Published online Dec 16, 2018. doi: 10.4253/wjge.v10.i12.392
Peer-review started: August 27, 2018
First decision: October 5, 2018
Revised: November 20, 2018
Accepted: December 10, 2018
Article in press: December 11, 2018
Published online: December 16, 2018
Processing time: 117 Days and 12.1 Hours
Immunotherapy is any treatment aimed at boosting or enhancing the immune system. It includes a wide range of options, from vaccines to treatment for conditions such as allergy and cancer. In the case of cancer, unlike other available treatments, immunotherapy is not aimed at destroying the tumor cells but at stimulating the patient’s immune system so that it attacks the tumor. In cancer, immunotherapy provides a series of advantages. Nevertheless, immunotherapy administered for treatment of cancer is associated with immune-mediated enterocolitis. Colitis mediated by monoclonal anti-cytotoxic T lymphocyte–associated antigen 4 and to programmed cell death protein 1 and its ligand PDL1 shares characteristics with chronic inflammatory bowel disease (IBD), and similar findings have been reported for both the endoscopy images and the segment involved. The most frequent lesions on endoscopy are ulcer and erythema, and the most frequently affected site is the sigmoid colon. A segmental pattern has been reported to be slightly more frequent than a continuous pattern. In addition, upper gastrointestinal lesions have been reported in up to half of patients, with the most frequent findings being gastritis and erosive duodenitis. As is the case in IBD, systemic corticosteroids and immunosuppressive treatment (anti-TNF agents) are the approaches used in patients with a more unfavorable progression. Immunotherapy must be suspended completely in some cases.
Core tip: Widespread use of immunotherapy in various types of cancer has led to reports of new associated adverse effects resulting from increased stimulation of the immune system, which can confuse the body’s own tissues and organs with foreign matter, thus leading it to attack the body’s healthy tissue. The most frequent immune-mediated adverse effects include asthenia, general malaise, fever, gastrointestinal toxicity (abdominal pain, diarrhea, and colitis), cutaneous toxicity, hypothyroidism and hepatitis. This review of the endoscopic evaluation of immunotherapy-induced toxicity presents the most typical endoscopic images, the differential diagnosis based on these images, and the initial management.
- Citation: Iranzo I, Huguet JM, Suárez P, Ferrer-Barceló L, Iranzo V, Sempere J. Endoscopic evaluation of immunotherapy-induced gastrointestinal toxicity. World J Gastrointest Endosc 2018; 10(12): 392-399
- URL: https://www.wjgnet.com/1948-5190/full/v10/i12/392.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i12.392
Immunotherapy is any treatment aimed at boosting or enhancing the immune system. It includes a wide range of options, from vaccines to treatment for conditions such as allergy and cancer. In the case of cancer, unlike other available treatments, immunotherapy is not aimed at destroying the tumor cells but at stimulating the patient’s immune system so that it attacks the tumor. In cancer, immunotherapy provides a series of advantages, such as targeted treatment, which only acts on tumor cells without damaging healthy cells, and immunological memory, which can subsequently be reactivated to recognize and attack the tumor once the immune system is stimulated. Immunotherapy is also subject to disadvantages, such as the time necessary for it to take effect-the immune response is not immediate but gradual-and the associated adverse effects. There are 2 main types of immunotherapy: Specific immunotherapy, which causes a response to a specific cell or antigen and includes vaccines and adaptive cell therapy; and nonspecific immunotherapy, which is aimed at stimulating the whole immune system and includes cytokines and regulatory proteins such as antibodies to cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) or the programmed cell death protein 1 and its ligand PDL1 (PD1/PDL1) pathway. Also of interest are monoclonal antibodies, which are included in another group, known as passive immunotherapy, and comprise molecules designed to recognize tumor cells or substances that the tumor needs for growth. These are administered intravenously and can destroy tumor cells or deprive them of the essential components they require for growth. They are sometimes combined with other treatments in order to enhance their effect[1,2].
Ipilimumab is a monoclonal anti-CTLA-4 immunoglobulin 1 (IgG1) antibody that activates destruction of regulatory T cells by stimulating antibody-mediated cytotoxicity, thus halting the immunosuppressive effect. Ipilimumab is currently approved for the treatment of advanced melanoma (unresectable or metastatic) in monotherapy or in combination with nivolumab (anti-PD1)[3-8].
The most advance therapy attempts to inhibit the PD-1/PD-L1 checkpoint pathway. The PD1 receptor is found in lymphocytes and acts as an inhibitory-type checkpoint by interacting with its ligands PDL1 and PDL2. Tumor cells are capable of expressing PDL1, thus inhibiting the action of the cytotoxic T cell on them and generating tolerance. In this way, anti-PD1 drugs (nivolumab, pembrolizumab, durvalumab) and anti-PDL1 drugs (avelumab, atezolizumab) overcome tumor immune tolerance and enable the action of cytotoxic T cells. Anti-PD1 and anti-PDL1 agents have several indications in various scenarios, both in monotherapy and in combination therapy in patients with melanoma, lung cancer, renal cell carcinoma, urothelial carcinoma, classic Hodgkin lymphoma, and head and neck tumors[9].
Toxicity of the various types of immunotherapy, or immune-related adverse effects, is closely associated with the mechanism of action of the different treatments, for example, the toxicity of interleukin 2 (IL-2) results from release of nitric oxide, IL-1, tumor necrosis factor alpha, and interferon gamma. In adoptive cell therapy, the initial toxicity is caused by lymphodepleting chemotherapy. After T-cell infusion, immunotoxicity manifests as fever, tachycardia, vascular hyperpermeability followed by multiorgan failure. In the most severe cases, this is due to cytokine release syndrome[10]. Blockade of CTLA-4 suppresses the function of regulatory T cells, which contribute to local inflammation in the gastrointestinal mucosa. It has been suggested that colitis associated with CTLA4 could result, in part, from this immunosuppressive function[11]. PD-1 and CTLA-4 blockade can generate toxicity that mimics autoimmune diseases[12].
Adverse effects involving the digestive system are recorded in around one-third of all patients receiving immunotherapy, specifically monoclonal anti-CTLA-4 IgG1 and anti-PD1 IgG4 antibodies[13].
Immune-mediated enterocolitis is one of the most common adverse effects, especially with ipilimumab. Up to one-third of patients treated with ipilimumab experience diarrhea, and immune-mediated colitis is observed in 7%-22% of cases[14]. In contrast, immune-mediated enterocolitis associated with nivolumab is less common, affecting around 10% of all patients who receive it[15]. The combination of ipilimumab and nivolumab is even more toxic than when each agent is used separately[8].
Colonoscopy with biopsies is the standard diagnostic approach for patients with lower digestive symptoms (e.g., diarrhea, hematochezia). It is recommended in patients who receive immunotherapy and have persistent diarrhea or associated poor prognostic factors (hospitalization due to oral intolerance or absence of response to corticosteroids). Upper gastrointestinal symptoms (e.g., dysphagia, gastroesophageal reflux, epigastralgia) are not uncommon and necessitate gastroscopy[16].
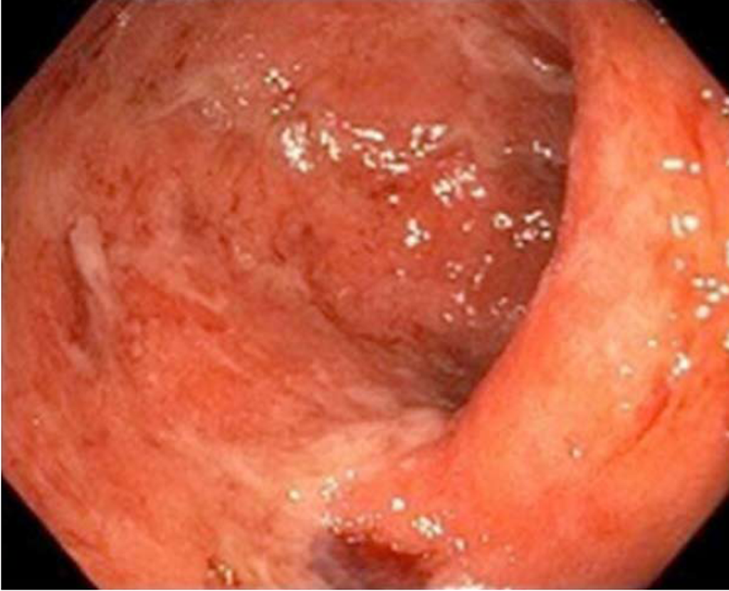
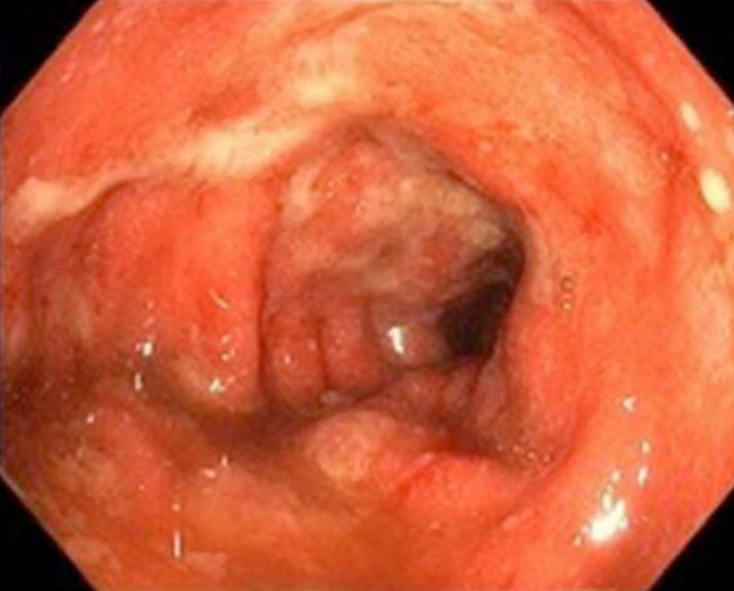
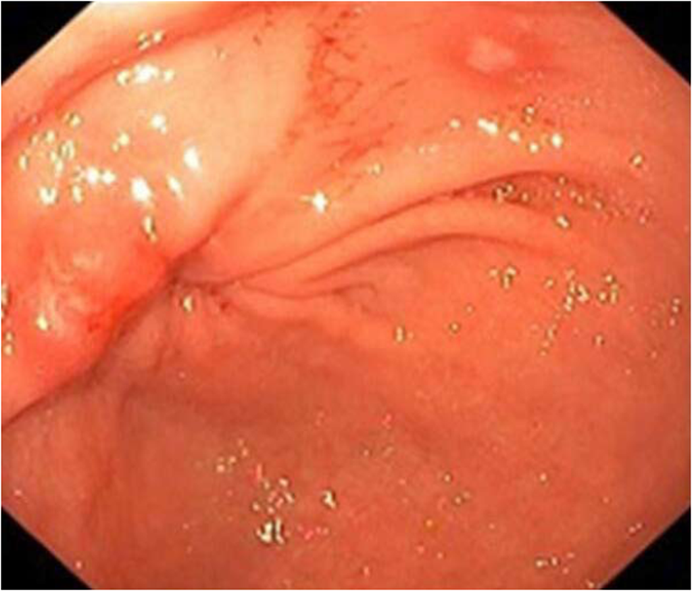
In the colon: In their series of 39 patients receiving anti-CTLA-4, Marthey et al[17] reported the most common lesions in endoscopy to be ulcer (79%) (Figures 1 and 2), erosion (13%), and erythema (8%) (Figure 3). The rectum and/or sigmoid colon were involved in 97% of cases, with extensive colitis being observed in 66% of patients. The distribution of the lesions was patchy in 55% of cases. The ileum was affected in only 5 patients.
In their study of 40 patients receiving treatment with anti-CTLA-4 agents who developed diarrhea and underwent flexible sigmoidoscopy or colonoscopy, Beck et al[18] reported findings for 36 cases. Again, the most common findings were erythema and ulcer (in 63% of patients). Endoscopy revealed inflammation several months after onset of enterocolitis, thus suggesting that in some cases, enterocolitis induced by anti-CTLA-4 agents can progress to inflammatory bowel disease (IBD).
We can find similar results for the type of involvement in immune-mediated colitis caused by anti-PD1 agents, which is less frequent than that associated with anti-CTLA-4 agents. Collins et al[15] studied a series of 20 patients with diarrhea who were receiving treatment with anti-PD1 agents and in 12 of whom colonoscopy findings were abnormal. The most frequent location was the descending colon (83%), and a patchy pattern, rather than a continuous pattern, was the most common (found in approximately 73% of patients). The most common lesions are also the same as those described above, namely, erythema, erosion, and ulceration.
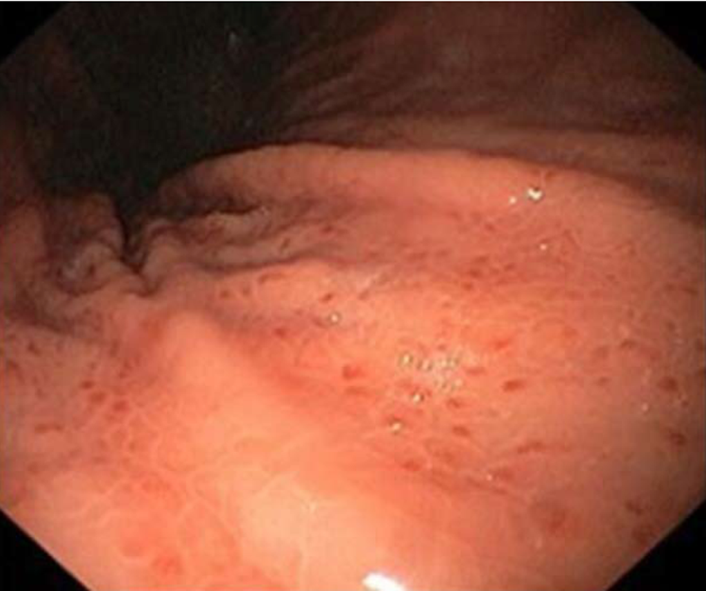
Esophagus-stomach-duodenum: In their study of 22 patients who underwent gastroscopy, Marthey et al[17] found lesions in 13 (60%), the most common being gastritis (Figures 4 and 5) and erosive duodenitis.
Similar results were reported by Collins et al[15], who found that 63% of patients also presented upper gastrointestinal lesions. Erythematous gastritis was reported in most cases.
Biliary tract: The scientific literature also contains references to biliary involvement, more specifically in cases of extrahepatic cholangitis[19] and toxic hepatitis[20]. In such cases, endoscopy can be based on retrograde cholangiopancreatography or cholangioscopy. To our knowledge, no endoscopic images of the biliary tract obtained by cholangioscopy have been reported.
Small intestine: To our knowledge, there are no published capsule endoscopy-based data on involvement of the small intestine as a result of toxicity induced by immunological therapy.
Other lesions seen in endoscopy include exudates, granularity, and loss of vascular pattern. These findings are similar to those of IBD (ulcerative colitis and Crohn disease). Endoscopy findings from the first 2 wk of treatment with the drug and before the onset of symptoms have not been shown to predict the development of immune-mediated colitis. Endoscopy-confirmed involvement and the need for infliximab are not more common in patients receiving high-dose anti-CTLA-4 agents, thus suggesting that the severity of enterocolitis is not dose-dependent[21,22].
Suspicion of toxicity due to immunotherapy should be based on the presenting complaints, of which diarrhea is the most common. Therefore, immune-mediated colitis should be taken into consideration in any patient receiving treatment with anti-CTLA-4 and/or anti-PD1 agents and who presents compatible symptoms. Other possible presenting complaints are abdominal pain, vomiting, hematochezia, weight loss, and/or fever. Onset of symptoms may be at any time during treatment and even several months after the last dose. The main conditions in the differential diagnosis are tumor progression, the infectious causes of diarrhea, and the development of IBD (Table 1). Therefore, imaging tests should also form part of the extension study for the primary tumor. Feces should also be tested for parasites and Clostridium difficile, and other tests, such as diagnostic colonoscopy, should be performed[21].
| Disease | Endoscopy findings | Clinical characteristics |
| IBD | UC: Continuous and circumferential mucosal inflammation starting in the rectum | Rectal bleeding, abdominal pain, diarrhea, chronic anemia |
| CD: Deep fissures, cobblestoning, segmental distribution, relative rectal sparing, and terminal ileal involvement | ||
| Radiation colitis | Similar to IBD | Rectal bleeding, chronic anemia |
| Infectious colitis | Diffuse effects on the colon | Dysentery-like diarrhea, different agents, Clostridium difficile and CMV to be ruled out |
| Colitis associated with diverticulosis | Segmental distribution, peridiverticular, sigmoid colon affected, rectum and proximal colon are normal | Rectal bleeding, abdominal pain, diarrhea |
| NSAID-induced colitis | Any part of the intestine, isolated lesions | Recurrent abdominal pain, obstruction, perforation, hemorrhage, chronic anemia |
| Microscopic colitis | Normal endoscopy findings | Watery diarrhea |
| Ischemic colitis | Segmentary colitis (sigmoid /left colitis) | Acute onset of abdominal pain and rectal bleeding |
The main laboratory abnormalities in patients receiving immunotherapy are anemia, increased C-reactive protein, and low levels of serum albumin, all of which are nonspecific and play no role in the differential diagnosis. Therefore, endoscopy is the key to diagnosis. However, the result of a macroscopically normal endoscopy does not rule out the diagnosis, and biopsy specimens should be taken throughout the colon and assessed according to the segment they came from. Furthermore, infection by cytomegalovirus should also be ruled out by immunohistochemical staining of the biopsy specimens.
Histopathology findings are compatible with acute colitis, which is characterized by a marked inflammatory cellular infiltrate in the lamina propria consisting of neutrophils, lymphocytes, plasma cells, and eosinophils. Occasional findings include foci of neutrophilic cryptitis, crypt abscess, gland destruction, and erosions of the mucosal surface[23].
The histological characteristics of immune-mediated colitis are often nonspecific and may mimic those of other types of colitis. However, a variety of histologic characteristics that can act as useful pointers have been reported. Active colitis, together with major apoptosis of the epithelial cells in the crypt, has been recognized as the most useful characteristic. Other, less common associated patterns include lymphocytic and collagenous colitis. The correlation with the clinical history and, in particular, exposure to the drug plays an essential role in enabling the pathologist to differentiate immune-mediated colitis from infectious colitis, IBD, and drug-related colitis[24].
Management of the patient with suspected immune-mediated enterocolitis should be multidisciplinary, involving oncologists, gastroenterologists, endoscopists, and the intensive care unit.
Treatment is mainly medical, and endoscopy is used only for diagnosis. Treatment of mild diarrhea (fewer than 3 watery stools per day) is based initially on oral antidiarrheal drugs together with fluid-electrolyte replacement. In moderate cases or absence of response, treatment should be started with oral corticosteroids (prednisone or equivalent at 0.5-1 mg/kg per day). In cases of severe diarrhea (more than 6 watery stools per day), treatment with anti-CTLA-4 and/or anti-PD1 agents should be suspended permanently, and intravenous corticosteroids should be started (methylprednisolone or equivalent 1-2 mg/kg per day). Patients who do not have a clinical response to intravenous corticosteroids after 3 d of treatment should start biologics (infliximab in a single dose of 5 mg/kg). The response to infliximab is generally fast, although some patients may require a second dose after 2 wk[21,25]. Marthey et al[17] reported that 37% of patients were treated successfully with corticosteroids. Biologic therapy was necessary owing to resistance to corticosteroids in 30% of cases (12 of 39 patients); infliximab was successful in 83% of cases (10 of 12 patients). Given the favorable response to infliximab, this therapy should be intensified rapidly in patients who do not respond to corticosteroids and whose clinical course is indolent. Treatment with corticosteroids during the first 5 d after onset of symptoms can enable more rapid resolution of symptoms than later initiation of treatment[26].
Perforation of the colon, while potentially fatal, is uncommon (< 1%). However, when surgery is necessary, colectomy should be subtotal and not segmental, since in most cases, enterocolitis induced by anti-CTLA-4 agents affects the whole colon[27-29].
Immune-mediated colitis is an emerging condition, given that the indications for immunotherapy, specifically anti-CTLA-4 and anti-PD1 agents, are expected to increase over time and for different types of tumor. Therefore, it is important to know the symptoms and determine the degree of involvement of immune-mediated colitis using endoscopy in order to initiate appropriate treatment early. The differential diagnosis should be based on infection, tumor progression, and IBD, with which the disease shares symptoms, endoscopy-confirmed lesions, and treatment.
The most common endoscopy-confirmed lesions of immune-mediated colitis are ulcers and erosions on edematous and erythematous mucosa. The most common location is the sigmoid colon, and a segmental pattern is slightly more common than a continuous pattern. Although histopathology is not specific in immune-mediated colitis, a biopsy must be taken to rule out other diseases and make a definitive diagnosis. Treatment includes systemic corticosteroids, although biologic therapy with infliximab may be necessary in some cases. Lastly, we believe that it is of the utmost importance to perform new studies that provide a detailed description of the adverse effects of regulatory proteins and of new, forthcoming agents in order to improve recognition and treatment of immune-mediate colitis in daily clinical practice.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fiori E, Kamimura K, Tseng PH S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 3604] [Article Influence: 450.5] [Reference Citation Analysis (0)] |
| 2. | Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 384] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 3. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1709] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 4. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 5. | Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 810] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 6. | Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712-4717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 751] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 7. | Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 532] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 8. | Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3331] [Cited by in RCA: 3409] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 9. | Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1367] [Cited by in RCA: 1243] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 10. | Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33:2092-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 524] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 11. | West NR, Powrie F. Immunotherapy Not Working? Check Your Microbiota. Cancer Cell. 2015;28:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1572] [Article Influence: 174.7] [Reference Citation Analysis (0)] |
| 13. | Ibrahim RA, Berman DM, DePril V, Humphrey RW, Chen T, Messina M, Chin KM, Liu HY, Bielefield M, Hoos A. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29:15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1108] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 15. | Collins M, Michot JM, Danlos FX, Mussini C, Soularue E, Mateus C, Loirat D, Buisson A, Rosa I, Lambotte O. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF, Bourdier de Beauregard M. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 18. | Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 647] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 19. | Kashima J, Okuma Y, Shimizuguchi R, Chiba K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother. 2018;67:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 20. | Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, Parkinson CA, Corrie PG. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2:e000268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119-iv142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1504] [Article Influence: 188.0] [Reference Citation Analysis (1)] |
| 22. | Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S, Targan SR, Wang HL. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PubMed] |
| 23. | García-Varona A, Odze RD, Makrauer F. Lymphocytic colitis secondary to ipilimumab treatment. Inflamm Bowel Dis. 2013;19:E15-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Vieth M, Montgomery E. Medication-associated gastrointestinal tract injury. Virchows Arch. 2017;470:245-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Andrews S, Holden R. Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag Res. 2012;4:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | O’Day S, Weber JS, Wolchok JD, Richards JM, Lorigan P, McDermott DF, Urba WJ, DePetril V, Heller KN, Ibrahim RA. Effectiveness of treatment guidance on diarrhea and colitis across ipilimumab studies. J Clin Oncol. 2011;29:8554-8554. |
| 27. | Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 819] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 29. | Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |