Published online Nov 16, 2018. doi: 10.4253/wjge.v10.i11.367
Peer-review started: August 7, 2018
First decision: August 24, 2018
Revised: September 26, 2018
Accepted: October 6, 2018
Article in press: October 7, 2018
Published online: November 16, 2018
Processing time: 102 Days and 18.2 Hours
To investigate the role of a novel minimally invasive endoscopic technique in the management of tight near-total corrosive strictures of the proximal esophagus involving the hypopharynx.
Two patients with near-total corrosive strictures of the proximal esophagus involving the hypopharynx were managed with the novel endoscopic technique. The technique involved passing a 0.025-inch flexible guide-wire across the stricture, and stricture dilatation, using 10F coaxial diathermy and balloon dilators, followed by electro-incision of the proximal aspect of the residual eccentric stricture by means of a novel approach using a wire-guided sphincterotome.
Both patients were successfully managed on an outpatient department basis with the complete relief of symptoms and resolution of strictures on endoscopy and an esophagogram. No adverse events were seen during or after the procedure. There was no recurrence of symptoms at a follow-up of over a year in both cases. There was a significant improvement in the body mass index of both patients after the procedure.
We report a novel flexible endoscopic technique for the management of complex hypopharyngo-esophageal strictures. In experienced hands, the procedure is relatively simple, safe and effective with a durable response.
Core tip: In this study, we evaluated the minimally invasive endoscopic management of near-total benign fibrotic strictures of the proximal esophagus involving the hypopharynx across the pharyngo-esophageal junction. Both patients were successfully treated using a novel approach: stricture dilatation (with a 10F co-axial diathermic dilator and through-the-scope balloon) followed by the electroincision of residual adhesions at the hypopharyngeal base with a wire-guided sphincterotome. To the best of our knowledge, this report represents the tightest esophageal or hypopharyngeal strictures ever opened endoscopically and reported in the literature.
- Citation: Dhaliwal HS, Kumar N, Siddappa PK, Singh R, Sekhon JS, Masih J, Abraham J, Garg S. Tight near-total corrosive strictures of the proximal esophagus with concomitant involvement of the hypopharynx: Flexible endoscopic management using a novel technique. World J Gastrointest Endosc 2018; 10(11): 367-377
- URL: https://www.wjgnet.com/1948-5190/full/v10/i11/367.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i11.367
The ingestion of corrosives leading to severe gastrointestinal (GI) injury is a common problem in India due to the relatively easy access to acids[1]. After acute ingestion, progressive cicatrisation sets in over the next 4-6 wk in cases with a severe injury, leading to benign fibrotic strictures of the upper GI tract[2]. Fibrotic strictures after acid ingestion affect both the esophagus and the stomach in almost equal proportions, with esophageal involvement mostly limited to the mid and distal esophagus[1]. Because of the socio-economic conditions prevailing in India, occasional neglected cases of acid ingestion may present late with tight near-total strictures of the esophagus. The situation is even more challenging if the near-total stricture afflicts the proximal esophagus with contiguous involvement of the hypopharynx. Surgery remains the only viable management option in this difficult scenario; however, because of extreme malnutrition, many patients may even be unfit for the surgery. The surgical options include a muscle flap inlay, gastric pull-up and esophageal substitute (colon or ileo-colon) interposition, all of which are associated with high morbidity and mortality[3,4,5]. The surgical outcomes are particularly worse if there is hypopharyngo-esophageal involvement, compared to the scenario if both hypopharynx and cervical esophagus are spared[4]. We report for the first time the successful flexible endoscopic management of near-total corrosive strictures of the proximal esophagus involving the hypopharynx, using a novel technique.
This study was a retrospective analysis of the prospectively collected records of the demographic data, radiological findings, endoscopic procedural details, post-procedural course and follow-up of 2 patients with one or more near-total strictures of the proximal esophagus along with hypopharyngeal involvement (as defined below). These cases were managed by us between January 2016 and December 2017.
A near-total stricture was defined as a very thin streak of oral contrast crossing the stricture on an esophagogram, and endoscopically, while a flexible guide-wire could be negotiated with difficulty across the stricture, a balloon dilator could not be entered into the stricture over the guide-wire even with best efforts.
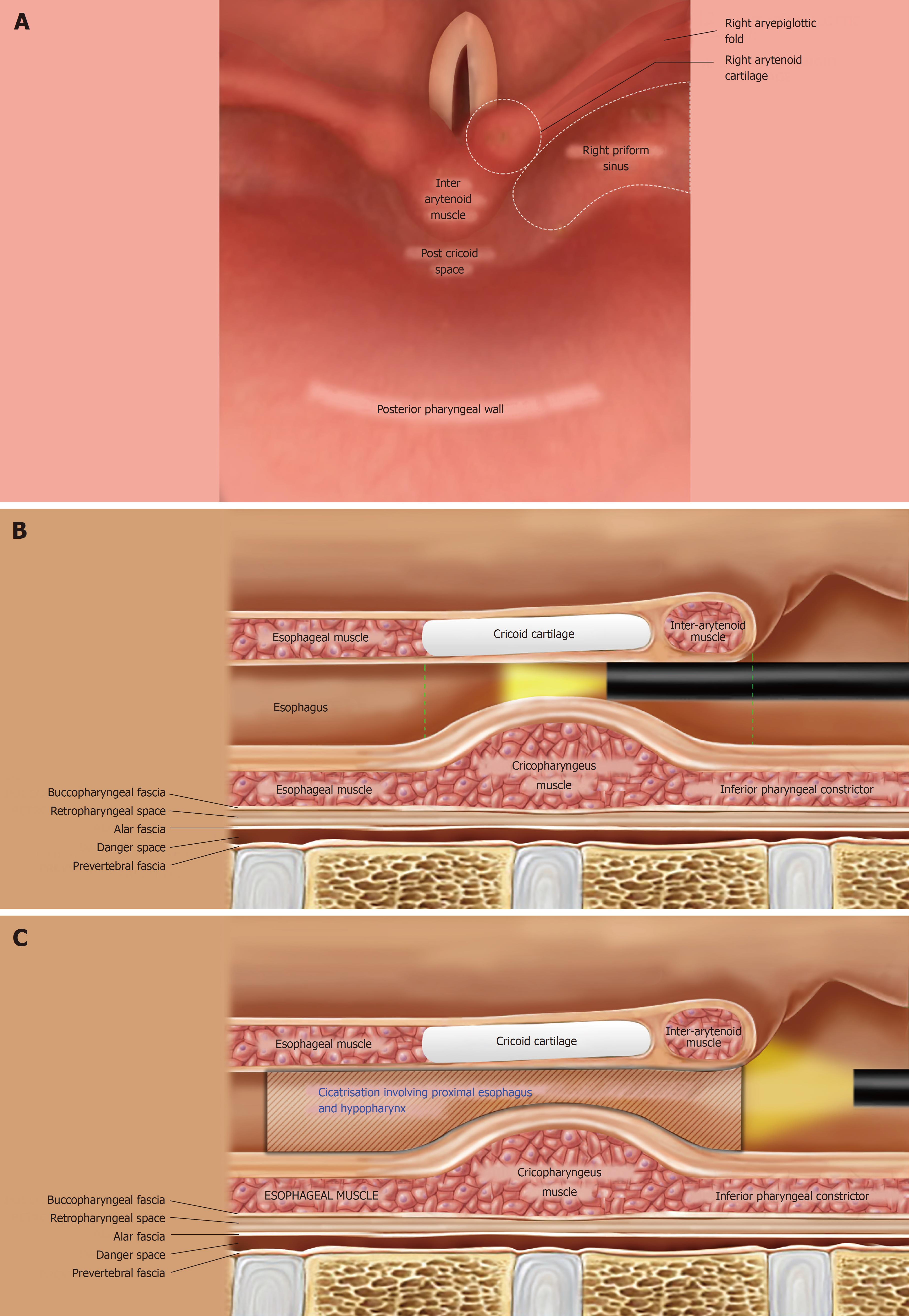
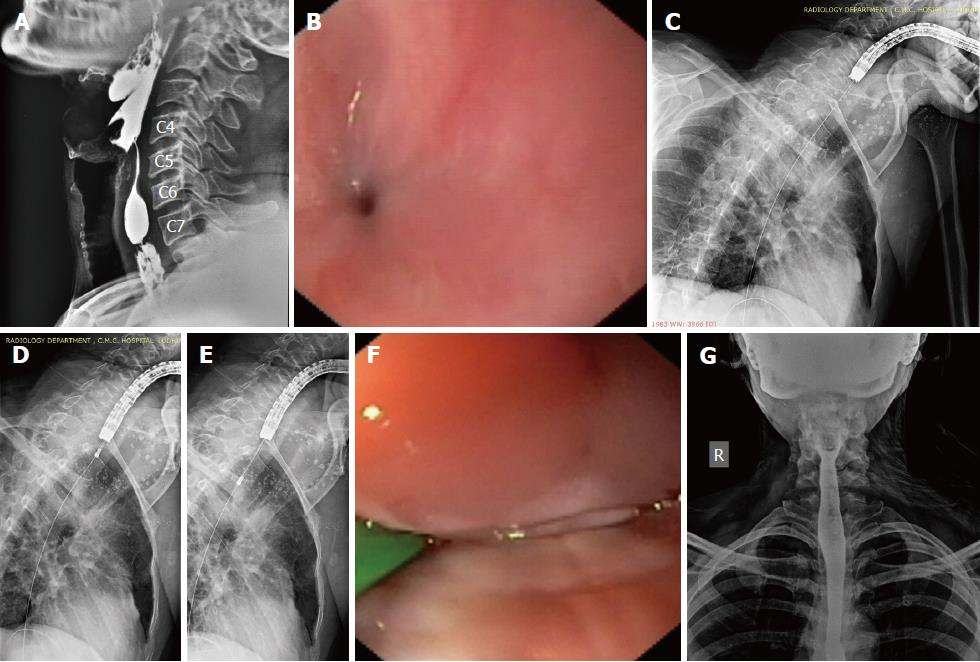
The hypopharynx, the most inferior part of the pharynx, extends from the level of the upper border of the epiglottis to the lower border of the cricoid cartilage and comprises 3 parts: the piriform sinuses, the post-cricoid region and the posterior pharyngeal wall (Figure 1A and 1B). The cricopharyngeus muscle (CP) is a circular muscle attached anteriorly to the cricoid cartilage (Figure 1B); within the 2-4 cm long upper esophageal sphincter (UES), the CP constitutes the zone of maximal UES pressure, which is approximately 1 cm in length. The apices of the piriform sinuses open into the proximal esophagus across the relaxed CP. The CP normally lies at the level of the C5-C6 vertebrae on a lateral neck X-ray film; while the piriform sinuses lie immediately above this level. The esophagus begins at the level of the C6 vertebra.
The cicatrisation in our cases had sufficient hypopharyngeal involvement such that it extended almost to the proximal (cranial) aspect of the post-cricoid region (Figure 1C) and the adjoining parts of the apices of both piriform sinuses (in other words, the stricture involved the CP and extended a few millimeters proximal to the CP as well). Furthermore (based on our previous experience as well), near-total strictures with such a proximal extension communicate with only one of the 2 piriform sinuses (generally the right piriform sinus) while the post-cricoid region and the contralateral piriform sinus are completely obliterated. As a result, the proximal part of the stricture appeared eccentric on the antero-posterior (AP) view of an esophagogram.
Both patients presented to us with absolute dysphagia. The initial management at presentation consisted of administration of intravenous fluids to correct the dehydration, basic blood investigations including complete blood counts, serum electrolytes, renal function tests, chest radiographs and correction of any underlying dyselectrolytemia or acid-base imbalance. Any history of chronic cough, hoarseness of voice and respiratory distress was probed and an otolaryngology (ENT) evaluation was performed to rule out any significant tracheal involvement or airway compromise.
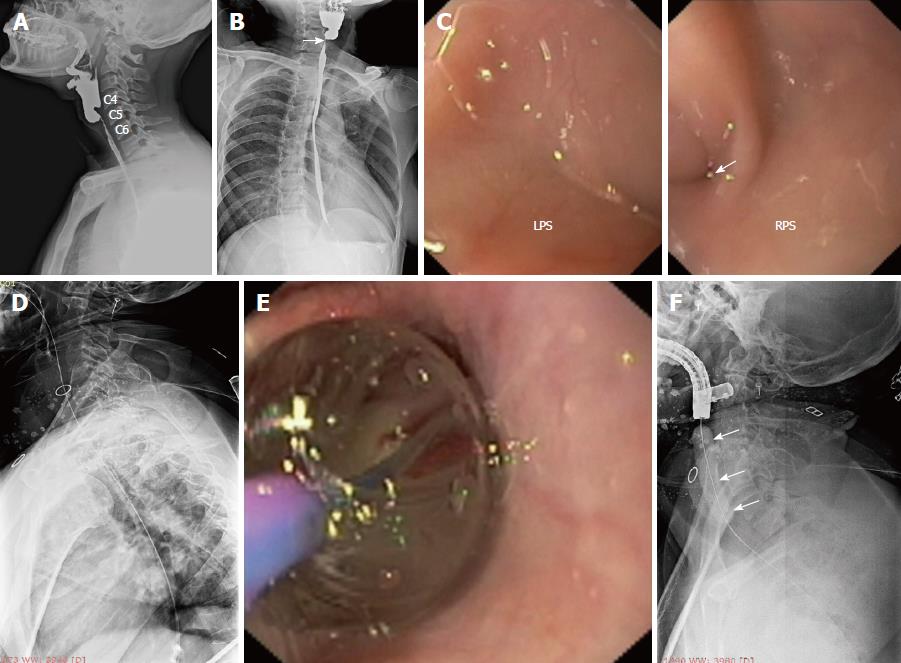
Both patients underwent multidisciplinary discussion, and a minimally invasive endoscopic therapy was suggested for the relief of absolute dysphagia. The therapeutic procedures were carried out in accordance with the Helsinki declaration. Institutional review board approval was not obtained due to the “time-sensitive nature” of the endoscopic therapy; any further delay while obtaining approval was likely to allow the conversion of the near-total stricture (Figure 2A, 2B) into a total stricture and, hence, potentially depriving the patients of life-saving minimally invasive therapy. Furthermore, both patients were severely malnourished and were also at risk of aspiration pneumonitis due to oro-pharyngeal secretions; hence an immediate action was highly desirable. As part of the informed consent process, the investigational nature of the endoscopic procedure and the potential complications (as discussed below) were explained to the patients. The discussion and the patient’s understanding that the endoscopic therapy was being utilized in an off-label fashion were documented in the medical records.
Both patients were severely malnourished at presentation and were considered high risk candidates for deep sedation. The procedure was performed under conscious sedation in the left lateral position with oxygen supplementation via nasal prong and the careful monitoring of vital parameters. The oropharyngeal secretions were adequately suctioned to prevent aspiration and to gain a clear view. Intermittent suctioning of oropharyngeal secretions with a catheter was also performed by a nurse during the procedure. Once the minute opening was identified at the apex of a piriform sinus (Figure 2C, arrow), a 0.025-inch flexible guide-wire with a straight tip (Jagwire; Boston Scientific, Natick, MA, the United States) was passed across the stricture (Figure 2D). The correct placement of the guide-wire was confirmed fluoroscopically. Subsequently, an attempt was made to pass a 6-8 mm, wire-guided, through-the-scope (TTS) balloon dilator over the wire. When this was not possible, the stricture was defined as near-total.
Stricture dilatation: The near-total strictures were dilated using a co-axial diathermic dilator, followed by a balloon dilator. Under endoscopic guidance, a 10F co-axial diathermic dilator (Cysto-Gastro-Set, Endo-Flex, Voerde, Germany) was threaded over the guide-wire [taken within the channel of a therapeutic gastroscope (GIF1TQ140 or GIF1TQ160, Olympus, Tokyo, Japan)] to the proximal part of the stricture. At this point, an intermittent diathermy current (cut mode, 50W, ERBE electrosurgical unit, Tubingen, Germany) was applied in steps while the endoscopist gently pushed forward the diathermic dilator under fluoroscopic guidance (Video 1) and the assistant held the wire in traction. In this way, the whole stricture segment was traversed by the tip of the diathermic dilator. Subsequently, the diathermic dilator was removed and a 6-8 mm, wire-guided, TTS balloon (CRE; Boston Scientific, Natick, MA, the United States) was passed over the guide-wire to further dilate the strictured segment (Figure 2E, 2F). A nasogastric tube was placed to initiate feeding and subsequent dilatations were attempted every week in an incremental manner with the wire-guided balloon dilators (up to 14 mm).
The strictures in both cases were felt undilatable as the balloon dilators could not open up the adhesions in the post-cricoid space and in the blocked piriform sinus (Video 2). A repeat esophagogram was performed to evaluate the status of the residual stricture (Figure 3A). In both cases, the esophagogram showed (as expected) the passage of oral contrast from one piriform sinus, while the other piriform sinus was still blocked. Both cases were subsequently subjected to electroincision therapy using a wire-guided sphincterotome, as described below.
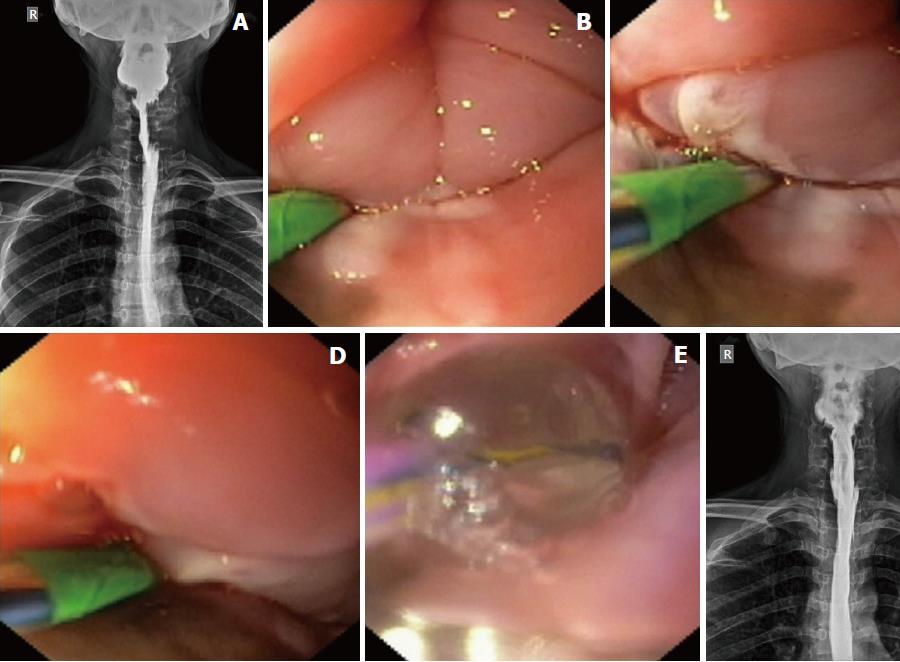
Electroincision therapy: The platelet count and coagulation parameters were checked before the procedure. A flexible guide-wire was passed across the stricture into the stomach, and a sphincterotome (Ultratome XL, Boston Scientific, MA, the United States) was threaded over it such that the cutting-wire of the instrument faced the side of the blocked piriform sinus and few millimeters of the cutting-wire were left above the proximal aspect of the residual stricture. At this point, a current (Endocut I, effect 2, 60W, VIO 300D; ERBE, Tubingen, Germany) was applied in steps to cut the adhesions, beginning at the adhesioned post-cricoid space and slowly cutting towards the blocked piriform sinus until the lateral pharyngeal wall was encountered (Figure 3B-D; Video 3). Care was taken to cut along the line of the expected endoluminal space without hitting the anterior laryngeal structures or the posterior pharyngeal wall. The scope (along with sphincterotome) was also pushed distally through the electroincised piriform sinus to cut the deeper (distal) adhesions (of the proximal esophagus) under vision (shown in the last part of Video 3). Subsequently, a balloon dilator (12-15 mm) was inflated within the electroincised piriform sinus (Figure 3E) to disrupt any residual fibrotic strands, followed by the removal of both the wire and balloon.
The scope could now be easily pushed into the esophagus from both piriform sinuses. A careful evaluation of the hypopharynx, vocal cords and proximal esophagus was done to rule out any complication arising from the procedure. The patients were observed in the hospital until evening. They were kept nil per oral for 6 h and were given intravenous fluids and antibiotics. In the absence of any complication, they were discharged from the hospital the same evening. Repeat endoscopic evaluation (Video 4) and esophagography (Figure 3F) were performed after 2 wk to confirm the disappearance of the stricture. An ENT evaluation was also performed at this visit to rule out any injury to the laryngeal nerves during the procedure.
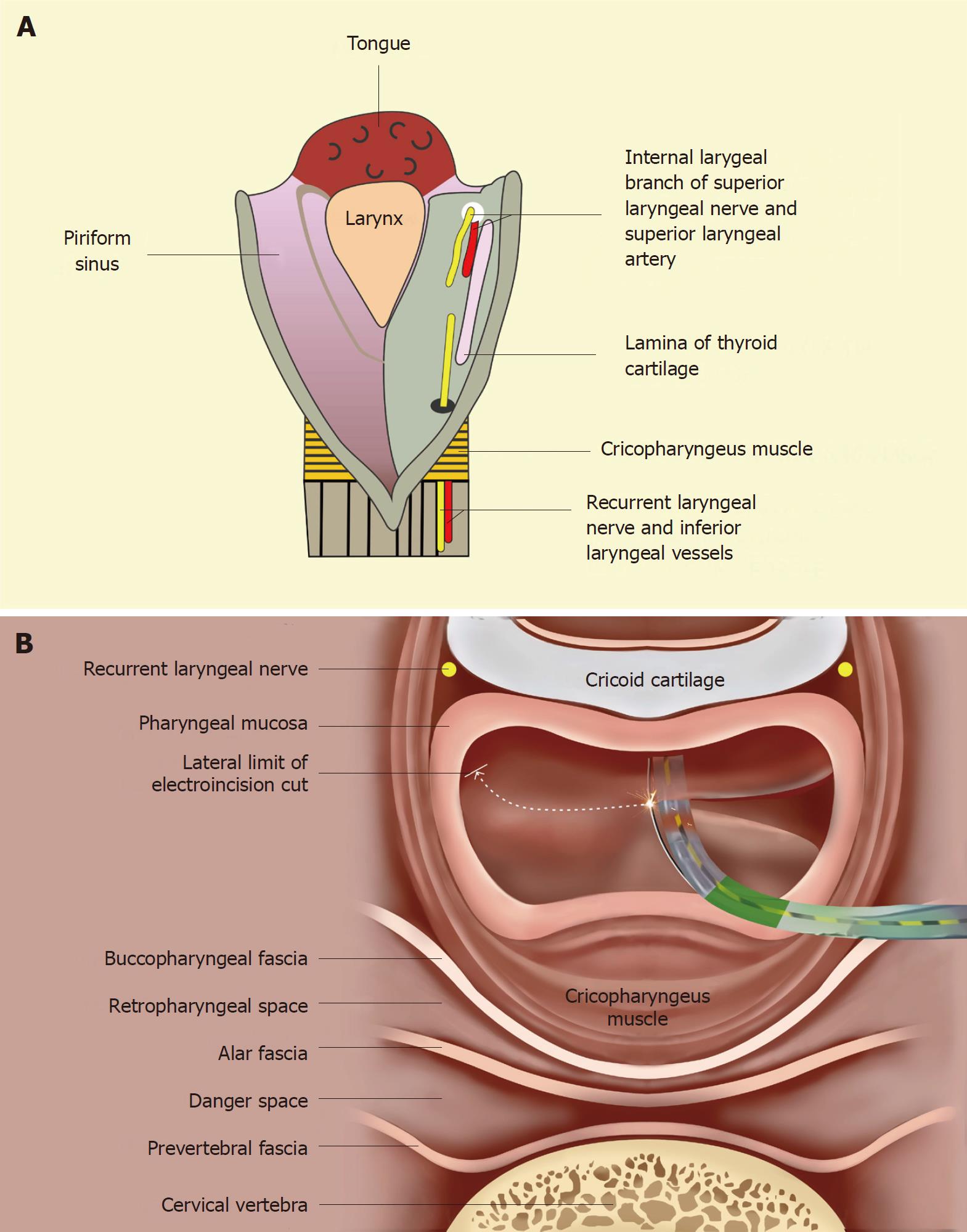
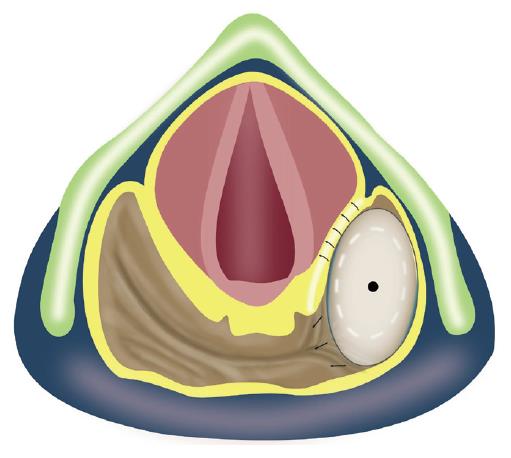
The dilatation and electroincision of GI strictures can potentially result in several complications, such as perforation, bleeding, pulmonary aspiration and pain. The standard measures used to prevent these complications have been elaborately discussed elsewhere[6] and were followed in this study. Besides the aforesaid complications, there is an additional concern while addressing hypopharyngeal strictures with the technique described above. As shown in Figure 4A, the laryngeal nerves and vessels lie submucosally in the anterior aspect of the piriform sinuses. The internal branch of the superior laryngeal nerve lies cranially (proximally), while the recurrent laryngeal nerve lies caudally (inferiorly). An injury to the former may result in anesthesia of the laryngeal mucous membrane as far inferiorly as the vocal cords. An injury to the recurrent laryngeal nerve may lead to ipsilateral vocal cord paralysis. Hence, as shown in Figure 4B, the line of electro-incision should stop several millimeters before reaching the anterior aspect of the piriform sinus.
The critical step was to pass the guide-wire across the stricture under combined endoscopic and fluoroscopic guidance. Because the strictures were very tight, this was a cumbersome step and required multiple attempts in both the cases. The guide-wire could not be passed in the first session in the first patient; the procedure was deferred for 3 d so that the tissue edema (because of multiple attempts) could resolve before a repeat attempt was made.
After the initial dilatation with the diathermy dilator and TTS balloon, the esophagograms showed residual eccentric stricture in both of the patients. Hence both of them were subjected to electro-incision. However, the distal esophageal stricture in case 2 was concentric (Figure 5) and hence was managed with dilatation alone (no electroincision was employed). There were no intra-procedural or post-procedural complications. The patients were followed up through regular outpatient department visits (every 3-4 mo) and various outcome measures were noted (Table 1). There was no recurrence of symptoms during the follow-up (22 mo in the first case and 14 mo in the second case). The body mass index improved in both of these patients after the endoscopic intervention.
| Case 1 | Case 2 | ||
| Demographic details | Age/Sex | 37/F | 42/M |
| BMI (kg/m2) at presentation | 13.2 | 15.6 | |
| Acid consumed | Sulphuric acid | Nitric acid | |
| Time since acid was consumed | 4 mo | 11 mo | |
| No. of strictures on esophagogram | 1 | 2 | |
| Approximate length of stricture | 3.5 cm | 4 cm (proximal stricture) | |
| 5 mm (distal stricture) | |||
| Near-total stricture communicated with which piriform sinus | Right piriform sinus | Right piriform sinus | |
| Concomitant gastric stricture | No | No | |
| Whether feeding jejunostomy was performed? | No | No | |
| Endoscopic procedural details | Time taken for the passage of guide-wire across the stricture | First session: Failed | 14 min |
| Second session: 22 min | |||
| Dilatation details | |||
| First balloon dilatation till | 6 mm | 6 mm | |
| No. of dilatations to reach 14 mm | 3 | 4 | |
| Residual stricture after dilatation on esophagogram | Yes | Proximal stricture: Yes | |
| Distal stricture: No | |||
| Time taken for electro-incision | 12 min | 10 min | |
| Primary outcome | Complete relief of dysphagia along with the resolution of stricture(s) on esophagogram and endoscopy, performed after 2 wk of full endoscopic therapy | Yes | Yes |
| Secondary outcomes | Intraprocedural complication | None | None |
| Post-procedural complication | None | None | |
| Duration of the follow-up | 22 mo | 14 mo | |
| Improvement in activities after the procedure | Yes | Yes | |
| Recurrence of dysphagia during the follow-up or any need of additional therapy | No | No | |
| Any regurgitation episode during the follow-up | No | No | |
| Any aspiration episode during the follow-up | No | No | |
| BMI at last follow-up (kg/m2) | 21.6 | 23.8 | |
The tight near-total corrosive stricture of the proximal esophagus with hypopharyngeal involvement is a uniquely challenging situation. Surgical intervention, the only viable management option to date, is associated with high morbidity and mortality. Minimally invasive therapeutic options, including endoscopic techniques, are an unmet yet highly desirable need for such difficult-to-treat patients. In this study, we evaluated the minimally invasive endoscopic management of near-total benign fibrotic strictures of the proximal esophagus involving the hypopharynx across the pharyngo-esophageal junction. Both patients were successfully treated using a novel approach: stricture dilatation (with a 10F co-axial diathermic dilator and TTS balloon) followed by the electroincision of residual adhesions at the hypopharyngeal base with a wire-guided sphincterotome. To the best of our knowledge, this report represents the tightest esophageal or hypopharyngeal strictures ever opened endoscopically and reported in the literature.
A diathermic dilator (with an 8.5F or 10F tip) was first used for cysto-gastrostomy or cysto-duodenostomy[7]. Subsequently, Kawakami et al[8] reported the successful management of severe biliary or pancreatic strictures using a 6F diathermic dilator. Siddappa et al[9] used a 6F diathermic dilator for negotiating near-total antropyloric corrosive strictures in 3 patients. The present study is the first report on managing near-total esophageal or hypopharyngeal strictures using a diathermic dilator. In contrast to Siddappa et al[9], we used a 10F diathermic dilator (instead of a 6F size), as we believe that this is quite safe for GI tract strictures and that a larger diameter diathermic dilator ensures the easy performance of subsequent balloon dilatation. In our opinion, a 6F diathermic dilator is only to be preferred for strictures of thin-walled structures such as the pancreatic or bile ducts.
In both cases, dilatation (with a diathermic dilator followed by a balloon dilator) did not completely open up the stricture, especially the proximal part of the stricture. The residual adhesions obliterating the post-cricoid space and the apex of the contralateral piriform sinus needed to be addressed to prevent the recurrence of symptoms as well as the pooling of secretions (which may lead to aspiration pneumonitis). These adhesions were electroincised using a wire-guided sphincterotome. Both the endoscopic electroincision of strictures in the pharyngo-esophageal region as well as the use of the wire-guided sphincterotome for the electroincision of GI strictures are described for the first time in this report. The electroincision of short-segment benign strictures in the more distal locations of the GI tract using a needle-knife has been extensively described[10,11,12]. The electroincision of adhesions in the pharyngo-esophagus is more challenging because of the surrounding vital structures and the lack of adequate space and visualization. At this location, we preferred electroincision with the wire-guided sphincterotome over a free-hand incision with a needle-knife. The guide-wire helps to orient the sphincterotome so that the plane of incision takes the right vertical (cranio-caudal) path [the curve of the horizontal path (Figure 4B) being assessed while looking down from the unfibrosed proximal hypopharynx] without inadvertently injuring the undesired tissue, which is quite possible with a needle-knife. The guide-wire also provides better control over the movement of the cutting-wire than does a free-hand technique. More-over, the longer length of the cutting-wire of the sphincterotome helps in cutting the deeper (distal) adhesions as well, compared to the relatively small length of a needle-knife. Both of our patients required only 1 session of electroincision with the wire-guided sphincterotome, despite the relatively long length of the strictures (Table 1). Hordijk et al[10] previously showed that with a needle-knife, anastomotic strictures 1.5-5 cm in length required a mean of 3 electroincision sessions to become symptom-free. It remains to be seen based on future randomized prospective studies whether the sphincterotome actually has an edge over a needle-knife in reducing the number of electroincision sessions for longer strictures.
Strictures of the proximal esophagus with concomitant hypopharyngeal involvement are challenging to manage endoscopically and are associated with a high recurrence rate. Tharavej et al[13] reported a favorable outcome in only 1 out of 28 such patients. One possible explanation for such high failure rates could be the lack of instituting electroincision after dilatation. Dilatation alone is insufficient to completely open up the hypopharyngeal strictures due to the interposition of laryngeal structures at the medial aspect of the dilating balloon placed in one of the piriform sinuses during dilatation (Figure 6). In that study[13], the detailed information on the appearance of the hypopharyngeal strictures was not provided; therefore the length and severity of the single case of hypopharyngeal stricture that responded to dilatation alone are not clear.
For the anatomical reasons illustrated in Figure 4, injury to the laryngeal nerves (particularly the recurrent laryngeal nerve) remains a potential complication of the technique described in this report. In the literature, there are isolated reports of recurrent laryngeal nerve injury after endoscopic procedures[14,15]. The placement of a self-expanding metal stent in the upper esophagus led to bilateral nerve injury by causing persistent compression of the bilateral nerves in the tracheo-esophageal groove[14]. However, injury to this nerve in the piriform sinus during dilatation is unlikely as there is only transient compression on the nerve for a few minutes. There is only one recent report of recurrent nerve injury in the piriform sinus after a bougie dilator (Savary Gillard dilator) was used[15]; the bougie dilator might have caused a longitudinal shearing force to avulse the mucosa of the piriform sinus and, subsequently, caused submucosal injury to the nerve. On the other hand, a balloon dilator only produces a transient radial force which should not avulse the mucosa, and nerve injury after balloon dilatation has never been reported, thus justifying the approach used by us to prevent injury to the laryngeal nerves, without leaving any residual stricture behind. The electro-incision cut was stopped a few millimeters before reaching the anterior aspect of the piriform sinus, and the remaining adhesions in this region were disrupted using a balloon dilator through the electroincised piriform sinus.
Traditionally, open surgical techniques had been the only viable management option for near-total hypopharyngo-esophageal strictures. However, surgery is associated with unacceptable morbidity and mortality. Wu et al[4] described the results of the surgical reconstruction of corrosive hypopharyngo-esophageal strictures in their retrospective series of 50 patients. The esophageal substitute was pulled up and anastomosed to the hypopharynx. One patient died intraoperatively and 8 patients (16%) had major early post-operative complications (leakage of the anastomosis, intestinal obstruction, obstruction of the esophageal substitute, graft failure and pneumonia). There were 10 major late complications: 6 had late stenosis of the hypopharyngeal anastomosis, 3 had intestinal obstruction and 1 had failure of the transplanted ileo-colon. Post-operatively, the swallow functions were unsatisfactory in 16% of the patients. The authors compared these results with another retrospective cohort of 102 patients of corrosive ingestion with an unstenosed hypopharynx who underwent esophageal substitute anastomosis with the cervical esophagus. The surgical outcomes in the 50 patients with hypopharyngeal involvement were significantly worse; on the other hand, among the patients with an unstenosed hypopharynx, only 6.8% (7/102) had major early complications and 93% (95/102) had normal swallow functions post-operatively.
In the past, short-segment cricopharyngeal strictures have also been managed by the endoscopic surgical techniques (rigid endoscopy) employed by ENT surgeons[16,17]. Similar to the endoscopic cricopharyngeal myotomy for Zenker’s diverticulum, there are important differences in the management of cricopharyngeal strictures as well, using flexible versus rigid endoscopic techniques. The potential advantages of flexible endoscopy over rigid endoscopy in this context have been discussed in detail elsewhere[18]. Moreover, the rigid endoscopic technique requires expensive equipment (e.g., a CO2 laser), while flexible endoscopy utilizes the routine accessories used for stricture dilatation and endoscopic retrograde cholangiopancreatography (ERCP). The post-operative recovery period is a few days to weeks in rigid endoscopy, while the patient can be discharged the same day after flexible endoscopic management. Lastly, ENT surgeons can only handle cricopharyngeal strictures; cases with adjacent esophageal cicatrisation (as was seen in both of our cases) would be best managed by gastroenterologists using the flexible endoscopic technique. Table 2 enumerates the important differences between the open surgical, rigid endoscopic and flexible endoscopic techniques for the management of hypopharyngeal strictures.
| Surgery | Rigid Endoscopy | Flexible Endoscopy | |
| Hospital admission | Required | Required | Not required |
| Performed by | Gastro-surgeons in the operation theatres | ENT surgeons in the operation theatres | Gastroenterologists or surgical endoscopists in the endoscopy suites |
| Hyper-extension of the patient’s neck | Not required | Required | Not required |
| Type of anesthesia given | General anesthesia | General anesthesia | Conscious sedation |
| Anesthetic and procedural time | Longest | Long | Short |
| External incision over the neck or chest wall | External incision is given. This predisposes to post-operative complications like fistula, wound infection and hematoma formation | Not given | Not given |
| Concomitant esophageal cicatrisation | Can be tackled | Cannot be tackled | Can be tackled |
| Clinical recovery after the procedure | Slow | Intermediate | Quick |
| Morbidity and mortality associated with the technique | High | Low | Least |
| Contraindications | Elderly patients with comorbidities | Short neck | None |
| Severe malnutrition | Retrognathia | ||
| Inability to give general anesthesia | Inability to give general anesthesia | ||
| Experience with the procedure till date | Maximum | Limited | Limited |
The main limitations of our study are the limited number of patients included and the retrospective nature of the study. Given the rarity of near-total strictures of the pharyngo-esophagus, a large sample size for conducting a prospective study is not expected. We have shown that the minimally invasive endoscopic management of this complex problem is feasible and can be offered to such patients, rather than blindly subjecting them to surgery.
The ingestion of corrosives may lead to tight near-total strictures of the esophagus with concomitant involvement of the hypopharynx. This is an extremely challenging situation for the clinicians and there are limited treatment options for addressing such complex strictures.
The only viable management option for complex hypopharyngo-esophageal strictures is surgery. However, surgical interventions are associated with high morbidity and mortality. Moreover, patients may even be unfit for undergoing surgery due to extreme malnutrition. Because of these reasons, many patients ultimately succumb to their illness. The development of minimally invasive endoscopic techniques would be a highly desirable step to salvage this subset of patients.
In this study, we evaluated a novel flexible endoscopic technique for the management of tight near-total corrosive strictures of the esophagus with concomitant involvement of the hypopharynx.
Two patients with near-total hypopharyngo-esophageal strictures were managed by the novel technique, under conscious sedation. A flexible 0.025-inch guide-wire was passed across the stricture, followed by dilatation of the stricture with a 10F coaxial diathermy and balloon dilators. The residual eccentric stricture was electroincised by a novel approach, using a wire-guided sphincterotome.
Both patients were successfully managed on an OPD basis with the complete relief of symptoms and resolution of strictures on endoscopy and an esophagogram. No complication was seen during or after the procedure. In the follow-up, patients remained symptom-free, along with a significant improvement in the body mass index.
The complex hypopharyngo-esophageal strictures were successfully opened up with the novel flexible endoscopic technique, without any complications.
We report a novel minimally invasive flexible endoscopic technique for addressing the tight near-total hypopharyngo-esophageal strictures. The technique is relatively simple, safe and effective with a durable response. But larger studies are required to validate the efficacy and safety of the technique across different endoscopic set-ups.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Zhu YL, Chen JQ S- Editor: Dou Y L- Editor: A E- Editor: Song H
| 1. | Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of corrosive acids. Spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 115] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Di Costanzo J, Noirclerc M, Jouglard J, Escoffier JM, Cano N, Martin J, Gauthier A. New therapeutic approach to corrosive burns of the upper gastrointestinal tract. Gut. 1980;21:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ananthakrishnan N, Parthasarathy G, Maroju NK, Kate V. Sternocleidomastoid muscle myocutaneous flap for corrosive pharyngoesophageal strictures. World J Surg. 2007;31:1592-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Wu MH, Tseng YT, Lin MY, Lai WW. Esophageal reconstruction for hypopharyngoesophageal strictures after corrosive injury. Eur J Cardiothorac Surg. 2001;19:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Yannopoulos P, Lytras D, Paraskevas KI. Esophageal reconstruction with intraoperative dilatation of the hypopharynx for the management of chronic corrosive esophageal strictures. A technical tip. Eur J Cardiothorac Surg. 2006;30:940-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sami SS, Haboubi HN, Ang Y, Boger P, Bhandari P, de Caestecker J, Griffiths H, Haidry R, Laasch HU, Patel P. UK guidelines on oesophageal dilatation in clinical practice. Gut. 2018;67:1000-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Cremer M, Devière J, Baize M, Matos C. New device for endoscopic cystoenterostomy. Endoscopy. 1990;22:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kawakami H, Kuwatani M, Kawakubo K, Eto K, Haba S, Kudo T, Abe Y, Kawahata S, Sakamoto N. Transpapillary dilation of refractory severe biliary stricture or main pancreatic duct by using a wire-guided diathermic dilator (with video). Gastrointest Endosc. 2014;79:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Siddappa P, Reddy YR, Gupta P, Gulati A, Gupta V, Sinha SK, Kochhar R. Application of a diathermic dilator for negotiating near-total antropyloric strictures. Endoscopy. 2016;48:E365-E366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Hordijk ML, Siersema PD, Tilanus HW, Kuipers EJ. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointest Endosc. 2006;63:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Maple JT. Hitch your wagon to a star-shaped incision? A closer look at electro-incision for benign gastroesophageal anastomotic strictures. Gastrointest Endosc. 2009;70:856-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Samanta J, Dhaka N, Sinha SK, Kochhar R. Endoscopic incisional therapy for benign esophageal strictures: Technique and results. World J Gastrointest Endosc. 2015;7:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Tharavej C, Pungpapong SU, Chanswangphuvana P. Outcome of dilatation and predictors of failed dilatation in patients with acid-induced corrosive esophageal strictures. Surg Endosc. 2018;32:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Gellad ZF, Hampton D, Tebbit CL, Puscas L, Pavey DA. Bilateral vocal cord paralysis following stent placement for proximal esophageal stricture. Endoscopy. 2008;40 Suppl 2:E150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Abushahin A, Lorenz RR, Bhatt A. Hoarseness of Voice After Endoscopic Dilation of an Esophageal Stricture. Gastroenterology. 2018;155:e20-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Chitose SI, Sato K, Fukahori M, Hamakawa S, Koga A, Sueyoshi S, Umeno H. Endoscopic surgical technique for benign fibrotic strictures of the upper esophageal sphincter. Dig Endosc. 2017;29:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Silver N, Gal TJ. Endoscopic CO2 laser management of chemoradiation-related cricopharyngeal stenosis. Ann Otol Rhinol Laryngol. 2014;123:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Dhaliwal HS, Sinha SK, Kochhar R. Endoscopic management of Zenker’s diverticulum. J Dig Endosc. 2015;6:45-54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |