Published online Oct 16, 2018. doi: 10.4253/wjge.v10.i10.259
Peer-review started: May 18, 2018
First decision: June 4, 2018
Revised: June 10, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: October 16, 2018
Processing time: 151 Days and 15.8 Hours
Acute pancreatitis (AP) is common gastrointestinal disease of varied aetiology. The most common cause of AP is gallstones, followed by alcohol abuse as an independent risk factor. With the increased need for invasive techniques to treat pancreatic and bile duct pathologies such as endoscopic retrograde cholangiopancreatography (ERCP), AP has emerged as the most frequent complication. While severe AP following ERCP is rare (0.5%), if it does develop it has a greater severity index compared to non-ERCP AP. Development of a mild form of AP after ERCP is not considered a clinically relevant condition. Differences in the clinical presentation and prognosis of the mild and severe forms have been found between non-ERCP AP and post-endoscopic pancreatitis (PEP). It has been proposed that AP and PEP may also have different immunological responses to the initial injury. In this review, we summarise the literature on clinical and inflammatory processes in PEP vs non-ERCP AP.
Core tip: Acute pancreatitis (AP) is the most frequent complication after endoscopic retrograde cholangiopancreatography (ERCP) and although low prevalence is found, if it develops it has greater severity index compared to non-ERCP AP. The differences in factors influencing appearance, clinical presentation and prognosis of ERCP induced and non ERCP induced AP were found, lead to opinion that mechanism by which they induce inflammation, may also be different. It would be of great importance to find immunological components that can distinguish patients with tendency to develop severe AP from patients with mild form, especially in ERCP induced AP where organ failure occurs half time earlier.
- Citation: Plavsic I, Žitinić I, Mikolasevic I, Poropat G, Hauser G. Endoscopic retrograde cholangiopancreatography-induced and non-endoscopic retrograde cholangiopancreatography-induced acute pancreatitis: Two distinct clinical and immunological entities? World J Gastrointest Endosc 2018; 10(10): 259-266
- URL: https://www.wjgnet.com/1948-5190/full/v10/i10/259.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i10.259
Acute pancreatitis (AP) is a common gastrointestinal disease with a reported incidence of 13-45 cases per 100000 persons annually[1]. According to the revised Atlanta classification, diagnosis of AP requires two of three following features: upper abdominal pain of acute onset, often radiating through to the back; serum amylase or lipase activity greater than three-times the normal level; and findings on cross-sectional abdominal imaging consistent with AP[2]. The severity of AP can be divided into mild, moderately severe or severe forms based on the presence or absence of persistent organ failure and local and systemic complications (Table 1). The mild form of AP is characterised by inflammation and the synthesis of proinflammatory cytokines in the affected area. The moderate and severe forms are characterised by the release of proinflammatory molecules into the circulation, causing systemic inflammatory response syndrome (SIRS)[3].
| Mild | Absence of both (peri) pancreatic necrosis and organ failure |
| Moderate | Presence of sterile (peri) pancreatic necrosis and transient organ failure |
| Severe | Infected (peri) pancreatic necrosis or persistent organ failure |
Gallstones are most common cause of AP, followed by alcohol abuse as an independent risk factor[2].
Invasive techniques used for the treatment of pancreatic and bile duct pathologies, such as endoscopic retrograde cholangiopancreatography (ERCP), carry a certain risk of complications. The most frequent of these is AP. Large variations in the reported incidence and severity of post-endoscopic pancreatitis (PEP) has led to unobjective risk evaluation, mostly consisting of retrospective studies. Kochar et al[4] reported an overall PEP incidence of 9.7%, while in high-risk patients the incidence was 14.7%. It is important to record why ERCP is performed, whether for therapeutic or diagnostic reasons, as patients may have an underlying condition that may affect the incidence of complications[5]. Most records report increased PEP after therapeutic ERCP[6].
AP is a disease of varied aetiology. Each produces a similar disease pattern, indicating that they all converge at a common point to initiate a cascade of events resulting in AP[7,8]. Messmann et al[5] found that people with AP are usually admitted to hospital several hours or even days after the initiation of symptoms. Therefore, it is impossible to determine the exact time of injury and initiation of the inflammatory phase. Instead, studies use PEP as a human model to examine the initial cytokine and acute-phase response in the first hours after initiation. It has been reported that PEP can serve as an ideal model for investigating the initial inflammatory phase in non-ERCP-induced AP.
An alternate opinion is that AP and PEP may actually be different disorders. This assumption is based on the differences in clinical presentation and prognosis of the mild and severe forms[9,10]. The triggers for the two disorders differ, and consequently, the mechanism by which they induce inflammation may also differ[11].
Different clinical outcomes of non-ERCP-induced AP and PEP have been found in several studies[9,10,12] (Table 2).
| PEP | non-ERCP-induced AP | Conclusion | |
| Fung et al[10] ERCP-induced acute necrotising pancreatitis vs ANP induced by other causes | Higher APACHE II scores on admission | Lower APACHE II scores on admission | ANP is more severe when ERCP-induced |
| More extensive pancreatic necrosis | Less extensive pancreatic necrosis | ||
| Higher rate of infected necrosis | Lower rate of infected necrosis | ||
| Testoni et al[12] ERCP induced AP vs non ERCP induced AP | No statistical difference: severity of the pancreatitis mortality rate (double in severe PEP) hospitalisation | ||
| In mild form serum amylase fell 50% in 38.9 h. Peak serum amylase halved within 48 h in 92% | In mild form serum amylase fell 50% in 46, 4 h. Peak serum amylase halved within 48 h in 73.6% | Statistical difference (P < 0.001) Mild form of PEP a sort of pancreatic reaction, instead of true episode of acute pancreatitis | |
| Abid et al[9] Mild form: ERCP induced AP vs non ERCP induced AP | Shorter duration of pain; Shorter time of intravenous hydration; Shorter time to resumption of oral diet; Shorter hospital stay (P < 0.001) | ERCP-induced AP mild attacks run a significantly shorter and milder course than non-ERCP related mild attacks | |
Patients that developed post-ERCP pancreatitis initially had a higher APACHE II score (key prognostic factor in predicting mortality) compared to AP of other aetiologies[10]. The APACHE II score takes approximately 48 h to achieve a good predictive index. Therefore, whether this score represents a good method to differentiate initial disease severity prognosis (within 24 h), and if it can be reliably used to compare non-ERCP AP and PEP, remain questionable[9].
As mentioned earlier, severe AP following ERCP is rare (0.5%), but if it does develop, it does so with a greater severity index when compared to non-ERCP AP. Fung et al[10] reported that the extent of parenchymal necrosis is greater in PEP patients. There was also a higher rate of infected necrosis in the PEP group in their study. In PEP, the infection occurs earlier than in acute non-ERCP-induced pancreatitis. Due to small number of patients with ERCP induced acute necrotising pancreatitis (ANP) and low statistical power of their study, results should be interpreted with caution. All the same, these results should be taken into consideration, since the presence of infection and its extent is more important for disease prognosis than pancreatic necrosis[10]. Organ failure develops early in the severe form of AP, either present at admission or 24 h later. In PEP, organ failure occurs twice as fast as in non-ERCP AP[3].
The mild form of ERCP-induced pancreatitis has a shorter and milder disease course with only a temporary increase in the level of enzymes in the blood (up to 48 h), suggesting a non-specific pancreatic reaction to injury, not necessary inflammation. Patients with mild post-ERCP pancreatitis have been reported to have a significantly shorter duration of pain and need for analgesia and parenteral hydration. All patients involved in this study, indicated for ERCP, were studied after they had been discharged from hospital because the acute condition can influence the intensity of inflammation[9].
Studies on drug effectiveness on the prevention of post-ERCP AP use the reduction in total post-ERCP AP incidence as the final measurement. So far, results have shown a reduction in the mild form but not the severe form. The primary goal should be a reduced incidence of severe PEP, as the mild form is not a clinically relevant condition[13-16].
As previously mentioned, the most common causes of non-ERCP AP are gallstones and alcohol abuse[2]. The primary location of injury for both causes are acinar cells[17]. Gallstones lead to duct obstruction and blocking of acinar exocytosis, leading to the colocalization of zymogen and lysosomal granules and early activation of pancreatic enzymes. Alcohol leads to oxidative and non-oxidative damage. The non-oxidative pathway involves increased levels fatty acid ethyl ester, whereas the oxidative pathway is characterised by the accumulation of acetaldehyde, acetate and NADH. Alcohol also modifies the intracellular redox state by diminishing the NAD/NADH ratio and increasing the lactate/pyruvate ratio, ultimately leading to metabolic alterations and acinar cell injury[18].
The factors influencing PEP incidence are multifactorial. These include patient-related factors, operator-related factors and method-related factors. Patient-related factors involve age, sex, pre-existing pancreatitis, prior history of post-ERCP pancreatitis, sphincter of Oddi dysfunction, and small bile duct and pancreatic divisum. Operator-related factors are associated with the experience of the endoscopist. The method-related factors are the most important because in them lies the greatest possibility for controlled intervention. Method-related factors cause mechanical injury a number of different ways. Combined operator and method- related factor as repeated and difficult papilla cannulation can lead to oedema and obstruction of free juice flow and sphincter of Oddi spasm. This mechanism may resemble the damage caused by gallstone obstruction. Furthermore, osmolarity and the ionic nature of the contrast media can cause chemical injury. Injecting contrast media are responsible for hydrostatic injury, which is one of the main causes of pancreatitis after ERCP[19]. Another factor is increased duct pressure, which can cause early activation of pancreatic enzymes[20]. However, microbiological factors related to contaminated endoscope and translocation from the intestines is not considered to play a major role.
It is considered that the first pancreatic event, in any of these circumstances, occurs at the level of acinar cells[21]. Intrapancreatic trypsinogen activation and NFκB activation represent the two main initial triggers for AP[8,22]. Sah et al[22] reviewed studies that used animal models to show that NFκB activates and induces inflammation without the need for trypsinogen activation. Therefore, these two events represent two independent cellular events.
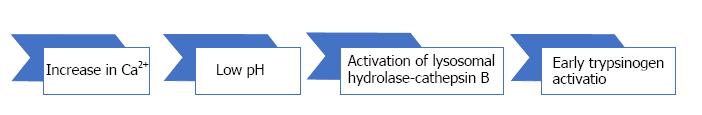
The early events in AP include inhibition of zymogen secretion, altered intracellular Ca2+ homeostasis that modifies pH values (Figure 1), intrapancreatic activation of trypsinogen and other zymogens and activation of cell death pathways (NFκB)[8,18].
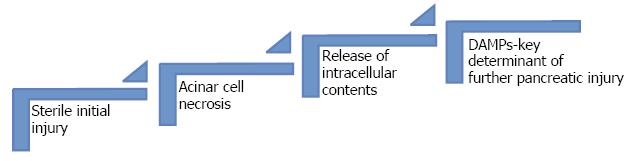
The initial injury of the acinar cells caused by zymogens is sterile[23] (Figure 2).
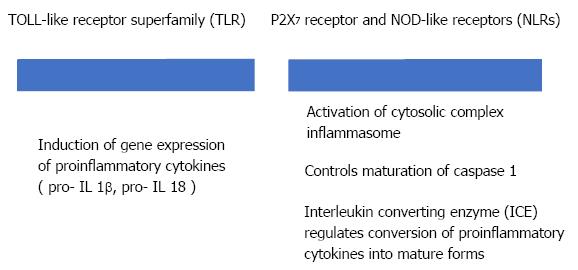
Sterile inflammation requires two distinct signals through the activation of pattern recognition receptors (PRRs) (Figure 3). PRRs, like Toll like receptor (TLR) and NOD like receptor (NLR), are part of the innate immune response[23].
Randomised controlled trials have been used to study the use of allopurinol in the prevention of post-ERCP AP. Allopurinol reduces the production of uric acid. Uric acid uses DAMPs (NLR receptors) to trigger an inflammatory response. These studies found that allopurinol decreases the incidence of post-ERCP AP[24,25], indicating that the innate immune cells play a role in AP after ERCP[21]. Shamoon et al[26] in their study, emphasise the importance of innate immune cells and derived inflammatory mediators as therapeutic targets in AP in early phase of the disease (24-48 h).
The balance between pro- and anti-inflammatory immune response determines the prognosis in AP. A fall in the co-expression of HLA-DR on CD14+ monocytes is considered a standard laboratory indicator of compensatory anti-inflammatory immune response syndrome (CARS)[27]. The severe form of AP is frequently associated with immune suppression, which increases the risk of infection, organ failure and death[28]. Kylanpaa et al[3] reported that impaired cellular immunity causes complications related to infection in AP at a later stage of the disease. Furthermore, Testoni et al[12] reported that infection in PEP occurs during or immediately after the procedure. For this reason, infection in non-ERCP AP is considered a secondary event, while in PEP it is considered the primary event.
While the role of different cytokines in AP has been extensively studied, the role of cellular immunity is poorly evaluated[28]. Innate immune cells are the major leukocyte population in the inflamed pancreas[29].
Monocytes and macrophages are the main inflammatory cell populations in AP, and both play active roles in AP progression. The production of proinflammatory factors like tumour necrosis factor (TNF)-α in pancreatic cell stimulates the activation of macrophages in distal organs including the peritoneum, spleen, liver and lungs. Monocyte chemoattractant protein (MCP)-1 and macrophage migration inhibitory factor (MIF) play important roles in AP. Bhatia et al[30] reported that blocking MCP-1 synthesis reduces the severity of AP. Furthermore, antibodies against MIF improve survival in rats with AP[31]. The expression of HLA-DR on monocytes gives a good indication of monocyte function. In cases of immunosuppression, decreased monocyte HLA-DR expression predicts the development of organ failure[32].
Neutrophils play a central role in the development of local and systemic complications, therefore, researchers have investigated the depletion of neutrophils as a therapeutic option for AP. Anti-neutrophil serum (ANS) exhibited a marked attenuation in intrapancreatic trypsin activation, ameliorated choline-deficient ethionine supplemented (CDE) diet-induced pancreatitis and completely prevented lung injury[33,34]. The depletion of neutrophils associated with ANS did not influence macrophage infiltration, but it did decrease the number of lymphocytes in the pancreas[29].
Progression of AP is accompanied by a change in the number and ratio of CD4+ and CD8+ lymphocytes[35]. CD4+ lymphocytes are especially important as they act as co-stimulators of macrophage activation via antigen presentation and the release of proinflammatory cytokines. They have been reported to have a direct cytotoxic effect on acinar cells through Fas ligand expression[36]. Depletion of CD4+ lymphocytes reduces the severity of AP[21]; however, CD4+ lymphocytes are a heterogeneous population and some release IL-22, which has an anti-inflammatory effect[37].
Natural killer (NK) cells are predominantly studied in response to infection and immunosurveillance against tumours. They are part of the innate immune system, giving them the ability to respond without prior sensitisation. They also carry certain abilities of adaptive immunity, as they are primed during development, their receptors can exhibit antigen specificity, they undergo clonal expansion during infection and generate long-lived memory cells[38]. Natural killer cells can undergo clonal-like expansion through specific and non-specific immune responses. While the specific response occurs via interaction of their activating receptors with viral antigens, the non-specific response is driven by the production of cytokines and proliferation following exposure to proinflammatory cytokines in the absence of TCR signals and co-stimulation[39,40]. Natural killer cells have immunological memory, which enables them to react faster and more aggressively in familiar surroundings. The most important cytokines produced by NK cells after activation are TNF-α and IFN-γ[41]. It is thought that NK cells that produce proinflammatory cytokines can contribute to dysregulation of the immune response as seen in sepsis[42]. The cytokine IL-15 pays a role in the maintenance of NK cells. The half-life of mature NK cells is about 1 wk, but in the absence of IL-15 they disappear in 48 h. These cells can also serve as an immunotherapeutic target.
Dabrowski et al[28] reported significant depletion of the NK cell population on the first day of severe AP, while there was no significant change in NK cell number in mild AP. These findings are consistent with the idea that severe forms of AP are related to immune suppression. Profound inhibition of innate cell immunity can be explained by the migration of NK cells and natural killer T (NKT) cells to the site of inflammation.
Natural killer T cells are generally autoreactive and can recognise both exogenous and endogenous ligands. There are two types of NKT cells, type I and type II. Type I is more prevalent in mice and can be either pathogenic or protective, although they have a greater propensity to be pathogenic. Type II is prevalent in humans, and predominantly protect against inflammation and autoimmune disease. Different self-antigens can stimulate type I NKT cells, and some of these antigens are present at elevated levels during inflammation[43].
In patients with severe AP there is a reduction in the number of peripheral lymphocytes, especially monocytes and cytotoxic T lymphocytes[28,44].
The most important anti-inflammatory cytokine is interleukin (IL)-10. It down-regulates the production of proinflammatory cytokines and the expression of HLA-DR on monocytes. If the compensatory anti-inflammatory response is too intense, however, it may lead to immunosuppression and complications including infection. The concentration of IL-10 is highest in the early phase of severe AP. As infection is considered to be one of the prognostic factors related to disease severity, IL-10 may be a promising predictive marker of organ failure[45]. There are conflicting reports for the use of IL-10 in the prevention of post-ERCP AP. In a randomised double-blind study, Deviere et al[46] showed a reduced incidence of post-ERCP AP after IL-10 usage, although this was not supported by a study by Dumot et al[47].
As a key proinflammatory mediator, IL-6 regulates the synthesis of acute-phase proteins in the liver as well as macrophage-conditioned tissue damage[48]. It reaches its peak value 24-48 h after clinical expression. In necrotising pancreatitis, the peak levels of IL-6 occur after 24 h[5]. Minkov et al[48] concluded that IL-6 represents an independent factor for predicting severity in acute non-ERCP pancreatitis.
The highest values of C-reactive protein (CRP) are recorded after 48–72 h, which is later than that of IL-6[5]. Although CRP has been identified as a late marker in laboratory monitoring[49], Messman et al[5] found that both IL-6 and CRP peak earlier in patients with ANP.
IL-1β-mediated signalling is required for full pancreatic and distal organ injury and inflammation[50], and is the pivotal inflammatory mediator in cell death associated with sterile inflammation[51]. Serum levels of IL-1β do not correlate with AP severity in humans, although it has been found that the values peak after 24 h and are greater in patients with severe AP compared to mild AP[52]. In animal models, peak serum IL-1β precede peak serum IL-6 values[50,53]. It is possible that IL-1β is required for the induction of IL-6 production, which is strongly correlated with disease severity in humans[54]. IL-1β and TNF-α are considered the primary cytokines that initiate and propagate most of the consequences of the SIRS in AP[55,56]. IL-6 prevents the synthesis of IL-1β and TNF-α[57].
Kilciner et al[49] compared early changes (within 24 h) in the serum levels of IL-2, IL-4, TNF-α and IL-6 in the development of post-ERCP pancreatitis. They used patients who underwent ERCP as well as a control group consisting of patients with non-ERCP AP caused by gallstones, drugs or alcohol. They found that IL-4, an anti-inflammatory cytokine, was significantly lower in post-ERCP and non-ERCP AP patients compared to patients who did not develop pancreatitis. The TNF-α level was not significantly different after 24 h in patients who developed PEP compared to those who did not develop pancreatitis after ERCP. After 24 h, the IL-6 levels did not differ from the control group, but they were significantly higher compared to patients who did not go on to develop pancreatitis after ERCP.
The role of IL-18 may depend on the presence of other cytokines. It plays an important role in the local immune response to pancreatic injury[23], and can also be found in serum. It has been described to prime NK cells, and NK cells that were unable to receive IL-18 signals were found to have defective cytotoxicity and cytokine secretion after stimulation[38].
AP is the most frequent complication after the ERCP procedure. Although the incidence of AP after ERCP is low, it is reported to occur in 0.5% of patients, PEP has a greater severity index compared to non-ERCP AP[10]. As the mild form of PEP is not a clinically relevant condition, it would be useful to identify early markers to predict whether a patient will develop the severe form of PEP.
The serial changes in amylase and lipase levels in patients without PEP suggest the existence of subclinical pancreatic damage. Messmann found that amylase and lipase levels increased equally among all patients after ERCP[5]. Amylase and lipase are released into the systemic circulation due to disturbed transport and increased ductal permeability; however, they are not thought to be responsible for inducing further inflammation. Based to these findings, we conclude that serum amylase values can’t serve as an adequate future therapeutic goal.
The role of cytokines, especially IL-10, IL-6 and TNF-α, have been extensively studied for the prediction of disease severity[45,48,55,56]. These cytokines can be used to predict the severity of PEP after 12-24 h; however, measurements taken 4 h after the procedure showed no significant difference between patients who developed PEP and those who did not develop PEP[51,58].
Further research on the initial inflammatory response is necessary, particularly as organ failure has been reported to occur earlier in severe forms of AP, either at admission or 14 h later. Furthermore, in PEP, organ failure occurs twice as fast than in non-ERCP AP[44]. Direct comparison of the initial inflammatory response between PEP and non-ERCP AP would be of significant importance to clarify these statements. Found difference in clinical response to initial injury might be explained by different initial immune response[59].
Infection is considered to be the most important prognostic factor for disease severity. Similarities between cytokines and inflammatory mediators in sepsis and AP are often compared. Kjaergaard et al[60] reported that the expression of NKG2D receptors on NK cells and CD14 on monocytes can be valuable prognostic markers of an unbalanced immune response, and may predict a worse outcome for critically ill patients. Also, Guo et al[61] presented natural killer cells as critical to eliminate pathogens during the early phase of sepsis and prevent patients from developing secondary infection. We suggest that similar components should be used in PEP and non ERCP AP.
In addition to searching for adequate biomarkers to assess disease severity, it is our opinion that novel therapeutic strategies for both of these conditions lie in uncovering the immune pathways.
The most frequent complication after ERCP is AP. In most cases, it is not a clinically relevant condition, but in 0.5% of patients it has a greater severity index compared to non-ERCP AP. In severe PEP, infection occurs earlier than in acute non-ERCP-induced pancreatitis, and organ failure occurs twice as fast. Treatment of AP, regardless of the cause, is primarily supportive and implies a certain economic burden in the healthcare system worldwide. More thorough clarification of disease pathogenesis is needed, in order to find adequate immune target to predict and consequently prevent severe form of the disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Gkekas I, Mohamed AA, Tahiri MJH, Venu RP S- Editor: Cui LJ L- Editor: A E- Editor: Wu YXJ
| 1. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1347] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 2. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 3. | Kylänpää L, Rakonczay Z Jr, O’Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflam. 2012;2012:360685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, Khashab MA, Kalloo AN, Singh VK. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143-149.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Messmann H, Vogt W, Holstege A, Lock G, Heinisch A, von Fürstenberg A, Leser HG, Zirngibl H, Schölmerich J. Post-ERP pancreatitis as a model for cytokine induced acute phase response in acute pancreatitis. Gut. 1997;40:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1690] [Article Influence: 58.3] [Reference Citation Analysis (2)] |
| 7. | Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 8. | Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Abid GH, Siriwardana HP, Holt A, Ammori BJ. Mild ERCP-induced and non-ERCP-related acute pancreatitis: two distinct clinical entities? J Gastroenterol. 2007;42:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Fung AS, Tsiotos GG, Sarr MG. ERCP-induced acute necrotizing pancreatitis: is it a more severe disease? Pancreas. 1997;15:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Mine T. Is post-endoscopic retrograde cholangiopancreatography pancreatitis the same as acute clinical pancreatitis? J Gastroenterol. 2007;42:265-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Testoni PA, Vailati C, Giussani A, Notaristefano C, Mariani A. ERCP-induced and non-ERCP-induced acute pancreatitis: Two distinct clinical entities with different outcomes in mild and severe form? Dig Liver Dis. 2010;42:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Murray B, Carter R, Imrie C, Evans S, O’Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | De Palma GD, Catanzano C. Use of corticosteriods in the prevention of post-ERCP pancreatitis: results of a controlled prospective study. Am J Gastroenterol. 1999;94:982-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Budzyńska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Andriulli A, Clemente R, Solmi L, Terruzzi V, Suriani R, Sigillito A, Leandro G, Leo P, De Maio G, Perri F. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002;56:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Steer ML. Early events in acute pancreatitis. Baillieres B. est Pract Res Clin Gastroenterol. 1999;13:213-225. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Pérez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol. 2015;5:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Trap R, Adamsen S, Hart-Hansen O, Henriksen M. Severe and fatal complications after diagnostic and therapeutic ERCP: a prospective series of claims to insurance covering public hospitals. Endoscopy. 1999;31:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Saluja A, Saluja M, Villa A, Leli U, Rutledge P, Meldolesi J, Steer M. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest. 1989;84:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Demols A, Deviere J. New frontiers in the pharmacological prevention of post-ERCP pancreatitis: the cytokines. JOP. 2003;4:49-57. [PubMed] |
| 22. | Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol. 2013;29:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Comert B, Isik AT, Aydin S, Bozoglu E, Unal B, Deveci S, Mas N, Cinar E, Mas MR. Combination of allopurinol and hyperbaric oxygen therapy: a new treatment in experimental acute necrotizing pancreatitis? World J Gastroenterol. 2007;13:6203-6207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Martinez-Torres H, Rodriguez-Lomeli X, Davalos-Cobian C, Garcia-Correa J, Maldonado-Martinez JM, Medrano-Muñoz F, Fuentes-Orozco C, Gonzalez-Ojeda A. Oral allopurinol to prevent hyperamylasemia and acute pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2009;15:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Shamoon M, Deng Y, Chen YQ, Bhatia M, Sun J. Therapeutic implications of innate immune system in acute pancreatitis. Expert Opin Ther Targets. 2016;20:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Mylona V, Koussoulas V, Tzivras D, Makrygiannis E, Georgopoulou P, Koratzanis G, Giamarellos-Bourboulis EJ, Tzivras MD. Changes in adaptive and innate immunity in patients with acute pancreatitis and systemic inflammatory response syndrome. Pancreatology. 2011;11:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Dabrowski A, Osada J, Dabrowska MI, Wereszczynska-Siemiatkowska U. Monocyte subsets and natural killer cells in acute pancreatitis. Pancreatology. 2008;8:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Xue J, Sharma V, Habtezion A. Immune cells and immune-based therapy in pancreatitis. Immunol Res. 2014;58:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259-G1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Sakai Y, Masamune A, Satoh A, Nishihira J, Yamagiwa T, Shimosegawa T. Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology. 2003;124:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Mentula P, Kylänpää-Bäck ML, Kemppainen E, Takala A, Jansson SE, Kautiainen H, Puolakkainen P, Haapiainen R, Repo H. Decreased HLA (human leucocyte antigen)-DR expression on peripheral blood monocytes predicts the development of organ failure in patients with acute pancreatitis. Clin Sci (Lond). 2003;105:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Bhatia M, Saluja AK, Hofbauer B, Lee HS, Frossard JL, Steer ML. The effects of neutrophil depletion on a completely noninvasive model of acute pancreatitis-associated lung injury. Int J Pancreatol. 1998;24:77-83. [PubMed] |
| 35. | Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol. 2010;16:3995-4002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 36. | Pezzilli R, Billi P, Gullo L, Beltrandi E, Maldini M, Mancini R, Incorvaia L, Miglioli M. Behavior of serum soluble interleukin-2 receptor, soluble CD8 and soluble CD4 in the early phases of acute pancreatitis. Digestion. 1994;55:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 39. | Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 40. | Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 866] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 41. | Ussat S, Scherer G, Fazio J, Beetz S, Kabelitz D, Adam-Klages S. Human NK cells require caspases for activation-induced proliferation and cytokine release but not for cytotoxicity. Scand J Immunol. 2010;72:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Leung B, Harris HW. NKT cells in sepsis. Clin Dev Immunol. 2010;2010:20953368. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014;142:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol. 2010;16:2867-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 45. | Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Early prediction of organ failure by combined markers in patients with acute pancreatitis. Br J Surg. 2005;92:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Devière J, Le Moine O, Van Laethem JL, Eisendrath P, Ghilain A, Severs N, Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Dumot JA, Conwell DL, Zuccaro G Jr, Vargo JJ, Shay SS, Easley KA, Ponsky JL. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol. 2001;96:2098-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Minkov GA, Halacheva KS, Yovtchev YP, Gulubova MV. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. 2015;44:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Kilciler G, Musabak U, Bagci S, Yesilova Z, Tuzun A, Uygun A, Gulsen M, Oren S, Oktenli C, Karaeren N. Do the changes in the serum levels of IL-2, IL-4, TNFalpha, and IL-6 reflect the inflammatory activity in the patients with post-ERCP pancreatitis? Clin Dev Immunol. 2008;2008:481560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Norman JG, Fink G, Franz M, Guffey J, Carter G, Davison B, Sexton C, Glaccum M. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg. 1996;223:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 645] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 52. | Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Fink GW, Norman JG. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997;9:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Stimac D, Fisić E, Milić S, Bilić-Zulle L, Perić R. Prognostic values of IL-6, IL-8, and IL-10 in acute pancreatitis. J Clin Gastroenterol. 2006;40:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Pereda J, Sabater L, Aparisi L, Escobar J, Sandoval J, Viña J, López-Rodas G, Sastre J. Interaction between cytokines and oxidative stress in acute pancreatitis. Curr Med Chem. 2006;13:2775-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Escobar J, Pereda J, Arduini A, Sandoval J, Sabater L, Aparisi L, López-Rodas G, Sastre J. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des. 2009;15:3027-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1115] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 58. | Concepción-Martín M, Gómez-Oliva C, Juanes A, Mora J, Vidal S, Díez X, Torras X, Sainz S, Villanueva C, Farré A. IL-6, IL-10 and TNFα do not improve early detection of post-endoscopic retrograde cholangiopancreatography acute pancreatitis: a prospective cohort study. Sci Rep. 2016;6:33492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Zitinic I, Plavsic I, Poropat G, Hauser G. ERCP induced and non-ERCP-induced acute pancreatitis: Two distinct clinical entities? Med Hypotheses. 2018;113:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Kjaergaard AG, Nielsen JS, Tønnesen E, Krog J. Expression of NK cell and monocyte receptors in critically ill patients--potential biomarkers of sepsis. Scand J Immunol. 2015;81:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Guo Y, Patil NK, Luan L, Bohannon JK, Sherwood ER. The biology of natural killer cells during sepsis. Immunology. 2018;153:190-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |