Published online Nov 16, 2018. doi: 10.4253/wjge.v10.i11.340
Peer-review started: July 5, 2018
First decision: July 19, 2018
Revised: August 21, 2018
Accepted: October 8, 2018
Article in press: October 9, 2018
Published online: November 16, 2018
Processing time: 134 Days and 14.9 Hours
To investigate that polysomnographic monitoring can accurately evaluate respiratory disturbance incidence during sedation for gastrointestinal endoscopy compare to pulse oximetry alone.
This prospective observational study included 10 elderly patients with early gastric cancer undergoing endoscopic submucosal dissection (ESD) under propofol sedation. Apart from routine cardiorespiratory monitoring, polysomnography measurements were acquired. The primary hypothesis was tested by comparing the apnea hypopnea index (AHI), defined as the number of apnea and hypopnea instances per hour during sedation, with and without hypoxemia; hypoxemia was defined as the reduction in oxygen saturation by ≥ 3% from baseline.
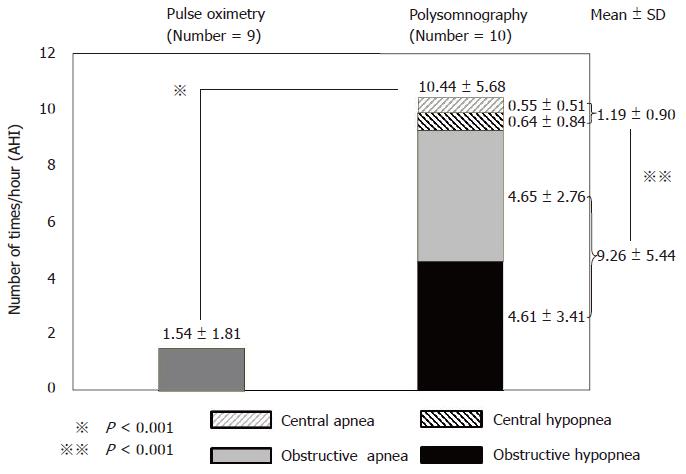
Polysomnography (PSG) detected 207 respiratory disturbances in the 10 patients. PSG yielded a significantly greater AHI (10.44 ± 5.68/h) compared with pulse oximetry (1.54 ± 1.81/h, P < 0.001), thus supporting our hypothesis. Obstructive AHI (9.26 ± 5.44/h) was significantly greater than central AHI (1.19 ± 0.90/h, P < 0.001). Compared with pulse oximetry, PSG detected the 25 instances of respiratory disturbances with hypoxemia 107.4 s earlier on average.
Compared with pulse oximetry, PSG can better detect respiratory irregularities and thus provide superior AHI values, leading to avoidance of fatal respiratory complications during ESD under propofol-induced sedation.
Core tip: Our aim was to demonstrate respiratory disturbances using polysomnography (PSG) during propofol sedation for gastric endoscopic submucosal dissection. Among the ten patients, 207 respiratory disturbances were identified by PSG. Apnea hypopnea index (AHI), defined as the number of apnea and hypopnea per hour, detected by PSG was significantly greater than that detected by pulse oximeter. Obstructive AHI was significantly greater than central AHI. The 25 instances of respiratory disturbances with hypoxemia were detected on an average of 107.4 s before they were detected by pulse oximetry. PSG would be useful for monitoring respiratory conditions with better detectability of AHI.
- Citation: Urahama R, Uesato M, Aikawa M, Yamaguchi Y, Hayano K, Matsumura T, Arai M, Kunii R, Isono S, Matsubara H. Polysomnographic assessment of respiratory disturbance during deep propofol sedation for endoscopic submucosal dissection of gastric tumors. World J Gastrointest Endosc 2018; 10(11): 340-347
- URL: https://www.wjgnet.com/1948-5190/full/v10/i11/340.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i11.340
Sedation is widely used to acquire a stable surgical field, better endoscopic images, and to reduce patient discomfort during gastrointestinal (GI) endoscopy[1-3]. Contrary to light conscious sedation usually used in short diagnostic GI endoscopy, deep sedation is required to minimize patient movement during extended and painful endoscopic procedures, such as endoscopic submucosal dissection (ESD) or endoscopic retrograde cholangiopancreatography. Propofol sedation has been reported to improve outcomes after ESD surgery and shorten procedure time[4]. However, propofol has dose-dependent respiratory depressant effects[5]; therefore, the incidence of fatal respiratory complications associated with deep sedation is of significant concern when ensuring the safety of the GI endoscopic procedures[6].
Recent guidelines on GI endoscopy strongly recommend pulse oximetry and careful monitoring of breathing during sedation[7,8]. Unlike the low incidence of hypoxemia (0.13%–0.46%) during conscious sedation for short GI endoscopy procedures[9,10], a relatively large prospective study including 799 patients undergoing propofol sedation for advanced GI endoscopic procedures reported that hypoxemia (arterial oxygen saturation, SaO2 < 90%), detected by pulse oximeter, occurred in 12.8% of the participants and that respiratory disturbances detected by a capnometer and requiring airway maneuvers, such as chin lift, occurred in 14.4% patients, even when under supervision by an anesthesiologist[11]. Because these studies only assessed the incidence of critical hypoxemia in the study population, it is unclear as to how many non-critical respiratory disturbances occurred in addition to these critical events. Thus, we hypothesized that pulse oximetry alone may underestimate the incidence of adverse respiratory episodes during propofol sedation, particularly in patients who receive supplemental oxygen. Furthermore, propofol can depress both inspiratory pump muscles and upper airway dilating muscles, thereby leading to either central or obstructive disordered breathing[12].
Although strategies for preventing respiratory disturbances significantly depend on the type of breathing abnormality encountered (central or obstructive), to the best of our knowledge, no previous study has systematically characterized breathing patterns and disturbances under sedation during GI endoscopy. Therefore, primarily, we tested the hypothesis that pulse oximetry underestimates respiratory disturbances during propofol sedation in patients undergoing ESD surgery; we also aimed to characterize breathing patterns under sedation. We employed polysomnography to assess state of consciousness, nature of breathing abnormalities, and oxygenation during sedation for GI endoscopy.
This prospective, observational study was approved by the institutional Ethics Committee (#1902-2014, Graduate School of Medicine, Chiba University, Chiba, Japan), and written informed consent was obtained from each patient after the aim and potential risks of the study were completely explained to each patient. Inclusion criteria were adult patients undergoing ESD surgery for early gastric cancer under propofol sedation with expected procedure duration of < 2 h. Exclusion criteria were patients with severe comorbidities, including presence of high risk of aspiration and allergies to propofol and pentazocine. Totally, 10 elderly patients (6 males and 4 females; mean age 71.4 years,) were enrolled between 2014 and 2015.
Prior to propofol sedation, electrodes for standard polysomnography (PSG) were attached to all patients (PSG-1100, Nihon Kohden, Tokyo, Japan), in addition to routine patient monitors for GI endoscopy (pulse oximetry, electrocardiogram, and intermittent blood pressure measurements). Bilateral central and occipital electroencephalograms, bilateral electrooculograms, submental electromyogram, airflow measurement with a nasal pressure prong and an oro-nasal thermistor, thoraco-abdominal wall motions with piezo-respiratory effort sensors, SaO2, and snoring over a microphone were recorded and relevant data were stored in a computer for further analyses. The patients, lying on their left side, received 2 L/min of oxygen through a nasal prong. Following a slow intravenous injection of propofol (1-2 mg/kg) until loss of consciousness, propofol was continuously infused at a rate of 1–4 mg/kg per hour so as to maintain a Ramsey score of 5-6 (loss of responses to verbal commands and light tapping on the shoulder, but arousable by painful stimulation)[13]. Pentazocine (7.5 mg) was intravenously administered for analgesia. Cardiorespiratory abnormalities or instabilities detected by the patient monitors were treated by altering the propofol infusion rate and/or using airway maneuvers following standard institutional protocols.
PSG data were manually analyzed by a certified sleep technician (Kunii R) and investigators using dedicated computer software (Polysmith, Nihon Kohden, Tokyo, Japan). For the PSG data, we focused on the following two sensors: (1) airflow measurement using the nasal pressure prong and the oro-nasal thermistor; and (2) thoraco-abdominal wall motion uses piezo-respiratory effort sensors (RIP-chest and/or RIP-abdomen). Apnea was defined as the absence of airflow for ≥ 10 s, determined using the nasal pressure signal. Hypopnea was defined as a ≥ 50% reduction in the nasal pressure signal for ≥ 10 s. State of consciousness (awake or sleep) was determined from the 30-s PSG recording using criteria defined by Rechtschaffen and Kales[14]. Apnea and hypopnea episodes were systematically classified based on the presence or absence of hypoxemia, which was defined as a ≥ 3% reduction in SaO2 from baseline, conscious states (awake and/or sleep), and presence or absence of thoraco-abdominal respiratory movements (obstructive and/or central). Apnea hypopnea index (AHI), the primary outcome variable, was defined as the frequency of apnea and hypopnea episodes per hour of sedation.
In primary analysis, the hypothesis was tested by comparing the AHI detected using PSG and pulse oximetry. The predominant pattern of respiratory disturbance was determined by comparing obstructive AHI and central AHI using the paired t-test. Summary statistics were calculated as frequencies and proportions for categorical data and as means and SD for continuous variables. P < 0.05 was considered statistically significant, and all p-values were two sided. All statistical analyses were performed using the SigmaPlot software (ver.12.0; Systat Software Inc., Point Richmond, CA).
Table 1 presents the patient characteristics and ESD indications. Majority of the patients were non-obese and elderly. All ESD procedures were completed without complications.
| Characteristic/indication | Value (mean ± SD) |
| Age (yr) | 71.4 ± 6.6 |
| Sex (male/female) | 6/4 |
| Height (cm) | 159.9 ± 8.9 |
| Body weight (kg) | 59.2 ± 8.2 |
| Body mass index (kg/m2) | 23.6 ± 3.5 |
| Histological type | |
| Well differentiated tubular adenocarcinoma | n = 7 |
| Moderately differentiated tubular adenocarcinoma | n = 1 |
| Signet-ring cell carcinoma | n = 2 |
| Invasion depth: mucosa | n = 10 |
| Ulceration: none | n = 10 |
| Longer axis of resected specimen size (mm) | 35.1 ± 10.2 |
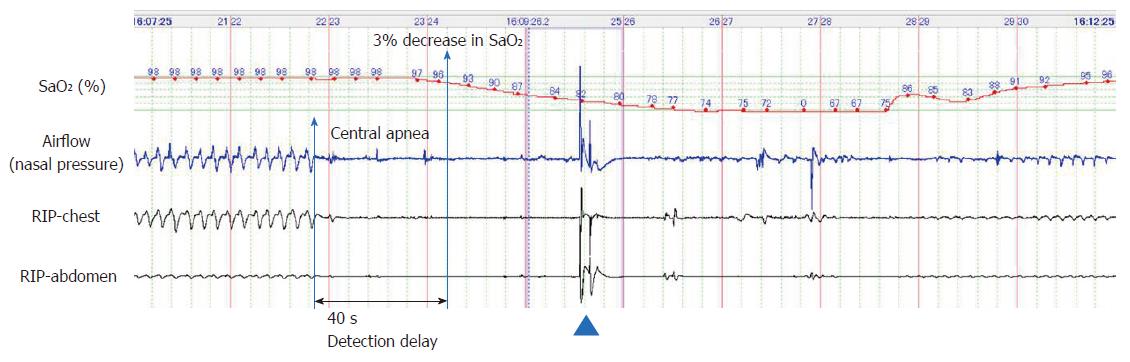
Figures 1, 2 and 3 represent polysomnographic recordings obtained during propofol sedation. Figure 1 depicts a long episode of central apnea that occurred immediately after initiation of the propofol sedation in a 67-year-old female. The chin-lift airway maneuver (arrowhead) restored breathing once; however, central apnea recurred, resulting in severe hypoxemia (SaO2, 67%). The hypoxemia gradually reversed along with recovery of breathing efforts. Notably, detection of central apnea by the nasal pressure signal preceded the 3% decrease in oxygen saturation by 40 s.
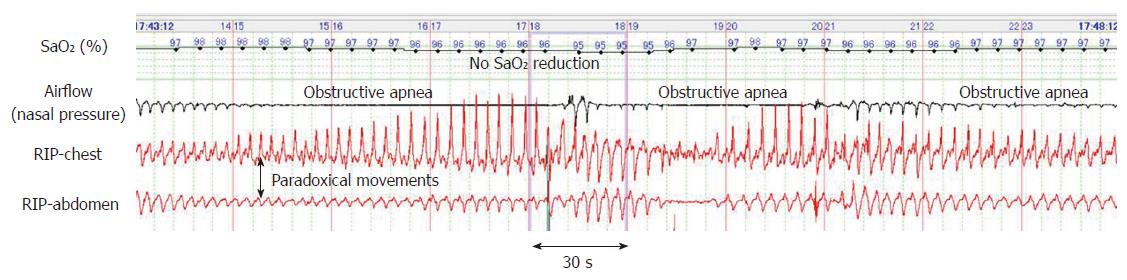
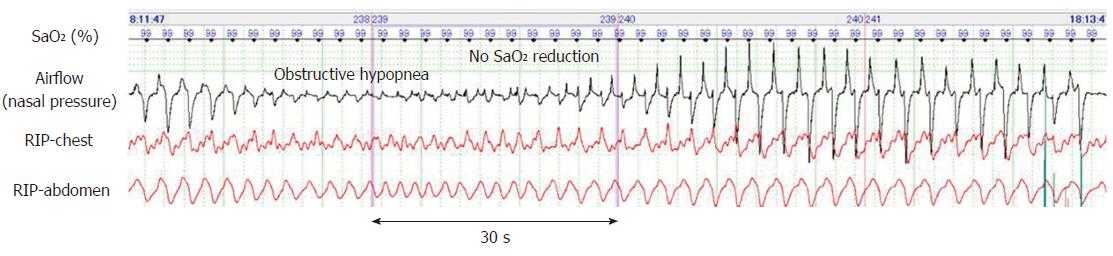
Figure 2 depicts a typical example of obstructive apnea periodically occurring in sleep state. Despite these long apnea episodes lasting for more than one minute, the SaO2 level remained > 95%. Similarly, periodic obstructive hypopnea occurred during the sleep state and without resulting in hypoxemia (Figure 3). Further, obstructive hypopnea diagnosed based on paradoxical thoraco-abdominal wall movements and flattened nasal pressure waves resolved spontaneously. Unlike such an abrupt resolution of obstructive hypopnea during natural sleep, obstructive hypopneas during sedation-induced sleep only improved gradually with an increase in breathing effort.
The results of PSG analysis are presented in Table 2 and Figure 4, and 207 respiratory disturbances were identified in total. While the frequency of the events in individual patients varied, all patients showed respiratory disturbance(s) during propofol sedation (total AHI: 10.44 ± 5.68/h). Based on the classification of the severity of sleep disordered breathing, 9 patients were categorized as having mild respiratory disturbances (AHI > 5 and AHI < 15), whereas 1 patient had moderate (AHI ≥ 15 and AHI < 30) respiratory disturbance. Although the average duration of apnea and hypopnea episodes was 38 s, the longest episode lasted for > 120 s. Even though the SaO2 level predominantly remained at >90% during sedation, 5 of 10 patients (50%) had respiratory disturbances that led to SaO2 levels falling to <90% at least once.
| Value (mean ± SD) | |
| Initial dose of propofol (mg/kg) | 1.2 ± 0.4 |
| Total dose of propofol (mg/kg) | 9.8 ± 3.8 |
| Sedation period (min) | 113.8 ± 35.8 |
| Total apnea hypopnea index (AHI) (/h) | 10.4 ± 5.7 |
| Mean duration of apnea hypopnea (s) | 38.1 ± 48.9 |
| Longest apnea and hypopnea (s) | 159.1 ± 147.9 |
| Patients with SaO2 < 70% event (s) | 20% |
| Patients with SaO2 < 90% event (s) | 50% |
| Cumulative time spent SaO2 less than 90% | 3.7% ± 9.1% |
| Detection earlier than SaO2 less (s) | 107.4 ± 67.0 |
Among the 207 respiratory disturbances identified by PSG, 87.9 % did not result in hypoxemia, whereas 12.1 % did, as detected by pulse oximetry. Total AHI, detected by PSG (10.44 ± 5.68/h), was significantly greater than that detected by pulse oximetry (1.54 ± 1.81/h, P < 0.001), thereby supporting our primary hypothesis that pulse oximetry alone underestimates respiratory disturbances during propofol sedation in patients undergoing ESD surgery (Figure 4).
While obstructive apnea and hypopnea episodes were common during propofol sedation (Figures 2 and 3), central apnea and hypopnea typically occurred immediately after a bolus injection of propofol and during the initial half of sedation, as depicted in Figure 1. The incidence of obstructive AHI (9.26 ± 5.44/h) was significantly greater than that of central AHI (1.19 ± 0.90/h, P < 0.001), thereby indicating the predominance of obstructive respiratory disturbances during propofol sedation (Figure 4).
Figure 1 depicts that PSG could detect apnea 40 s earlier than a manifest reduction in the SaO2 levels. Respiratory disturbance with hypoxemia occurred 25 times in 9 patients, and all such instances were detected by PSG. Importantly, these 25 instances of respiratory disturbances were, on average, detected by PSG 107.4 ± 67.0 s earlier than that by pulse oximetry (Table 2).
We measured consciousness, breathing, and oxygenation using PSG during propofol sedation for ESD surgery and observed that respiratory disturbances with SaO2 falling to < 90% occurred in 50% of the patients. Importantly, a majority of the respiratory disturbances were episodes of non-hypoxemic obstructive apneas and hypopneas, and our data indicate that pulse oximetry underestimates the incidence of respiratory disturbances. To the best of our knowledge, this is the first study of its kind.
We used AHI as an index to characterize severity and nature of respiratory disturbances during propofol sedation. AHI was calculated using the incidence of apnea and hypopnea identified based on their standard definitions widely used in PSG studies[15]. Contrary to a previous prospective study that assessed the incidence of respiratory disturbances or hypoxemia and reported a value of 12.8%[11], the incidence of SaO2 of < 90% was higher in our study (50%). This divergence can be attributed to older age, longer sedation period, and different body position adopted by us. Further, the use of AHI allowed us to quantify the number of apnea and hypopnea episodes in individual patients; thus, obstructive and central events could be clearly distinguished. Notably, although the severity of respiratory disturbance differed among patients, they all occurred during propofol sedation. Further, apnea and hypopnea episodes were predominantly obstructive in nature, and central events were also observed. These results indicate that devising a uniform strategy to prevent respiratory disturbances during sedation may be difficult and imply that reliable respiratory monitoring that can identify respiratory disturbances without delay and categorize them as either obstructive or central are essential for choosing appropriate treatment strategies. We demonstrated that combined monitoring of nasal pressure and thoraco-abdominal movement is both reliable and accurate; however, the clinical usefulness of this combination is questionable owing to its complexity and the level of respiratory physiology knowledge required. Thus, the nasal pressure waveform alone also reflects inspiratory flow limitation caused by airway obstruction[16], and unlike capnography, this parameter is not affected by carbon dioxide insufflation. Also, the nasal pressure waveform can detect not only the respiratory rate but can also identify the decrease in ventilation, like hypopnea. Therefore, we believe that nasal pressure measurement is potentially useful for respiratory monitoring during sedation and that it must be tested in future clinical studies.
Our results corroborate with those of previous studies wherein pulse oximetry was found to underestimate apnea and hypopnea incidence during propofol sedation[11,17]. However, this does not imply that pulse oximetry is not a suitable cardiorespiratory monitor during sedation for GI endoscopy. In fact, we found that hypoxemic episodes were accurately identified by pulse oximetry alone (Figure 1). Further, it should be noted that severe desaturation was caused by long duration of central apnea in association with a deeper level of sedation immediately after a bolus injection of propofol, and it has been shown during propofol sedation that, a higher loading dose, rather than total propofol dose, is associated with severe sedation-related adverse events[18]. Although more evidence is necessary, it is possible that unexpected deeper sedation during propofol sedation for GI endoscopy can impair respiratory compensatory mechanisms and lead to rare but critical cardiorespiratory complications that require intensive intervention or treatment[19]. Furthermore, our results indicate that critical events constitute a small proportion of the greater incidence of non-hypoxemic apnea and hypopnea episodes observed here, and currently, we lack an understanding about the pathological significance of these non-hypoxemic apneas and hypopneas. Unlike hypoxic events caused by long duration of central apnea just after a bolus injection of propofol, non-hypoxemic obstructive events tended to happen during continuous infusion of propofol. Therefore, they could be early markers for effective prevention of critical events during and/or immediately after sedation. More severe hypoxemia can develop when oxygen therapy is immediately terminated after endoscopy, because residual sedatives could worsen respiratory disturbances. In fact, deaths in patients undergoing GI endoscopy during and after propofol sedation have been reported[20]. Clearly, future studies need to explore the clinical significance of non-hypoxemic respiratory disturbances.
Pulse oximetry monitors oxygenation rather than ventilation, and several physicians use pulse oximetry alone for monitoring respiration during ESD. Specifically, in patients requiring oxygenation, oxygen saturation is often used as a delayed index for ventilation, and it has been reported that when respiratory arrest occurs, it takes 1-2 min for the decrease in oxygen saturation to become evident[21]. This time lag can be crucial in patients requiring prompt medical intervention.
In ambient air, decreased ventilation increases the partial pressure of carbon dioxide in arterial blood, thereby gradually decreasing oxygen saturation. However, oxygen saturation does not immediately reflect changes in supplemental oxygen provided. In cases of hypercapnia caused by hypoventilation, the oxygen saturation level is usually between 90%–99%, and it is possible that by the time the oxygen saturation decreases, the patient may have entered a state of respiratory arrest[22-24]. Importantly, cardiac arrest usually occurs 4-5 min after respiratory arrest, with a gap of only 1-2 min between the decrease in SaO2 and the occurrence of cardiac arrest. Thus, the key to safely performing endoscopy in patients under deep sedation is to quickly detect and address respiratory disturbances. Finally, the fact that PSG can detect respiratory disturbances approximately 107.4 s before the decrease in oxygen saturation is important. Therefore, in procedures performed with the patient under sedation, real-time respiration monitoring, such as using PSG based on respiration management for general anesthesia, is considered necessary.
There are several limitations in this study. First, the sample size is small and the patient population is limited to the elderly; thus, generalizing the findings presented here is difficult. Further randomized controlled trials need to be confirmed. However, we believe that our primary hypothesis has been quantitatively tested using AHI rather than just the number of episodes during the sedation. Second, propofol sedation was performed by a trained physician; however, he was not an anesthesiologist. Although whether the involvement of an anesthesiologist increases the safety during sedation for GI endoscopy is unknown[1,18,25,26], we did not aim to test the safety of propofol sedation. However, it was actually difficult to keep the patient’s Ramsey score at all times during ESD. The depth of sedation may have influenced the outcome. Third, this study did not assess in detail patient risks for developing upper airway obstruction when unconscious. Particularly, the greater number of participants with obstructive sleep apnea might have increased the rate of respiratory disturbance with severe hypoxemia, and this aspect should have been addressed before initiating the study. Thus, it would be interesting to explore the differences in the nature of respiratory disturbances during sedation for GI endoscopy between patients with and without obstructive sleep apnea[27].
In conclusion, episodes of non-hypoxemic obstructive apnea and hypopnea, which are undetectable by pulse oximetry, are common in elderly patients undergoing ESD under propofol-induced sedation. Careful respiratory monitoring using both pulse oximetry and nasal pressure monitors may be helpful for preventing critical cardiorespiratory events during relatively deep sedation for advanced GI endoscopy.
Endoscopic treatments often take long time, however procedures are better tolerated in terms of patient satisfaction and safety when sedation is administered.
Recent guidelines on gastrointestinal endoscopy strongly recommend pulse oximetry and careful monitoring of breathing during sedation. But it is unclear as to how many non-critical respiratory disturbances occurred in addition to critical events.
The objectives are to reveal that polysomnography (PSG) can accurately evaluate respiratory disturbance incidence during sedation for gastric endoscopic submucosal dissection (ESD) compare to pulse oximetry alone and to characterize breathing patterns.
This study included 10 elderly patients with early gastric cancer undergoing ESD under propofol sedation. PSG measurements were acquired. The comparison of respiratory disturbances between PSG and pulse oximetry was tested by the apnea hypopnea index (AHI), defined as the number of apnea and hypopnea instances per hour during sedation, with and without hypoxemia. The breathing pattern was characterized by the waveform of PSG.
PSG detected 207 respiratory disturbances in the 10 patients. PSG yielded a significantly greater AHI (10.44 ± 5.68/h) compared with pulse oximetry (1.54 ± 1.81/h, P < 0.001). Obstructive AHI (9.26 ± 5.44/h) was significantly greater than central AHI (1.19 ± 0.90/h, P < 0.001). Compared with pulse oximetry, PSG detected the 25 instances of respiratory disturbances with hypoxemia 107.4 s earlier on average.
PSG can better detect respiratory irregularities in detail compared with pulse oximetry and thus provide superior AHI values, leading to distinguish between obstructive and central events clearly.
It is not necessary to take all kinds of PSG monitoring for the patients under sedation. Among PSG monitoring, nasal pressure measurement is potentially useful for respiratory monitoring and that it must be tested in future clinical studies. Moreover, we will clarify what characters of patients require strict monitoring before endoscopic procedures under sedation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Amornyotin S, Hosoe N, Skok P S- Editor: Wang JL L- Editor: A E- Editor: Song H
| 1. | Park CH, Shin S, Lee SK, Lee H, Lee YC, Park JC, Yoo YC. Assessing the stability and safety of procedure during endoscopic submucosal dissection according to sedation methods: a randomized trial. PLoS One. 2015;10:e0120529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Uesato M, Nabeya Y, Akai T, Inoue M, Watanabe Y, Kawahira H, Mamiya T, Ohta Y, Motojima R, Kagaya A. Salivary amylase activity is useful for assessing perioperative stress in response to pain in patients undergoing endoscopic submucosal dissection of gastric tumors under deep sedation. Gastric Cancer. 2010;13:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Uesato M, Nabeya Y, Akai T, Inoue M, Watanabe Y, Horibe D, Kawahira H, Hayashi H, Matsubara H. Monitoring salivary amylase activity is useful for providing timely analgesia under sedation. World J Gastrointest Endosc. 2014;6:240-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Park CH, Min JH, Yoo YC, Kim H, Joh DH, Jo JH, Shin S, Lee H, Park JC, Shin SK. Sedation methods can determine performance of endoscopic submucosal dissection in patients with gastric neoplasia. Surg Endosc. 2013;27:2760-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Eikermann M, Malhotra A, Fassbender P, Zaremba S, Jordan AS, Gautam S, White DP, Chamberlin NL. Differential effects of isoflurane and propofol on upper airway dilator muscle activity and breathing. Anesthesiology. 2008;108:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: a risk factor analysis. Scand J Gastroenterol. 2008;43:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Obara K, Haruma K, Irisawa A, Kaise M, Gotoda T, Sugiyama M, Tanabe S, Horiuchi A, Fujita N, Ozaki M. Guidelines for sedation in gastroenterological endoscopy. Dig Endosc. 2015;27:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | ASGE Ensuring Safety in the Gastrointestinal Endoscopy Unit Task Force, Calderwood AH, Chapman FJ, Cohen J, Cohen LB, Collins J, Day LW, Early DS. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc. 2014;79:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Sieg A; bng-Study-Group, Beck S, Scholl SG, Heil FJ, Gotthardt DN, Stremmel W, Rex DK, Friedrich K. Safety analysis of endoscopist-directed propofol sedation: a prospective, national multicenter study of 24 441 patients in German outpatient practices. J Gastroenterol Hepatol. 2014;29:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Goudra BG, Singh PM, Gouda G, Borle A, Gouda D, Dravida A, Chandrashakhara V. Safety of Non-anesthesia Provider-Administered Propofol (NAAP) Sedation in Advanced Gastrointestinal Endoscopic Procedures: Comparative Meta-Analysis of Pooled Results. Dig Dis Sci. 2015;60:2612-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Coté GA, Hovis RM, Ansstas MA, Waldbaum L, Azar RR, Early DS, Edmundowicz SA, Mullady DK, Jonnalagadda SS. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Hillman DR, Walsh JH, Maddison KJ, Platt PR, Kirkness JP, Noffsinger WJ, Eastwood PR. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology. 2009;111:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1845] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 14. | Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington: Public Health Service 1968; . |
| 15. | Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2718] [Cited by in RCA: 3727] [Article Influence: 286.7] [Reference Citation Analysis (0)] |
| 16. | Isono S, Feroah TR, Hajduk EA, Brant R, Whitelaw WA, Remmers JE. Interaction of cross-sectional area, driving pressure, and airflow of passive velopharynx. J Appl Physiol (1985). 1997;83:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Cacho G, Pérez-Calle JL, Barbado A, Lledó JL, Ojea R, Fernández-Rodríguez CM. Capnography is superior to pulse oximetry for the detection of respiratory depression during colonoscopy. Rev Esp Enferm Dig. 2010;102:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Mehta PP, Kochhar G, Kalra S, Maurer W, Tetzlaff J, Singh G, Lopez R, Sanaka MR, Vargo JJ. Can a validated sleep apnea scoring system predict cardiopulmonary events using propofol sedation for routine EGD or colonoscopy? A prospective cohort study. Gastrointest Endosc. 2014;79:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229-37; quiz 1518-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 284] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Goudra B, Nuzat A, Singh PM, Gouda GB, Carlin A, Manjunath AK. Cardiac arrests in patients undergoing gastrointestinal endoscopy: A retrospective analysis of 73,029 procedures. Saudi J Gastroenterol. 2015;21:400-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Fu ES, Downs JB, Schweiger JW, Miguel RV, Smith RA. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126:1552-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Keidan I, Gravenstein D, Berkenstadt H, Ziv A, Shavit I, Sidi A. Supplemental oxygen compromises the use of pulse oximetry for detection of apnea and hypoventilation during sedation in simulated pediatric patients. Pediatrics. 2008;122:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Lynn LA, Curry JP. Patterns of unexpected in-hospital deaths: a root cause analysis. Patient Saf Surg. 2011;5:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Suzuki T. Considerations Regarding Monitored Anesthesia Care under Endoscopic Sedation: Endoscopic Procedure Rooms Currently at Risk. J Jpn Soc Clin Anesth. 2014;34:151–160. [DOI] [Full Text] |
| 25. | Buxbaum J, Roth N, Motamedi N, Lee T, Leonor P, Salem M, Gibbs D, Vargo J. Anesthetist-Directed Sedation Favors Success of Advanced Endoscopic Procedures. Am J Gastroenterol. 2017;112:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Goudra BG, Singh PM, Gouda G, Borle A, Gouda D, Dravida A, Chandrashakhara V. Safety of Non-anesthesia Provider-Administered Propofol (NAAP) Sedation in Advanced Gastrointestinal Endoscopic Procedures: Comparative Meta-Analysis of Pooled Results. Dig. Dis. Sci. 2015;60:2612-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Andrade CM, Patel B, Gill J, Amodeo D, Kulkarni P, Goldsmith S, Bachman B, Geerken R, Klein M, Anderson W. Safety of Gastrointestinal Endoscopy With Conscious Sedation in Patients With and Without Obstructive Sleep Apnea. J Clin Gastroenterol. 2016;50:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |