INTRODUCTION
Linear echoendoscope, introduced in the 1990s, opened the era of interventional endoscopic ultrasound (IEUS). The linear echoendoscope enabled EUS guided Fine Needle Aspiration (EUS-FNA) allowing the path of the needle to be traced during the puncture process. Tissue acquisition was the first EUS-guided interventional procedure and the higher diagnostic quality of EUS-FNA has undoubtedly been established for other tissue acquisition modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) guided biopsy. After EUS-FNA, other procedures, including celiac plexus neurolysis (CPN) and block (CPB), pancreatic pseudocyst drainage, abdominal and mediastinal collections/abscesses drainage, and, in selected cases, pancreatic and biliary ductal system drainage, were introduced in clinical practice. Recently, EUS-guided fine needle injection with local delivery of antitumor agents (cellular therapy, gene therapy, alcohol injection, and radiofrequency ablation) has been considered a promising modality to treat solid and cystic tumors of the pancreas or other abdominal or mediastinal malignancies. This review summarizes the diagnostic capabilities of endoscopic ultrasound and elaborates in detail its therapeutic capability and potential.
EUS-FNA
The diagnostic utility of EUS-FNA has been clearly established. EUS-FNA is a simple, cost-effective, and versatile technique that has been adapted for the diagnosis of gastrointestinal tract lesions as well as other organ sites[1]. Compared to other techniques, EUS-FNA has the advantage of being able to detect lesions not clearly visualized by other imaging tests[2]. Indications for EUS-FNA are numerous: intra-abdominal and mediastinal lymph nodes[3], pancreatic masses[4], pancreatic cysts[5,6], and gastrointestinal sub-mucosal masses[7]. The major advantage is documented in case of failure of other biopsy techniques[1]: EUS-FNA performed after other unsuccessful diagnostic attempts can provide a diagnosis in 85%-95% of cases[4,7,8]. Today, EUS-FNA is recommended as the primary modality in diagnosis of pancreatic masses, pancreatic cysts, and posterior mediastinal lymph nodes. As mentioned above, FNA performed under EUS requires a linear array echoendoscope, because the full length of the needle can be tracked in real time into the lesion. The use of color-Doppler avoids the accidental puncture of intervening vessels. Needles are designed to have a tip visualized by ultrasound. The needle comprises a protective plastic cover of the working channel, a handle at the proximal end allowing the needle to move out from the cover into the lesion, and a needle with a stylet that prevents contaminations with gut cells. Their are available in arrange of sizes (e.g. 19G, 22G and 25G). Trucut needles (19G) are also available and used for gastric sub-mucosal tumors and solid and cystic lesion of the pancreas[9]. The use of trucut needles is limited in the stomach due its stiffness. The real value of trucut needles is not well established, although in cases of insufficient sample with the EUS-FNA alone, the use of sequential trucut biopsy seems to improve the diagnostic power[10]. More recently, the echo-brush, a device that allows brushing on the EUS guide, has become available, although evidence of its effectiveness has not been published.
EUS-FNA is performed by positioning the target lesion on top of the ultrasound image. The correct positioning of the needle in the lesion is monitored in real time, intervening vessels are also visualized to avoid accidental puncture. The stylet is withdrawn, aspiration with a 10 mL syringe applied, and the needle moved back and forward several times (Figure 1A). Many centers offer on-site cytopathological adequacy evaluation; the main reason being the reduction of non-diagnostic specimens. Apparently, on-site evaluation increases the diagnostic yield by 10%-15%[11]. In a recent comparison between trained endosonographers in sample adequacy assessment and cytotechnologists, there was a significant difference in favor of cytotechnologists for adequacy assessment (P = 0.004) and preliminary diagnosis of benign vs. malignant (P = 0.001)[12]. The endosonographer’s and pathologist’s expertise, the type of lesion, the vascularization, and needle size all represent factors that impact on the diagnostic yield. However, the continuous feedback between the endosonographer and dedicated cytopathologist is mandatory to obtain diagnostic FNAs. Information on clinical condition and lesion characteristics are necessary for subsequent processing of the specimen, as are performance of ancillary studies to reach the definitive diagnosis, such as immunochemical staining, molecular analysis or genetic evaluation by PCR.
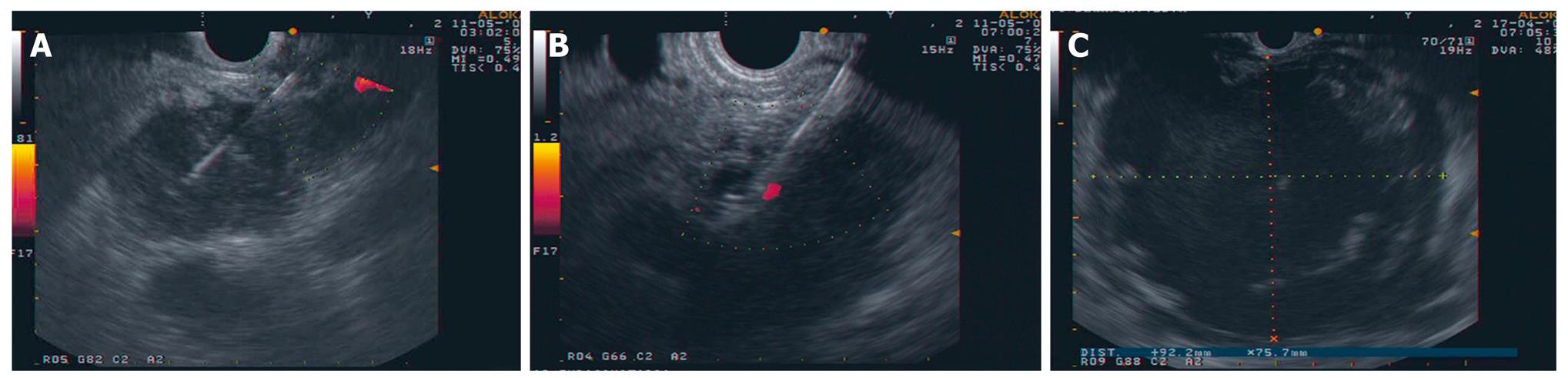
Figure 1 Images of interventional EUS.
A: EUS FNA with a 22G needle on a solid pancreatic mass; B: CPN: the needle (19G) is inserted immediately adjacent to the lateral aspect of the aorta at the level of the celiac trunk; C: Large pancreatic collection seen from the posterior gastric wall.
EUS-GUIDED CELIAC PLEXUS NEUROLYSIS AND CELIAC PLEXUS BLOCK
In the same way as FNA, the close proximity between the tip of the echoendoscope and the target organ allows therapeutic procedures, such as injection therapies, to be performed safely and effectively, when compared with radiologic or surgical procedures. The term CPN refers to the injection of alcohol in the celiac ganglion to induce neurolysis in patients with pancreatic cancer or other malignancies. The term CPB refers to the injection of steroids to inhibit celiac ganglion function in benign conditions, such as the chronic pancreatitis. However, many use the term without distinction. CPN has been established as an effective technique to improve pain control in patients with pancreatic carcinoma. The effectiveness of surgical CPN with injection of absolute alcohol in the celiac plexus was confirmed by prospective randomized trials[13]. Similar results were demonstrated for percutaneous injection guided by CT[14,15]. There are several advantages of using EUS vs a CT-guided percutaneous approach: the proximity of the posterior lesser curve of the stomach to the celiac plexus, the use of continuous real time visualization of the target area, and the color-Doppler to avoid accidental puncture of vascular structures. The needle (19G or 20G) is inserted under EUS guidance immediately adjacent to the lateral aspect of the aorta at the level of the celiac trunk. For CPN, 10 mL of bupivacaine (0.25%) followed by 10 mL of alcohol (98%) are injected. The process can be repeated in the other side of the aorta. For CPB, a steroid (triamcinolone suspension 40 mL bilateral or 80ml unilateral) is used in place of alcohol (Figure 1B). Evidence suggest EUS-guided CPN is a safe and effective procedure with a significant response in term of reduction of pain and opioid intake[16]. The efficacy data for CPB in treating pain for chronic pancreatitis are less well established, with a transient response in about 50% of patients[17]. However, recent recognition that celiac ganglia can be visualized by EUS in almost 81%[18] of patients, allows the performance of direct CPN and CPB on the ganglia thus significantly improving results for both CPN and CPB[19]. Further studies are needed to establish the real efficacy of CPB in chronic pancreatitis with this direct injection.
EUS-GUIDED PANCREATIC COLLECTIONS DRAINAGE
The therapeutic options for treating pancreatic pseudocysts, abscesses and necrosis, include surgery, percutaneous drainage, and trans-mural non-EUS-guided or EUS-guided drainage. The success rates and complications of these different approaches were analyzed in a review: the success rate for surgical, percutaneous, non EUS-guided and EUS-guided trans-mural drainage were 100%, 84%, 90%, and 94%, respectively. The rate of complications were 28%-34% with 1%-8.5% mortality for surgery, 18% with 2% mortality for percutaneous drainage, 15% with 0% mortality for trans-mural non EUS-guided drainage, and 1.5% with 0% mortality for EUS-guided trans-mural drainage[20]. In the 1980s, reports by Sahel et al[21] and Cremer et al[22] showed the feasibility and efficacy of trans-mural drainage for treating pancreatic fluid collection. The limitation of trans-mural non-EUS-guided drainage is its relatively blind approach: in this way, no bulging collection can be drained due to the risk of perforation, and hemorrhages take place in about 6% of cases[23]. EUS-guided trans-mural drainage improves both the safety of the trans-mural procedure and the number of candidates for this treatment. Real time EUS visualization with Doppler allows the puncture of collection in patients without bulging and in patients with portal hypertension with multiple collateral vessels[24-28]. The positioning is also selected based on EUS evaluation of the distance between the gastrointestinal wall and cyst wall that should be less of one cm. EUS-guided drainages are performed with a linear array echoendoscope, the color Doppler used to identify regional vessels, the puncture is performed with a 19G needle and a guide-wire introduced through the needle and coiled within the collection under fluoroscopic guidance. The tract is sequentially dilated and finally the stents are placed (double pigtail, nose-cystic tube, plastic stents) (Figure 1C). The higher technical quality of EUS-guided over non-EUS-guided trans-mural drainage of pancreatic collections has been clearly demonstrated by two reports[28,29]. More recently, a randomized trial comparing EUS and EGD for trans-mural drainage showed a higher technical success rate of EUS than EGD and a superior safety profile of EUS-guided drainage, although it was not statistically significant. Finally, major complications were observed in the group who underwent non-EUS-guided drainage[30].
With the same technique, other EUS-guided procedures, such as the trans-rectal drainage of pelvic abscess, are considered useful and safe[31].
EUS-GUIDED BILIARY/PANCREATIC DRAINAGE
Endoscopic Retrograde Cholangiopancreatography (ERCP) is the most appropriate technique for treating common bile duct and pancreatic duct stenosis secondary to benign and malignant diseases. Biliary and/or pancreatic duct cannulation and visualization are successful with ERCP in a high percentage of cases managed by experienced hands. Common causes of failure include complex peripapillary diverticula, prior surgery procedures (such as gastrectomy with Billroth II anastomosis), tumor involvement of the papilla, biliary sphincter stenosis, and impacted stones[32]. Percutaneous transhepatic cholangiography (PTC) and surgery are alternative approaches to access and drain obstructed ducts. Since 1996, when Wiersema et al[33] first described EUS-guided bile duct puncture, several case reports have been published on EUS-guided biliary and pancreatic duct puncture and drainage[34-42]. These case reports illustrate different techniques to approach bile and pancreatic ducts: transgastric or transduodenal puncture, rendezvous after positioning a guide wire through the papilla, or creating a new papilla by fistula formation.
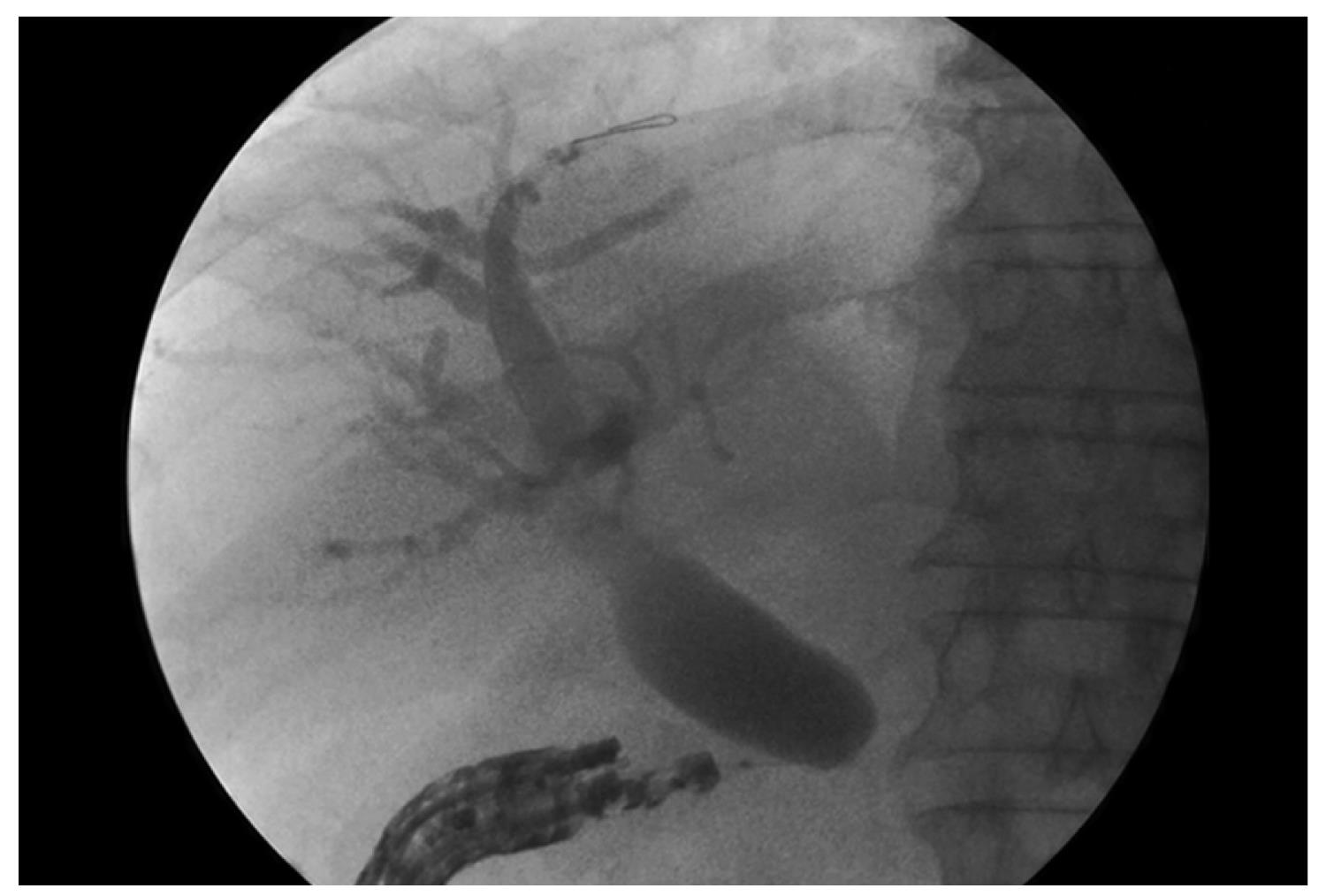
The puncture is performed with 19G or 22G needles and the positioning is selected based on EUS evaluation of the distance between the gastrointestinal wall and the bile or pancreatic duct over the stricture (Figure 2). After puncture, bile or pancreatic juice is aspirated and iodine contrast injected to obtain a cholangiogram/pancreatogram; a 0.018-inch guide wire is positioned in the duct and the rendezvous procedure is attempted. The wire is captured with a snare and pulled out of the EUS-scope, which is replaced with a standard duodenoscope. When the wire fails to pass through the papilla in the duodenal lumen, a new papilla can be created by precut, and pneumatic dilatation is performed with a biliary balloon dilatation catheter. Finally, a plastic stent is placed. In all these reports, interventional EUS proved feasible and safe with a low complications rate; however, only few series have been published. Kahaleh et al[43] recently described a series of 23 patients treated with interventional endoscopic ultrasound cholangiography (IEUC). Biliary decompression was accomplished in over 90% of the cases that had previously received ERCP without success. In one of such case, bile leakage occurred using the extrahepatic approach. We describe a series of consecutive patients in whom ERCP failed for different reasons and IEUC was applied instead of the traditional percutaneous approach. Our series of nine patients confirmed the feasibility and safety of EUS-assisted procedures[44]. In our opinion, in the extrahepatic approach, the stent placement prevents the bile leakage.
Figure 2 EUS-guided cholangiography through the duodenal wall.
Recently, EUS-guided transmural cholecystostomy was described in nine high-risk patients with severe acute cholecystitis at high operative risk for immediate cholecystectomy. The report showed that the procedure was feasible and safe as an initial, interim, or even definitive treatment of patients[45].
These techniques however, are currently restricted to expertise centers dedicated to biliopancreatic therapy.
EUS-GUIDED ANTI-TUMORAL THERAPY
EUS-guided FNI is emerging as an attractive delivery method for antitumor agents. This technique is the latest development in interventional ultrasound and seems particularly promising in the treatment of cystic and solid pancreatic lesion. Two reports described this EUS-guided treatment by alcohol injection on adrenal gland metastasis[46] and by TNFerade injection on metastatic lymph nodes[47]. The first study on the treatment of solid pancreatic lesions assessed the feasibility and safety of EUS-direct injection of allogenic mixed lymphocyte culture (cytoimplant) in locally advanced pancreatic adenocarcinoma[48]. The technique of EUS-guided FNI was also applied to the delivery of antitumor viral therapy using ONYX-015, a replication selective adenovirus, with a deletion in the E1B-55kDa gene, which preferentially replicates in and kills malignant cells[49]. These experimental studies showed the feasibility and safety of these therapeutic procedures. More recent studies on TNFerade, used in combination with chemo-radiation in the treatment of locally advanced pancreatic cancer[50] and in patients with locally advanced esophageal cancer[47], showed good tolerability and seem to optimize long-term outcomes.
The ablation of pancreatic tissue by EUS-guided ethanol injection was recently proposed as an effective therapy for cystic pancreatic lesions. Despite recent advances in diagnostic modalities and molecular studies, substantial morphological overlap restricts the accuracy of differentiation of each cystic lesion, and consequently drafting a management plan. To date, surgical resection is recommended for malignant and potentially malignant lesions. Surgical resection carries significant morbidity. A pilot study showed that the ethanol lavage on cystic lesions of the pancreas allowed complete resolution in one third of patients, but with complete epithelial lining ablation in all resected specimen[51]. In an experimental study on a porcine model, Matthes et al[52] showed how the efficacy of the ethanol lavage is concentration-dependent. Recently, a prospective study on EUS-guided ethanol lavage with paclitaxel injection indicated that this method is safe, feasible and effective to treat pancreatic cystic tumors of the pancreas. This study found that 11 of 14 patients showed complete resolution and highlighted no significant complications[53]. These promising results indicate further studies are warranted involving larger populations and longer follow up. Ablative modalities such as radiofrequency (RF), cryotechnology and brachytherapy are widely used in oncology and an EUS-guided approach has been proposed. Goldberg et al[54] investigated the feasibility and safety of performing radiofrequency ablation in the normal pancreas of pigs. The results were positive in term of necrosis, and the authors concluded that management of small neuroendocrine tumors and unresectable solid tumors might be potential indications of this technique. More recently, Carrara et al[55,56] investigated the ability of a new flexible ablation device that combines bipolar radiofrequency with cryotechnology, into the porcine pancreas, liver and spleen. These studies demonstrated the feasibility, efficacy, and safety of EUS-guided transgastric application of the hybrid cryotherm probe in the porcine models.
However, these positive results need further systematic studies investigating the effect of EUS-guided RF in tumor tissues. Jin et al[57] evaluated the clinical efficacy and safety of EUS-guided interstitial implantation of radioactive iodine 125 seeds in twenty patients with advanced pancreatic cancer. There were no obvious complications following therapy, however, their preliminary data suggested improvement in pain, but no long-term survival benefit.
CONCLUSION
The role of EUS in therapeutic procedures continues to expand. The principles behind endoscopic ultrasound-guided fine needle aspiration paved the way for the development of therapeutic endoscopic ultrasound. The endoscopic ultrasound-guided puncture of fluid collections, abscesses and obstructed biliary and pancreatic ductal systems facilitated the passage of guide-wires, thus allowing therapeutic drainage procedures to be performed. Substances can be delivered by endoscopic ultrasound into targeted areas; an example where the EUS has a clear and define role is the celiac plexus block and neurolysis. There is also a potentially important role for EUS FNI therapies as part of the management strategy for unresectable cancer and for pancreatic cystic lesions.
Finally, EUS-guided techniques, such as the radiofrequency ablation, might evolve into routine procedures as soon as the necessary basic tools become commercially available.
Peer reviewers: Shyam Varadarajulu, MD, Director of Interventional Endoscopy, Division of Gastroenterology-Hepatology, University of Alabama at Birmingham School of Medicine, Birmingham, AL 35294-0007, United States; Gen Tohda, MD, PhD, Department of Gastroenterology and Endoscopy, Fukui Kosei Hospital 201 Shimo-Rokujyo cho, Fukui 918-8537, Japan
S- Editor Li JL L- Editor Stewart GJ E- Editor Ma WH