Published online Oct 16, 2018. doi: 10.4253/wjge.v10.i10.308
Peer-review started: April 28, 2018
First decision: May 18, 2018
Revised: July 13, 2018
Accepted: August 26, 2018
Article in press: August 27, 2018
Published online: October 16, 2018
Processing time: 171 Days and 10.9 Hours
To systematically review safety/efficacy of therapeutic endoscopic-retrograde-cholangiopancreatography (ERCP) performed during pregnancy, considering fetal viability, fetal teratogenicity, premature delivery, and future postpartum development of the infant.
Systematic computerized literature search performed using PubMed with the key words “ERCP” and “pregnancy”. Two clinicians independently reviewed the literature, and decided on which articles to incorporate in this review based on consensus and preassigned priorities. Large clinical trials, meta-analyses, systematic reviews, and controlled trials were assigned higher priority than review articles or small clinical series, and individual case reports were assigned lowest priority. Dr. Cappell has formal training and considerable experience in conducting systematic reviews, with 4 published systematic reviews in peer-reviewed journals indexed in PubMed during the last 2 years, and with a PhD in neurophysiology that involved 5 years of training and research in biomedical statistics.
Advances in imaging modalities, including abdominal ultrasound, MRCP, and endoscopic ultrasound, have generally obviated the need for diagnostic ERCP in non-pregnant and pregnant patients. Clinical experience with performing ERCP during pregnancy is burgeoning, with > 500 cases of therapeutic ERCP reported in the literature, aside from a national registry study of 58 patients. These studies show that therapeutic ERCP has a very high rate of technical success in clearing the bile duct of gallstones, and has a relatively low and acceptable rate of maternal and fetal complications. The great majority of births after therapeutic ERCP are full-term, have normal birth weights, and are healthy. A recent trend is performing ERCP without radiation to eliminate radiation teratogenicity. Systematic literature review reveals 147 cases of ERCP without fluoroscopy in 8 clinical series. These studies demonstrate extremely high technical success in endoscopically removing choledocholithiasis, favorable maternal outcomes with rare maternal ERCP complications, and excellent fetal outcomes. ERCP without fluoroscopy generally confirms proper biliary cannulation by aspiration of yellow bile per sphincterotome or leakage of yellow bile around an inserted guide-wire.
This systematic literature review reveals ERCP is relatively safe and efficacious during pregnancy, with relatively favorable maternal and fetal outcomes after ERCP. Recommendations are provided about ERCP indications, special ERCP techniques during pregnancy, and prospects for future research.
Core tip: This work systematically reviews safety/efficacy of therapeutic endoscopic-retrograde-cholangiopancreatography (ERCP) performed during pregnancy, considering fetal viability, fetal teratogenicity, premature delivery, and future development of the infant after parturition. Systematic computerized literature search was performed using PubMed with key words “ERCP” and “pregnancy”. Two clinicians independently reviewed the literature, and decided on which articles to incorporate in this review based on pre-arranged prioritization and consensus. Clinical experience with performing ERCP during pregnancy is burgeoning, with > 500 cases of therapeutic ERCP reported in the literature, plus a national registry study of 58 patients.
- Citation: Cappell MS, Stavropoulos SN, Friedel D. Systematic review of safety and efficacy of therapeutic endoscopic-retrograde-cholangiopancreatography during pregnancy including studies of radiation-free therapeutic endoscopic-retrograde-cholangiopancreatography. World J Gastrointest Endosc 2018; 10(10): 308-321
- URL: https://www.wjgnet.com/1948-5190/full/v10/i10/308.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i10.308
Endoscopic-retrograde-cholangiopancreatography (ERCP) is currently the standard technique for treating choledocholithiasis and associated complications, such as cholangitis and biliary stricture, in the non-pregnant population. The approach to pregnant women with suspected choledocholithiasis, however, differs somewhat from that for non-pregnant patients because of concerns about the pregnant mother and the fetus, including procedure time, teratogenicity of intra-procedural medications, and fetal radiation exposure. This work systematically reviews ERCP during pregnancy, with a particular focus on differences between the pregnant vs non-pregnant patient in patient indications, patient preparation, procedural medications, complications, reducing fetal radiation exposure, and maternal and fetal outcomes.
Systematic computerized literature search was performed using PubMed with the key words “ERCP” and “pregnancy”. Two clinicians independently reviewed the literature, and decided on which articles to incorporate in this review based on consensus. Large clinical trials, meta-analyses, systematic reviews, and controlled trials were assigned higher priority than review articles or small clinical series, and individual case reports were assigned the lowest priority. Data were extracted independently by 2 authors to prevent errors in data extraction. Dr. Cappell has formal training and considerable experience in conducting in conducting systematic reviews, with 4 published systematic reviews in peer-reviewed journals indexed in PubMed during the last 2 years, and with a Ph.D. in neurophysiology that involved 5 years of training and research in biomedical statistics.
Up to 20% of American adults have cholelithiasis, of whom about 20% develop symptoms or complications during their life-time[1,2]. About 750000 cholecystectomies are performed annually in America. Risk factors for cholelithiasis include advanced age, female gender, obesity, hyperlipidemia, pregnancy, and physical inactivity[2]. Symptoms and complications increase in frequency when gallstones are present > 5 years, and when they are > 10 mm in diameter[3]. The pathophysiology of pregnancy-related lithogenicity includes bile supersaturated with cholesterol, increased gallbladder volume, diminished gallbladder motility, and changes in the bile salt pool[4-7]. These gestational changes are largely mediated by increased levels of the gestational hormones of estrogen and progesterone[4].
The prevalence of cholelithiasis during pregnancy varies with the study population. A study performed in India noted only a 1% prevalence[8], whereas a study performed in a Californian Hispanic cohort reported a 5% prevalence[9]. Both cohorts were asymptomatic at study initiation. A prospective study of abdominal ultrasound among > 3000 pregnant subjects without cholelithiasis detected at baseline showed 5% developed cholelithiasis by the second trimester, and 10% developed cholelithiasis by six weeks postpartum[10]. About 1% of this cohort developed symptoms from cholelithiasis. A Mexican study noted that symptomatic gallstone disease during pregnancy usually manifests as acute cholecystitis, even though 19% had choledocholithiasis[11]. Cholelithiasis and hypertriglyceridemia are the primary etiologies of pancreatitis during pregnancy[12,13], whereas alcohol-induced pancreatitis is unusual during pregnancy because expectant mothers generally abstain from alcohol due to its fetal toxicity[14]. Cholelithiasis and choledocholithiasis are sometimes encountered during pregnancy because female gender, concurrent pregnancy, and prior pregnancy are risk factors for cholelithiasis. Fortunately, the endoscopist is infrequently required to perform ERCP, with its attendant risks during pregnancy, because ERCPs can often be delayed to postpartum because patients have minimal clinical findings or can directly undergo cholecystectomy without antecedent ERCP for acute cholecystitis.
The unique maternal and fetal physiologic requirements during pregnancy affect the usual practice of ERCP. The unique maternal and fetal physiologic requirements during pregnancy affect the usual practice of ERCP. ERCP in non-pregnant patients is usually performed with the patient in the prone position to aid in selective bile cannulation and to provide better fluoroscopic imaging compared to other positions. However, this position is not recommended during advanced pregnancy for the following reasons: to avoid patient discomfort from the enlarged, gravid uterus pressing against the hard X-ray platform, to avoid decreased systemic and uterine perfusion from the enlarged gravid uterus compressing the aorta, and to avoid decreased venous return from the enlarged gravid uterus compressing the inferior vena cava[15]. Patients may also require supporting cushions during advanced pregnancy to minimize patient discomfort. Rapid intra-procedural infusion of IV fluids is generally recommended to promote pancreatic perfusion and decrease the incidence and severity of post-ERCP pancreatitis, but may be inadvisable during pregnancy because of the already expanded extravascular space and salt retention during pregnancy[16]. However, the fetus poorly tolerates maternal systemic hypotension because blood flow is shunted away from the uterus during maternal hypotension[17], and maternal hypotension should, therefore, be aggressively treated, if feasible, before performing ERCP. As for all patients undergoing ERCP, the pregnant patient should have her vital signs stabilized, electrolyte disorders corrected, and major disorders such as sepsis, hypovolemia, and hypoxemia addressed before undergoing ERCP. As in the general population all pregnant patients undergoing anticipated therapeutic ERCP should have a complete hemogram and prothrombin/international normal ratio determination. It is important to test for pregnancy with a beta-HCG determination in women who are undergoing ERCP, are of childbearing age, and have a recent pregnancy history that is uncertain or suggestive of early pregnancy to avoid inadvertent fetal radiation exposure[18].
The mother should be maintained nil per os (NPO) for at least 6 h before ERCP to reduce risks of aspiration of gastric contents. Elective endotracheal intubation should be strongly considered before ERCP, especially during advanced pregnancy, because the gravid uterus, impinges upon the stomach and increases the risk of aspiration of gastric contents[19]. It may, moreover, be necessary to perform ERCP in the supine position, especially during advanced pregnancy, which can further increase aspiration risks[20]. The mother can typically be extubated soon after ERCP in the absence of chronic pulmonary disease.
The American Society for Gastrointestinal Endoscopy promulgated guidelines for endoscopy during pregnancy, including ERCP, which incorporate safety data for commonly used endoscopic medications during pregnancy[21,22], as classified by the United States Food and Drug Administration (FDA) from A (most safe) to D (least safe), with a special category of X, for drugs contraindicated during pregnancy. The general principle is to avoid FDA category X and restrict FDA category D drugs, and substitute FDA category B or C drugs for category D drugs, if feasible, during pregnancy. Indomethacin suppositories are recommended for ERCP in patients at risk for pancreatitis, but indomethacin is an FDA category C drug, with concern about premature closure of a patent ductus arteriosus (PDA) in late pregnancy[22]. Propofol is considered safe (FDA category B), even though it crosses the placenta and causes transient fetal sedation. Meperidine is considered safer (FDA category B) than either fentanyl or morphine (both FDA category C). Moreover, meperidine causes minimal spasm of the sphincter of Oddi, whereas other narcotics may cause problematic spasm of this sphincter during ERCP. Midazolam is considered safer than diazepam, even though both are category D drugs because diazepam has been occasionally associated with cleft palate[23]. Glucagon is used to reduce intestinal spasm and is believed to be generally safe during pregnancy (FDA category B)[24]. Glucagon administration may be justifiable during ERCP if needed to cannulate the choledochus during therapeutic ERCP to prevent maternal cholangitis from choledocholithiasis, but glucagon administration can usually be obviated by prompt choledochal cannulation by an expert endoscopist. Simethicone is used to eliminate troublesome intraluminal bubbles and is believed to be relatively safe during pregnancy (FDA category C)[25]. It should, however, be used only if necessary during ERCP. Informed patient consent for ERCP should include a discussion regarding fetal safety during pregnancy, including fetal toxicity from radiation exposure. In terms of antibiotics, penicillins/cepholosporins/macrolides are generally safe, provided no hypersensitivity occurs, but quinolones/tetracyclines/sulfonamides/Flagyl are not safe[25].
The management of pregnant women with pancreaticobiliary disease requires a multidisciplinary approach, with a clinical team including a gastroenterologist, obstetrician/perinatologist, radiation safety officer, and anesthesiologist, who preferably specializes in obstetric anesthesiology. The requisite experience and expertise is typically found in a tertiary, academic medical center. The gastroenterologist should have significant expertise and experience in ERCP to be best equipped to deal with the challenges and risks of ERCP during pregnancy. The qualifications of an experienced advanced therapeutic endoscopist have not been standardized, but may include both a > 90% bile duct cannulation rate[26], and an adequate annual volume of therapeutic ERCPs (> 40 sphincterotomies per year)[27]. One study demonstrated that low volume ERCP-endoscopists exposed their patients to significantly more radiation during ERCP than high volume ERCP-endoscopists[28]. An experienced endoscopist is more likely to minimize procedural time, anesthesia dosages, and radiation time. An inexperienced gastroenterology fellow should play a limited role in this situation. The anesthesiologist should be in attendance during the entire ERCP, and not rely on a nurse anesthetist for administering sedation. The surgeon plays a critical role in the timing of cholecystectomy, and in providing backup for emergency CBD exploration or for complications after ERCP[29].
Electrocautery is a concern during pregnancy. Amniotic fluid readily conducts electricity which can reach the fetus[21,30]. Biliary sphincterotomy should use only bipolar current to decrease scatter of electricity. Biliary sphincterotomy, if necessary during ERCP, should use minimal cautery with the grounding pad placed on the right side, such as the right arm or right posterior thorax, to minimize electrical conduction to the fetus[22,31]. Strategies to avoid electrocautery include inserting a biliary stent without cautery, but this can be problematic unless delivery is imminent because of a long-term potential for stent clogging. Balloon sphincteroplasty is an alternative to sphincterotomy, but this maneuver can induce pancreatitis[32]. General principles of ERCP during pregnancy are summarized in Table 1.
| 1. Counsel patient, husband, and family on risks vs benefits of ERCP for mother as well as fetus |
| 2. Obtain written informed consent from pregnant patient (not the father) |
| 3. Endoscopist should assess whether his/her experience and skill is adequate for dealing with anticipated biliary pathology in a pregnant patient with this medical history |
| 4. Position patient on left side or supine, if possible, especially during advanced pregnancy |
| 5. Preferentially perform ERCP during second trimester, if possible |
| 6. During late third trimester, delay elective ERCP to after delivery |
| 7. Use safety guidelines (see Table 2) to minimize fetal radiation exposure and risks |
| 8. Consider performing EUS prior to ERCP to assess CBD diameter as well as number, size, and shape of gallstones |
| 9. Multidisciplinary input involving a perinatologist, high-risk obstetrician, obstetric anesthesiologist, radiation safety officer, and surgeon prior to ERCP |
| 10. Administer parenteral fluids consistent with clinical status and pregnancy requirements |
| 11. Reverse metabolic derangements and appropriately intervene to correct abnormalities in vital signs before scheduling ERCP |
| 12. Administer antibiotics and other drugs during ERCP that are considered relatively safe during pregnancy |
| 13. Endoscopist should be familiar with and prepared to use full armamentarium of endoscopic techniques including needle-knife sphincterotomy, transeptal sphincterotomy, choledochoscopy, and IDUS |
| 14. Counsel patients regarding requirements for follow-up visits, especially with stent placement |
| 15. Avoid pancreatic endotherapy during ERCP because this entails a higher risk than biliary endotherapy |
| 1. Highly qualified and experienced ERCP endoscopist |
| 2. Limited (solely observational) role of inexperienced gastroenterology fellow during ERCP |
| 3. Informed consent to include discussion of radiation teratogenicity |
| 4. Consult perinatologist |
| 5. Consult radiation safety officer and medical physicist, if available, to minimize fetal radiation exposure |
| 6. Endoscopist performing ERCP should become familiar with fluoroscopy equipment, especially with options to minimize radiation exposure |
| 7. Formal consultation of anesthesiologist before ERCP |
| 8. Anesthesiologist to attend during entire ERCP, even if nurse-anesthetist is present |
| 9. Consider using an obstetric anesthesiologist rather than a general anesthesiologist for ERCP |
| 10. Avoid ERCP for weak indications |
| 11. Avoid solely diagnostic ERCP |
| 12. Strongly consider MRCP as an alternative for diagnostic ERCP in low yield indications |
| 13. Obtain informed, written consent that includes discussion of risks of fetal radiation |
| 14. Perform ERCP at a hospital endoscopy unit rather than an ambulatory center in order to better manage procedural complications |
| 15. Perform ERCP at a tertiary hospital rather than a community hospital where highly specialized consultants are likely to be present |
| 16. Perform ERCP as expeditiously as possible to minimize radiation exposure and anesthesia medications |
| 17. Employ modern and highly collimated radiation unit with the smallest possible field |
| 18. Position patient as far as possible from radiation source consistent with reasonable images |
| 19. If possible, employ “low-dose” radiation protocol in terms of kvp, field size, and frame rate |
| 20. Place lead shield underneath patient between likely fetal area and radiation tube |
| 21. Place dosimeters on patient above expected uterine location and record fluoroscopy time and total radiation dosage |
| 22. Minimize procedure time, procure all anticipated endoscopy equipment within endoscopy room before beginning the procedure |
| 23. Employ static images as opposed to continuous fluoroscopy to reduce radiation exposure |
| 24. Use digital image acquisition technology if possible, instead of film-screen radiography |
| 25. Position patient to permit anterior-posterior beam projection |
| 26. Avoid image magnification |
| 27. Employ last image-hold or fluoroscopy loop recording feature when possible rather than additional fluoroscopy |
| 28. Consider radiation-free ERCP in conjunction with other techniques such as temporary stenting and, if needed, needle-knife and transpapillary sphincterotomy |
| 29. Document ductal clearance without radiation using IDUS or choledochoscopy |
| 30. X-ray image receptor should be placed as close as possible to the patient |
| 31. Adjust patient position between choices of supine, prone, or lateral to minimize fetal radiation exposure |
Fetal radiation exposure is a significant concern because of its potential teratogenic effects and subsequent carcinogenetic effects. Fetal radiation exposure and toxicity depends upon multiple factors, including maternal size, maternal distribution of fat, volume of amniotic fluid, fetal gestational age, and radiation delivery method. The most important factors determining fetal exposure are total radiation time and dosage, both of which should be minimized. Draping the lower abdomen and pelvis of patients with lead shields helps minimize uterine exposure[21]. Lead shielding is best placed below the patient because radiation typically emanates from below[21]. However, radiation scatter within the mother is likely the main source of fetal radiation exposure[33]. Static (spot) films are recommended instead of continuous fluoroscopy to decrease radiation exposure[34]. Also recommended are a modern radiation source, a well collimated unit, and avoidance of “hard-copy” images that require higher radiation dosage[21]. A radiation safety officer can provide valuable input. Dosimetry monitors can be placed externally on top of the uterus to monitor fetal radiation exposure. In one case, this device demonstrated low radiation exposure to the fetus, and higher radiation exposure to the maternal placenta and spleen[35]. Radiation exposure often exceeds 10 millisievert (mSv) during prolonged ERCP[33]. With recommended precautions, fetal radiation exposure during ERCP should be uniformly < 50-100 mSv, which is considered the radiation threshold for teratogenesis[21,36]. Techniques to reduce radiation exposure are summarized in Table 2.
Fetal radiation exposure is particularly concerning during early pregnancy. Radiation exposure to > 200 mGy could result in growth restriction and congenital anomalies, especially of the eyes, skeleton, and genitalia[31]. Thus, semi-elective ERCP should be deferred to the second trimester when feasible. Untoward outcomes of ERCP–related radiation exposure is not well studied, and they may conceivably manifest only later in childhood. Regardless, radiation exposure should be well documented, if feasible, for retrospective analysis[37]. One study suggested this documentation was unnecessary because of low teratogenicity risk, but this study used limited fluoroscopy time[38].
Outcome analysis regarding ERCP during pregnancy should consider technical procedural success, fetal outcomes, neonatal health, and birth weight. In a relatively large, retrospective, study of 68 ERCPs during 65 pregnancies, technical success was uniformly achieved[39]. Although 11 patients (16%) developed pancreatitis after ERCP, no other major complications occurred, including maternal hemorrhage, gastrointestinal perforation, or ascending cholangitis; maternal or fetal deaths; and fetal malformations. ERCPs performed during the first trimester had relatively worse fetal outcomes. Fifty-three patients (90%) had a full–term pregnancy after ERCP, but mothers undergoing ERCP during the first trimester had only 73% of deliveries at term, a higher risk of preterm delivery (20%), and higher risk of low-birth-weight infants (21%). In a series of 20 patients undergoing therapeutic ERCPs during pregnancy, there was one neonatal death 26 h after delivery that occurred in a patient who had undergone three therapeutic ERCPs during pregnancy with pancreatic duct stenting at each session for pancreatic duct stenosis after surgical sphincteroplasty[15]. This patient had developed acute pancreatitis after each of her 3 ERCPs. Another mother suffered spontaneous abortion 3 wk after ERCP. There were no other significant maternal or fetal complications.
A national cohort study of 58 pregnant women undergoing ERCP vs a three-fold larger control population of non-pregnant women demonstrated that the major ERCP complications of gastrointestinal perforation, hemorrhage, or infection were not more common during pregnancy, but post-ERCP pancreatitis was significantly increased during pregnancy at 12% vs 5% (adjusted odds ratio: 2.8, 95%CI: 2.1-3.8). This increased rate is attributed to avoiding fluoroscopy to verify wire and catheter position and to time pressure to expeditiously perform ERCP during pregnancy[40-42]. This work is important in that it represents the largest study heretofore on ERCP during pregnancy, but is subject to limitations including lack of data on patient comorbidities, maternal alcohol or illicit drug use, endoscopic complications, type of ERCP (diagnostic vs therapeutic), ERCP indications, and use or lack of monitored anesthesia care[43]. Also, as aforementioned, usual measures to minimize pancreatitis after ERCP, such as high volume IV fluid infusion, indomethacin suppositories, and pancreatic stents are infrequently used during pregnancy. A recent large, multicenter, study demonstrated that endoscopy during pregnancy is associated with an increased risk of preterm birth or small size for gestational age, but no increased risk of stillbirths or congenital malformations[40-42].
In a series of 18 women undergoing ERCP with biliary sphincterotomy for choledocholithiasis, one patient had a postsphincterotomy bleed and one patient had mild pancreatitis after ERCP and had preterm labor, but fetal outcomes were all favorable[44]. Scant data exist on long term postpartum follow-up after intrapartum ERCP, but this study of 18 women reported normal child development at 6 years[44]. Generally, therapeutic ERCP is believed to be relatively safe and effective during pregnancy, though safety concerns are increased during the first trimester, and there appears to be an increased risk of maternal pancreatitis after ERCP during pregnancy.
Two relatively large systematic reviews, one published in full[45], and the other published as an abstract[46], show that ERCP during pregnancy is relatively safe. In a systematic literature review performed by Cappell in 2011[45], 296 pregnant patients underwent therapeutic ERCP. Fetal outcomes as reported in 254 cases (86%) included: healthy infants at birth in 237, prematurely born infants with low birth weight in 11, late spontaneous abortions in 3, infant death soon after birth in 2, and voluntary abortion in 1. Perinatal mortality was only about 1% despite pregnant mothers undergoing therapeutic ERCP mostly for major gallstone complications, such as obstructive jaundice, ascending cholangitis, or gallstone pancreatitis. Moreover, no congenital anomalies were reported in the infants. However, these very favorable outcomes must be interpreted cautiously because most of the reviewed studies reported outcome only at parturition without subsequent follow-up, and fetal outcome data was absent in 15% of the pooled study patients.
A systematic literature review of 214 ERCP’s during pregnancy, published only as an abstract, reported a 5% pancreatitis rate, a 5% preterm birth rate, and about a 1% rate of spontaneous abortions[46]. Technical success of ERCP was high, even though >10% had to undergo stent placement and/or multiple ERCPs. These data on the largest individual studies and prior systematic reviews are summarized in Table 3.
| First author, yr, reference | Study characteristics | Findings |
| Tang SJ, 2009[39] | Large retrospective study of 68 ERCPs performed during 65 pregnancies. | Pancreatitis occurred in 11 pregnant patients (16%) after ERCP. No other major maternal complications occurred during pregnancy. No fetal deaths and no fetal malformations occurred. After ERCP 53 patients had deliveries at term (90% rate for known delivery outcomes). However, ERCP performed during first trimester had less favorable outcomes: preterm delivery = 20%, and low-birth-weight infants = 21% |
| Ludvigsson JF, 2017[42] | National cohort study in Sweden of 58 pregnant patients undergoing ERCP included in a much larger study of 3052 patients undergoing any gastrointestinal endoscopy during pregnancy. | Of 58 pregnant patients undergoing ERCP unfavorable fetal outcomes included: 3 (5.2%) preterm births, 0 (0%) stillbirths, 0 (0%) neonatal deaths, 12 (20.7%) Cesarean sections, 1 (1.7%) Apgar score < 7 at 5 min, 1 (1.7%) small for gestational age, and 3 (5.2%) with any major congenital malformation. All these pregnancy outcomes were similar to that of pregnancy outcomes for mothers not undergoing endoscopy during pregnancy |
| Jamidar PA, 1995[15] | Retrospective study of therapeutic ERCPs performed during 20 pregnancies. | Two significant complications: one spontaneous abortion 3 wk after ERCP, and 1 neonatal death 26 h. post-partum that occurred after the expectant mother underwent 3 therapeutic ERCPs during pregnancy with pancreatic stenting at each session complicated by post-ERCP pancreatitis. No other significant maternal or fetal complications |
| Gupta R, 2005[44] | Retrospective study of therapeutic ERCPs performed during 18 pregnancies for choledocholithiasis. | Complications: 1 mild postsphincterotomy bleed; and 1 mild pancreatitis and preterm labor after ERCP. All fetal outcomes were favorable. This study had long-term follow-up after intra-partum ERCP: all 18 infants had normal child development at 6 yr |
| Cappell MS, 2011[45] | Systematic literature review of 296 pregnant patients undergoing therapeutic ERCP including 254 (86%) in which fetal outcome was reported. | Fetal outcomes as reported in 254 cases included: healthy infants at birth in 237, prematurely born infants with low-birth-weight in 11, late spontaneous abortions in 3, infant death soon after birth in 2, and voluntary abortion in 1. Perinatal mortality was only about 1% despite pregnant mothers undergoing therapeutic ERCP mostly for major gallstone complications, such as obstructive jaundice, ascending cholangitis, or gallstone pancreatitis. No congenital anomalies were reported in the infants. These favorable data must be interpreted cautiously: in this literature review, fetal outcome data were missing in 42 (15%) of reported mothers undergoing ERCP during pregnancy |
In the general population solely diagnostic ERCP is not recommended anymore, and has been replaced by less invasive tests such as endoscopic ultrasound (EUS); and magnetic resonance cholangiopancreatography (MRCP)[47]. ERCP is not recommended unless it is most likely to be therapeutic. The same principle applies during pregnancy: solely diagnostic ERCP is not recommended during pregnancy.
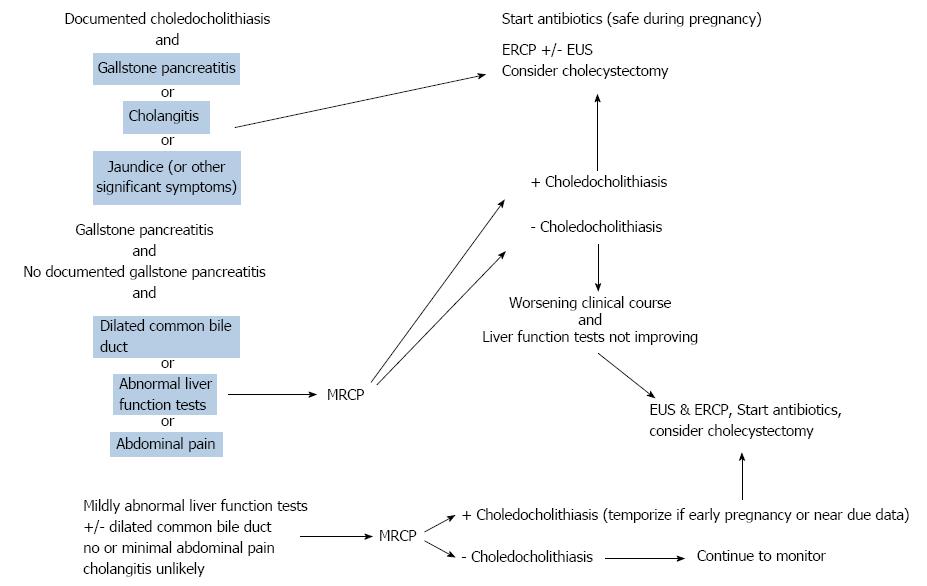
During the past 30 years, therapeutic ERCP during pregnancy has evolved from a novelty described in case reports to accepted practice with refinement of endoscopic techniques paralleling greater clinical experience, better technology, and greater technical expertise[21,31,48-51]. Progress in ERCP has been paralleled by advances in laparoscopic cholecystectomy. The first ERCP during pregnancy was a report in 1990 of five successful cases of biliary sphincterotomy and gallstone extraction for choledocholithiasis or cholangitis[48]. An estimated 500 or more women have been reported undergoing ERCP during pregnancy, aside from a national registry study of 58 patients[42]. Considerations in performing ERCP during pregnancy include clinical indication, maternal clinical status, laboratory results, ancillary radiologic studies, fetal age, endoscopist expertise, and hospital support. Risks vs benefits should be assessed for every high risk endoscopic procedure during pregnancy, especially ERCP[45]. Patients with documented choledocholithiasis associated with gallstone pancreatitis, cholangitis, jaundice, significant abdominal pain, pyrexia, leukocytosis, common bile duct dilatation on imaging studies, or grossly abnormal liver function tests need urgent ERCP, just like non-pregnant patients[52]. Patients with significantly elevated liver enzymes and/or a dilated CBD are more likely to harbor choledocholithiasis than patients without these features[53]. Preoperative ERCP is preferred over the alternative of direct cholecystectomy for these indications to avoid the increased morbidity and mortality from complex biliary surgery during cholecystectomy[54]. However, the indication for ERCP is more ambiguous in minimally symptomatic or asymptomatic patients with choledocholithiasis. Evaluation and therapy for uncomplicated cholelithiasis discovered during pregnancy is generally deferred until postpartum. Most patients with acute cholecystitis during pregnancy undergo cholecystectomy without preoperative ERCP[55]. Indeed. cholecystectomy for acute cholecystitis is the third most common non-obstetric operation performed during pregnancy[56].
The diagnostic armamentarium for suspected choledocholithiasis in pregnancy differs from the general approach in non-pregnant patients in that radiation-based imaging, such as abdominal CT, is not employed. Transabdominal ultrasound is relatively inexpensive and safe during pregnancy and is typically the initial imaging test. MRCP is especially useful during pregnancy, but raises a concern about a negative exam in the face of disparate clinical and laboratory findings[57]. In one small series, MRCP obviated the need for ERCP in pregnant women with pancreatobiliary abnormalities[58]. EUS is safe in pregnancy and highly accurate, but commits the patient to an endoscopy during pregnancy with its inherent procedural and sedation risks. However, a negative EUS examination can obviate ERCP with its greater attendant risks[59]. EUS also provides data on number, size, location, and morphology of choledocholithiasis for patients requiring ERCP.
Pregnancy stage and fetal development are paramount considerations in the timing of ERCP. ERCPs and cholecystectomies are generally best performed during the second trimester, after organogenesis during the first trimester and before the third trimester with its increased risk of premature delivery[45,60]. Postpartum ERCP is the best option if delay is feasible.
The prospect of ERCP often promotes anxiety in both the mother and endoscopist. Recent studies still show some risks of ERCP during pregnancy[48,61]. The large series by Tang et al[39] reported that ERCP can be safely performed throughout pregnancy, but may somewhat impact fetal health when performed during early gestation. An early multicenter series, including 15 first trimester ERCPs (FTE), demonstrated technical success, but had complications of one spontaneous abortion and one neonatal death[15]. Another series with dedicated obstetric input and lead shielding demonstrated good technical success and good fetal outcome, though only one FTE was performed[62]. An Indian series had 4 FTE’s, trivial fluoroscopy time, and a six year child follow-up[46]. The two series by Smith et al[38] and Kahaleh et al[63] were notable for limited fluoroscopy time, technical success, and good fetal outcomes, though two women developed eclampsia during the third trimester after undergoing ERCP. These series noted a slightly higher rate of post-ERCP pancreatitis than in the general population, in accord with cumulative data[40,41].
Most studies of ERCP during pregnancy are limited by relatively small study size, absence of controls, retrospective design, and lack of comparative statistics[45]. Some studies focus on the technical success of the ERCP, without reporting fetal outcome altogether[64,65].
A recent trend is performing radiation-free ERCP (RFE) during pregnancy[34]. Transabdominal ultrasound has guided subsequent RFE, but this technique is cumbersome. In RFE a two-stage procedure may be performed, where the initial ERCP during pregnancy is temporizing, uses minimal or no fluoroscopy, and typically incorporates biliary sphincterotomy and stent placement; the subsequent postpartum ERCP is definitive[64,65]. In a patient presenting in late pregnancy, it is reasonable to perform a moderate sphincterotomy and insert a biliary stent to defer more definitive therapy to postpartum[64]. Performance of ERCP in the first trimester may involve risks of termination of pregnancy, especially if the ERCP is prolonged and entails considerable radiation exposure. Visualization of bile drainage after wire insertion or bile aspiration after catheter cannulation is currently usually used to confirm successful selective biliary cannulation. Shortcomings of this method include wires or catheters can inadvertently enter the cystic duct, chronically obstructed biliary systems may yield “white bile”, and curled wires may cause bile duct injury that renders stent insertion difficult.
The initial case of RFE was inadvertent use of a needle-knife for an impacted common bile duct stone[66]. The first clinical series of RFE after ultrasound consisted of 6 pregnant women with acute pancreatitis or cholangitis[67]. The 6 patients underwent selective bile duct cannulation, biliary sphincterotomy, and successful gallstone removal, but two infants were born prematurely, including one with significant complications. Altogether 147 ERCP’s have been performed during pregnancy without fluoroscopy in 8 clinical series, reflecting endoscopist ingenuity and technological progress (Table 4)[64,68-75]. These clinical data are extremely promising, with a very high rate of technical success (clearing of CBD stones), low rate of maternal complications, delivery of predominantly healthy babies, mostly normal birth weights, and typical delivery at term (Table 4)[64,68-75]. However, case series from tertiary academic centers may not be extrapolated to community hospitals. Radiation–free ERCP is ideal, but should not be pursued if this unduly prolongs the ERCP and increases the risks of complications, especially pancreatitis. Moreover, brief fluoroscopy with “ultra-short” (< 60 s) radiation exposure may produce as favorable fetal results as radiation-free ERCP.
| First author, yr, reference | Number reported | Indications | Technique of radiation-free ERCP | Outcomes |
| Shah 2016[75] | Non-radiation ERCP attempted-31 non-pregnant subjects. 26 successfully underwent ERCP without fluoroscopy. 5 required fluoroscopy during ERCP | Adult patients with suspected biliary stones based on abnormal serum liver tests, abdominal imaging, and/or abdominal pain. Underwent EUS per protocol. Patients with suspected large stone burden, complicated stone disease, or difficult anatomy were excluded | Antecedent EUS used as a guide before ERCP. Selective cannulation confirmed by aspirating visible bile in 26 patients. 5 patients required radiation for double wire or precut papillotomy. All patients had EUS. 4 others had ERCP obviated by EUS | No adverse events among patients who underwent bile cannulation, sphincterotomy, and stone removal without fluoroscopy. One patient undergoing ERCP with fluoroscopy had moderated post-ERCP pancreatitis |
| Ersoz 2016[74] | 22 patients: first trimester-2, second trimester-3, third trimester-17 | Abdominal ultrasound demonstrates stone/sludge in gallbladder-22 (100%), choledocholithiasis-12, mean total bilirubin = 5.49 ± 1.66 mg/dL, acute cholangitis-2, acute cholecystitis-2 | Selective biliary cannulation attempted with sphincterotome and confirmed by bile aspiration. Biliary sphincterotomy and balloon dilation-18/22 had visible gallstones, 3 required transpancreatic papillary septotomy | 5 complications after ERCP: epigastric pain without elevated lipase elevation-2, mild pancreatitis treated conservatively-2, minor post-sphincterotomy bleeding successfully treated with epinephrine injection without blood transfusions. All delivered healthy infants at term |
| Sethi S, 2015[73] | 3 patients: 14, 7, or 28 wk pregnant | 1 and 2-Dilated CBD and total bilirubin > 5.0 mg/dL after laparoscopic cholecystectomy, 3-Dilated CBD, multiple gallstones and increased total bilirubin level | All cases: EUS-guided ERCP with selective biliary cannulation confirmed by bile aspiration. Biliary sphincterotomy and stone extraction(s) using balloon sweeps or Spyglass technology | Uncomplicated. All mothers did well-rapidly discharged from hospital. Fetal outcomes not reported |
| Agcaoglu O, 2013[72] | 5 patients: mean gestational age = 20 wk, range 12-32 wk | Gallstone pancreatitis and obstructive jaundice-3, cholangitis and obstructive jaundice-2 | Selective cannulation confirmed by aspiration or direct visualization of bile. After CBD cannulated guide-wire passed, sphincterotomy completed, and stones extracted by basket or balloon sweep | No maternal or fetal adverse events or short term complications. No long-term follow-up available |
| Yang J, 2013[71] | 24 patients: first or second trimester-9, third trimester-15 | All patients had severe biliary pancreatitis. Leukocyte count 15000-29000 × 106/L, serum amylase: 500-2000 units/L, increased bilirubin in 20 | All patients underwent emergency ERCP without fluoroscopy and endoscopic biliary drainage. 15 patients in third trimester had pregnancy terminated: induced delivery-7, cesarean section-6, full-term normal delivery-2. Then underwent second ERCP with fluoroscopy to remove gallstones. 9 patients in early pregnancy underwent endoscopic retrograde biliary drainage in second ERCP without fluoroscopy. Had biliary stent for average of 3.8 mo | 100% technical success rate: CBD stones removed in all 24 patients. Only 2 maternal complications: mild hemorrhage during second ERCP. All infants born healthy. At term births-20, premature births-4 with cesarean section (for severe intrauterine distress) |
| Huang P, 2017[70] | 86 patients (largest series): no fluoroscopy-81 ultra-short duration of fluoroscopy-5. Mean gestational age = 22.5 wk, Range: 15-35 wk | Acute biliary pancreatitis-32, acute cholangitis-23, dilated CBD-20, severe nonbiliary acute pancreatitis-11 | Underwent antecedent abdominal ultrasound or MRCP. CBD cannulated using a guide-wire and then catheter over guide-wire. CBD cannulation confirmed by aspiration or oozing of bile. Then endoscopic biliary sphincterotomy and endoscopic nasobiliary drainage or retrograde biliary drainage. 51 had biliary stents | Technical success: 81 without fluoroscopy.Complications in 8.1%:Biliary bleeding-2, acute cholecystitis-1, post-ERCP pancreatitis-2. All babies were healthy at up to 12 mo. follow-up. All babies had normal birth weights (> 3 kg). Mean Apgar score at 5 min = 9 |
| Akcakaya A, 2009[69] | 6 patients: mean gestational age = 23 wk, range: 14-34 wk | Choledocholithiasis-4, Cholangitis-1, Persistent biliary fistula after hydatid disease surgery-1 (undergoing 2 ERCPs) | All patients had biliary sphincterotomy and balloon sweeps. Precut sphincterotomy performed with needle-knife for 1 patient with impacted stone | Complete stone extraction confirmed by abdominal ultrasound. No post-ERCP complications, premature birth, abortion or intrauterine growth retardation were observed |
| Shelton J, 2008[68] | 21 patients: first trimester-7, second trimester-9, third trimester-5 | Jaundice and biliary colic-11, biliary pancreatitis-8, cholecystitis-1, abnormal intraoperative cholangiogram-1 | Guide-wire inserted into CBD followed by sphincterotome over guide-wire. CBD cannulation then confirmed by suction of yellow bile via catheter in first 10 cases. In next 11 cases CBD cannulation confirmed by leakage of yellow bile around guide-wire. Then wire-guided biliary sphincterotomy performed followed by balloon sweeps to extract stones. Choledochoscopy used for bile duct clearance in 5 last cases | 100% technical success without fluoroscopy. One case of moderate pancreatitis. All then became asymptomatic. Follow-up of 18 pregnancies: Uneventful delivery of healthy babies-17, premature delivery at 35 wk with low birth weight-1 |
| Sharma SS, 2008[64] | 11 patients: first trimester-2, second trimester-6, third trimester-3 | Abdominal pain and jaundice-11, cholangitis-2, dilated CBD-11, gallstones-8 | All had 2-stage procedures. First stage during pregnancy: biliary sphincterotomy and stenting without radiation, bile aspirated to confirm biliary cannulation. Second stage ERCP postpartum: Stents removed, cholangiogram performed. Stones removed by Dormia basket-8, mechanical lithotripsy-1, or open surgery-1, no residual stones-1 | Marked symptomatic improvement after first stage of therapy. All had normal, full-term delivery. “Good” maternal and fetal outcomes |
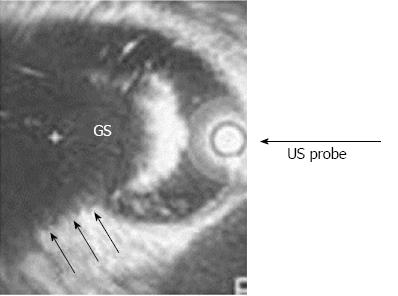
EUS is now readily available and should be considered prior to RFE. EUS is especially useful to gauge CBD diameter; number, size, and morphology of gallstones; and may occasionally obviate the need for ERCP. Intraductal stone clearance can be demonstrated by a balloon pull-through. Intraductal ultrasound (IDUS) (Figure 1) is an underutilized modality to assess ductal clearance. Particularly expert endoscopists can perform trans-septal sphincterotomy; especially after inserting a low-profile stent into the presumptive pancreatic duct.
Choledochoscopy (cholangioscopy) is very useful to disrupt choledocholithiasis via laser therapy or lithotripsy and confirm ductal clearance[68,73,75]. It is less useful and potentially dangerous for selective duct cannulation because the 10 French insertion catheter is somewhat stiff and may not smoothly negotiate an angulated pancreatic duct. The procedure is selected according to the clinical scenario and physician expertise (Figure 2). ERCP is particularly challenging and potentially involves some risk during the first trimester.
Decisions regarding cholecystectomy in pregnant women with biliary disease is entwined with ERCP concerns. As aforementioned, the second trimester is usually the most favorable time for both ERCP and cholecystectomy. Cholecystectomy timing is determined by the patient’s clinical course, with or without ERCP. Patients with concomitant cholecystitis should undergo surgery as soon as feasible. A series of seven pregnant patients had good maternal and fetal outcomes after undergoing ERCP with biliary sphincterotomy, and stone extraction, followed by immediate cholecystectomy for biliary pancreatitis[76]. Delaying cholecystectomy may result in biliary complications later during pregnancy or postpartum[77,78].
A first trimester pregnant woman underwent concurrent laparoscopic cholecystectomy and ERCP via a rendezvous technique wherein a wire was inserted by the surgeon via the cystic duct, through the CBD, and into the small intestine; the endoscopist accessed this wire for cannulation at ERCP[79]. This combined procedure resulted in technical success and favorable fetal outcome. This combined method should minimize risks of pancreatitis, but requires prolonged operative time and extra anesthesia medications for the twin procedures. One endoscopist performed his own rendezvous technique via EUS after failed biliary cannulation during standard ERCP, with good results for the mother and the fetus[80].
Pancreatic ERCP during pregnancy may be reported in the future[81]. Magnetic technology currently applied to detect endoscope position during endoscopy (especially colonoscopy) may conceivably be applied to wires and catheters during ERCP[82]. A meta-analysis would be clinically beneficial; it would likely demonstrate comparable maternal and fetal outcomes with minimal radiation vs radiation-free ERCP. Clinical studies on efficacy of fetal heart rate monitoring during ERCP would be helpful. Data are sparse for ERCP during the first trimester. Long term follow-up data would be helpful on outcomes of children who received ERCP radiation in utero. Future technological improvements in ERCP may prove beneficial to the pregnant population.
A limitation of this review is that some of the data are from case reports which may be anecdotal and may be subject to reporting bias in that ERCP endoscopists may be more likely to report successful cases of ERCP during pregnancy. However, biases were minimized by systematically reviewing the literature. Errors in abstracting data from the literature were eliminated by two investigators independently reviewing all the analyzed publications. In conclusion, performance of ERCP during pregnancy is a substantial undertaking requiring endoscopist forethought, with potential use of multiple modalities including EUS. ERCP is generally safe during pregnancy. It should generally be avoided during the first trimester, and performed in the first trimester only for urgent and strong indications such as gallstone pancreatitis with documented choledocholithiasis, cholangitis, symptomatic choledocholithiasis, or jaundice. The endoscopist should frankly discuss procedural risks vs benefits with the patient. Radiation safety measures are paramount, as is the endoscopist’s experience and technical skills. Various strategies and technologies may enhance biliary cannulation and ductal clearance during ERCP. Radiation–free ERCP is ideal, but should not unduly increase procedural time and risk of complications, especially pancreatitis.
Endoscopic retrograde cholangiopancreatography (ERCP) is currently the standard technique for treating choledocholithiasis and associated complications, such as cholangitis, biliary pancreatitis, and biliary stricture, in the non-pregnant population. The approach in pregnant women with suspected choledocholithiasis, however, differs somewhat from that for non-pregnant patients because of concerns about the pregnant mother and the fetus, including procedure time, teratogenicity of intraprocedural medications, and fetal radiation exposure.
This work systematically collates the clinical data from the clinical studies, including the numerous small clinical series, to render these data accessible to clinicians. This work provides a systematic review of the rapidly evolving literature in this clinically booming field to provide highly important and clinically relevant updates on ERCP safety, efficacy, and recent technical improvements in pregnant patients.
This work reports numerous techniques to reduce radiation exposure and other safety precautions to decrease fetal risk from ERCP during pregnancy. Indeed, this work discusses in detail radiation free ERCP during pregnancy to completely eliminate teratogenic risks of radiation.
This review encompassed more than 500 cases published in small clinical series and scattered reports, in addition to 58 cases recently reported in a retrospective Swedish registry study.
This work focuses on techniques to improve ERCP safety during pregnancy, including analysis of the relatively recently introduced radiation-free ERCP to completely eliminate the potential for radiation teratogenicity. Radiation-free ERCP is shown to be a relatively safe, and efficacious technique. However, more clinical data are required on this promising technique.
This work shows that therapeutic ERCP is a reasonably safe therapy for the mother and the fetus during pregnancy, and it should be performed when indicated for symptomatic choledocholithiasis and its associated complications (including ascending cholangitis, gallstone pancreatitis, and biliary stricture) during pregnancy. This work confirms that solely diagnostic ERCP should generally not be performed during pregnancy due to the risks of fetal radiation teratogenesis and induction of early labor, and should be replaced by diagnostic MRCP or endoscopic ultrasound. ERCP should not be performed during pregnancy for asymptomatic stones because of potential fetal risks; ERCPs can often be delayed to postpartum because patients have minimal clinical findings, or patients can directly undergo cholecystectomy during pregnancy without antecedent ERCP for acute cholecystitis.
More data are needed on radiation-free ERCPs. This work describes technique modifications for therapeutic ERCP during pregnancy to improve procedural safety. It is hoped that clinicians adapt these technique modifications during ERCP to further improve ERCP safety and efficacy during pregnancy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gkekas I, Khoury T, Sugimoto M, Wang ZY, Zhang QS S- Editor: Cui LJ L- Editor: A E- Editor: Wu YXJ
| 1. | Pak M, Lindseth G. Risk Factors for Cholelithiasis. Gastroenterol Nurs. 2016;39:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 3. | Shabanzadeh DM, Sørensen LT, Jørgensen T. A Prediction Rule for Risk Stratification of Incidentally Discovered Gallstones: Results From a Large Cohort Study. Gastroenterology. 2016;150:156-167.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Van Bodegraven AA, Böhmer CJ, Manoliu RA, Paalman E, Van der Klis AH, Roex AJ, Kruishoop AM, Devillé WL, Lourens J. Gallbladder contents and fasting gallbladder volumes during and after pregnancy. Scand J Gastroenterol. 1998;33:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kern F Jr, Everson GT, DeMark B, McKinley C, Showalter R, Erfling W, Braverman DZ, Szczepanik-van Leeuwen P, Klein PD. Biliary lipids, bile acids, and gallbladder function in the human female. Effects of pregnancy and the ovulatory cycle. J Clin Invest. 1981;68:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 117] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bolukbas FF, Bolukbas C, Horoz M, Ince AT, Uzunkoy A, Ozturk A, Aka N, Demirci F, Inci E, Ovunc O. Risk factors associated with gallstone and biliary sludge formation during pregnancy. J Gastroenterol Hepatol. 2006;21:1150-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | de Bari O, Wang TY, Liu M, Paik CN, Portincasa P, Wang DQ. Cholesterol cholelithiasis in pregnant women: pathogenesis, prevention and treatment. Ann Hepatol. 2014;13:728-745. [PubMed] |
| 8. | Anita C, Kumar P, Malathi S, Udayakumar N, Jayanthi V. Gallstones in pregnancy--a prevalence study from India. J Clin Gastroenterol. 2008;42:1065-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 9. | Hansen GC, Duerinckx AJ, Fymat A, Wong L, Ngo C. Cholelithiasis in the gravid Hispanic population. J Clin Ultrasound. 1994;22:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Ko CW, Beresford SA, Schulte SJ, Matsumoto AM, Lee SP. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology. 2005;41:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Álvarez-Villaseñor AS, Mascareño-Franco HL, Agundez-Meza JJ, Cardoza-Macías F, Fuentes-Orozco C, Rendón-Félix J, Chávez-Tostado M, Irusteta-Jiménez L, García-Rentería J, Contreras-Hernández GI. Cholelithiasis during pregnancy and postpartum: prevalence, presentation and consequences in a Referral Hospital in Baja California Sur. Gac Med Mex. 2017;153:159-165. [PubMed] |
| 12. | Luo L, Zen H, Xu H, Zhu Y, Liu P, Xia L, He W, Lv N. Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet. 2018;297:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Xu Q, Wang S, Zhang Z. A 23-year, single-center, retrospective analysis of 36 cases of acute pancreatitis in pregnancy. Int J Gynaecol Obstet. 2015;130:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Pruett D, Waterman EH, Caughey AB. Fetal alcohol exposure: consequences, diagnosis, and treatment. Obstet Gynecol Surv. 2013;68:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Jamidar PA, Beck GJ, Hoffman BJ, Lehman GA, Hawes RH, Agrawal RM, Ashok PS, Ravi TJ, Cunningham JT, Troiano F. Endoscopic retrograde cholangiopancreatography in pregnancy. Am J Gastroenterol. 1995;90:1263-1267. [PubMed] |
| 16. | Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol. 2014;306:R91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Cappell MS. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol Clin North Am. 2003;32:123-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | ASGE Standards of Practice Committee, Pasha SF, Acosta R, Chandrasekhara V, Chathadi KV, Eloubeidi MA, Fanelli R, Faulx AL, Fonkalsrud L, Khashab MA, Lightdale JR, Muthusamy VR, Saltzman JR, Shaukat A, Wang A, Cash B. Routine laboratory testing before endoscopic procedures. Gastrointest Endosc. 2014;80:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Cohen-Kerem R, Railton C, Oren D, Lishner M, Koren G. Pregnancy outcome following non-obstetric surgical intervention. Am J Surg. 2005;190:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Ferreira LE, Baron TH. Comparison of safety and efficacy of ERCP performed with the patient in supine and prone positions. Gastrointest Endosc. 2008;67:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | ASGE Standard of Practice Committee, Shergill AK, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Evans JA, Early DS, Fanelli RD, Fisher DA, Foley KQ, Fukami N, Hwang JH, Jain R, Jue TL, Khan KM, Lightdale J, Pasha SF, Sharaf RN, Dominitz JA, Cash BD. Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2012;76:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Available from: https://www.fda.gov/Drugs/DrugSafety/default.htm. |
| 23. | Safra MJ, Oakley GP Jr. Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet. 1975;2: 478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 155] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Jacobs ML, Verhoog S, van der Linden WH, Huisman WM, Wallenburg HC, Weber RF. Glucagon stimulation test: assessment of beta-cell function in gestational diabetes mellitus. Eur J Obstet Gynecol. Reprod Biol. 1994;56; 27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Philadelphia, Lippincott Williams and Wilkins, 2011. . |
| 26. | Huibregtse K. Complications of endoscopic sphincterotomy and their prevention. N Engl J Med. 1996;335:961-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Rabenstein T, Schneider HT, Nicklas M, Ruppert T, Katalinic A, Hahn EG, Ell C. Impact of skill and experience of the endoscopist on the outcome of endoscopic sphincterotomy techniques. Gastrointest Endosc. 1999;50:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Liao C, Thosani N, Kothari S, Friedland S, Chen A, Banerjee S. Radiation exposure to patients during ERCP is significantly higher with low-volume endoscopists. Gastrointest Endosc. 2015;81:391-398.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Tuech JJ, Binelli C, Aube C, Pessaux P, Fauvet R, Descamps P, Arnaud JP. Management of choledocholithiasis during pregnancy by magnetic resonance cholangiography and laparoscopic common bile duct stone extraction. Surg Laparosc Endosc Percutan Tech. 2000;10:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Einarson A, Bailey B, Inocencion G, Ormond K, Koren G. Accidental electric shock in pregnancy: a prospective cohort study. Am J Obstet Gynecol. 1997;176:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Magno-Pereira V, Moutinho-Ribeiro P, Macedo G. Demystifying endoscopic retrograde cholangiopancreatography (ERCP) during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2017;219:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Jeong S, Ki SH, Lee DH, Lee JI, Lee JW, Kwon KS, Kim HG, Shin YW, Kim YS. Endoscopic large-balloon sphincteroplasty without preceding sphincterotomy for the removal of large bile duct stones: a preliminary study. Gastrointest Endosc. 2009;70:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Samara ET, Stratakis J, Enele Melono JM, Mouzas IA, Perisinakis K, Damilakis J. Therapeutic ERCP and pregnancy: is the radiation risk for the conceptus trivial? Gastrointest Endosc. 2009;69:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Baron TH, Schueler BA. Pregnancy and radiation exposure during therapeutic ERCP: time to put the baby to bed? Gastrointest Endosc. 2009;69:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Huda A, Garzón WJ, Filho GC, Vieira B, Kramer R, Xu XG, Gao Y, Khoury HJ. Evaluation of staff, patient and foetal radiation doses due to endoscopic retrograde cholangiopancreatography (ERCP) procedures in a pregnant patient. Radiat Prot Dosimetry. 2016;168:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Johlin FC, Pelsang RE, Greenleaf M. Phantom study to determine radiation exposure to medical personnel involved in ERCP fluoroscopy and its reduction through equipment and behavior modifications. Am J Gastroenterol. 2002;97:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Dumonceau JM, Garcia-Fernandez FJ, Verdun FR, Carinou E, Donadille L, Damilakis J, Mouzas I, Paraskeva K, Ruiz-Lopez N, Struelens L. Radiation protection in digestive endoscopy: European Society of Digestive Endoscopy (ESGE) guideline. Endoscopy. 2012;44:408-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Smith I, Gaidhane M, Goode A, Kahaleh M. Safety of endoscopic retrograde cholangiopancreatography in pregnancy: Fluoroscopy time and fetal exposure, does it matter? World J Gastrointest Endosc. 2013;5:148-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Tang SJ, Mayo MJ, Rodriguez-Frias E, Armstrong L, Tang L, Sreenarasimhaiah J, Lara LF, Rockey DC. Safety and utility of ERCP during pregnancy. Gastrointest Endosc. 2009;69:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Inamdar S, Berzin TM, Sejpal DV, Pleskow DK, Chuttani R, Sawhney MS, Trindade AJ. Pregnancy is a Risk Factor for Pancreatitis After Endoscopic Retrograde Cholangiopancreatography in a National Cohort Study. Clin Gastroenterol Hepatol. 2016;14:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Petersen BT. Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis During Pregnancy: More Questions Than Answers From Administrative Databases. Clin Gastroenterol Hepatol. 2016;14:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 42. | Ludvigsson JF, Lebwohl B, Ekbom A, Kiran RP, Green PH, Höijer J, Stephansson O. Outcomes of Pregnancies for Women Undergoing Endoscopy While They Were Pregnant: A Nationwide Cohort Study. Gastroenterology. 2017;152:554-563.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Cappell MS. Evaluating the Safety of Endoscopy During Pregnancy: The Robust Statistical Power vs Limitations of a National Registry Study. Gastroenterology. 2017;152:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Gupta R, Tandan M, Lakhtakia S, Santosh D, Rao GV, Reddy DN. Safety of therapeutic ERCP in pregnancy - an Indian experience. Indian J Gastroenterol. 2005;24:161-163. [PubMed] |
| 45. | Cappell MS. Risks versus benefits of gastrointestinal endoscopy during pregnancy. Nat Rev Gastroenterol Hepatol. 2011;8:610-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Tiwari P, Khan AS, Nass JP, Rivera RE, Romero RV, Antillon MR, Roy PK. ERCP in pregnancy: A systematic review. Gastrointest Endosc. 2011;73:AB392-AB393. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Moffatt DC, Yu BN, Yie W, Bernstein CN. Trends in utilization of diagnostic and therapeutic ERCP and cholecystectomy over the past 25 years: a population-based study. Gastrointest Endosc. 2014;79:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Baillie J, Cairns SR, Putman WS, Cotton PB. Endoscopic management of choledocholithiasis during pregnancy. Surg Gynecol Obstet. 1990;171:1-4. [PubMed] |
| 49. | Goldschmiedt M, Wolf L, Shires T. Treatment of symptomatic choledocholithiasis during pregnancy. Gastrointest Endosc. 1993;39:812-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Talamini MA. Controversies in laparoscopic cholecystectomy: contraindications, cholangiography, pregnancy and avoidance of complications. Baillieres Clin Gastroenterol. 1993;7:881-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Akcakaya A, Koc B, Adas G, Kemik O. The use of ERCP during pregnancy: is it safe and effective? Hepatogastroenterology. 2014;61:296-298. [PubMed] |
| 52. | ASGE Standards of Practice Committee, Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, Ikenberry SO, Jain R, Khan K, Krinsky ML, Strohmeyer L, Dominitz JA. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 53. | Vaynshtein J, Sabbag G, Pinsk I, Rahmani I, Reshef A. Predictors for choledocholitiasis in patients undergoing endoscopic ultrasound. Scand J Gastroenterol. 2018;53:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Vitale GC. Endoscopic retrograde cholangiopancreatography (ERCP) and the surgeon. Interventional endoscopy in the management of complex hepatobiliary and pancreatic disease. Surg Endosc. 1998;12:387-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Al-Hashem H, Muralidharan V, Cohen H, Jamidar PA. Biliary disease in pregnancy with an emphasis on the role of ERCP. J Clin Gastroenterol. 2009;43:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Ramin KD, Ramsey PS. Disease of the gallbladder and pancreas in pregnancy. Obstet Gynecol Clin North Am. 2001;28:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Polistina FA, Frego M, Bisello M, Manzi E, Vardanega A, Perin B. Accuracy of magnetic resonance cholangiography compared to operative endoscopy in detecting biliary stones, a single center experience and review of literature. World J Radiol. 2015;7:70-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Oto A, Ernst R, Ghulmiyyah L, Hughes D, Saade G, Chaljub G. The role of MR cholangiopancreatography in the evaluation of pregnant patients with acute pancreaticobiliary disease. Br J Radiol. 2009;82:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Vohra S, Holt EW, Bhat YM, Kane S, Shah JN, Binmoeller KF. Successful single-session endosonography-based endoscopic retrograde cholangiopancreatography without fluoroscopy in pregnant patients with suspected choledocholithiasis: a case series. J Hepatobiliary Pancreat Sci. 2014;21:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Hedström J, Nilsson J, Andersson R, Andersson B. Changing management of gallstone-related disease in pregnancy - a retrospective cohort analysis. Scand J Gastroenterol. 2017;52:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Savas N. Gastrointestinal endoscopy in pregnancy. World J Gastroenterol. 2014;20:15241-15252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (4)] |
| 62. | Tham TC, Vandervoort J, Wong RC, Montes H, Roston AD, Slivka A, Ferrari AP, Lichtenstein DR, Van Dam J, Nawfel RD. Safety of ERCP during pregnancy. Am J Gastroenterol. 2003;98:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Kahaleh M, Hartwell GD, Arseneau KO, Pajewski TN, Mullick T, Isin G, Agarwal S, Yeaton P. Safety and efficacy of ERCP in pregnancy. Gastrointest Endosc. 2004;60:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Sharma SS, Maharshi S. Two stage endoscopic approach for management of choledocholithiasis during pregnancy. J Gastrointestin Liver Dis. 2008;17:183-185. [PubMed] |
| 65. | Farca A, Aguilar ME, Rodriguez G, de la Mora G, Arango L. Biliary stents as temporary treatment for choledocholithiasis in pregnant patients. Gastrointest Endosc. 1997;46:99-101. [PubMed] |
| 66. | Binmoeller KF, Katon RM. Needle knife papillotomy for an impacted common bile duct stone during pregnancy. Gastrointest Endosc. 1990;36:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Simmons DC, Tarnasky PR, Rivera-Alsina ME, Lopez JF, Edman CD. Endoscopic retrograde cholangiopancreatography (ERCP) in pregnancy without the use of radiation. Am J Obstet Gynecol. 2004;190:1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Shelton J, Linder JD, Rivera-Alsina ME, Tarnasky PR. Commitment, confirmation, and clearance: new techniques for nonradiation ERCP during pregnancy (with videos). Gastrointest Endosc. 2008;67:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 69. | Akcakaya A, Ozkan OV, Okan I, Kocaman O, Sahin M. Endoscopic retrograde cholangiopancreatography during pregnancy without radiation. World J Gastroenterol. 2009;15:3649-3652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Huang P, Zhang H, Zhang XF, Zhang X, Lv W. Individualized endoscopic treatment for pregnant patients with acute pancreaticobiliary diseases. J Health Res Rev. 2017;4:13-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 71. | Yang J, Zhang X, Zhang X. Therapeutic efficacy of endoscopic retrograde cholangiopancreatography among pregnant women with severe acute biliary pancreatitis. J Laparoendosc Adv Surg Tech A. 2013;23:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Agcaoglu O, Ozcinar B, Gok AF, Yanar F, Yanar H, Ertekin C, Gunay K. ERCP without radiation during pregnancy in the minimal invasive world. Arch Gynecol Obstet. 2013;288:1275-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Sethi S, Thosani N, Banerjee S. Radiation-Free ERCP in Pregnancy: A “Sound” Approach to Leaving No Stone Unturned. Dig Dis Sci. 2015;60:2604-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Ersoz G, Turan I, Tekin F, Ozutemiz O, Tekesin O. Nonradiation ERCP with endoscopic biliary sphincterotomy plus papillary balloon dilation for the treatment of choledocholithiasis during pregnancy. Surg Endosc. 2016;30:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Shah JN, Bhat YM, Hamerski CM, Kane SD, Binmoeller KF. Feasibility of nonradiation EUS-based ERCP in patients with uncomplicated choledocholithiasis (with video). Gastrointest Endosc. 2016;84:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Polydorou A, Karapanos K, Vezakis A, Melemeni A, Koutoulidis V, Polymeneas G, Fragulidis G. A multimodal approach to acute biliary pancreatitis during pregnancy: a case series. Surg Laparosc Endosc Percutan Tech. 2012;22:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Veerappan A, Gawron AJ, Soper NJ, Keswani RN. Delaying cholecystectomy for complicated gallstone disease in pregnancy is associated with recurrent postpartum symptoms. J Gastrointest Surg. 2013;17:1953-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Othman MO, Stone E, Hashimi M, Parasher G. Conservative management of cholelithiasis and its complications in pregnancy is associated with recurrent symptoms and more emergency department visits. Gastrointest Endosc. 2012;76:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Shirah BH, Mikwar ZA, Ahmad AN, Dahlan YM. Laparoendoscopic Rendezvous for Concomitant Cholecystocholedocholithiasis: A Successful Modality Even in the Most Difficult Presentations Including Pregnancy. Case Rep Surg. 2016;2016:8618512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Singla V, Arora A, Tyagi P, Sharma P, Bansal N, Kumar A. Failed common bile duct cannulation during pregnancy: Rescue with endoscopic ultrasound guided rendezvous procedure. Endosc Ultrasound. 2016;5:201-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Khan J, Ylinen J, Victorzon M. Pancreatic rupture during childbirth treated successfully by endoscopic drainage. Endoscopy. 2012;44 Suppl 2:E65-E66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Leung JW, Thai A, Yen A, Ward G, Abramyan O, Lee J, Smith B, Leung F. Magnetic endoscope imaging (ScopeGuide) elucidates the mechanism of action of the pain-alleviating impact of water exchange colonoscopy - attenuation of loop formation. J Interv Gastroenterol. 2012;2:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |