Published online Nov 16, 2018. doi: 10.4253/wjge.v10.i11.354
Peer-review started: February 28, 2018
First decision: July 9, 2018
Revised: July 17, 2018
Accepted: August 21, 2018
Article in press: August 21, 2018
Published online: November 16, 2018
Processing time: 154 Days and 16 Hours
To investigate indications and outcomes of endoscopic retrograde cholangiopancreatography (ERCP) in cirrhotics, especially adverse events. Patients with cirrhosis undergoing ERCP are believed to have increased risk. However, there is a paucity of literature describing the indications and outcomes of ERCP procedures in patients with cirrhosis, especially focusing on adverse events.
We performed a systematic appraisal of major literature databases, including PubMed and EMBASE, with a manual search of literature from their inception until April 2017.
A total of 6,505 patients from 15 studies were analyzed (male ratio 59%, mean age 59 years), 11% with alcoholic and 89% with non-alcoholic cirrhosis, with 56.2% Child-Pugh class A, and 43.8% class B or C. Indications for ERCP included choledocholithiasis 60.9%, biliary strictures 26.2%, gallstone pancreatitis 21.1% and cholangitis 15.5%. Types of interventions included endoscopic sphincterotomy 52.7%, biliary stenting 16.7% and biliary dilation 4.6%. Individual adverse events included hemorrhage in 4.58% (95%CI: 2.77-6.75%, I2 = 85.9%), post-ERCP pancreatitis (PEP) in 3.68% (95%CI: 1.83-6.00%, I2 = 89.5%), cholangitis in 1.93% (95%CI: 0.63-3.71%, I2 = 87.1%) and perforation in 0.00% (95%CI: 0.00-0.23%, I2 = 37.8%). Six studies were used for comparison of ERCP-related complications in cirrhosis vs non-cirrhosis, which showed higher overall rates of complications in cirrhosis patients with pooled OR of 1.63 (95%CI: 1.27-2.09, I2 = 65%): higher rates of hemorrhage with OR of 2.05 (95%CI: 1.62-2.58, I2 = 2.1%) and PEP with OR of 1.33 (95%CI: 1.04-1.70, I2=65%), but similar cholangitis rates with OR of 1.23 (95%CI: 0.67-2.26, I2 = 44.3%).
There is an overall higher rate of adverse events related to ERCP in patients with cirrhosis, especially hemorrhage and PEP. A thorough risk/benefit assessment should be performed prior to undertaking ERCP in patients with cirrhosis.
Core tip: Patients with cirrhosis undergoing endoscopic retrograde cholangiopancreatography (ERCP) are considered to have increased risk. However, there is a paucity of literature describing the indications and outcomes of ERCP procedures in these patients. Our meta-analysis included 6,505 patients from 15 studies, with indications including choledocholithiasis, biliary strictures, gallstone pancreatitis and cholangitis. Types of interventions included sphincterotomy, stenting and dilation. Individual adverse events included hemorrhage, post-ERCP pancreatitis (PEP), and cholangitis. Comparison of ERCP-related complications in cirrhosis vs non-cirrhosis suggested higher overall rates of complications in cirrhosis patients with pooled (especially hemorrhage and PEP) but similar cholangitis rates.
- Citation: Mashiana HS, Dhaliwal AS, Sayles H, Dhindsa B, Yoo JW, Wu Q, Singh S, Siddiqui AA, Ohning G, Girotra M, Adler DG. Endoscopic retrograde cholangiopancreatography in cirrhosis - a systematic review and meta-analysis focused on adverse events. World J Gastrointest Endosc 2018; 10(11): 354-366
- URL: https://www.wjgnet.com/1948-5190/full/v10/i11/354.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i11.354
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most commonly performed endoscopic procedures and is known for its high-risk nature[1]. Performing ERCP in patients with cirrhosis is not only challenging, but may even be a high-risk procedure in this setting[2]. There is a known increased incidence of gallstones and choledocholithiasis in patients with cirrhosis, potentially requiring frequent ERCP procedures[2,3]. ERCP inherently carries risks of usual adverse events, including post-ERCP pancreatitis (PEP), hemorrhage, infection, perforation, and anesthesia-related events[4]. In addition, risks of adverse events in patients are believed to be higher in patients with cirrhosis requiring ERCP due to a poor synthetic function of the liver and resulting portal hypertension, ascites, varices, coagulopathy, and encephalopathy[5].
Surgery may not always be an option for pancreatobiliary disorders in patients with cirrhosis because of the high rates of morbidity and mortality due to underlying liver disease. As a general rule, minimally-invasive approaches, including ERCP, are favored in these patients[6]. Even though the increased risk of ERCP-related adverse events in cirrhosis patients is recognized, there is a relative paucity of literature, as well as some conflicting literature, describing the indications and outcomes of ERCP procedures in patients with cirrhosis.
We thus performed the present systematic review to evaluate the ERCP indications and characteristics, as well as a meta-analysis of ERCP outcomes in patients with cirrhosis. The important outcomes that we focused upon include pooled incidence rates of patient characteristics, ERCP indications, ERCP-related interventions and individual ERCP-related adverse events: (1) hemorrhage; (2) PEP; (3) cholangitis; and (4) perforation. The secondary outcomes included a comparison of ERCP complications in cirrhosis vs non-cirrhosis patients with pooled odds ratio (OR).
The preferred reporting items for systematic reviews and meta-analyses statement and the meta-analysis of observational studies in epidemiology guidelines were followed[7,8]. The objectives, primary outcomes, search strategy, inclusion criteria, and methods for study selection, data extraction, and data synthesis of this meta-analysis were defined in a protocol in advance. Data fields were pre-defined, and sensitivity analysis and subgroup analysis were also pre-specified in the protocol.
We performed a literature search using the keywords “endoscopic retrograde cholangiopancreatography”, “ERCP”, “cirrhosis”, “adverse events”, or “complications” in various combinations to identify original studies published from MEDLINE using both Ovid and PubMed without language restrictions. Other databases that were explored included EMBASE and Scopus. The reference lists of included papers and related review articles were manually searched. A literature search was conducted by two authors (HSM and ASD) in consultation with an experienced medical librarian.
We included original prospective, cohort, retrospective, case-control and, when possible, randomized control studies that evaluated the ERCP complications in cirrhosis patients. We also included the studies that provided a comparison of ERCP complications in cirrhosis and non-cirrhosis patients. We included the studies in English and any studies in other languages found through the manual search of references from inception until April 2017. We excluded studies that described the ERCP complications only in non-cirrhosis patients, and did not define clearly the number of ERCPs or their outcomes.
In the initial screening stage, simple relevance criteria were employed for study selection: (1) human participants; and (2) ERCP complications in cirrhosis patients as an outcome measure. Each title and abstract of the articles obtained through the electronic search was independently reviewed by two investigators (HSM and ASD). Citations were excluded only if deemed to be obviously irrelevant by both reviewing investigators, however those with reviewer disagreement were included for full review.
In the second stage of study selection, the full content of each article obtained during the screening stage was reviewed and evaluated. Using predetermined selection criteria and assessment methods, two investigators (HSM and ASD) independently evaluated the full content of each English language article. Articles in other languages were reviewed and evaluated by multilingual investigators as well as google translation tools using the same criteria and assessment methods.
We included studies that reported the ERCP complications in cirrhosis patients and that described hazard ratio (HR), relative risk (RR), or OR of comparison of ERCP complications in cirrhosis and non-cirrhosis patients. In addition, cohort and case-control studies that reported data on ERCP complications in cirrhosis patients were included if no related randomized controlled trials were found.
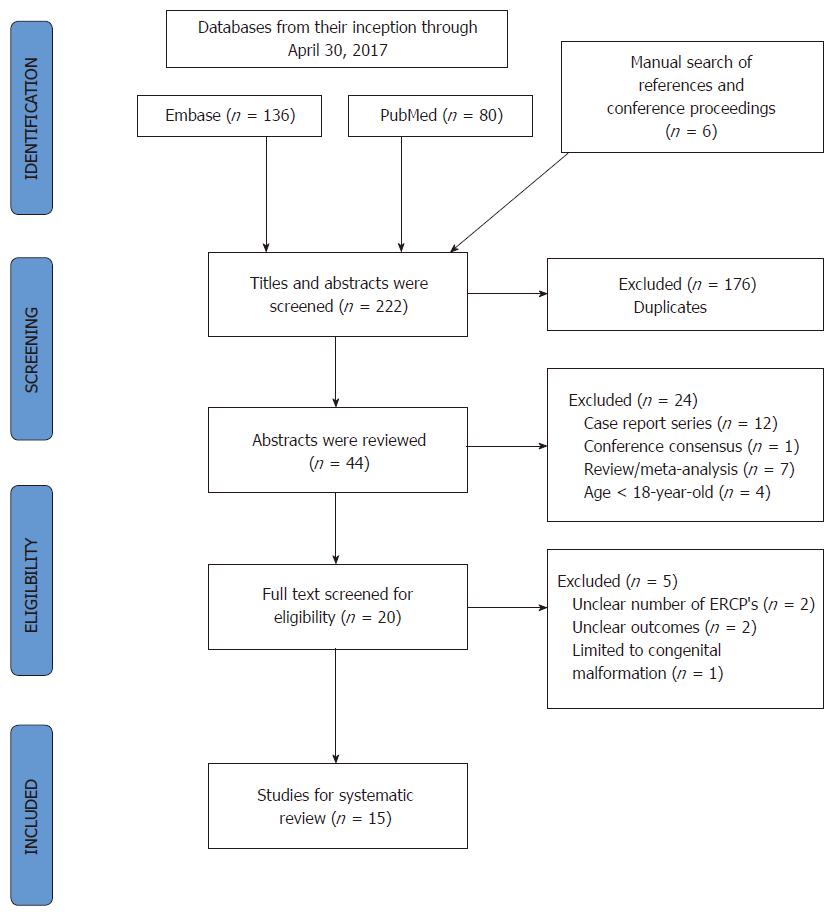
Twenty-one studies relevant to the inclusion criteria were identified. The actual numbers of ERCP cases were collected from tables and manuscript text in each study. Since data was from previously published studies, an institutional review board approval was waived. Figure 1 presents the study selection process in accordance with the preferred reporting items for systematic reviews and meta-analyses statement[7]. A summary of studies is shown in Table 1. After excluding six studies for various reasons, including unclear information on a number of ERCPs, outcomes, consensus statements or ERCP in congenital malformation patents, 15 studies were selected for final analysis. These 15 studies included the six studies that were separately used to perform a subset analysis to compare ERCP adverse events in cirrhosis and non-cirrhosis patients.
| Ref. | Yr of publication | Country | Study type | Cohort/ Case-control | Yr | No. of patients |
| Navaneethan et al[5] | 2017 | United States | Retrospective | Case-control | 2010 | 3228 |
| Jagtap et al[20] | 2017 | India | Retrospective | Cohort | 2014-2016 | 134 |
| Adler et al[16] | 2016 | United States | Retrospective | Cohort | 2003-2014 | 328 |
| Inamdar et al[13] | 2016 | United States | Retrospective | Case-control | 2009 | 1930 |
| Gill et al[14] | 2016 | Pakistan | Retrospective | Case-control | 2008-2014 | 100 |
| Churrango et al[24] | 2016 | United States | Retrospective | Cohort | 2008-2015 | 194 |
| Leal et al[19] | 2015 | Spain | Retrospective | Case-control | 2002-2014 | 158 |
| Zhang et al[2] | 2015 | China | Retrospective | Cohort | 2000-2014 | 77 |
| Li et al[17] | 2014 | China | Retrospective | Cohort | 2000-2008 | 46 |
| Ma et al[22] | 2013 | China | Retrospective | Cohort | 2002-2013 | 41 |
| Artifon et al[21] | 2011 | Brazil | Prospective | Case-control | Not specified | 105 |
| Park et al[18] | 2004 | South Korea | Prospective/Retrospective | Case-control | 1998-2003 | 41 |
| Prat et al[25] | 1996 | France | Retrospective | Cohort | 1988-1993 | 52 |
| Freeman et al[23] | 1995 | United States | Prospective | Case-control | Not specified | 64 |
| Sugiyama et al[15] | 1993 | Japan | Prospective | Cohort | Not specified | 7 |
Data from the eligible studies were independently abstracted by the two investigators (HSM and BD) using the Microsoft Excel program. Any disagreement or uncertainty was resolved by discussion and rechecking original articles, and, if still unresolved, then contacting the authors and consulting external experts. Information such as authors, title, published year, country of study, study design, sample size, and sampling methods, socio-demographic characters such as age, sex, race, exposures and their measurement methods, outcomes and their validation methods, duration of follow-up, adjusted risk factors, and HR or RR of ERCP in cirrhosis and non-cirrhosis patients were duly recorded.
The overall proportions of patients experiencing any post-procedure adverse events or specific complications were estimated using random effects methods designed for the pooling of proportions. The actual proportions were estimated after the Freeman-Tukey double arcsine transformation had been applied to the individual study proportions and standard errors were calculated using the scoring method[9,10]. For the subset of studies that provided separate reports of adverse events for patients with or without cirrhosis, we combined individual study results to calculate the pooled OR and 95% confidence intervals (CI) using random-effects meta-analysis for a dichotomous outcome[11]. Between-study heterogeneity was assessed using the I2 statistic, which is an estimate of the percentage of variation across studies that is due to true heterogeneity and not due to chance[12]. Baseline characteristics of study participants were aggregated from 15 analyzed studies as shown in Table 2. All analyses were performed using STATA version 14.2 (StataCorp, College Station, TX). A two-sided P-value < 0.05 was considered statistically significant.
| Ref. | Yr published | Country | Study period | Study type |
| Navaneethan et al[5] | 2017 | United States | 2010 | Retrospective (NIS), Multicenter |
| Inamdar et al[13] | 2016 | United States | 2009 | Retrospective (NIS), Multicenter |
| Gill et al[14] | 2016 | Pakistan | 2008-2014 | Retrospective, Single center |
| Leal et al[19] | 2015 | Spain | 2002-2014 | Retrospective, Single center |
| Li et al[17] | 2014 | China | 2000-2008 | Retrospective, Single center |
| Freeman et al[23] | 1995 | United States | NS | Retrospective, Multicenter |
The Newcastle–Ottawa score was used to assess the quality of nonrandomized studies by two authors (BD and HSM). Any discrepancies were resolved by a third reviewer (DGA).
A total of 6505 patients from 15 studies were analyzed. A description of the studies is reported in Table 1. Adverse events secondary to ERCP in these patients are reported in Table 3. From the demographic information that was provided in various studies, male ratio was 59% and mean age was 59.26 years in ten studies. Out of the nine studies that described the etiology of cirrhosis, 11% had alcoholic cirrhosis and 89% had non-alcoholic causes. Data from 13 studies described 56.2% of the patients belonging to Child-Pugh class A, and the remainding 43.8% were Child-Pugh class B or C.
| Ref. | Total no. of patients (cirrhotics) | Number of ERCPs | PEP | Hemorrhage | Cholangitis | Perforation | % of complications |
| Navaneethan et al[5] | 3228 | 3228 | 3871 | 681 | 10 | 6 | 14.5 |
| Jagtap et al[20] | 134 | 134 | 21 | 41 | 10 | 0 | 11.9 |
| Adler et al[16] | 328 | 538 | 251 | 61 | 15 | 2 | 14.6 |
| Inamdar et al[13] | 1930 | 1930 | 1601 | 441 | 15 | N/A | 11.3 |
| Gill et al[14] | 100 | 100 | 31 | 61 | 3 | 0 | 12 |
| Churrango et al[24] | 194 | 194 | 31 | 51 | N/A | 0 | 4.1 |
| Leal et al[19] | 158 | 158 | 71 | 91 | 10 | 1 | 17 |
| Zhang et al[2] | 77 | 77 | 42 | 242 | 1 | 0 | 37.6 |
| Li et al[17] | 46 | 46 | 43 | 23 | 3 | 0 | 19.5 |
| Ma et al[22] | 41 | 41 | 04 | 24 | 0 | 0 | 4.8 |
| Artifon et al[21] | 105 | 105 | 35 | 75 | 0 | 5 | 14.2 |
| Park et al[18] | 41 | 41 | 36 | 66 | 4 | 0 | 31.7 |
| Prat et al[25] | 52 | 52 | 01 | 31 | 3 | 1 | 13.4 |
| Freeman et al[23] | 64 | 64 | N/A1 | 51 | N/A | N/A | 7.8 |
| Sugiyama et al[15] | H/B | 7 | 0* | 0* | 0 | 0 | 0 |
A total of 6735 ERCP procedures were performed. The indications for the ERCP included choledocholithiasis in 60.9% (4006/6571) of the procedures in 13 studies, cholangitis 15.5% (1021/6571) in 13 studies, biliary strictures 26.2% (1740/6635) in 14 studies and gallstone pancreatitis 21.1% (916/4338) in nine studies. The type of intervention during the ERCP was described in ten studies, which included endoscopic sphincterotomy in 52.7% of the procedures, biliary stenting in 16.7% and biliary dilation in 4.6% of the cases.
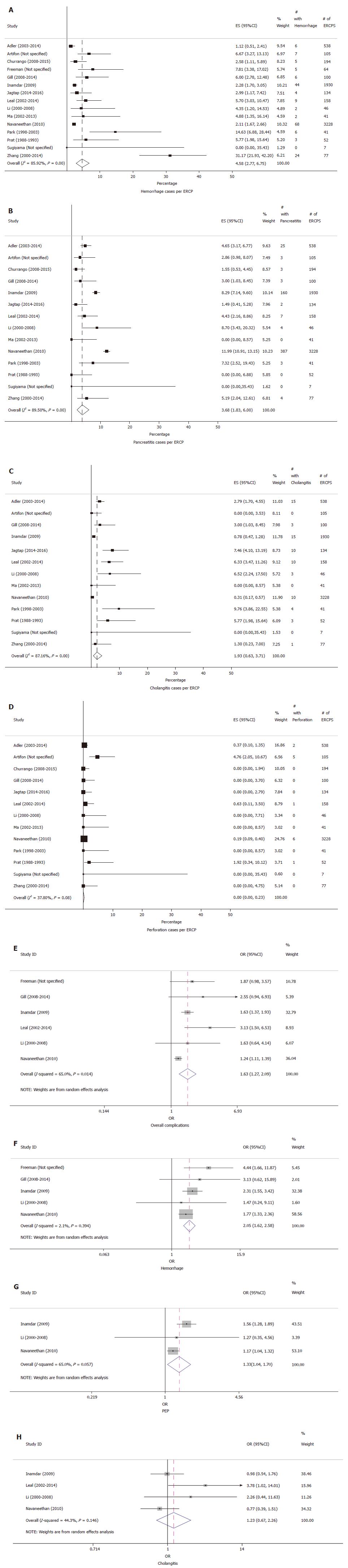
The individual adverse event rates were as follows: incidence of ERCP-related hemorrhage in 15 studies was 4.58% (95%CI: 2.77-6.75%, P < 0.01, I2 = 85.92%) (Figure 2A), PEP in 14 studies was 3.68% (95%CI: 1.83-6.00%, P < 0.01, I2 = 89.50%) (Figure 2B), cholangitis in 13 studies was 1.93% (95%CI: 0.63-3.71%, P < 0.01) (Figure 2C) and perforation in 13 studies was 0.00% (95%CI: 0.00-0.23%, P = 0.08, I2 = 37.8%) (Figure 2D).
Six out of 15 studies also compared adverse events in cirrhosis vs non-cirrhosis patients. Table 3 provides a description of the studies used for comparing the adverse events. Figure 2E looks at the meta-analysis of the comparison of overall complications in these six studies. Patients with cirrhosis had higher overall rates of complications compared to non-cirrhosis patients, and this difference was statistically significant. Pooled OR for overall complications was 1.63 (95%CI: 1.27-2.09, P < 0.0001, I2 = 65%). Hemorrhage rate for patients with cirrhosis was higher than non-cirrhosis, from a comparison in five studies, with a pooled OR 2.05 (95%CI: 1.62-2.58, P < 0.0001, I2 = 2.1%) (Figure 2F). PEP rate comparison from three studies showed a higher incidence in patients with cirrhosis, with a pooled OR 1.33 (95%CI: 1.04-1.70, P = 0.021, I2 = 65%) (Figure 2G). Cholangitis rate comparison between patients with or without cirrhosis, as evaluated from four studies was not statistically significant, with a pooled OR of 1.23 (95%CI: 0.67-2.26, P = 0.511, I2 = 44.3%) (Figure 2H). A perforation rate comparison was described in only two studies, and hence comparison analysis could not be obtained.
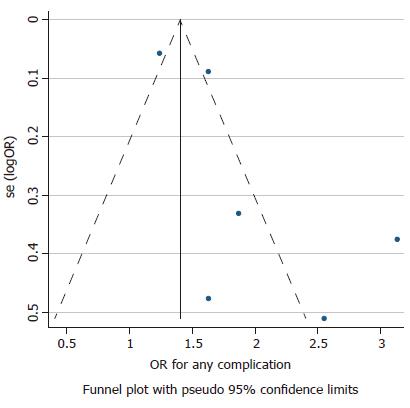
The power to detect publication bias is low due to the small number of studies for comparison. Nevertheless, the P-values were found to be statistically significant for overall complications, hemorrhage and PEP. Figure 3 presents a symmetrical funnel plot for the studies used in comparing overall complications. Heterogeneity is high due to the different sizes of the studies, with some studies being small and others being large. The actual percentage of I2 is described in the results above. The details regarding the methodological quality of studies using the Newcastle-Ottawa scale are provided in Table 4.
| Ref. | Country | Study type | Cohort/ Case-control | Yr | No. of patients | Newcastle-Ottawa Scale | Outcome | |
| Selection | Comparability | |||||||
| Navaneethan et al[5] | United States | Retrospective | Case-control | 2010 | 3228 | A | C | *** |
| Jagtap et al[20] | India | Retrospective | Cohort | 2014-2016 | 134 | A | ** | |
| Adler et al[16] | United States | Retrospective | Cohort | 2003-2014 | 328 | A | C | *** |
| Inamdar et al[13] | United States | Retrospective | Case-control | 2009 | 1930 | A | B | ** |
| Gill et al[14] | Pakistan | Retrospective | Case-control | 2008-2014 | 100 | A | C | ** |
| Churrango et al[24] | United States | Retrospective | Cohort | 2008-2015 | 194 | A | C | ** |
| Leal et al[19] | Spain | Retrospective | Case-control | 2002-2014 | 158 | A | C | *** |
| Zhang et al[2] | China | Retrospective | Cohort | 2000-2014 | 77 | A | C | *** |
| Li et al[17] | China | Retrospective | Cohort | 2000-2008 | 46 | A | C | *** |
| Ma et al[22] | China | Retrospective | Cohort | 2002-2013 | 41 | B | C | ** |
| Artifon et al[21] | Brazil | Prospective | Case-control | Not specified | 105 | B | C | *** |
| Park et al[18] | South Korea | Prospective/Retrospective | Case-control | 1998-2003 | 41 | A | C | *** |
| Prat et al[25] | France | Retrospective | Cohort | 1988-1993 | 52 | A+ | C | *** |
| Freeman et al[23] | United States | Prospective | Case-control | Not specified | 64 | A | C | *** |
| Sugiyama et al[15] | Japan | Prospective | Cohort | Not specified | 7 | B | C | *** |
In this meta-analysis of studies describing ERCP-related adverse events in patients with cirrhosis, we observed a statistically significant higher rate of overall adverse events related to ERCP, particularly of PEP and hemorrhage. Similar results were observed in the subset analysis of studies, which allowed a comparison of ERCP-related adverse events in cirrhosis vs non-cirrhosis patients. Additionally, the subset analysis showed a trend towards higher rates of post-procedure cholangitis in patients with cirrhosis, although that was not significantly higher than that in non-cirrhosis patients.
Prior studies have presented variable results when evaluating adverse events in patients with cirrhosis undergoing ERCP. Most of the studies in the past have shown higher rates of hemorrhage in patients with cirrhosis compared to non-cirrhosis, likely due to a poor synthetic function of the liver, portal hypertension, prolonged coagulation times, etc.[5,13-15]. The lowest rates of hemorrhage (1.1%) in cirrhosis patients were reported by Adler et al[16] in a large retrospective study performed at two large centers, including over 500 ERCP procedures, as compared to 4.58% seen in our meta-analysis. Two major factors potentially contributing to those lower rates are 1) a smaller percentage (15%) of patients receiving sphincterotomy when compared with other studies that could have confounded the results, and 2) performance of ERCP by very experienced operators with a particularly long history of performing these complicated procedures in patients with advanced liver disease.
A retrospective matched cohort study of the 2009 National Inpatient Sample with 3228 patients by Inamdar et al[13] showed an overall ERCP–related hemorrhage rate of 2.3% in cirrhosis patients, which is once again lower than the rate demonstrated in our meta-analysis. However, on the subset analysis, ERCP-associated hemorrhage for decompensated cirrhosis was 4.3% when compared to 1.3% in patients with compensated cirrhosis, and 1% in non-cirrhosis patients. Another retrospective matched case-control study by Navaneethan et al[5] using the 2010 National Inpatient Sample database showed an ERCP-associated hemorrhage of 2.1% in cirrhosis vs 1.2% in non-cirrhosis patients. The results from our meta-analysis clearly demonstrate higher rates of hemorrhage in cirrhosis patients than previously reported, with a pooled OR of 2.05.
Li et al[17] reported no statistically significant difference between ERCP-associated hemorrhage in cirrhosis (4.3%) and non-cirrhosis (3%) patients, but those with Child-Pugh class C had statistically significant higher rates of hemorrhage at 25%. Nevertheless, further information on whether these bleeds were clinically significant or not was provided. Similarly, a study by Park et al[18] described higher rates of ERCP-related hemorrhage in patients with Child-Pugh class C (35%) as compared to class A (0%) and B (16%).
Endoscopic sphincterotomy (EST) has been shown to independently increase the risk of hemorrhage in cirrhosis as well as non-cirrhosis patients[5,14,19]. The Navaneethan et al[5] study showed that performing EST in both compensated and decompensated cirrhosis patients was an independent risk factor of post-ERCP bleeding. In the study by Park et al[18], the rates of bleeding were significantly lower for endoscopic papillary balloon dilation in comparison to EST. In addition, one study also observed lower rates of bleeding when the ERCPs in cirrhosis patients were performed in medium- and large-sized hospitals[5]. Since only a limited number of studies have described hemorrhage or other adverse events in terms of Child-Pugh class or the type of intervention, no separate analysis could be obtained in our meta-analysis[16-18,20].
In terms of PEP, our meta-analysis shows the overall incidence of cirrhosis to be 3.68% (95%CI: 1.83-6%), as evaluated from 14 studies. The comparative meta-analysis using three available studies reveal a higher rate of PEP in cirrhosis when compared to non-cirrhosis patients, with a pooled OR of 1.33, which was statistically significant as well. While some of the comparison studies failed to demonstrate a statistically significant difference for PEP in cirrhosis vs non-cirrhosis patients, the study by Navaneethan et al[5] described a higher rate of PEP in cirrhosis patients on univariate analysis, although this difference fell away once they adjusted other factors that increased the risk of PEP. These authors did demonstrate that performing EST was associated with an increased risk of PEP, although the cause was unclear, while at the same time placing prophylactic pancreatic stents was associated with a decreased risk of PEP[5,14,17,19]. Notably, cirrhosis alone did not increase the risk of PEP. Patients with alcoholic cirrhosis were noted to have a higher rate of PEP vs non-alcoholic cirrhosis[5]. Similarly, increased rates of PEP with EST were seen by Adler et al[16]. Artifon et al[21] showed that the risk of PEP was decreased with supra-papillary technique (0%) in comparison with standard cannulation technique (4.8%). Park et al[18] suggested lower rates of PEP with endoscopic papillary balloon dilation in comparison to EST, but the results did not reach statistical significance. A possible argument explaining the higher rates of PEP is the conservative intravenous hydration approach adopted by physicians, due to concerns of volume overload in decompensated cirrhosis patients[13].
The rate of post-ERCP cholangitis in cirrhosis patients from our meta-analysis of 13 studies was 1.93% (95%CI: 0.63-3.71%), and the comparison analysis from four studies showed an OR of 1.23 in cirrhosis patients when compared to non-cirrhosis patients, but it was not statistically significant. In the study by Adler et al[16], the overall rate of post-ERCP cholangitis was 2.8%. However, on the sub-group analysis, the rate was 5.8% in patients receiving EST as compared to 2.3% in patients with no sphincterotomy, although the difference was not statistically significant. There was no comparison group of patients without cirrhosis in this study. When looking at literature that included a comparison group of non-cirrhosis patients, the study by Navaneethan et al[5] demonstrated lower rates of post-ERCP cholangitis in cirrhosis when compared to non-cirrhosis, although the difference was not statistically significant. The reason for this trend is believed to be the consistent use of prophylactic antibiotics in cirrhosis patients for spontaneous bacterial peritonitis or other indications. No statistically significant difference in cholangitis rates was appreciated in any other studies[13,14,18,22]. The only study showing higher rate of cholangitis in the cirrhosis (6.3%) vs non-cirrhosis group (1.8%) was by Leal et al[19], however the authors could not provide a plausible explanation for their observation, and suggested performing further studies that implement preventive strategies to avoid cholangitis in patients with cirrhosis.
The perforation rate per our meta-analysis of 13 studies was 0% (95%CI: 0.00-0.23%), and, as described above, there was no comparison analysis between the cirrhosis and non-cirrhosis group due to the small number of studies describing it. Adler et al[16] reported an overall perforation rate of 0.4%, and Navaneethan et al[5] reported a perforation rate of 0.2% in patients with cirrhosis and 0.1% in patients without cirrhosis, although with no statistically significant difference.
A small number of studies have described the relationship of adverse events with the Child-Pugh score. These studies consistently demonstrated that the patients with higher Child-Pugh class scores had more complications overall[16-18,20]. Inamdar et al[13] demonstrated a similar risk of adverse events between the non-cirrhosis group and patients with compensated cirrhosis. However, higher rates of adverse events were observed in patients with decompensated cirrhosis. Similarly, Adler et al[16] described the post-procedure adverse events to be lower in Child-Pugh class A (6.1%) as compared to class B and C combined (11.3%), which was statistically significant. Zhang et al[2] noted no association between the rates of adverse events when correlated to Child-Pugh class, but elucidated that patients with higher MELD scores had higher rates of adverse events.
Higher rates of adverse events have also been reported depending on maneuvers performed during the ERCP. Performing EST has been associated with higher rates of adverse events in comparison to performing stenting alone or endoscopic papillary balloon dilation[5,14,19,23]. Adler et al[16] described the overall post-ERCP adverse events to be higher after EST (23.3%), when compared to patients who did not undergo sphincterotomy (5.6%). Moreover, Freeman et al[23] indicated EST in cirrhosis patients was associated with excess morbidity and mortality related to bleeding, with poor outcomes primarily reported in Child-Pugh class C patients. Freeman further suggested that ERCP-related mortality could be reduced by avoiding EST where dilation or stenting alone is adequate.
Even with the higher rates of overall adverse events seen in patients with cirrhosis, as described in our comparison meta-analysis of six studies with an OR of 1.63 (95%CI: 1.27-2.09), the cholangitis rates surprisingly did not show a statistically significant difference amongst the two groups as has been described above.
Our present meta-analysis has a few limitations. First is that the maximum number of cases are derived from only three studies by Navaneethan et al[5], Inamdar et al[13] and Adler et al[16]. Secondly, only a few studies describe adverse events in terms of indications, the severity of cirrhosis or the type of ERCP-related interventions. Due to these reasons, we were unable to obtain a separate sub-group analysis based in relation to these. The heterogeneity of the overall complication comparison in cirrhosis vs non-cirrhosis patients is high, which makes it hard to draw specific conclusions from the meta-analysis when combined with the low power to detect bias. This suggests the need for better-controlled prospective studies in the future for improved clarity of post-ERCP adverse events in cirrhosis patients. Based on our experience with ERCP in cirrhosis, we believe that the adverse events seen in patients with cirrhosis are similar overall to those seen among unselected patients undergoing ERCP, although patients with Childs classes B and C have higher adverse event rates when compared with those with Childs class A. Patients with cirrhosis without PSC have significantly greater adverse event rates when compared with patients with PSC, which runs somewhat counter to prevailing thought.
In summary, our meta-analysis clearly demonstrates that there is a higher rate of adverse events related to ERCP (particularly of hemorrhage and PEP) in patients with cirrhosis than that of patients without cirrhosis, especially in patients with Child-Pugh class B or C, and when receiving interventions like EST. Despite the increased adverse event rates, ERCP remains the least invasive therapeutic approach for appropriate indications in pancreatobiliary pathologies for patients with cirrhosis[13]. A thorough risk/benefit assessment should be performed in cirrhosis patients prior to ERCP.
Patients with cirrhosis undergoing endoscopic retrograde cholangiopancreatography (ERCP) are believed to have increased risks. However, there is a paucity of literature describing the indications and outcomes of ERCP procedures in patients with cirrhosis, especially focusing on adverse events.
ERCP is one of the most commonly performed endoscopic procedures and is known for its high-risk nature. Performing ERCP in patients with cirrhosis is not only challenging, but may even be a high-risk endeavor in this setting. There was therefore a need for a meta-analysis to estimate adverse events associated with ERCP in cirrhosis patients.
To assess the adverse events associated with ERCP in cirrhosis patients.
The preferred reporting items for systematic reviews and meta-analyses statement and the meta-analysis of observational studies in epidemiology guidelines were followed. The overall proportion of patients experiencing any post-procedure adverse events or experiencing specific complications were estimated using random effects methods designed for the pooling of proportions. The actual proportions were estimated after the Freeman-Tukey double arcsine transformation had been applied to the individual study proportions and standard errors were calculated using the scoring method.
Individual adverse events included hemorrhage in 4.58% (95%CI: 2.77-6.75%, I2 = 85.9%), post-ERCP pancreatitis (PEP) in 3.68% (95%CI: 1.83-6.00%, I2 = 89.5%), cholangitis in 1.93% (95%CI: 0.63-3.71%, I2 = 87.1%) and perforation in 0.00% (95%CI: 0.00-0.23%, I2 = 37.8%).
There is an overall higher rate of adverse events related to ERCP in patients with cirrhosis, especially hemorrhage and PEP.
In the future, a thorough risk/benefit assessment should be performed in cirrhosis patients prior to ERCP.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D, D
Grade E (Poor): E
P- Reviewer: Chow WK, Gonzalez-Ojeda A, Kitamura K, Nakai Y, Sharma SS, Sun SY, Tantau A, Tsuyuguchi T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Song H
| 1. | Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ, Fanelli R. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Zhang J, Ye L, Zhang J, Lin M, He S, Mao X, Zhou X, Zhi F. MELD scores and Child-Pugh classifications predict the outcomes of ERCP in cirrhotic patients with choledocholithiasis: a retrospective cohort study. Medicine (Baltimore). 2015;94:e433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Acalovschi M. Gallstones in patients with liver cirrhosis: incidence, etiology, clinical and therapeutical aspects. World J Gastroenterol. 2014;20:7277-7285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 4. | Alkhatib AA, Hilden K, Adler DG. Comorbidities, sphincterotomy, and balloon dilation predict post-ERCP adverse events in PSC patients: operator experience is protective. Dig Dis Sci. 2011;56:3685-3688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Navaneethan U, Njei B, Zhu X, Kommaraju K, Parsi MA, Varadarajulu S. Safety of ERCP in patients with liver cirrhosis: a national database study. Endosc Int Open. 2017;5:E303-E314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Higashi H, Matsumata T, Adachi E, Taketomi A, Kashiwagi S, Sugimachi K. Influence of viral hepatitis status on operative morbidity and mortality in patients with primary hepatocellular carcinoma. Br J Surg. 1994;81:1342-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8058] [Article Influence: 537.2] [Reference Citation Analysis (2)] |
| 8. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16802] [Article Influence: 672.1] [Reference Citation Analysis (0)] |
| 9. | Miller JJ. The Inverse of the Freeman – Tukey Double Arcsine Transformation. Am Stat. 1978;32:138-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Stat. 1950;21:607-611. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1674] [Cited by in RCA: 1682] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 12. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46547] [Article Influence: 2115.8] [Reference Citation Analysis (3)] |
| 13. | Inamdar S, Berzin TM, Berkowitz J, Sejpal DV, Sawhney MS, Chutanni R, Pleskow DK, Trindade AJ. Decompensated cirrhosis may be a risk factor for adverse events in endoscopic retrograde cholangiopancreatography. Liver Int. 2016;36:1457-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Gill M, Yousuf SGN, Nawaz A, Nazir R. Outcomes of endoscopic retrograde cholangiopancreatography in patients with cirrhosis. J Hepatol. 2016;64:S278. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Sugiyama M, Atomi Y, Kuroda A, Muto T. Treatment of choledocholithiasis in patients with liver cirrhosis. Surgical treatment or endoscopic sphincterotomy? Ann Surg. 1993;218:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Adler DG, Haseeb A, Francis G, Kistler CA, Kaplan J, Ghumman SS, Laique SN, Munigala S, Taylor LJ, Cox K. Efficacy and safety of therapeutic ERCP in patients with cirrhosis: a large multicenter study. Gastrointest Endosc. 2016;83:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Li DM, Zhao J, Zhao Q, Qin H, Wang B, Li RX, Zhang M, Hu JF, Yang M. Safety and efficacy of endoscopic retrograde cholangiopancreatography for common bile duct stones in liver cirrhotic patients. J Huazhong Univ Sci Technolog Med Sci. 2014;34:612-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Song MH, Seo DW, Min YI. Endoscopic sphincterotomy vs. endoscopic papillary balloon dilation for choledocholithiasis in patients with liver cirrhosis and coagulopathy. Gastrointest Endosc. 2004;60:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Leal C, Colan J, Mendez-Bocanegra A, Fernandez A, Sendino O, De Miguel CR, Roura-Poch P, Fernandez J, Llach J, Guarner-Argente C. Cholangitis is a leading complication of endoscopic retrograde cholangiopancreatography in patients with cirrhosis: An analysis of two high volume centers. Hepatology. 2015;62:1224A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Jagtap N, Tandon M, Sharma M, Gupta R, Lakhtakia S, Ramchandani M, Kalapala R, Rao GV, Reddy DN. Safety and efficacy of ERCP in patients with cirrhosis. Dig Endosc. 2017;29:145. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Artifon EL, da Silveira EB, Aparicio D, Takada J, Baracat R, Sakai CM, Garcia RT, Teich V, Couto DS. Management of common bile duct stones in cirrhotic patients with coagulopathy: a comparison of supra-papillary puncture and standard cannulation technique. Dig Dis Sci. 2011;56:1904-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Ma MY, Jiang GB, Wang X, Mu L, Ji GZ, Wang M. ERCP for treatment of choledocholithiasis in patients with liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 2013;21:3736-3741. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1690] [Article Influence: 58.3] [Reference Citation Analysis (2)] |
| 24. | Churrango J, Doppalapudi K, A Hsieh A, Ahlawat S. Sa1171 Predictors of Post-ERCP Complications in Patients with Liver Cirrhosis. Gastrointest Endosc. 2016;83:AB240-AB241. [DOI] [Full Text] |
| 25. | Prat F, Tennenbaum R, Ponsot P, Altman C, Pelletier G, Fritsch J, Choury AD, Bernades P, Etienne JP. Endoscopic sphincterotomy in patients with liver cirrhosis. Gastrointest Endosc. 1996;43:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |