Published online Jun 16, 2018. doi: 10.4253/wjge.v10.i6.109
Peer-review started: January 5, 2018
First decision: January 31, 2018
Revised: March 5, 2018
Accepted: April 11, 2018
Article in press: April 11, 2018
Published online: June 16, 2018
Processing time: 161 Days and 5.9 Hours
To compare the adenoma detection rate (ADR) between gastroenterologists and colorectal surgeons at Box Hill Hospital, Melbourne, Australia.
A total of 300 colonoscopies performed by gastroenterologists and colorectal surgeons at Box Hill Hospital were retrospectively reviewed from May 2016 to June 2017. Exclusion criteria were: Patients ≤ 50 years old, colonoscopies with failure of caecal intubation, patients who previously had colon cancer and/or a colonic resection, history of polyposis syndromes or inflammatory bowel disease, or a colonoscopy within the last 10 years. Patient demographics, indications, symptoms and procedural-related outcomes were measured.
The ADR was not significantly different between gastroenterologists and colorectal surgeons (34% vs 34.67%; P = 0.90). The adjusted odds ratio correcting for gender, age, 1st degree relative with colorectal cancer, previous colonoscopy, trainee involvement and caecal or terminal ileum intubation rate was 1.19 (0.69-2.05).
Both specialties at our institution exceed benchmark standards suggested by published Australian and American guidelines. An association between endoscopist specialty and ADR was not observed.
Core tip: Our study concludes that there is no association between specialty (gastroenterology and colorectal surgeons) and proficiency in colonoscopy, using adenoma detection rate as a quality indicator. The adenoma detection rate in both specialties at our institution exceed benchmark standards suggested by published Australian and American guidelines, reflecting the high standards of care and efficacy of the common training pathway for both specialties.
- Citation: Lee AHH, Lojanapiwat N, Balakrishnan V, Chandra R. Is there a difference in adenoma detection rates between gastroenterologists and surgeons? World J Gastrointest Endosc 2018; 10(6): 109-116
- URL: https://www.wjgnet.com/1948-5190/full/v10/i6/109.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i6.109
Colorectal cancer (CRC) poses a significant health burden in Australia. In 2018, it is estimated to become the second most commonly diagnosed cancer with an incidence of 17004 new cases[1]. Colonoscopy is the gold standard screening tool for CRC, allowing for the detection and removal of precursor lesions. To ensure high standards for colonoscopy in Australia, gastroenterologists and colorectal surgeons are required to complete similar training requirements under the Gastroenterological Society of Australia (GESA).
ADR is the primary quality measure in colonoscopy, having been proven to accurately predict effective CRC prevention. It is defined as the proportion of screening colonoscopies that detect at least one histologically confirmed colorectal adenoma. Meeting the standard ADR is crucial in reducing CRC incidence and minimising CRC-related mortality[2]. The performances of gastroenterologists and colorectal surgeons in colonoscopy have been compared in the literature, with varied results. Several studies have demonstrated that gastroenterologists are more effective than non-gastroenterologists at preventing CRC by colonoscopy whilst other studies have showed no difference between the two specialties[3-6].
Comparing the ADR between gastroenterologists and colorectal surgeons in Australia is of significant interest. Although both specialties have similar training requirements, they remain completely separate specialties. This study aims to compare the ADR between gastroenterologists and colorectal surgeons at a single centre in Melbourne, Australia.
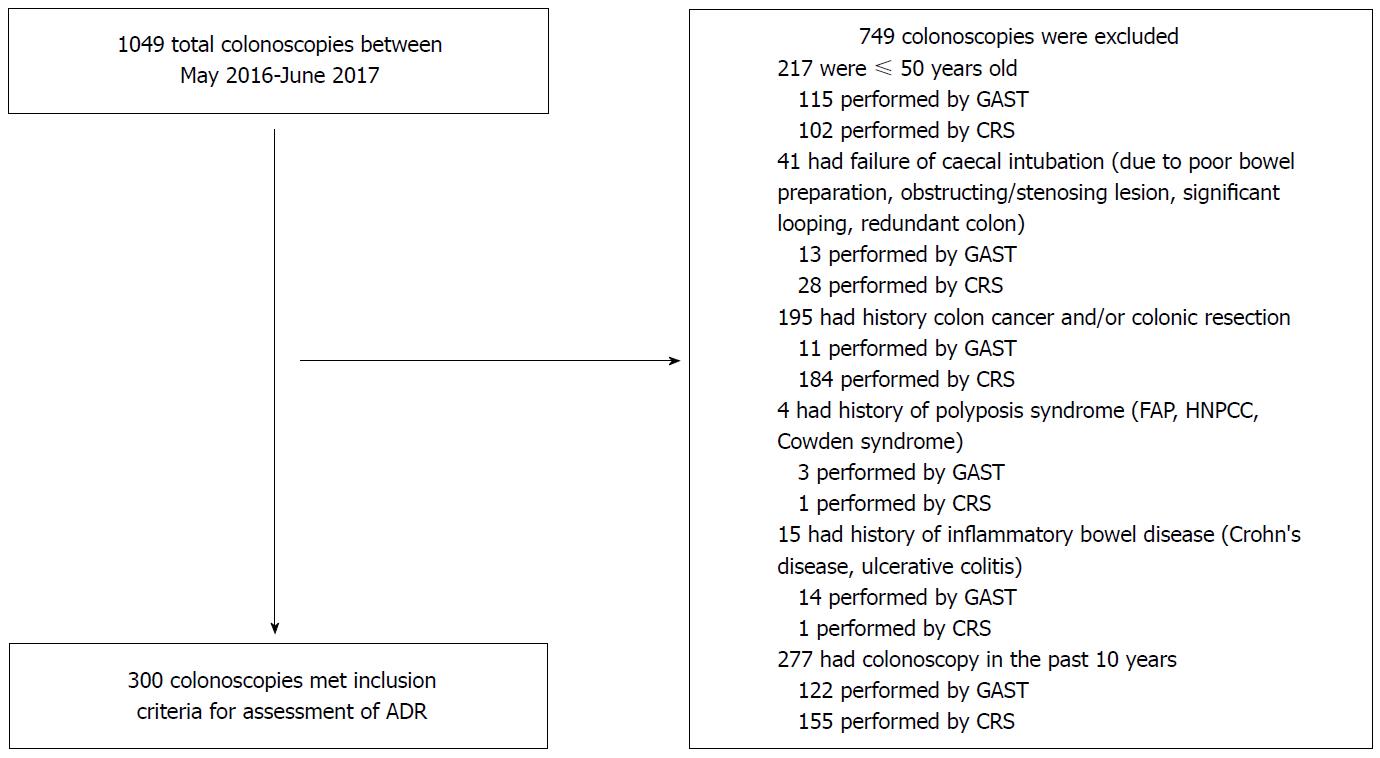
Consecutive patients undergoing colonoscopies by gastroenterologists and colorectal surgeons were identified from the endoscopy database at Box Hill hospital (Melbourne, Australia) between May 2016 and June 2017. Exclusion criteria included patients aged 50 and younger, colonoscopies with failure of caecal intubation, previous CRC and/or a colonic resection, history of polyposis syndromes or inflammatory bowel disease, or a colonoscopy within the last 10 years.
Excluding such patients was for ease of comparison of our results with guidelines published by the Gastroenterological Society of Australia (GESA), American Society for Gastrointestinal Endoscopy(AGSE) and American College of Gastroenterology (ACG) discussed in detail below. These guidelines were specific to patients ≥ 50 years old of “average-risk”, and patients with previous pathologies would not lie within this bracket. Exclusion of cases with failure of caecal intubation ensured that proficiency was strictly based on the ability to detect adenomas, and ADR was not affected by pre-existing patient-related factors impacting caecal intubation (e.g., poor bowel preparation, obstructing/stenosing lesion, significant looping, redundant colon). Rex et al[7] also recommends that failed caecal intubation due to poor bowel preparation, severe colitis or known stricture or polyp for treatment, need not be counted in determining caecal intubation rates when assessing effectiveness of colonoscopy.
Information regarding cases was obtained from the electronic medical record system. All participating endoscopists were either certified by the GESA or supervised by an endoscopist certified by the GESA[1].
The study was approved by the Office of Research and Ethics at Eastern Health.
Data were collected by two investigators and included patient demographics, indication for colonoscopy (screening versus non-screening), trainee involvement, and caecal and terminal ileum (TI) intubation rate. Screening colonoscopies were those performed for a positive faecal occult blood test in the absence of any other indications. Indications for non-screening colonoscopies included investigation of symptoms, 1st degree relative with CRC, abnormal imaging and iron deficiency anaemia. Colonoscopies performed for symptoms included abdominal pain, bloating, change in bowel habits, macroscopic per rectal bleeding, loss of weight, anorexia, anal symptoms such as pruritus or pain, and symptomatic anaemia such as syncope or shortness of breath. It was recorded if an adenoma was detected when at least one polyp was removed during the colonoscopy.
Trainees were registered surgical or gastroenterology trainees, under The Royal College of Surgeons or The Royal College of Physicians, who have not attained certification by the GESA. When trainees were involved, they performed the colonoscopy with direct supervision by consultants, who only intervened when there was difficulty traversing a part of the colon. Caecal intubation was recorded if reported or on viewing photo documentation of caecal landmarks such as the triradiate fold, ileocaecal valve and appendiceal orifice. TI intubation was recorded if reported.
The primary outcome was adenoma detection rate (ADR), the definition of which was extended to include colonoscopies for all indications. Secondary outcomes included polypectomy rate, polyp detection rate, tumour detection rate, hyperplastic polyp detection and adenocarcinoma detection rate.
Comparative statistics were performed using Student’s t test and Pearson’s Chi-squared analysis. Separate analyses of ADRs by gender, indication and age were performed to control for different patient populations. Multivariate logistic regression was performed to control for patient-level confounders (gender, age, 1st degree relative with CRC, 1st colonoscopy, trainee involvement, caecal or TI intubation). Associations were quantified by odds ratios and 95% confidence intervals (CI). A significant P-value was defined as < 0.05. All analyses were conducted using Stata IC Version 15.
A total of 300 colonoscopies performed at Box Hill Hospital were found to have met inclusion criteria (Figure 1). 150 colonoscopes performed by 16 gastroenterologists and 150 colonoscopes performed by 8 colorectal surgeons were obtained from May 2016 to June 2017.
Baseline demographics are summarised in Table 1. Gastroenterologists were more likely to perform colonoscopies on patients who had never had a colonoscopy (98.0% vs 89.33%, P = 0.002) and were more likely to intubate the TI (70.00% vs 36.00%, P = 0.000), whereas colorectal surgeons had a higher trainee involvement rate (12.00% vs 36.67%, P = 0.0001).
| Gastroenterologists (n = 150) | Colorectal surgeons (n = 150) | P-value | |
| Female (%) | 50.67 | 52 | 0.817 |
| Patient age (mean, yr) | 66.59 | 67.18 | 0.61 |
| 1st degree relative with CRC (%) | 6.67 | 10 | 0.296 |
| 1st colonoscopy (%) | 98 | 89.33 | 0.002 |
| Trainee involved (%) | 12 | 36.67 | < 0.0001 |
| Caecal intubation rate (%) | 100 | 100 | - |
| TI intubation rate (%) | 70 | 36 | < 0.0001 |
In both specialties, the majority of colonoscopies were indicated for non-screening purposes-84.67% and 86.67% of colonoscopies performed by gastroenterologists and colorectal surgeons respectively.
There were no significant differences identified between gastroenterologists and colorectal surgeons for ADR (34.00 vs 34.67, P = 0.903), hyperplastic polyp detection rate (14.00 vs 8.67, P = 0.145), polyp detection rate (51.33 vs 46.00, P = 0.355), tumour detection rate (2.00 vs 4.67, P = 0.198), adenocarcinoma detection rate (2.67 vs 4.00, P = 0.520) and polypectomy rate (51.33 vs 46.00, P = 0.3555).
We analysed ADR according to gender, indication and age for each specialty (Table 2). While controlling for various population groups, no statistically significant difference was detected between specialties.
| Adenoma detection rate (%) | |||
| Gastroenterologists | Colorectal surgeons | P-value | |
| Gender | |||
| Male | 41.89 | 38.89 | 0.712 |
| Female | 26.32 | 30.77 | 0.541 |
| P-value | 0.044 | 0.297 | |
| Indication | |||
| Screening | 52.17 | 30 | 0.142 |
| Non-screening | 30.71 | 35.38 | 0.426 |
| P-value | 0.046 | 0.638 | |
| Age group | |||
| 50-54 | 31.58 | 26.32 | 0.721 |
| 55-59 | 31.82 | 33.33 | 0.91 |
| 60-64 | 33.33 | 28.57 | 0.724 |
| 65-69 | 20.83 | 33.33 | 0.362 |
| 70-74 | 38.46 | 47.83 | 0.509 |
| 75-79 | 56.25 | 36.84 | 0.251 |
| 80-84 | 37.5 | 38.46 | 0.965 |
| 85-89 | 28.57 | 30 | 0.949 |
| 90-94 | 0 | 0 | - |
| P-value | 0.606 | 0.89 | |
Within each speciality, ADR was higher in males compared to females. There was also a peak in ADR for colonoscopies performed in those 70 to 84 years of age. ADR was higher in the screening colonoscopies compared to non-screening in gastroenterologists, but the opposite was observed for colorectal surgeons. Only the differences in ADR between genders (41.89 vs 26.32, P = 0.440) and between indications (52.17 vs 30.71, P = 0.046) within gastroenterologists were statistically significant.
The logistic regression results with ADR are demonstrated in Table 3. The odds ratio for ADR with surgeons as compared to gastroenterologists, adjusted for sex, age, 1st degree relative with CRC, previous colonoscopy, trainee involvement and caecal or TI intubation rate was 1.19 (95%CI: 0.69-2.05). A significant difference in ADR was not demonstrated even after adjusting for potential confounders.
| Variable | Odds ratio (95%CI) |
| Endoscopist level Specialty1 (SURC/GAST) | 1.19 (0.69-2.05) |
| Patient level Gender2 (F/M) | 0.57 (0.34-0.93) |
| Age | 1.02 (0.99-1.04) |
| 1st degree relative with CRC (N/Y) | 1.21 (0.48-3.11) |
| 1st colonoscopy (N/Y) | 0.89 (0.32-2.54) |
| Trainee involvement (N/Y) | 1.44 (0.78-2.65) |
| Caecal intubation (N/Y) | 1 |
| Terminal ileum intubation (N/Y) | 0.89 (0.53-1.49) |
This is the first study in Australia to compare the ADR between gastroenterologists and colorectal surgeons. In our institution, we found no significant difference in the ADR between the two specialities.
It is important to monitor ADR performance in order to optimise CRC prevention. Corley et al[2] found that each 1% increase in ADR predicted a 3% decrease in the risk of cancer. Similarly, Kaminski et al[8] found that an increased ADR was associated with a reduced risk of CRC and death. Low ADRs not only reflect the failure of detecting precancerous lesions at colonoscopy, but also result in an inappropriately increased length of time to the next colonoscopy, thereby increasing the risk of interval cancers[9].
Our findings parallel other studies that found no significant differences for ADRs between specialties. Ollington et al[3] compared the ADR between gastroenterologists and colorectal surgeons in California. 180 and 119 colonoscopies were performed by 8 gastroenterologists and 16 colorectal surgeons respectively. No significant difference was detected between both specialties (33% gastroenterologists vs 29% colorectal surgeons; P = 0.38). Bhangu et al[4] prospectively reviewed 10, 026 colonoscopies performed by physicians (general physicians or gastroenterologists) and surgeons (general or colorectal), from a United Kingdom hospital endoscopy service. After adjusting for age, sex and indication, it concluded that accreditation and procedural volume, but not endoscopic specialty, were predictors of ADR. Most recently, a study by Kozbial et al[10] analysing 59901 screening colonoscopies performed in Austria concluded that there was no significant difference in ADRs in relation to specialty or setting.
In contrast, 3 studies demonstrated higher ADRs in gastroenterologists compared to non-gastroenterologists. Pox et al[11] performed a prospective cross-sectional study on 2821392 colonoscopies in Germany for individuals 55 years and older. He reported ADRs of 25.1% and 22.3% for gastroenterologists and non-gastroenterologists (internists and colorectal surgeons) respectively (adjusted OR 1.18; 95%CI: 1.16-1.21). Though this is a statistically significant result, its clinical significance is arguable and the colonoscopies analysed were indicated for screening purposes only. De jonge et al[12] found that surgeons were 80% less likely to find an adenoma as compared to gastroenterologists (OR 0.2; 95%CI: 0.1-0.6). However, there was not an equal representation of specialties, with surgeons representing 1% of endoscopists. Additionally, a lower caecal intubation rate was found in surgeons and internists as compared to gastroenterologists (after adjusting for poor bowel preparation, severe colitis and an intervention as an indication), which could have accounted for the significant difference in ADR. Leyden et al[13] assessed colonoscopies performed by gastroenterologists and surgical trainees and reported ADRs of 14% and 9% respectively (P = 0.0065). Given the low ADRs in this study, the results may not be an accurate representation of each specialty.
There is evidence in the literature postulating the superior performance of gastroenterologists in colonoscopies, utilising other outcome measures such as incidence of post-colonoscopy CRC[14,15], mortality secondary to CRC[5] and polypectomy rate[6,16]. However, most results were reported against non-gastroenterologists and not colorectal surgeons[5,6,14,15]. The difference reported was not significant[15] or if significant, was usually small and may not be clinically significant[16].
Both specialties at our institution exceed the recertification criteria set by the GESA, i.e., an ADR of at least 25% in patients 50 years or older, having intact colons, with no findings of acute IBD, and with intubation to the caecum or terminal ileum. They also exceed benchmark standards suggested by the ASGE and ACG. As of 2015, ADR targets of 30% in men and 20% in women over the age of 50 are endorsed[9]. In our study, gastroenterologists demonstrated ADRs of 41.89% and 26.32% in males and females over the age of 50 while colorectal surgeons demonstrated ADRs of 38.89% and 30.77% in males and females over the age of 50.
Although the differences in the ADR between both specialties were not significant in our study, a higher ADR in colorectal surgeons was observed with a higher trainee involvement. This finding is mirrored by Qayed et al[17], who observed a significantly greater ADR with trainee participation than without, and attributed this to the presence of an additional observer and more focused examination behind each colonic fold during withdrawal of the colonoscope due to active supervision. Although this association was not statistically analysed for in our study, greater trainee involvement may increase ADRs, and may be implemented to increase ADRs.
Quality of colonoscopy is a pertinent issue. The National Bowel Cancer Screening program in Australia has plans of expansion, offering free screening FOBT, followed by colonoscopy if FOBT positive, to Australians aged 50 to 74 years old biannually by 2020. Our study reflects that high standards are upheld in colonoscopy, regardless of specialty.
Ways to improve ADR has been explored due to large variations in ADRs in the literature[18]. Interventions targeting endoscopist performance have varied effects on ADRs. Performance report cards could be used to improve ADR, especially among physicians with low ADR < 25%[19]. Video recording led to the increase in inspection time and quality, however its impact on ADR was equivocal[20]. In contrast, a multi-intervention program involving personalised feedback and financial penalties, showed no significant improvements in ADR[21].
Interventions directed at withdrawal time have been looked into. Recording or lengthening the withdrawal time was not associated with improvement of adenoma or polyp detection rates[22-24]. However, ADR improved significantly when implementation of a targeted 8-min withdrawal time with the use of an audible timer was combined with inspection training. This highlights the potential of continuous feedback in improving ADRs instead of addressing withdrawal time in isolation[25,26]. A repeat examination or increased observation time at the right side of the colon has been shown to increase ADR. Hence greater time could be spent examining the proximal colon, especially since small lesions located there are more frequently associated with advanced neoplasia[27,28].
Utilising technological adjuncts to augment ADRs have been explored[29,30]. High definition imaging and selective application of dyes are not useful in increasing ADRs[29,31]. Widespread use of dyes increase the detection of small flat adenomas but are time consuming[32]. Evidence around electronic highlighting of flat lesions are still lacking[33]. The use of full-spectrum colonoscopy, with a panoramic 330 degree view of the colon, has not been shown to be superior to standard colonoscopy with regards to ADR through a meta-analysis of eight randomised controlled trials[34]. Despite this, narrow band imaging has been demonstrated to be effective in endoscopic predictions of histology, reducing costs and avoiding risks associated with polypectomy[29].
The use of attachable add-on devices which increase exposure of mucosa has been introduced. Cap cuff-assisted colonoscopy has been tested, with 4 randomised studies demonstrating gains of 3%-9% in ADR, albeit carrying risks of mucosal erosions and lower ileal intubation rates[35-38]. Another novel idea is the use of behind-folds visualising colonoscopy technologies. Through the review of 3 randomised tandem studies, Brand et al[39] found that it reduced miss rates for 1 to 9 mm adenomas. However, the validity of this in reducing incidence of CRC and death has yet to be determined. Despite uncertainty surrounding efficacy, such devices show promise and could be used with discretion in daily practice[30].
The use of pre-operative simethicone has been shown to increase ADR. Simethicone is an anti-foaming agent which reduces the surface tension of bubbles, thereby reducing the need for intraoperative flushing which could reduce visualisation of the colon due to fluid accumulation. It has also been shown to reduce air accumulation and abdominal bloating, thereby improving patient compliance to bowel preparation[40,41].
Our study had limitations, such as its retrospective nature. Hence, several information was not able to be obtained. Withdrawal time was not recorded, hence no insight could be provided regarding withdrawal time and increased ADR. However as mentioned previously, evidence shows that isolated increase in withdrawal time does not increase ADR and hence its inclusion in analysis would not provide much insight. The level of consultant participation in colonoscopy when a trainee was involved, the level of experience armed by each trainee at the time of colonoscopy as well as the actual number of trainees involved were not recorded. Hence, an accurate association between trainee involvement and ADR could not be established. However, this is not the main aim of our study and this could be explored in further future studies. Despite these limitations, we applied strict exclusion criteria and all colonoscopies in this study were performed under similar conditions and with mandatory compliance to quality guidelines at an institutional and national level. Multivariate analysis controlling for age and gender was implemented as studies have shown that ADR is affected by these factors[11,18].
A larger sample size may have increased the power of the study and allowed the differences to reach statistical significance, but the clinical significance of such small differences come into question. Moreover, the sample size required to attain statistical significance would not be feasible for retrospective review. Finally, our study was performed at a single centre and a sample of colonoscopies during a certain time period were used to ascertain ADRs for both specialties. Therefore, this may not be a true representation of all gastroenterologists and colorectal surgeons across Australia.
In conclusion, both gastroenterologists and colorectal surgeons at our institution exceed benchmark standards suggested by the GESA, ASGE and ACG. An association between endoscopist specialty and ADR was not observed, even after controlling for patient-level factors. Our study reassures clinicians and patients that high standards are upheld in colonoscopy, regardless of specialty.
Colorectal cancer (CRC) poses a significant health burden in Australia. In 2017, it is estimated to become the second most commonly diagnosed cancer with an incidence of 16682 new cases. Colonoscopy is the gold standard screening tool for CRC, with the adenoma detection rate (ADR) as the primary quality measure. ADR is defined as the proportion of screening colonoscopies that detect at least one histologically confirmed colorectal adenoma. Meeting the standard ADR is crucial in reducing CRC incidence and minimising CRC-related mortality. The performances of gastroenterologists and colorectal surgeons in colonoscopy have been compared in the literature, with varied results.
Quality of colonoscopy is a pertinent issue, with the expansion of the National Bowel Cancer Screening program, offering free screening to Australians aged 50 to 74 years old every two years by 2020. ADR has been established as an important measure of endoscopist proficiency. At present, no study has compared the ADR between gastroenterologists and colorectal surgeons in Australia. Although both specialties have similar training requirements, they remain completely separate specialties. This study aims to compare the ADR between gastroenterologists and colorectal surgeons, and hence reflect the standards of colonoscopy of both specialties in Australia. This would propel higher quality research to be undertaken regarding ways to increase ADR in colonoscopy and hence ensure more effective prevention of CRC.
This study aims to compare the ADR between gastroenterologists and colorectal surgeons at a single centre in Melbourne, Australia.
A total of 300 colonoscopies performed by gastroenterologists and colorectal surgeons at Box Hill Hospital were retrospectively reviewed from May 2016 to June 2017. Exclusion criteria were: Patients ≤ 50 years old, colonoscopies with failure of caecal intubation, patients who previously had colon cancer and/or a colonic resection, history of polyposis syndromes or inflammatory bowel disease, or a colonoscopy within the last 10 years. Patient demographics, indications, symptoms and procedural-related outcomes were measured.
The ADR was not significantly different between gastroenterologists and colorectal surgeons (34% vs 34.67%, P = 0.90). The adjusted odds ratio correcting for gender, age, 1st degree relative with colorectal cancer, previous colonoscopy, trainee involvement and caecal or terminal ileum intubation rate was 1.19 (0.69-2.05).
Both gastroenterologists and colorectal surgeons at our institution exceed benchmark standards suggested by the GESA, ASGE and ACG. An association between endoscopist specialty and ADR was not observed, even after controlling for patient-level factors. Our study reassures clinicians and patients that high standards are upheld in colonoscopy, regardless of specialty. Ways to improve ADR has been explored, such as interventions targeted at endoscopists performance, increasing withdrawal time or observation time, technological adjuncts or add-on devices and the use of simethicone. Currently, there is a lack of high quality evidence that demonstrates increase in ADR with each of these interventions to support their routine use in colonoscopy. Despite this uncertainty, technological adjuncts such as narrow band imaging and cap cuff-assisted colonoscopy may be used with discretion in daily practice. Greater time spent examining the proximal colon could be considered.
The ADR in both specialties exceed benchmark standards reflecting the high standards of education and training in Australia. Higher quality evidence investigating patient and endoscopist-specific factors that increase ADR is warranted.
The authors thank the endoscopists for contributing to this data for analysis, Associate Nurse Unit Manager Tatiana Hendarto for assistance with obtaining colonoscopy records, and Associate Professor Tarik Sammour for assistance with statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Allaix ME, Aytac E, Biondi A S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Australian Government. Bowel cancer (Colorectal cancer) in Australia. Cancer Australia. 30 Jan. 2018; Available from: https://bowel-cancer.canceraustralia.gov.au/statistics Cited 11 Feb 2018. |
| 2. | Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1560] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 3. | Ollington KF, Brelian D, Morgan J, Le Q, Fleshner P, Melmed GY. Comparison of adenoma detection rates between gastroenterologists and colorectal surgeons. Am Surg. 2012;78:269-270. [PubMed] |
| 4. | Bhangu A, Bowley DM, Horner R, Baranowski E, Raman S, Karandikar S. Volume and accreditation, but not specialty, affect quality standards in colonoscopy. Br J Surg. 2012;99:1436-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Jiang M, Sewitch MJ, Barkun AN, Joseph L, Hilsden RJ. Endoscopist specialty is associated with colonoscopy quality. BMC Gastroenterol. 2013;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 384] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 8. | Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 370] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 836] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 10. | Kozbial K, Reinhart K, Heinze G, Zwatz C, Bannert C, Salzl P, Waldmann E, Britto-Arias M, Ferlitsch A, Trauner M. High quality of screening colonoscopy in Austria is not dependent on endoscopist specialty or setting. Endoscopy. 2015;47:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460-7.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | de Jonge V, Sint Nicolaas J, Cahen DL, Moolenaar W, Ouwendijk RJ, Tang TJ, van Tilburg AJ, Kuipers EJ, van Leerdam ME; SCoPE Consortium. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc. 2012;75:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Leyden JE, Doherty GA, Hanley A, McNamara DA, Shields C, Leader M, Murray FE, Patchett SE, Harewood GC. Quality of colonoscopy performance among gastroenterology and surgical trainees: a need for common training standards for all trainees? Endoscopy. 2011;43:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 15. | Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 16. | Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Specialty differences in polyp detection, removal, and biopsy during colonoscopy. Am J Med. 2010;123:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Qayed E, Shea L, Goebel S, Bostick RM. Association of trainee participation with adenoma and polyp detection rates. World J Gastrointest Endosc. 2017;9:204-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Sey MSL, Liu A, Asfaha S, Siebring V, Jairath V, Yan B. Performance report cards increase adenoma detection rate. Endosc Int Open. 2017;5:E675-E682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Rex DK, Hewett DG, Raghavendra M, Chalasani N. The impact of videorecording on the quality of colonoscopy performance: a pilot study. Am J Gastroenterol. 2010;105:2312-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Shaukat A, Oancea C, Bond JH, Church TR, Allen JI. Variation in detection of adenomas and polyps by colonoscopy and change over time with a performance improvement program. Clin Gastroenterol Hepatol. 2009;7:1335-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Velásquez J, Espinoza-Ríos J, Huerta-Mercado J, Pinto J, De los Ríos R, Piscoya A, OR C, Zegarra A, Bussalleu A. [Impact assessment of increasing the time of withdrawal of colonoscopy in the detection rate of polyps in our midst]. Rev Gastroenterol Peru. 2009;29:321-325. [PubMed] |
| 23. | Taber A, Romagnuolo J. Effect of simply recording colonoscopy withdrawal time on polyp and adenoma detection rates. Gastrointest Endosc. 2010;71:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Lin OS, Kozarek RA, Arai A, Gluck M, Jiranek GC, Kowdley KV, McCormick SE, Schembre DB, Soon MS, Dominitz JA. The effect of periodic monitoring and feedback on screening colonoscopy withdrawal times, polyp detection rates, and patient satisfaction scores. Gastrointest Endosc. 2010;71:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 27. | Klare P, Phlipsen H, Haller B, Einwächter H, Weber A, Abdelhafez M, Bajbouj M, Brown H, Schmid RM, von Delius S. Longer observation time increases adenoma detection in the proximal colon - a prospective study. Endosc Int Open. 2017;5:E1289-E1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Ai X, Qiao W, Han Z, Tan W, Bai Y, Liu S, Zhi F. Results of a second examination of the right side of the colon in screening and surveillance colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Hassan C, Pickhardt PJ, Rex DK. Performance improvements of imaging-based screening tests. Best Pract Res Clin Gastroenterol. 2010;24:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Gkolfakis P, Tziatzios G, Dimitriadis GD, Triantafyllou K. New endoscopes and add-on devices to improve colonoscopy performance. World J Gastroenterol. 2017;23:3784-3796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Pellisé M, Fernández-Esparrach G, Cárdenas A, Sendino O, Ricart E, Vaquero E, Gimeno-García AZ, de Miguel CR, Zabalza M, Ginès A. Impact of wide-angle, high-definition endoscopy in the diagnosis of colorectal neoplasia: a randomized controlled trial. Gastroenterology. 2008;135:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Facciorusso A, Del Prete V, Buccino V, Valle ND, Nacchiero MC, Muscatiello N. Full-spectrum versus standard colonoscopy for improving polyp detection rate: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2018;33:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Horiuchi A, Nakayama Y. Improved colorectal adenoma detection with a transparent retractable extension device. Am J Gastroenterol. 2008;103:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Horiuchi A, Nakayama Y, Kato N, Ichise Y, Kajiyama M, Tanaka N. Hood-assisted colonoscopy is more effective in detection of colorectal adenomas than narrow-band imaging. Clin Gastroenterol Hepatol. 2010;8:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | De Palma GD, Giglio MC, Bruzzese D, Gennarelli N, Maione F, Siciliano S, Manzo B, Cassese G, Luglio G. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc. 2018;87:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | González-Fernández C, García-Rangel D, Aguilar-Olivos NE, Barreto-Zúñiga R, Romano-Munive AF, Grajales-Figueroa G, Zamora-Nava LE, Téllez-Avila FI. Higher adenoma detection rate with the endocuff: a randomized trial. Endoscopy. 2017;49:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Brand EC, Dik VK, van Oijen MGH, Siersema PD. Missed adenomas with behind-folds visualizing colonoscopy technologies compared with standard colonoscopy: a pooled analysis of 3 randomized back-to-back tandem colonoscopy studies. Gastrointest Endosc. 2017;86:376-385.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Bai Y, Fang J, Zhao SB, Wang D, Li YQ, Shi RH, Sun ZQ, Sun MJ, Ji F, Si JM. Impact of preprocedure simethicone on adenoma detection rate during colonoscopy: a multicenter, endoscopist-blinded randomized controlled trial. Endoscopy. 2018;50:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Zhang S, Zheng D, Wang J, Wu J, Lei P, Luo Q, Wang L, Zhang B, Wang H, Cui Y. Simethicone improves bowel cleansing with low-volume polyethylene glycol: a multicenter randomized trial. Endoscopy. 2018;50:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |