Published online Oct 15, 2009. doi: 10.4253/wjge.v1.i1.72
Revised: March 2, 2009
Accepted: March 9, 2009
Published online: October 15, 2009
Glanzmann's thrombasthenia (GT) is a rare autosomal recessive bleeding syndrome characterized by abnormal Glycoprotein IIb/IIIa complex (GIIb/IIIa) on platelets with resultant abnormality in platelet aggregation. There is very little information regarding polypectomy management in GT. We report a single patient with this rare disease, who underwent sequential endoscopic management of large colon polyps. Polypectomy in our GT patient was complicated by immediate and delayed bleeding. Multiple clips used after standard cautery polypectomy for a polyp 10 mm or larger in our GT patient, was most effective in preventing immediate and delayed post-polypectomy bleeding than other known therapeutic approaches. We favor preemptive use of multiple clips in large polypectomy defects for GT patients and we may argue the added cost may be offset by the reduction in the need for blood products, and by averting or shortening potential hospitalizations.
- Citation: Raina D, Movva A, Rahhal F, Abderrahim K, Schade R, Chamberlain SM. Colonoscopy polypectomy management in Glanzmann’s thrombasthenia. World J Gastrointest Endosc 2009; 1(1): 72-75
- URL: https://www.wjgnet.com/1948-5190/full/v1/i1/72.htm
- DOI: https://dx.doi.org/10.4253/wjge.v1.i1.72
Glanzmann’s thrombasthenia (GT) is a rare autosomal recessive bleeding syndrome characterized by an absence of platelet aggregation secondary to an abnormality in the Glycoprotein IIb/IIIa complex (GIIb/IIIa)[1]. The disease is characterized by impaired platelet aggregation stemming from defective fibrinogen binding to GPIIb/IIIa and clinically manifests as a bleeding diathesis. We report a patient with Glanzmann’s thrombasthenia who bled following colonic polypectomy and review the role of clips in endoscopic hemostasis for post-polypectomy bleeding in GT.
A 52-year-old African American female was referred by her primary care physician for a diagnostic colonoscopy to evaluate anemia and hemoccult positive stools. This patient was diagnosed with GT at the age of 17 after experiencing frequent nosebleeds and menorrhagia. Since menopause at age 48, the patient had no further bleeding episodes. She had no previous surgery except for dental extraction for which she had received platelet transfusion. She reported no family history of colon cancer or polyps but had an older sister with a diagnosis of GT. She underwent four sequential colonoscopies over 9 mo as enumerated below.
All the colonoscopic examinations were performed in a standard fashion (Olympus CF-H180 A/L endoscopes) at our university medical center endoscopy suite under the direct supervision and assistance of a single gastroenterology-attending physician (SC). Anesthesia services provided propofol during all procedures. Blood products, antifibrinolytic agents and procedural timing were managed by the hematology service.
The first procedure was performed when the patient had baseline hemoglobin of 10 mg/dL. No blood products were given prior to the procedure. The colonoscopy revealed multiple sessile polyps. The two largest sessile polyps, 10 mm and 15 mm each, were noted in the right colon. The 10 mm sessile polyp was completely removed using a saline-assisted technique in combination with Endoloop and standard cautery (A blend of 10 watts of cutting power and 30 joules of coagulation energy, Valleylab electrosurgical unit, Tyco Healthcare, Boulder, CO). Immediate post- polypectomy bleeding was observed and successfully controlled with 2 Triclips (Cook Endoscopy Medical GI Endoscopy, Bloomington, IN). Further polypectomies were deferred. The histological examination of the polyp revealed a sessile serrated adenoma with no dysplasia. Subsequently the patient presented to the hospital with delayed (after 3 d) self-limiting hematochezia and a decrease in hemoglobin that responded adequately to two units of packed red blood cells (PRBC). Surgical opinion was obtained for further management of the residual large polyps. They recommended endoscopic surveillance (Table 1).
| Colonoscopy | Number of clips placed/polyp size | Delayed post procedure hematochezia | Pre procedure blood products and or AMAa | Post procedure blood products | Factor rVIIae | LOSf |
| Colonoscopy with polypectomy. Diagnostic (C1) | Two/10 mm | Self limiting | None | 2 units of PPd, 4 units of PRBCc, AMAa | None | 9 d |
| Colonoscopy with polypectomy (C2) | None/5-6 mm | None | 2 units of HLA Pb, 2 units of PRBCc, and AMAa | None | None | 3 d |
| Colonoscopy with polypectomy (C3) | Single/15 mm | Severe | 2 units of HLA Pb, 1 unit of PRBCc, AMAa | 16 units of PPd, 6 units of PRBCc, AMAa | RVIIae 80 mcg/kg, Q2 h for a total of 5 doses | 11 d |
| Colonoscopy for hematochezia (C4) | Four/prior 15 mm polyp site | None | 1 d |
The second colonoscopy was planned (10 d after C1) with pre-procedure blood products (Table 1). A day prior to the procedure the patient received aminocaproic acid and HLA matched platelets (Table 1). Hot biopsy polypectomies were performed to remove ten smaller approximately 4-5 mm polyps from the left side of the colon. The 15 mm polyp on the right side of the colon was left in place at this time to help localize any potential post-polypectomy bleeding. No immediate or delayed bleeding occurred. All polyps were hyperplastic on histology (Table 1).
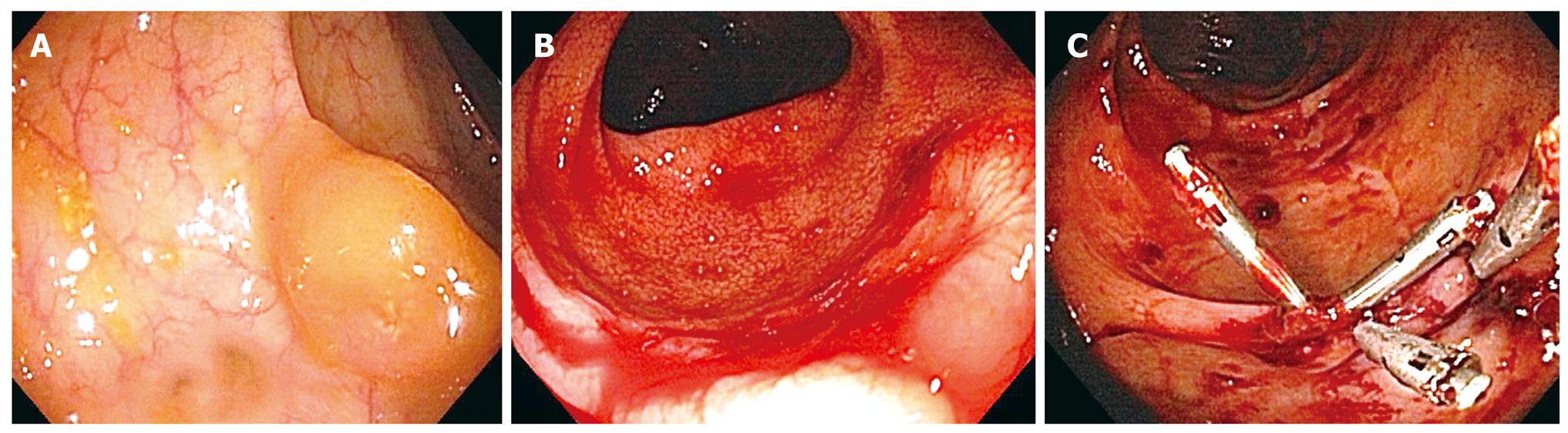
The pre-procedure HLA-matched platelets and antifibrinolytics were given the same day prior to the third colonoscopy performed 7 mo after C2. The 15 mm right-sided polyp was tattooed with 4cc of India ink and removed with a saline assisted technique (Figure 1A). No immediate bleeding noted, though a single QuickClip (QuickClip 2, Olympus, Center Valley, PA) was placed at the post-polypectomy site to close the defect. Two other smaller polyps 7 mm and 9 mm in size were removed from the left colon using standard electrocautery, with no clips applied. Three days later the patient was admitted with hematochezia and a three-gram drop in hemoglobin (Table 1).
Hematochezia was severe, presenting as intermittent large bloody 4 to 5 bowel movements per day. Over the next 7 d, bleeding failed to stop and the patient required daily replacement with several units of packed red blood cells, platelets and intravenous aminocaproic acid. The patient also received intravenous recombinant factor VIIa and prothrombin concentrate complex, but the hematochezia was persistent.
The fourth colonoscopy on day 10 post-procedure (C3) identified an active bleeding site in the right colon at the tattooed 15 mm post-polypectomy site (Figure 1B). Previously placed QuickClip was not identified. Dual therapy with epinephrine injection and placement of 4 Resolution Clips (Boston Scientific, Natick, MA) achieved immediate hemostasis (Figure 1C). No further episodes of colonic bleeding occurred (Table 1).
GT, originally described in 1918, is a rare disorder characterized by prolonged bleeding time, normal platelet count, and absent macroscopic platelet aggregation[2,3]. The basis for defective platelet function is a deficiency or dysfunction of platelet membrane GIIb/IIIa complex (α 2b β3 integrin)[2,3]. The bleeding noted in GT is predominantly mucocutaneous, while purpura, epistaxis, gingival hemorrhage, and menorrhagia are nearly constant features; gastrointestinal bleeding and hematuria are less common. In most cases, bleeding symptoms manifest rapidly after birth, but diagnosis is often delayed. Diagnosis may be suspected in patients with mucocutaneous bleeding with absent platelet aggregation in response to all physiologic stimuli, and a normal platelet count and morphology[4].
Transfusion of platelets is the standard of therapy for bleeding once conservative measures fail. It is however, limited by the development of alloantibodies to GIIb/IIIa complex and or /HLA complex[5]. HLA matched platelet transfusion or leukocyte depleted platelet transfusion may lessen that problem. Recombinant activated factor seven (rFVIIa; NovoSeven_; Nordisk A/S, Bagsvaerd, Denmark) is reported as an effective alternative in managing severe bleeding episodes especially in patients at risk or with a history of alloimmunization[6]. A review based on a large international survey included 103 GT patients who experienced severe bleeding episodes during 34 surgical and invasive procedures[6,7]. It was previously reported that rFVII was effective in achieving hemostasis in approximately two thirds of the cases[6,7]. However, on subgroup analysis it was determined that gastrointestinal bleeding was difficult to control with rFVII. A recent case report further highlighted that gastrointestinal bleeding can occur after polypectomy in GT patients, and may ultimately require surgical intervention[8].
This is in contrast to an older case report in which pre-procedure platelet transfusion and aminocaproic acid was effective in preventing post-polypectomy bleeding[9]. This suggests that one has to maximize local hemostatic therapy, especially in gastrointestinal bleeds arising from polypectomies in GT patients.
Post-polypectomy bleeding as a major complication after colonoscopy has been reported to occur after approximately 1%-6% of polypectomies[7,8]. Delayed bleeding can present within days up to 2 wk post procedure[10,11]. To minimize post-polypectomy hemorrhage, techniques such as submucosal injection of saline or the use of detachable nylon loop are employed before completion of polypectomy as was done in this case[1,14]. Prophylactic clip application for prevention of post-polypectomy hemorrhage by closure of mucosal defects after polypectomy has also been reported[15]. In our patient four colonoscopies were performed. When examined retrospectively this allows us to analyze the several techniques attempted to prevent and treat post-polypectomy bleeding in GT. Post-polypectomy bleeding was significant when a single clip was applied as compared to two clips when adjusted for polyp size and site. Our observation is that in a patient with GT, multiple versus single hemostatic clips may have a more significant role in preventing or limiting post-polypectomy bleeding and it is effectiveness in achieving hemostasis once that happens. Depth of bite or retention rate may differ between different types of clips, whether that can help explain the difference in severity of post-polypectomy hemorrhage would be unknown. Studies in animal models have shown that Resolution Clips retain longer at site of application compared to Tri-clips though no difference was noted in hemodynamic stability in bleeding ulcers[16,17]. Longer retention has been shown in clinical setting when Resolution Clips were used to close post-polypectomy defects[18]. Whether a certain type of clip can improve hemostasis is not known, but this needs to be weighed against the fact that longer retention may be a limitation for residual polyp removal.
In conclusion, colonic polypectomy in GT patients may be complicated by immediate or delayed bleeding. The single previous GT case report suggested a protective effect of platelet transfusion and aminocaproic acid in preventing post-polypectomy bleeding. Another study implied that severe bleeding could be stopped by use of rFVII. However, we conclude that for polyps
10 mm or larger the addition of mechanical therapy, with multiple clips after standard cautery polypectomy, may be effective in preventing immediate and delayed post-polypectomy bleeding in patients with GT. The cost of preemptive multiple clips at post-polypectomy site may be offset by a reduction in need for blood products and by averting or shortening potential hospitalizations.
Peer reviewers: Carlos Robles-Medranda, MD, Head of the Endoscopy Division, Ecuadorian Institute of Digestive Disease (IECED), San Antonio Clinic, Av. Reales Tamarindo y Tennis Club, Portoviejo-Manabi-Ecuador, Casilla 13-01-266, Ecuador; Rami Eliakim, Professor, Chair of Medicine, Head, Department of Gastroenterology, Rambam Health Care Campus, Haifa 31096, Israel
S- Editor Li JL L- Editor Alpini GD E- Editor Ma WH
| 1. | Iishi H, Tatsuta M, Kitamura S, Narahara H, Iseki K, Ishiguro S. Endoscopic resection of large sessile colorectal polyps using a submucosal saline injection technique. Hepatogastroenterology. 1997;44:698-702. |
| 2. | George JN, Caen JP, Nurden AT. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990;75:1383-1395. |
| 3. | Nurden AT. Inherited abnormalities of platelets. Thromb Haemost. 1999;82:468-480. |
| 4. | Nair S, Ghosh K, Kulkarni B, Shetty S, Mohanty D. Glanzmann's thrombasthenia: updated. Platelets. 2002;13:387-393. |
| 5. | Levy-Toledano S, Tobelem G, Legrand C, Bredoux R, Degos L, Nurden A, Caen JP. Acquired IgG antibody occurring in a thrombasthenic patient: its effect on human platelet function. Blood. 1978;51:1065-1071. |
| 6. | Poon MC, Demers C, Jobin F, Wu JW. Recombinant factor VIIa is effective for bleeding and surgery in patients with Glanzmann thrombasthenia. Blood. 1999;94:3951-3953. |
| 7. | Poon MC, D'Oiron R, Von Depka M, Khair K, Negrier C, Karafoulidou A, Huth-Kuehne A, Morfini M. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann's thrombasthenia: results of an international survey. J Thromb Haemost. 2004;2:1096-1103. |
| 8. | Bakdash S, Lyons JM, Bastacky SI, Pezzone MA, McGee JB, Schoen RE, Regueiro M, Lee KK, Bontempo FA. Management of persistent gastric bleeding in a patient with Glanzmann's thrombasthenia. Am J Hematol. 2008;83:411-415. |
| 9. | Orlando RC, White GC 2nd. Colonoscopic polypectomy in Glanzmann's thrombasthenia. Gastrointest Endosc. 1983;29:33-34. |
| 10. | Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J Clin Gastroenterol. 1992;15:347-351. |
| 11. | Rosen L, Bub DS, Reed JF 3rd, Nastasee SA. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum. 1993;36:1126-1131. |
| 12. | Gibbs DH, Opelka FG, Beck DE, Hicks TC, Timmcke AE, Gathright JB Jr. Postpolypectomy colonic hemorrhage. Dis Colon Rectum. 1996;39:806-810. |
| 13. | Macrae FA, Tan KG, Williams CB. Towards safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983;24:376-383. |
| 14. | Hachisu T. A new detachable snare for hemostasis in the removal of large polyps or other elevated lesions. Surg Endosc. 1991;5:70-74. |
| 15. | Iida Y, Miura S, Munemoto Y, Kasahara Y, Asada Y, Toya D, Fujisawa M. Endoscopic resection of large colorectal polyps using a clipping method. Dis Colon Rectum. 1994;37:179-180. |
| 16. | Shin EJ, Ko CW, Magno P, Giday SA, Clarke JO, Buscaglia JM, Sedrakyan G, Jagannath SB, Kalloo AN, Kantsevoy SV. Comparative study of endoscopic clips: duration of attachment at the site of clip application. Gastrointest Endosc. 2007;66:757-761. |
| 17. | Maiss J, Dumser C, Zopf Y, Naegel A, Krauss N, Hochberger J, Matthes K, Hahn EG, Schwab D. "Hemodynamic efficacy" of two endoscopic clip devices used in the treatment of bleeding vessels, tested in an experimental setting using the compact Erlangen Active Simulator for Interventional Endoscopy (compactEASIE) training model. Endoscopy. 2006;38:575-580. |
| 18. | Khashab M, Rex DK. Persistence of resolution clips on colorectal polypectomy sites. Gastrointest Endosc. 2007;66:635-636. |