Copyright
©The Author(s) 2025.
World J Gastrointest Endosc. Mar 16, 2025; 17(3): 99540
Published online Mar 16, 2025. doi: 10.4253/wjge.v17.i3.99540
Published online Mar 16, 2025. doi: 10.4253/wjge.v17.i3.99540
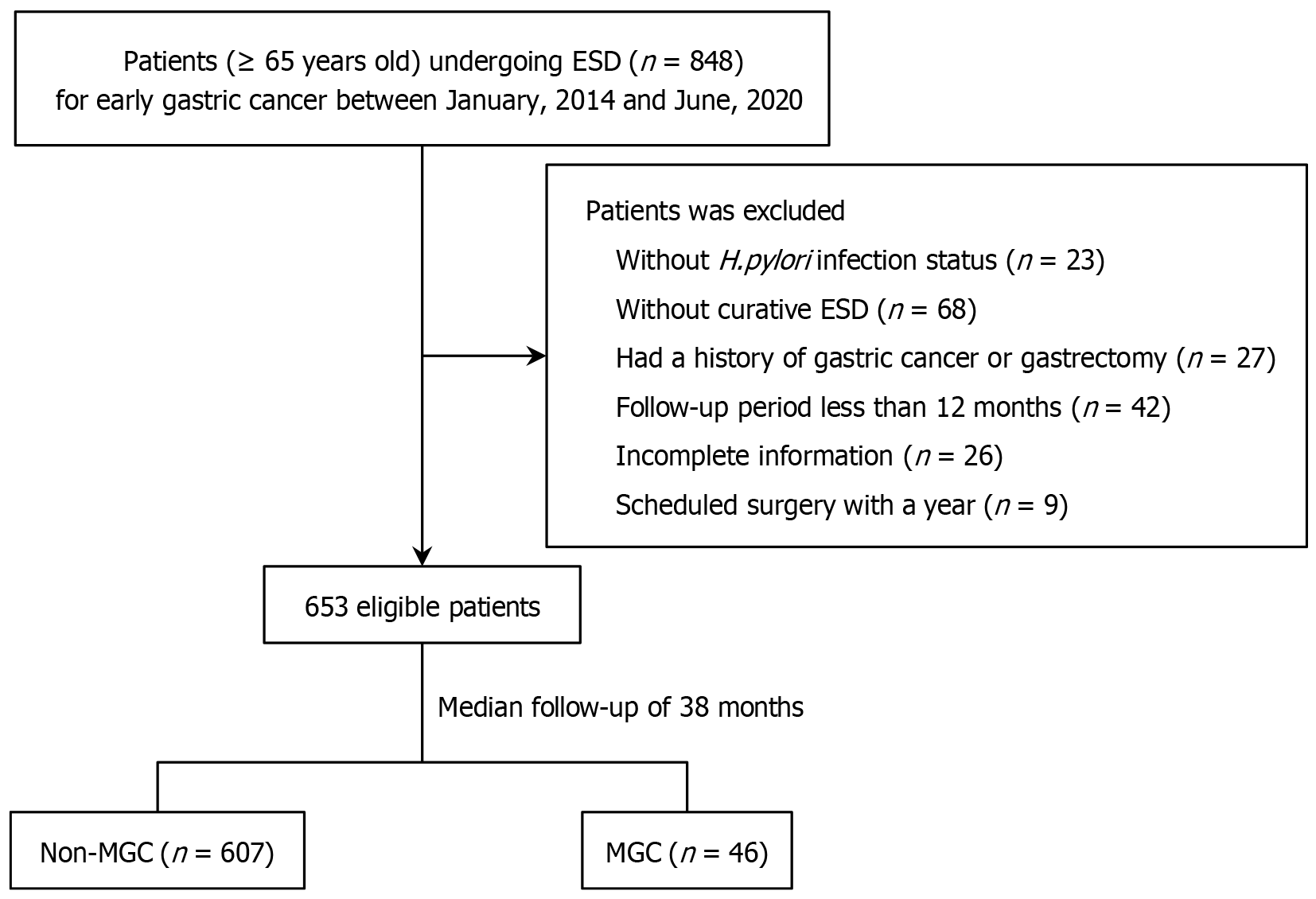
Figure 1 Flow diagram of the enrolled study patients.
ESD: Endoscopic submucosal dissection; MGC: Metachronous gastric cancer; H. pylori: Helicobacter pylori.
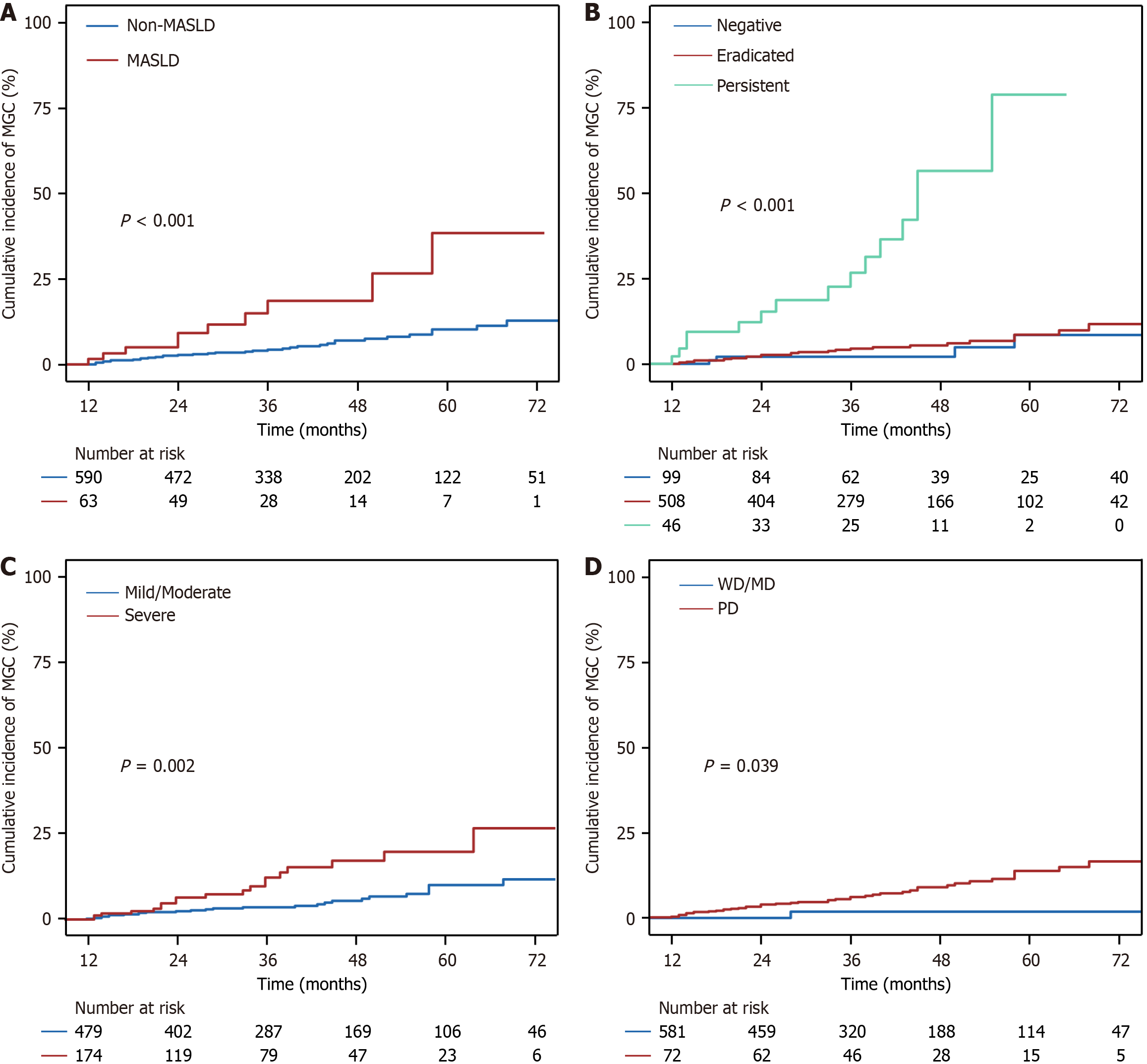
Figure 2 Cumulative incidences of metachronous gastric cancer after curative endoscopic submucosal dissection between different risk factors.
A: The presence or absence of metabolic dysfunction-associated steatotic liver disease; B: Different Helicobacter pylori infection status; C: Degrees of mucosal atrophy; D: Differentiation of the lesions. MASLD: Metabolic dysfunction-associated steatotic liver disease; MGC: Metachronous gastric cancer; PD: Poorly differentiated; WD: Well differentiated; MD: Moderately differentiated.
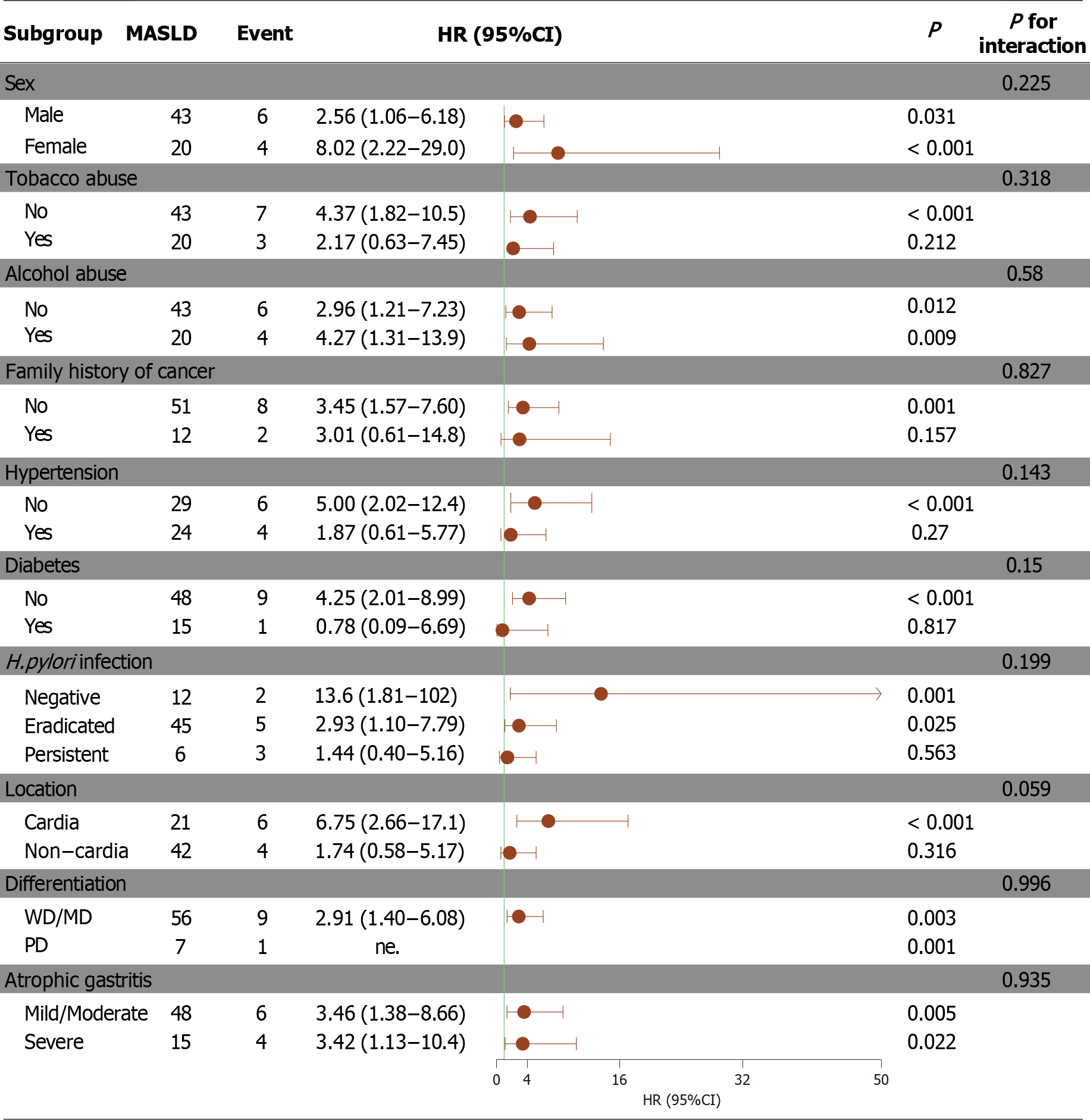
Figure 3 A subgroup analyses forest plot of the risk factors for metabolic dysfunction-associated steatotic liver disease associated with metachronous gastric cancer.
MASLD: Metabolic dysfunction-associated steatotic liver disease; PD: Poorly differentiated; WD: Well differentiated; MD: Moderately differentiated; HR: Hazard ratio; CI: Confidence interval; H. pylori: Helicobacter pylori.
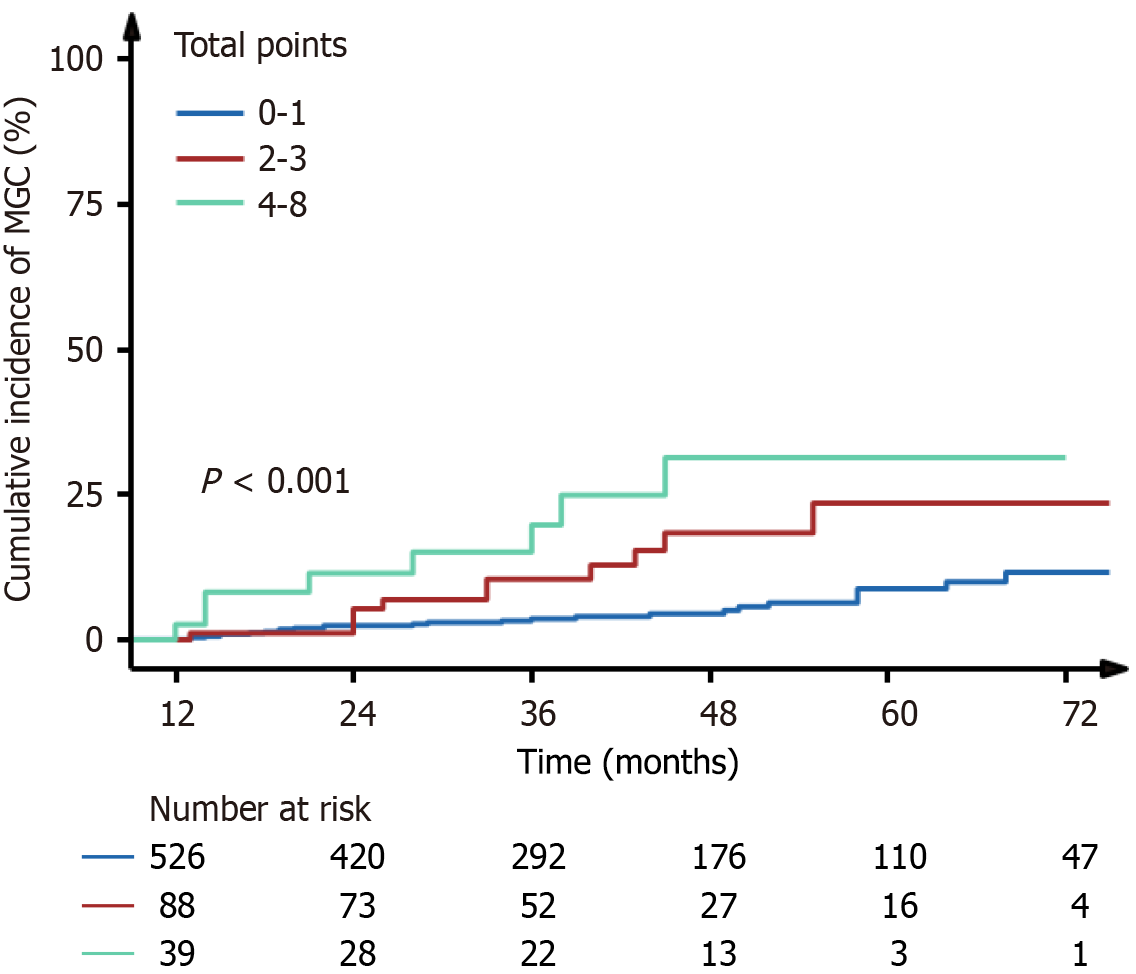
Figure 4 Cumulative incidence of metachronous gastric cancer according to the risk stratification based on the risk factors.
MGC: Metachronous gastric cancer.
- Citation: Xiang Y, Yuan Y, Wang ZY, Zhu YM, Li WY, Ye QG, Wang YN, Sun Q, Ding XW, Longi F, Tang DH, Xu GF. Comorbidities related to metachronous recurrence for early gastric cancer in elderly patients. World J Gastrointest Endosc 2025; 17(3): 99540
- URL: https://www.wjgnet.com/1948-5190/full/v17/i3/99540.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i3.99540