence for diagnosis and treatment of sporadic SNADETs.
FAP is known to be associated with the incidence of SNADETs[7,21]. Several other factors are believed to be associated with sporadic SNADETs, including smoking, colorectal neoplasm, and Helicobacter pylori (H. pylori) infection. Smoking was identified as a risk factor for SNADETs or small bowel adenocarcinoma (SBA)[22-24], with reported odds ratios of 2.7-4.6. Colorectal neoplasm was reported as a risk factor of SBA and sporadic duodenal adenoma[22,25-28]; the reported odds ratio for sporadic duodenal carcinoma among patients with a history of colorectal cancer was 3.74. In addition, H. pylori infection was identified as a risk factor for SNADETs[22]. A previous study reported that superficial non-ampullary duodenal epithelial carcinoma in patients infected with H. pylori was significantly located on the oral side of the major papilla compared to that in patients who were not infected with H. pylori[29]. Although gastric cancer and atrophic gastritis mainly result from H. pylori infection, the relationship between SNADETs and gastric cancer or atrophic gastritis remains controversial[22,25,29].
SNADETs mainly exist in the descending part of the duodenum[30-32], with 90% of treated SNADETs located from the first to second portion of the duodenum[31-35]. Of note, tumor location is not associated with final histological grade[31].
The gross type of SNADETs were classified according to the Paris endoscopic classification[36]. The gross morphology is based on endoscopic findings and divided into protruded pedunculated (Ip), protruded sessile (Is), semipedunculated (Isp), superficial elevated (IIa), or superficial shallow or depressed types (IIc). The elevated type was the most frequent gross type of SNADETs[20,31]. If two or more components were detected, the lesion was diagnosed as a mixed pattern, such as IIa + IIc or IIa + Is.
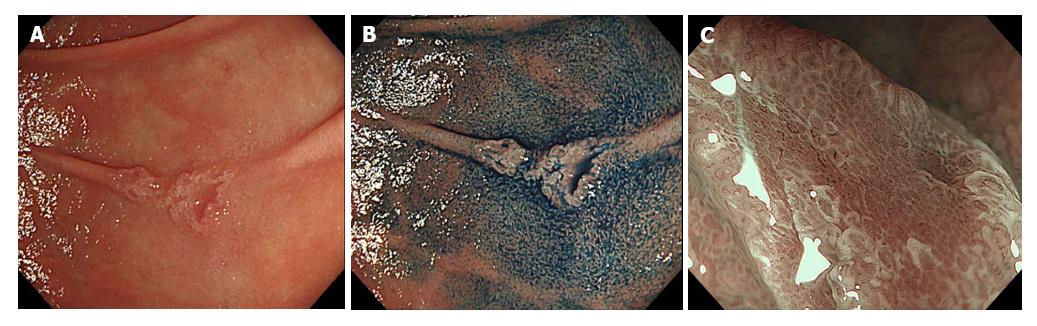
Endoscopic diagnoses were made by qualified endoscopists at the time of routine endoscopy, magnifying endoscopy (ME), and chromoendoscopy with indigo carmine (Figure 1). At present, there are no standard criteria for the endoscopic diagnosis of SNADETs and current practice includes obtaining biopsy specimens after endoscopic diagnoses. C4.1 or HGA lesions diagnosed by biopsy were reported to have the potential to progress to malignant lesions[9,10,37], especially for lesions ≥ 20 mm in size[13,38]. Another study stated that C4.1 tumors diagnosed by biopsy using the Vienna classification with nodular or rough surfaces with a red color were more likely to progress to adenocarcinoma during the follow-up period[13]. Malignant potential is quite different between C3 and C4.1 Vienna classified tumors and between LGA and HGA lesions diagnosed by biopsy. C3 or LGA lesion diagnosed by biopsy showed a low risk of progression to adenocarcinoma[13,39], for which follow-up without ER may be acceptable due to a high risk of adverse events.
The associations between endoscopic diagnoses and final pathological diagnoses of resected specimens were reported in duodenal lesions. With regard to lesion size, mean tumor diameter of high-grade adenoma (HGA) lesions or superficial adenocarcinoma (SAC) lesions was significantly larger than that of low-grade adenoma (LGA) lesions[20]. The rate of lesions > 5 mm in diameter in HGA or SAC lesions was significantly higher than that in LGA lesions. Further, all submucosal adenocarcinoma lesions are ≥ 10 mm in diameter[20]. According to pathological diagnosis based on the Vienna classification, category 4 (C4) tumors had significantly larger diameters than category 3 (C3) tumors[40]. With regard to the color of lesions, a solitary or predominantly red color was significantly more frequent in HGA or SAC lesions than those in LGA lesions[20]. A solely red colored lesion was reported as an indicator of carcinoma[31]. Furthermore, all submucosal cancers were reported to be red[20]. With regard to the macroscopic characteristics of SNADETs, depression and mixed-type morphology are reported to be associated with carcinoma[31,41]. Furthermore, submucosal cancers exhibited 0 -I or 0 - IIa + IIc types[20]. The features of these lesions on ME with narrow band imaging (NBI) was also reported, and have been described as consisting of a microsurface pattern and microvascular pattern, which assists endoscopic diagnosis[40,42,43]. In addition, Kikuchi et al[44] proposed a diagnostic algorithm using ME with NBI for SNADETs. However, the importance of pretreatment biopsy diagnoses of SNADETs remains controversial. Discordance between pretreatment biopsy diagnoses and final pathological diagnoses was reported in duodenal lesions[13,19,43,45,46], as well as gastric epithelial lesions[47]. Some patients with biopsy diagnoses of HGA before resection were reported to have their diagnoses upgraded from HGA to adenocarcinoma after resection[39]. Pretreatment biopsy diagnoses had greater specificity and similar accuracy, but lower sensitivity compared with pretreatment endoscopic diagnoses[20,31]. Furthermore, Kakushima et al[31] reported that pretreatment diagnoses of carcinomas via endoscopy or biopsy were limited to 88% (57/65) of carcinoma lesions. All lesions of carcinomas cannot be diagnosed before treatment, even if biopsy was conducted. Unintended fibrosis may be induced by the biopsy because the duodenal wall is thin, which may make ER more difficult[41,48,49]. Recently, Kakushima et al[50] suggested a useful scoring system to determine C3 and C4 lesions. This system was based on lesion diameter, color, macroscopic type, and nodularity that were easily observed via endoscopy. A lesion that scored ≥ 3 points was judged as C4 or higher. The scoring system’s diagnostic accuracy rate was 86%, and the scores of C4 or higher lesions were significantly higher than those of C3 lesions (P < 0.001). This system helps clinicians decide upon a suitable treatment strategy for SNADETs without biopsy diagnosis.
Some studies categorized SNADETs as LGA, HGA, or SAC based on histological diagnosis[20,39]. On the other hand, the revised Vienna classification was also used as the diagnostic classification for SNADETs in other reports[13,40,42,44]. These two classifications were inconsistent, and there remains difficulty in creating a unified classification.
Conventionally, surgical removal was conducted for SNADETs; however, high rates of morbidity and mortality were reported. ER was recently recommended as an alternative treatment for SNADETs. Cancer without lymph node metastasis may be indicated for ER. Previous case series have suggested that intramucosal carcinoma has no lymph node metastasis, whereas submucosal carcinoma carries a risk of lymph node metastasis of up to 25%[39,51]. Therefore, indications for ER should be limited to clinically confirmed intramucosal carcinomas, including HGA.
ER was applied to SNADETs as an alternative and less invasive treatment to conventional surgical resection. However, ER for SNADETs remains a challenging treatment because it is a technically difficult procedure with a high adverse events risk. The posterior wall of the duodenum sticks to the retroperitoneum at the superior and lower duodenal angles. It is often difficult to maintain an appropriate visual field during endoscopy while using the endo-knife because the duodenum is located deep within the abdomen and has a narrow and bent lumen. Double-balloon enteroscopy was reported as useful for maneuverability[52]. In addition, achieving mucosal lift via local injection is difficult because of numerous folds and Brunner glands. Therefore, although endoscopic muscosal resection (EMR) and endoscopic submucosal dissection (ESD) are mainly performed ER techniques for SNADETs, endoscopic techniques to resect superficial SNADETs have not yet been standardized.
EMR is a procedure that uses a snare and was the preferred technique used in majority of previous studies. This procedure was developed to resect sessile or flat lesions limited to the superficial layers. EMR was conventionally used not only for en bloc resection, but also for piecemeal resection. A meta-analysis verified the safety and effectiveness of EMR for non-ampullary duodenal polyps, including 90% of adenomas[53]. The mean size of specimens resected via EMR was 13-35 mm. The rate of complete ER without remnant part was 93% [95% confidence interval (CI): 89%-97%]; however, the en bloc resection rate was only 45% and the piecemeal resection rate was 55%, with 29% of cases requiring adjuvant argon plasma coagulation after EMR. Furthermore, 10% of cases required multiple procedures to achieve initial complete ER. EMR adverse events included delayed bleeding [5% (95%CI: 2%-7%)] and perforation [1% (95%CI: 1-3%)]. Endoscopic management could be achieved for all intraprocedural perforations, while surgery was required for delayed perforations. The recurrence rate after EMR was 15% over a 6-72 mo follow-up period. The rate of successful endoscopic removal of recurrent lesions was 62% (95%CI: 37%-87%). Surgical intervention was required in only 2.4% (95%CI: 0.6%-4.0%) of cases. There was no procedure-related mortality. Additionally, the safety and usefulness of EMR, as well as the favorable long-term prognosis of this technique, were previously reported[19,33,37,54-57]. However, other reports suggested that lesions > 2 cm in diameter tend to require a piecemeal resection via EMR, leading to higher recurrence[33,49,54,58-60]. Increasing tumor circumference in the duodenal lumen was reported as the strongest negative predictor of successful endoscopic treatment including EMR for SNADETs[61].
Recently, underwater EMR (UEMR) was invented as a new technique of EMR. This technique fills the duodenal lumen with physiological saline without a submucosal injection and is based on a similar principle as conventional EMR, which lifts the mucosa and submucosa away from the deeper muscularis propria layer to achieve successful ER. The effectiveness of UEMR for the treatment of small SNADETs within 20 mm was reported[62]. Closure with endoclip was achieved for all lesions due to small mucosal defects after UEMR, and this may have accounted to the lack of any associated adverse events (i.e., delayed perforation and bleeding).
Additionally, cold polypectomy, including cold snare polypectomy (CSP) and cold forceps polypectomy (which were originally used for colorectal neoplasms), was adopted as a method for small SNADETs. One study compared resection width and depth of polyps treated with CSP and HSP, and found that although the resection depth after CSP was more superficial compared to that after HSP, resection depth was adequate following both techniques, and suggested that CSP may have a superior safety profile to HSP for colorectal subcentimeter polyps[63]. In another study, the effectiveness of cold polypectomy in treating both sporadic and multiple SNADETs was reported without adverse events[64].
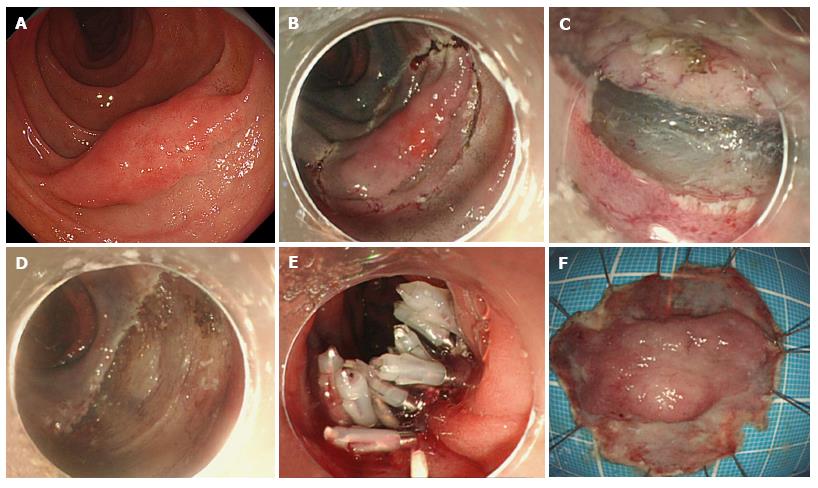
ESD was invented for en bloc resection of gastrointestinal lesions where it is frequently used for the treatment of gastric, colonic and esophageal lesions, but rarely used for duodenal lesions. This may be partly explained by the fact that ESD requires a high skill level and a qualified operator with thorough knowledge of duodenal anatomy, which is characterized by an abundance of blood vessels in the submucosal layer and a thin muscle layer[65]. However, in qualified hands, ESD has been reported to achieve complete resection (en bloc resection without positive margin) in 80%-100% of SNADETs[34,43,45,49,66,67] (Figure 2). The size of tumors that are treated via ESD is larger than that via EMR. The rate of en complete resection using ESD is higher than that using EMR, which contributes to accurate histopathological assessment of vertical and horizontal surgical margins, and results in a lower risk of local recurrence[49,58,68-70].
ESD has been reported to be associated with a higher rate of perforation than EMR, even among tumors of the same size[48,68,71,72]. Electrocauterization, which is a major risk factor for delayed perforation after endoscopic treatment, is more frequently required during ESD than during EMR[73]. The rates of intraoperative and delayed perforations were reported as 6.3%-50.0% in ESD cases and 0%-14.3% in EMR cases[34,58,66,67,74-76]. Moreover, emergency surgery has been performed in 3.3%-25.0% of patients who underwent duodenal ESD as a result of uncontrollable intraoperative or delayed perforations[46,49,66,67,77]. Perforations occurred in the anal portion of the ampulla of Vater because exposure of the duodenal wall to pancreatic juice and bile enzymes caused proteolysis or chemical irritation[34]. Therefore, ESD should be performed in clinically appropriate patients with SNADETs in order to avoid such serious adverse events. Moreover, clinicians should take into account the fact that the length of hospital stay is longer in patients that underwent ESD compared to EMR due to the higher incidence of adverse events in the former[58]. Therefore, lesions that are resectable using EMR should not be resected using ESD. ESD is recommended for lesions > 20 mm or those suggestive of carcinoma, which can likely be resected en bloc. Where duodenal ESD was indicated, cases tended to be performed under general anesthesia, and this was especially true for larger lesions, in order to ensure safety and to facilitate transition to surgery in the case of an adverse event[58,68]. Recently, the pocket-creation method using an ST hood was proposed as a safe and quick alternative for duodenal ESD[78], which facilitates access into the submucosal layer via the ST hood. The evidence summarized above demonstrates that ESD is still a challenging procedure due to its high adverse events rate; and therefore, regardless of the procedure used, an appropriate closure technique after ESD is required.
Closure of the mucosal defect after ER has been suggested as a countermeasure for duodenal perforation, which may reduce the risk of hazardous adverse events, as well as ESD in the colon[66,79]. Simple prophylactic closure using an endoclip after duodenal ER was reported to reduce the risk of delayed bleeding[80]. However, complete closure could not be achieved with a conventional clip, especially for a large ulcer after ESD, because the size of a conventional clip is too small. Furthermore, the grasp strength of a conventional clip is insufficient to maintain closure. In fact, some clips drop off, resulting in reports of delayed perforation[81]. The combination of endoclip and Endoloop using a double-channel endoscope was reported for closure of large mucosal defects after ER[82-84]. Recently, closure via the string clip suturing method was developed, which can be completed with a single-channel endoscope[85]. Furthermore, the over-the-scope clip (OTSC) (Ovesco Endoscopy AG, Tubingen, Germany), polyglycolic acid (PGA) sheets (Neoveil; Gunze Co., Kyoto, Japan) with fibrin glue (Beriplast P Combi-Set; CSL Behring Pharma, Tokyo, Japan), and laparoscopic-endoscopic cooperative surgery (LECS) were recently reported as possible measures of closure for large mucosal defects after duodenal ESD.
OTSC was invented as a device for closure of mucosal defects in acute gastrointestinal perforation and anatomic leaks, in addition to being a hemostatic device for bleeding lesions[86,87], and has been used for closure of gastrointestinal tract defects after ESD in the duodenum. Mori et al[88] reported the clinical outcome of prophylactic closure after ESD using OTSC, and found no occurrences of delayed bleeding and delayed perforation. If the mucosal defect after ER is > 20 mm, prophylactic closure with the OTSC is recommended for safe and reliable closure, in spite of its higher medical costs compared to other available closure devices.
The combination of PGA and fibrin glue has been generally used and proven safe in various surgeries[89-92]. These materials were applied to endoscopic treatment of the esophagus, stomach, and colon[93-95], and were found to reduce the risk of post-ESD bleeding[93]. Similarly, some case reports showed the efficacy of shielding over ulcers after ER in the duodenum[81,96,97]. These materials are naturally absorbable, but remain on the lesion for approximately 1 wk, which is when delayed bleeding and perforation are likely to occur[81]. This procedure may be particularly useful in lesions that are difficult to close endoscopically or through surgery because of their anatomical location.
LECS was developed as a treatment procedure for gastrointestinal tumors[98]. This procedure is also applied to duodenal tumors to reduce the risk of adverse events. SNADETs are mainly treated with laparoscopic reinforcement after ESD, which is called duodenal LECS (D-LECS)[99]. In this procedure, the mucosal defect is closed appropriately and tightly after laparoscopic suturing of the duodenal wall from the serosal side. No severe postoperative adverse events were reported. Laparoscopic surgery can also assist the ESD procedure by repositioning the duodenum. Furthermore, if perforation occurred during the ESD procedure, the perforation can be closed endoscopically and laparoscopically, which is easier than endoscopy alone. D-LECS was completed in a closed manner with no risk of tumor dissemination. However, ESD and LECS are most expensive than EMR, and LECS is not covered by the national insurance system. Although ESD and LECS may be more cost-effective in the long-term because of their associated low recurrence rates, we have to take into consideration the high cost of ESD and LECS.
Although SNADETs are rare, they have a potential of progression to cancer or further metastasis. Therefore, the development of diagnosis and treatment procedures at an early stage is important. However, these developments may have occurred slower than those in other gastrointestinal organs and are not yet standardized. Hence, developing unified criteria and algorithms for diagnosis and treatment of SNADETs is an important clinical priority.
EMR may be the current first-line treatment for SNADETs to prevent malignant progression. For smaller lesions, UEMR or cold polypectomy may be safer. Although ESD has a higher risk of adverse events, a higher en bloc resection rate can be achieved, which is suitable for larger lesions or lesions that are highly suspicious of carcinoma. Closure techniques and the shielding method for mucosal defect after ESD were reported as useful methods for preventing ESD adverse events. These new methods may overcome adverse events in ESD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Imaeda H, Watari J S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Moss WM, McCart PM, Juler G, Miller DR. Primary adenocarcinoma of the duodenum. Arch Surg. 1974;108:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Höchter W, Weingart J, Seib HJ, Ottenjann R. [Duodenal polyps. Incidence, histologic substrate and significance]. Dtsch Med Wochenschr. 1984;109:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Jung SH, Chung WC, Kim EJ, Kim SH, Paik CN, Lee BI, Cho YS, Lee KM. Evaluation of non-ampullary duodenal polyps: comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2010;16:5474-5480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Alwmark A, Andersson A, Lasson A. Primary carcinoma of the duodenum. Ann Surg. 1980;191:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Batra SK, Schuman BM, Reddy RR. The endoscopic variety of duodenal villous adenoma - an experience with ten cases. Endoscopy. 1983;15:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 8. | Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg. 1988;207:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Miller JH, Gisvold JJ, Weiland LH, McIlrath DC. Upper gastrointestinal tract: villous tumors. AJR Am J Roentgenol. 1980;134:933-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990;66:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87:37-42. [PubMed] |
| 13. | Okada K, Fujisaki J, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 14. | Barnes G Jr, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Santoro E, Sacchi M, Scutari F, Carboni F, Graziano F. Primary adenocarcinoma of the duodenum: treatment and survival in 89 patients. Hepatogastroenterology. 1997;44:1157-1163. [PubMed] |
| 16. | van Heumen BW, Mul K, Nagtegaal ID, van Kouwen MC, Nagengast FM. Management of sporadic duodenal adenomas and the association with colorectal neoplasms: a retrospective cohort study. J Clin Gastroenterol. 2012;46:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | van Heumen BW, Nieuwenhuis MH, van Goor H, Mathus-Vliegen LE, Dekker E, Gouma DJ, Dees J, van Eijck CH, Vasen HF, Nagengast FM. Surgical management for advanced duodenal adenomatosis and duodenal cancer in Dutch patients with familial adenomatous polyposis: a nationwide retrospective cohort study. Surgery. 2012;151:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Lee CHA, Shingler G, Mowbray NG, Al-Sarireh B, Evans P, Smith M, Usatoff V, Pilgrim C. Surgical outcomes for duodenal adenoma and adenocarcinoma: a multicentre study in Australia and the United Kingdom. ANZ J Surg. 2018;88:E157-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Goda K, Kikuchi D, Yamamoto Y, Takimoto K, Kakushima N, Morita Y, Doyama H, Gotoda T, Maehata Y, Abe N. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: Multicenter case series. Dig Endosc. 2014;26 Suppl 2:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Neugut AI, Jacobson JS, Suh S, Mukherjee R, Arber N. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomarkers Prev. 1998;7:243-251. [PubMed] |
| 22. | Kakushima N, Ono H, Yoshida M, Takizawa K, Tanaka M, Kawata N, Ito S, Imai K, Hotta K, Ishiwatari H. Characteristics and risk factors for sporadic non-ampullary duodenal adenocarcinoma. Scand J Gastroenterol. 2017;52:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Chen CC, Neugut AI, Rotterdam H. Risk factors for adenocarcinomas and malignant carcinoids of the small intestine: preliminary findings. Cancer Epidemiol Biomarkers Prev. 1994;3:205-207. [PubMed] |
| 24. | Wu AH, Yu MC, Mack TM. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int J Cancer. 1997;70:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Neugut AI, Santos J. The association between cancers of the small and large bowel. Cancer Epidemiol Biomarkers Prev. 1993;2:551-553. [PubMed] |
| 26. | Genta RM, Hurrell JM, Sonnenberg A. Duodenal adenomas coincide with colorectal neoplasia. Dig Dis Sci. 2014;59:2249-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut. 2004;53:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Ramsoekh D, van Leerdam ME, Dekker E, Ouwendijk RT, van Dekken H, Kuipers EJ. Sporadic duodenal adenoma and the association with colorectal neoplasia: a case-control study. Am J Gastroenterol. 2008;103:1505-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Maruoka D, Arai M, Ishigami H, Okimoto K, Saito K, Minemura S, Matsumura T, Nakagawa T, Katsuno T, Yokosuka O. Sporadic nonampullary duodenal adenoma/carcinoma is associated with not only colon adenoma/carcinoma but also gastric cancer: association of location of duodenal lesions with comorbid diseases. Scand J Gastroenterol. 2015;50:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Spira IA, Ghazi A, Wolff WI. Primary adenocarcinoma of the duodenum. Cancer. 1977;39:1721-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Kakushima N, Kanemoto H, Sasaki K, Kawata N, Tanaka M, Takizawa K, Imai K, Hotta K, Matsubayashi H, Ono H. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015;21:5560-5567. [PubMed] [DOI] [Full Text] |
| 32. | Mitsuishi T, Hamatani S, Hirooka S, Fukasawa N, Aizawa D, Hara Y, Dobashi A, Goda K, Fukuda T, Saruta M. Clinicopathological characteristics of duodenal epithelial neoplasms: Focus on tumors with a gastric mucin phenotype (pyloric gland-type tumors). PLoS One. 2017;12:e0174985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc. 2009;69:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Fanning SB, Bourke MJ, Williams SJ, Chung A, Kariyawasam VC. Giant laterally spreading tumors of the duodenum: endoscopic resection outcomes, limitations, and caveats. Gastrointest Endosc. 2012;75:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1320] [Article Influence: 60.0] [Reference Citation Analysis (4)] |
| 37. | Oka S, Tanaka S, Nagata S, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Haruma K, Chayama K. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Lépilliez V, Napoléon B, Ponchon T, Saurin JC. [Duodenal adenomas: diagnostic and treatment]. Gastroenterol Clin Biol. 2009;33:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Kakushima N, Ono H, Takao T, Kanemoto H, Sasaki K. Method and timing of resection of superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26 Suppl 2:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Mizumoto T, Sanomura Y, Tanaka S, Kuroki K, Kurihara M, Yoshifuku Y, Oka S, Arihiro K, Shimamoto F, Chayama K. Clinical usefulness of magnifying endoscopy for non-ampullary duodenal tumors. Endosc Int Open. 2017;5:E297-E302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501-12508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Yoshimura N, Goda K, Tajiri H, Ikegami M, Nakayoshi T, Kaise M. Endoscopic features of nonampullary duodenal tumors with narrow-band imaging. Hepatogastroenterology. 2010;57:462-467. [PubMed] |
| 43. | Endo M, Abiko Y, Oana S, Kudara N, Chiba T, Suzuki K, Koizuka H, Uesugi N, Sugai T. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Kikuchi D, Hoteya S, Iizuka T, Kimura R, Kaise M. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26 Suppl 2:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Takahashi T, Ando T, Kabeshima Y, Kawakubo H, Shito M, Sugiura H, Omori T. Borderline cases between benignancy and malignancy of the duodenum diagnosed successfully by endoscopic submucosal dissection. Scand J Gastroenterol. 2009;44:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Nonaka S, Oda I, Tada K, Mori G, Sato Y, Abe S, Suzuki H, Yoshinaga S, Nakajima T, Matsuda T. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Takao M, Kakushima N, Takizawa K, Tanaka M, Yamaguchi Y, Matsubayashi H, Kusafuka K, Ono H. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012;15:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Bourke MJ. Endoscopic resection in the duodenum: current limitations and future directions. Endoscopy. 2013;45:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26 Suppl 2:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Kakushima N, Yoshida M, Iwai T, Kawata N, Tanaka M, Takizawa K, Ito S, Imai K, Hotta K, Ishiwatari H. A simple endoscopic scoring system to differentiate between duodenal adenoma and carcinoma. Endosc Int Open. 2017;5:E763-E768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, Pawlik TM, Herman JM, Edil BH, Ahuja N. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Yamamoto H, Miura Y. Duodenal ESD: conquering difficulties. Gastrointest Endosc Clin N Am. 2014;24:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Navaneethan U, Hasan MK, Lourdusamy V, Zhu X, Hawes RH, Varadarajulu S. Efficacy and safety of endoscopic mucosal resection of non-ampullary duodenal polyps: a systematic review. Endosc Int Open. 2016;4:E699-E708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Kim HK, Chung WC, Lee BI, Cho YS. Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver. 2010;4:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 58. | Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol. 2014;20:8624-8630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Navaneethan U, Lourdusamy D, Mehta D, Lourdusamy V, Venkatesh PG, Sanaka MR. Endoscopic resection of large sporadic non-ampullary duodenal polyps: efficacy and long-term recurrence. Surg Endosc. 2014;28:2616-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Kedia P, Brensinger C, Ginsberg G. Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc. 2010;72:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Yamasaki Y, Uedo N, Takeuchi Y, Higashino K, Hanaoka N, Akasaka T, Kato M, Hamada K, Tonai Y, Matsuura N. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy. 2018;50:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Suzuki S, Gotoda T, Kusano C, Ikehara H, Sugita A, Yamauchi M, Moriyama M. Width and depth of resection for small colorectal polyps: hot versus cold snare polypectomy. Gastrointest Endosc. 2018;87:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 64. | Maruoka D, Matsumura T, Kasamatsu S, Ishigami H, Taida T, Okimoto K, Nakagawa T, Katsuno T, Arai M. Cold polypectomy for duodenal adenomas: a prospective clinical trial. Endoscopy. 2017;49:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Matsuda Y, Sakamoto K, Kataoka N, Yamaguchi T, Tomita M, Makimoto S. Perforation associated with endoscopic submucosal dissection for duodenal neoplasm without a papillary portion. World J Gastrointest Surg. 2017;9:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013;45:136-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Jung JH, Choi KD, Ahn JY, Lee JH, Jung HY, Choi KS, Lee GH, Song HJ, Kim DH, Kim MY. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 68. | Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc. 2015;7:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. 2016;22:600-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 70. | Sohn JW, Jeon SW, Cho CM, Jung MK, Kim SK, Lee DS, Son HS, Chung IK. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc. 2010;24:3195-3200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Marques J, Baldaque-Silva F, Pereira P, Arnelo U, Yahagi N, Macedo G. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;7:720-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Fujihara S, Mori H, Kobara H, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Masaki T. Management of a large mucosal defect after duodenal endoscopic resection. World J Gastroenterol. 2016;22:6595-6609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Kim TW, Kim GH, Park DY, Ahn S, Lim W, Lee BE, Song GA. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc. 2017;31:1936-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Klein A, Nayyar D, Bahin FF, Qi Z, Lee E, Williams SJ, Byth K, Bourke MJ. Endoscopic mucosal resection of large and giant lateral spreading lesions of the duodenum: success, adverse events, and long-term outcomes. Gastrointest Endosc. 2016;84:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Hoteya S, Yahagi N, Iizuka T, Kikuchi D, Mitani T, Matsui A, Ogawa O, Yamashita S, Furuhata T, Yamada A. Endoscopic submucosal dissection for nonampullary large superficial adenocarcinoma/adenoma of the duodenum: feasibility and long-term outcomes. Endosc Int Open. 2013;1:2-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Miura Y, Shinozaki S, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Otake Y, Saito Y, Sakamoto T, Aoki T, Nakajima T, Toyoshima N, Matsuda T, Ono H. New closure technique for large mucosal defects after endoscopic submucosal dissection of colorectal tumors (with video). Gastrointest Endosc. 2012;75:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Hoteya S, Kaise M, Iizuka T, Ogawa O, Mitani T, Matsui A, Kikuchi D, Furuhata T, Yamashita S, Yamada A. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc. 2015;27:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 82. | Matsuda T, Fujii T, Emura F, Kozu T, Saito Y, Ikematsu H, Saito D. Complete closure of a large defect after EMR of a lateral spreading colorectal tumor when using a two-channel colonoscope. Gastrointest Endosc. 2004;60:836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Fujii T, Ono A, Fu KI. A novel endoscopic suturing technique using a specially designed so-called “8-ring” in combination with resolution clips (with videos). Gastrointest Endosc. 2007;66:1215-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Sakamoto N, Beppu K, Matsumoto K, Shibuya T, Osada T, Mori H, Shimada Y, Konno A, Kurosawa A, Nagahara A. “Loop Clip”, a new closure device for large mucosal defects after EMR and ESD. Endoscopy. 2008;40 Suppl 2:E97-E98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Nishizawa T, Akimoto T, Uraoka T, Mitsunaga Y, Maehata T, Ochiai Y, Fujimoto A, Goto O, Kanai T, Yahagi N. Endoscopic string clip suturing method: a prospective pilot study (with video). Gastrointest Endosc. 2018;87:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 87. | Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752-2760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 88. | Mori H, Kobara H, Nishiyama N, Fujihara S, Kobayashi N, Masaki T. Simple but reliable endoscopic sliding closure with ring-shaped surgical thread after endoscopic submucosal dissection. Endoscopy. 2015;47 Suppl 1 UCTN:E428-E429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Takeuchi J, Suzuki H, Murata M, Kakei Y, Ri S, Umeda M, Komori T. Clinical evaluation of application of polyglycolic acid sheet and fibrin glue spray for partial glossectomy. J Oral Maxillofac Surg. 2013;71:e126-e131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Kawai H, Harada K, Ohta H, Tokushima T, Oka S. Prevention of alveolar air leakage after video-assisted thoracic surgery: comparison of the efficacy of methods involving the use of fibrin glue. Thorac Cardiovasc Surg. 2012;60:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Ueda K, Tanaka T, Hayashi M, Li TS, Tanaka N, Hamano K. Mesh-based pneumostasis contributes to preserving gas exchange capacity and promoting rehabilitation after lung resection. J Surg Res. 2011;167:e71-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Uemura K, Murakami Y, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Sueda T. Combination of polyglicolic acid felt and fibrin glue for prevention of pancreatic fistula following pancreaticoduodenectomy. Hepatogastroenterology. 2009;56:1538-1541. [PubMed] |
| 93. | Tsuji Y, Fujishiro M, Kodashima S, Ono S, Niimi K, Mochizuki S, Asada-Hirayama I, Matsuda R, Minatsuki C, Nakayama C. Polyglycolic acid sheets and fibrin glue decrease the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms (with video). Gastrointest Endosc. 2015;81:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Sakaguchi Y, Tsuji Y, Ono S, Saito I, Kataoka Y, Takahashi Y, Nakayama C, Shichijo S, Matsuda R, Minatsuki C. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy. 2015;47:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Tsuji Y, Ohata K, Gunji T, Shozushima M, Hamanaka J, Ohno A, Ito T, Yamamichi N, Fujishiro M, Matsuhashi N. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to cover wounds after colorectal endoscopic submucosal dissection (with video). Gastrointest Endosc. 2014;79:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Doyama H, Tominaga K, Yoshida N, Takemura K, Yamada S. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014;26 Suppl 2:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Takimoto K, Toyonaga T, Matsuyama K. Endoscopic tissue shielding to prevent delayed perforation associated with endoscopic submucosal dissection for duodenal neoplasms. Endoscopy. 2012;44 Suppl 2 UCTN:E414-E415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 99. | Otowa Y, Kanaji S, Morita Y, Suzuki S, Yamamoto M, Matsuda Y, Matsuda T, Oshikiri T, Nakamura T, Kawara F. Safe management of laparoscopic endoscopic cooperative surgery for superficial non-ampullary duodenal epithelial tumors. Endosc Int Open. 2017;5:E1153-E1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |