Published online Feb 8, 2017. doi: 10.4254/wjh.v9.i4.217
Peer-review started: July 9, 2016
First decision: September 7, 2016
Revised: October 12, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: February 8, 2017
Processing time: 215 Days and 8.8 Hours
To evaluate efficacy/safety of hepatitis C virus (HCV) protease inhibitor boceprevir with pegylated interferon (PEG-IFN) alfa and weight-based ribavirin (RBV) in a phase 3 trial.
A prospective, multicenter, phase 3, open-label, single-arm study of PEG-IFN alfa, weight-based RBV, and boceprevir, with a PEG-IFN/RBV lead-in phase was performed. The HCV/human immunodeficiency virus coinfected study population included treatment naïve (TN) and treatment experienced (TE) patients. Treatment duration ranged from 28 to 48 wk dependent upon response-guided criteria. All patients had HCV Genotype 1 with a viral load > 10000 IU/mL. Compensated cirrhosis was allowed. Sample size was determined to establish superiority to historical (PEG-IFN plus RBV) rates in sustained viral response (SVR).
A total of 257 enrolled participants were analyzed (135 TN and 122 TE). In the TN group, 81.5% were male and 54.1% were black. In the TE group, 76.2% were male and 47.5% were white. Overall SVR12 rates (HCV RNA < lower limit of quantification, target not detected, target not detected) were 35.6% in TN and 30.3% in TE. Response rates at SVR24 were 28% in TN and 10% in TE, and exceeded those in historical controls. The highest rate was observed in TN non-cirrhotic participants (36.8% and the lowest in TE cirrhotics (26.3%). Cirrhotic TN participants had a 27.8% SVR12 rate and 32.1% of TE non-cirrhotics achieved SVR12. Significantly lower response rates were observed among black participants; in the TE, SVR12 was 39.7% in white participants but only 13.2% of black subjects (P = 0.002). Among the TN, SVR12 was 42.1% among whites and 27.4% among blacks (P = 0.09).
The trial met its hypothesis of improved SVR compared to historical controls but overall SVR rates were low. All-oral HCV treatments will mitigate these difficulties.
Core tip: Approval of first generation hepatitis C virus (HCV) protease inhibitors has initiated a change in care of HCV infected patients. Phase 2 trials in HCV/human immunodeficiency virus coinfected patients have suggested improved efficacy and tolerability for regimens that combined pegylated interferon (PEG-IFN) + ribavirin (RBV) with either boceprevir or telaprevir. We evaluated an HCV treatment regimen using a first generation HCV protease inhibitor (boceprevir) with PEG-IFN, and weight-based RBV in a phase 3 treatment trial, including HCV treatment-naïve and treatment-experienced coinfected subjects. While sustained viral response rates were low overall they did exceed historical PEG-IFN/RBV rates. Use of new interferon-free direct acting antiviral agents modalities in this population is indicated.
- Citation: Sherman KE, Kang M, Sterling R, Umbleja T, Marks K, Kiser JJ, Alston-Smith B, Greaves W, Butt AA, the ACTG 5294 BIRTH Study Team. Phase 3 trial of first generation protease inhibitor therapy for hepatitis C virus/human immunodeficiency virus coinfection. World J Hepatol 2017; 9(4): 217-223
- URL: https://www.wjgnet.com/1948-5182/full/v9/i4/217.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i4.217
Hepatitis C virus (HCV) coinfection is a major cause of morbidity and mortality among those with human immunodeficiency virus (HIV) infection[1-4]. Prior to the emergence of new HCV targeted direct acting antiviral agents (DAAs) in 2011, response to standard therapy with pegylated interferon (PEG-IFN) and ribavirin (RBV) was poor, both in terms of efficacy and medication tolerability[5]. The approvals of first generation serine protease inhibitors of HCV replication initiated a revolution in terms of the care and management of HCV infected patients. Phase 2 trials in HCV/HIV coinfected patients suggested improved efficacy with moderate drug tolerability for treatment regimens that combined either boceprevir or telaprevir with PEG-IFN + RBV[6,7]. In an effort to define treatment efficacy with response- and cirrhosis-guided regimens in HCV/HIV coinfected, we conducted a prospective, multicenter, open-label Phase 3 trial in both HCV treatment naïve and treatment experienced participants with comparison to historical controls in the same clinical trials network.
The study was performed in the NIH AIDS Clinical Trials Group network (ACTG, National Institutes of Health Registration number NCT01482767) with enrollment of participants at 42 sites across the United States. All participants provided informed consent and the study was conducted with approval of Institutional Review Boards at each site. The study was monitored by an independent, NIH-chartered data safety and monitoring board.
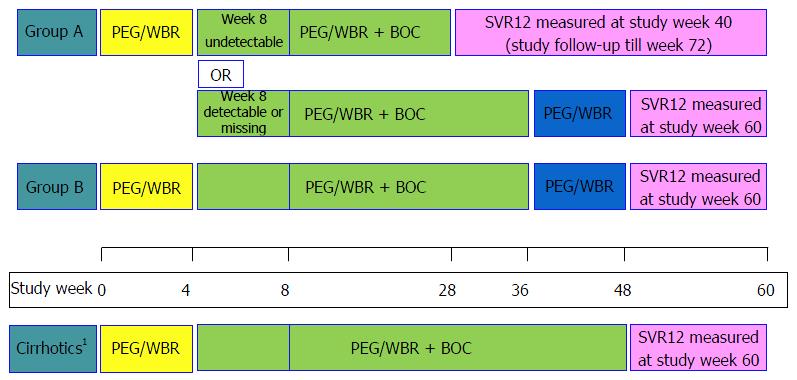
The overall study design is shown in Figure 1. Briefly, treatment naïve (TN) participants (Group A) were treated with PEG-IFN alfa 2b 1.5 μg/kg subcutaneously with weight-based ribavirin (800-1400 mg/d) for 4 wk (lead-in). Then boceprevir 800 mg tid was added to the treatment regimen. Cirrhotic participants received 44 wk of triple therapy. Among non-cirrhotics, the week 8 serum HCV RNA was used to determine total duration of therapy. Those who had undetectable HCV RNA at week 8 completed therapy at week 28. Those with detectable HCV RNA at week 8 received 32 wk of triple therapy followed by 12 additional weeks of double-drug therapy with PEG-IFN/RBV. Treatment experienced participants (TE) (Group B) also had lead-in followed by 32 wk of triple therapy and 12 wk of PEG-IFN/RBV double therapy if non-cirrhotic, or by 44 wk of triple therapy if cirrhotic. Treatment was to be discontinued due to failure if: (1) HCV RNA ≥ 100 IU/mL at week 12; (2) detectable HCV RNA at week 24; or (3) confirmed HCV RNA > 1000 IU/mL any time after week 12. HCV RNA was determined to be undetectable if below the lower limit of quantification (LLOQ) and target not detected (TND) by Roche COBAS® TaqMan® HCV Test v2.0.
Key inclusion criteria included HCV genotype 1 with HCV RNA ≥ 10000 IU/mL. All participants underwent either liver biopsy or non-invasive marker (FibroSure®) testing to determine whether or not cirrhosis was present. Cirrhotics were confirmed to have stage A Child-Pugh disease. HIV RNA viral load was required to be < 50000 copies/mL for participants not on antiretroviral therapy, or less than 50 copies/mL for those on an approved antiretroviral regimen. A CD4+ T-cell count of > 200 cells/mm3 was also required within 42 d of study entry. Approved regimens included efavirenz, raltegravir, lopinavir/ritonavir, atazanavir/ritonavir or darunavir/ritonavir plus a dual nucleoside reverse transcriptase inhibitor backbone that did not include zidovudine or didanosine. Key exclusion criteria were those with mixed HCV genotypes, prior use of HCV protease or polymerase inhibitors or the presence of decompensated liver disease. Also excluded were other known causes of significant liver disease including HBV, HAV, hemochromatosis, or alpha-1 antitrypsin deficiency.
Data were centrally submitted and analyzed using SAS 9.4 (SAS Institute, Cary, NC, United States). The key outcome measure was sustained viral response in each study group and how the estimates compared to those in historical controls from a prior study of PEG-IFN plus RBV therapy (ACTG 5178). The study was powered to conclude that sustained viral response (SVR) is greater than 28% in TN and 10% in treatment experienced participants, based on A5178 results on HCV genotype 1 participants. The SVR proportions were estimated with two-sided 95% Wilson confidence intervals (CI), and Fisher’s exact tests were conducted for comparisons between groups. The analyses included all participants who met the eligibility criteria and initiated the study treatment.
The baseline characteristics of the TN and TE participants as well as the historical controls are shown in Table 1. A total of 257 enrolled participants were analyzed: 135 TN (Group A) and 122 TE (Group B). The study included primarily middle-age males. There was a high representation of black/African-American participants, and this was accompanied by a similarly high percentage of IL28b genotypes carrying the “T” allele. Median CD4 counts were above 600 cell/mm3 in both groups, corresponding to the high rate of active antiretroviral therapy (> 95%). There were more participants with cirrhosis in TE than in TN, in both A5294 and historical controls.
| Characteristic | A5294 | Historical controls | ||
| Treatment naïve (n = 135) | Treatment Exp (n = 122) | Treatment naïve (n = 183) | Treatment Exp (n = 87) | |
| Age (yr) | ||||
| Median | 51 | 53 | 48 | 48 |
| Q1, Q3 | 44, 57 | 49, 57 | 41, 52 | 42, 51 |
| Sex | ||||
| Male | 110 (81.5) | 93 (76.2) | 151 (82.5) | 74 (85.1) |
| Female | 25 (18.5) | 29 (23.8) | 32 (17.5) | 13 (14.9) |
| IV drug history | ||||
| Never | 71 (52.6) | 70 (57.4) | 73 (39.9) | 39 (44.8) |
| Currently | 0 | 0 | 4 (2.2) | 1 (1.1) |
| Previously | 64 (47.4) | 52 (42.6) | 106 (57.9) | 47 (54.0) |
| Race | ||||
| Asian | 2 (1.5) | 2 (1.6) | 1 (0.5) | 0 |
| Black or African American | 73 (54.1) | 53 (43.4) | 91 (49.7) | 24 (27.6) |
| White | 57 (42.2) | 58 (47.5) | 79 (43.2) | 59 (67.8) |
| American Indian | 0 | 2 (1.6) | 3 (1.6) | 1 (1.1) |
| More than One Race | 2 (1.5) | 2 (1.6) | 5 (2.7) | 0 |
| Unknown | 1 (0.7) | 5 (4.1) | 4 (2.2) | 3 (3.4) |
| BMI (kg/m2) | ||||
| Median | 26.3 | 27.5 | 25.7 | 26 |
| Q1, Q3 | 22.6, 29.6 | 25.0, 31.0 | 22.9, 29.4 | 23.6, 30.1 |
| Missing | 1 | 0 | 0 | 0 |
| IL28b genotype (RS 12979860) | ||||
| c/c | 32 (25.2) | 31 (27.2) | 38 (33.9) | 19 (31.1) |
| c/t | 61 (48.0) | 39 (34.2) | 51 (45.5) | 30 (49.2) |
| t/t | 34 (26.8) | 44 (38.6) | 23 (20.5) | 12 (19.7) |
| Missing | 8 | 8 | 71 | 26 |
| CD4 (cells/mm3) | ||||
| Median | 646 | 621.5 | 495 | 520 |
| Q1, Q3 | 462, 818 | 488.5, 858.5 | 373, 697 | 368, 706 |
| Missing | 2 | 2 | 0 | 0 |
| HIV RNA quantitation | ||||
| Unquantifiable | 133 (100.0) | 113 (92.6) | 129 (70.5) | 71 (81.6) |
| Quantifiable | 0 | 9 (7.4%) | 54 (29.5) | 16 (18.4) |
| Missing | 2 | 0 | 0 | 0 |
| HCV RNA (log10 IU/mL) | ||||
| Median | 6.7 | 6.9 | 6.5 | 6.6 |
| Q1, Q3 | 6.2, 7.1 | 6.5, 7.3 | 6.1, 6.8 | 6.3, 7.0 |
| Missing | 1 | 0 | 0 | 0 |
| Cirrhosis | ||||
| Yes | 18 (13.3) | 38 (31.1) | 20 (10.9) | 18 (20.7) |
| No | 117 (86.7) | 84 (68.9) | 163 (89.1) | 69 (79.3) |
| Baseline cART regimen | ||||
| No ART | 2 (1.5) | 6 (4.9) | 40 (21.9) | 11 (12.6) |
| EFV + 2 NRTIs | 58 (43.0) | 51 (41.8) | NA | NA |
| RAL + 2 NRTIs | 47 (34.8) | 45 (36.9) | NA | NA |
| LPV/RTV + 2 NRTIs | 4 (3.0) | 4 (3.3) | NA | NA |
| ATV/RTV + 2 NRTIs | 18 (13.3) | 10 (8.2) | NA | NA |
| DRV/RTV + 2 NRTIs | 6 (4.4) | 6 (4.9) | NA | NA |
Overall SVR12 (HCV RNA < LLOQ, TND (target not detected) at 12 wk post treatment discontinuation) rates were 35.6% (95%CI: 28.0-43.9%) in TN and 30.3% (95%CI: 22.9%-39.0%) in TE (Table 2). Rates of response exceeded SVR24 in historical controls: 28% in TN and 10% in TE. The highest rate was observed in TN non-cirrhotic participants (36.8%, 95%CI: 28.6%-45.8%) and the lowest in TE cirrhotic participants (26.3%, 95%CI: 15.0%-42.0%). Cirrhotic TN participants had a 27.8% (95%CI: 12.5%-50.9%) SVR12 rate and 32.1% (95%CI: 23.1%-42.7%) of TE non-cirrhotics achieved SVR12. Race was a significant factor in treatment outcome. Indeed, among TE, SVR12 was noted to occur in 39.7% of white participants but in only 13.2% of those identified as black (P = 0.002). Among TN, SVR12 was 42.1% among whites and 27.4% among blacks (P = 0.09). Treatment discontinuation rates were high in all groups and were attributed to a mix of treatment failure per HCV viral load criteria or due to adverse events. Among TN, there was one death unrelated to the study, 42 (31%) treatment failures leading to early discontinuation, and additional 22 (16%) premature treatment discontinuations due to adverse events. In TE, there were 52 treatment failures (43%), additional 16 (13%) premature treatment discontinuations due to adverse events, and no deaths. The most commonly reported adverse events of grade 3 or higher included hematologic laboratory events (44% in TN and 48% in TE), and general body (chills, fatigue, pain, weight loss; 23% in TN and 22% in TE), gastrointestinal (4% in TN and in 3% TE) and neurologic (7% in TN and 5% in TE) symptoms. HIV breakthrough was rare and only two study participants (both on raltegravir regimen) met predetermined criteria for this event.
| A5294 participants (n = 257) | % SVR12 |
| Treatment naïve (n = 135) | 35.6 |
| Non-cirrhotic (n = 117) | 36.8 |
| Cirrhotic (n = 18) | 27.8 |
| Treatment experienced (n = 122) | 30.3 |
| Non-cirrhotic (n = 84) | 32.1 |
| Cirrhotic (n = 38) | 26.3 |
Among TN, the highest SVR rates were observed among participants whose cART regimen included ritonavir - boosted atazanavir with a 2 nucleoside/nucleotide backbone. Overall SVR12 rate in this group (n = 18) was 61.1% (95%CI: 38.6%-79.7%) which was significantly higher than SVR12 rates among participants receiving other cART regimens combined (P = 0.018) in a post-hoc analysis. However, we note that this was an exploratory analysis on a small subset not adjusted for baseline covariates, and this effect was not observed in TE.
HCV/HIV coinfection remains a serious medical problem characterized by a high global disease burden (4-5 million) of patients who are at risk for increased fibrotic progression, cirrhosis, and hepatocellular carcinoma[8]. Coinfected patients also have significant non-hepatic complications including increased cardiovascular risk[9]. Therefore, HCV cure is a priority in the management of coinfected HCV/HIV patients. The emergence of new DAAs for HCV has been a rapid and turbulent process which followed years of stagnation in the field. It is not surprising that new therapeutic regimens have been under investigation, even as earlier regimens were entering confirmatory clinical trials. The primary Phase 2 trial for boceprevir/PEG-IFN/RBV was initiated in 2010 and results were reported in July 2013[6]. Planning for the Phase 3 trial reported in this publication began in 2011, and the study completed in early 2015. During this brief interlude, even more effective, shorter duration regimens were studied and brought to the marketplace.
Despite this rapid advancement in therapy, the Phase 3 trial met its primary goals and moved the field forward in a number of key aspects. First, it again demonstrated the importance of Phase 3 trials which often reveal efficacy levels that fall short of their Phase 2 predecessors. The Phase 2 HCV/HIV coinfection trial of the boceprevir/PEG-IFN/RBV regimen yielded an SVR rate of 63%. This is significantly higher than what we observed in the Phase 3 trial which enrolled a population more representative of the United States HCV/HIV population at large in terms of racial distribution. Indeed, the proportion of black participants in this study (49%) is higher than the imputed racial distribution of HCV/HIV coinfected patients in United States (23%-33%) based upon a 2002 analysis[10]. It also exceeds the black representation in the previously reported Phase 2 trial[6]. Our treatment population was more male, more non-white, with a higher representation of the IL28b T allele and with more advanced fibrosis/cirrhosis than the population enrolled in the previously reported Phase 2 study. Our findings of a lower SVR in this population is similar to that reported in “real world” analyses using first generation protease inhibitors[11].
Interestingly, we observed a higher SVR12 among treatment-naïve subjects whose cART regimen consisted of ritonavir boosted atazanavir + a dual NRTI backbone. Pharmacokinetic data indicates that boceprevir AUC was reduced 32% when administered with ritonavir-boosted darunavir while atazanavir AUC decreased only 5%[12]. While we cannot categorically state that this difference affected overall SVR, we suspect it represents an important factor in treatment outcomes among treatment naïve patients. The lack of this finding in treatment experienced participants may represent the overall decreased effectiveness of the PEG-IFN component in that group which masks more subtle effects related to HCV protease inhibitor pharmacokinetics.
Interferon-based therapy is difficult to tolerate and this is clearly demonstrated by the high drop-out rate seen in our study cohort. Though some guidelines and insurers still encourage use of PEG-IFN in some treatment groups, this approach may be particularly detrimental in the HIV-infected patient where tolerability to interferon-based regimens seems to be lower than that observed in comparable Phase 3 trials in monoinfected patients.
Though the treatments utilized in this Phase 3 multicenter trial will not be utilized in general practice, our study provided several important principles and observations that may guide future trials in the field. First, we provide additional support to the concept that Phase 3 trials represent a more accurate representation of true response rates compared to Phase 2 trials. We also note that outcomes in HCV/HIV coinfected patients may be related to the background HIV antiretroviral regimen and that this effect may be a drug effect rather than a class effect. Finally, we note the systematic delays in initiation of clinical trials for those with underlying HIV infection vs those without HIV. Phase 3 trials of first generation HCV protease inhibitors lagged significantly behind drug approvals in HCV monoinfected patients. More recent drug development programs have attempted to remedy this situation, but the HIV research community should remain vigilant to reduce this bias going forward, particularly in rapidly moving developmental fields.
We are indebted to the study subjects and to the following at each of the sites for patient recruitment and their participation in this project: Princy N Kumar MD and Susan Vajda RN - Georgetown University (Site 1008) Grant N/A; Donna McGregor and Richard Green - Northwestern University CRS (Site 2701) Grant AI 069471, UL1 RR02574; MetroHealth CRS (Site 2503) Grant 1U01AI069501-01; Mark A Rodriguez RN BSN and Geyoul Kim RN BS - Washington University Therapeutics CRS (Site 2101) Grant AI69439; Graham Ray and Jacob Langness - University of Colorado CRS (Site 6101) Grant 2UM1AI069432, UL1 TR001082; Roger Bedimo and Holly Wise - Trinity Health and Wellness Center CRS (Site 31443) Grant U01 AI069471; Michelle Saemann RN BSN and Carl J Fichtenbaum MD - University of Cincinnati (Site 2401) Grant UM1AI068636; Jorge L Santana Bagur MD FIDSA and Daniel Casiano RN BSN - Puerto Rico AIDS/CRS (Site 5401) Grant 5UM1AI069415; UCSD Antiviral Research Center CRS (Site 701) Grant UM1AI069432; Valery Hughes FNP and Todd Stroberg RN - Weill Cornell Chelsea CRS (Site 7804) Grant 5UM1 AI069419, UL1 TR000457; Roberto C Arduino and Martine Diez - Houston AIDS Research Team CRS (Site 31473) Grant 2UM1 AI069503; Pola de la Torre MD and Yolanda Smith BA - Cooper University Hospital (Site 31476) Grant AI069503-01; Ioana Bica MD and Betsy Adams RN - Boston Medical Center (Site 104) Grant 5U01A1069472; Ilene Wiggins RN and Andrea Weiss BPharm - Johns Hopkins University CRS (Site 201) Grants 2UM1 AI069465 and UL1TR001079, Institute for Clinical and Translational Research; University of Washington AIDS CRS (Site 1401) Grant UM1AI069481; Pamela Poethke RN and Deborah Perez RN - the Miriam Hospital CRS (Site 2951) Harvard/Boston/Providence CTU Grant UM1-AI069412; Mary Adams RN and Christine Hurley RN - University of Rochester (Site 31787) Grant UM1 AI069511, UL1 TR000042; Debbie Slamowitz RN and Sandra Valle PA-C - Stanford University (Site 501) Grant AI 069556; Ramakrishna Prasad MD MPH and Lisa Klevens RN BSN - University of Pittsburgh (Site 1001) Grant UM1AI069494; Dr. Susan Koletar, MD and Kathy Watson RN - Ohio State University (Site 2301) Grant UM1AI069494; Benigno Rodriguez MD MSc FIDSA and Kristen Allen RN BSN - Case CRS (Site 2501) Grant AI69501; Peter Gordon MD and Jolene Noel-Connor RN - Columbia University P and S CRS (Site 30329) Grant 5UM1AI069470-10. Supported in part by Columbia University's CTSA grant UL1 TR000040 from NCATS/NIH; Bronx-Lebanon Hosp. Ctr. CRS (Site 31469) Grant 1U01AI069503-01; Daniel Nixon DO PhD and Vicky Watson RN - Virginia Commonwealth University CRS (Site 31475) Grant UM1-AI069503; Shobha Swaminathan and Baljinder Singh - Rutgers New Jersey Medical School CRS (Site 31786) Grant AI069419-10; Connie Funk RN MPH and Fred R Sattler MD - University of Southern California CRS (Site 1201) Grants AI069428 and AI27673; Beverly E Sha MD and Tondria Green RN BSN ACRN - Rush University Medical Center CRS (Site 2702) Grant U01 AI069471; Linda Makohon RN BSN and Leslie Faber RN BSN - Henry Ford Health System (Site 31472) Grant 5UM1A1069503, B40465; Susan Blevins RN MS ANP-C and Catherine Kronk BA - Chapel Hill CRS (Site 3201) Grants UM1 AI069423, CTSA: 1UL1TR001111, CFAR: P30 AI50410; Vicki Bailey RN and Fred Nicotera Vanderbilt Therapeutics CRS (Site 3652) Grant 2UM1AI069439-08 and supported in part by the Vanderbilt CTSA grant TR000445 from NIH; Alabama CRS (Site 31788) Grant 1U01AI069452-01; Dr. Debika Bhattacharya MD and Maria Palmer PA - UCLA Care Center CRS (Site 601) Grant AI069424; Jacquelin Granholm and Susanna Naggie - Duke University (Site 1601) Grant U01-AI069484; Eric S Daar and Sadia Shaik - Harbor-UCLA (Site 603) Grant AI 069424, UL1 TR000124; Denver Public Health CRS (Site 31470); Wayne State Univ. CRS (Site 31478) Grant 1U01AI069503-01; Annie Luetkemeyer MD and Anna Smith RN - UCSF AIDS CRS (Site 801) CTU Grant 5UM1AI069496; Pablo Tebas MD and Yan Jiang RN - Penn Therapeutics CRS (Site 6201) Grant ACTG: UM-IA-069534-08, CFAR: 5-P30-AI-045008-15; Amy Sbrolla RN and Teri Flynn ANP-BC - Massachusetts General Hospital CRS (Site 101) Grant UM1AI068636; Paul Sax MD and Cheryl Keenan RN BC - Brigham and Women’s Hospital (Site 107) Grant UM1AI069412; Clifford Gunthel MD and Ericka R Patrick RN MSN - Emory-CDC CTU The Ponce de Leon CRS (Site 5802) Grant 1U01AI069418-01 and Emory University Center For AIDS Research - P30AI050409; Weill Cornell Uptown CRS (Site 7803) Grant UM1AI069419.
Hepatitis C virus (HCV) coinfection is a major cause of morbidity and mortality among those with human immunodeficiency virus (HIV) infection. Prior to the emergence of new HCV targeted direct acting antiviral agents in 2011, response to standard therapy with pegylated interferon (PEG-IFN) and ribavirin (RBV) was poor, both in terms of efficacy and medication tolerability. The approvals of first generation serine protease inhibitors of HCV replication initiated a revolution in terms of the care and management of HCV infected patients. Phase 2 trials in HCV/HIV coinfected patients suggested improved efficacy with moderate drug tolerability for treatment regimens that combined either boceprevir or telaprevir with PEG-IFN + RBV. In an effort to define treatment efficacy with response- and cirrhosis- guided regimens in HCV/HIV coinfected, the authors conducted a prospective, multicenter, open-label Phase 3 trial in both HCV treatment naïve and treatment experienced participants with comparison to historical controls in the same clinical trials network.
The treatment of hepatitis C is a rapidly moving and dynamic field. Introduction of new agents has led to expansion of indications prior to completion of comprehensive Phase 3 trials in some cases. This study provides data regarding a large Phase 3 trial of a first generation protease inhibitor of HCV which was utilized in combination with PEG-IFN and RBV in HCV/HIV coinfected patients.
This is the largest study to investigate the efficacy and safety of this first generation protease inhibitor therapy in HCV/HIV coinfected patients. The treatment was not optimal, but it did meet criteria for treatment success compared to historical controls treated with PEG-IFN plus RBV.
While this study demonstrates efficacy of a first generation HCV protease inhibitor in the treatment of HCV/HIV coinfected patients, the regimen is unlikely to be widely used due to rapid development of all-oral regimens that have supplanted the used of PEG-IFN-based regimens. The importance of conducting Phase 3 trials was emphasized by the lower rates of efficacy than were observed in Phase 2 trials that included highly selected patients.
Treatment naïve patients are those who have never been treated with a hepatitis C active agent while treatment experienced are those who may have been exposed to interferon or PEG-IFN with or without RBV in the past. Therapies for HIV are collectively called cART which includes combinations of drugs used for antiretroviral therapy.
The authors report data on efficacy and safety of HCV protease inhibitor boceprevir with PEG-IFN alfa and weight-based RBV in a phase 3 trial in patients with HCV plus HIV. The result, in terms of RBV, is similar to that reported by other studies in the real world and reflects the limits of this treatment. The authors, correctly, described the chronology of their trial, born before the entry in the clinical practice of the new treatments.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Larrubia JR, Makara M, Pellicano R S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 833] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 2. | Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, Héripret L, Costagliola D, May T, Chêne G. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Kovari H, Ledergerber B, Cavassini M, Ambrosioni J, Bregenzer A, Stöckle M, Bernasconi E, Kouyos R, Weber R, Rauch A. High hepatic and extrahepatic mortality and low treatment uptake in HCV-coinfected persons in the Swiss HIV cohort study between 2001 and 2013. J Hepatol. 2015;63:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Shire NJ, Welge JA, Sherman KE. Response rates to pegylated interferon and ribavirin in HCV/HIV coinfection: a research synthesis. J Viral Hepat. 2007;14:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, Rivero A, Mak C, Thompson S, Howe AY. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, Gharakhanian S, McCallister S, Henshaw J, Girard PM. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 637] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 9. | Fernández-Montero JV, Barreiro P, de Mendoza C, Labarga P, Soriano V. Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat. 2016;23:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 520] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Sterling RK, Kuo A, Rustgi VK, Sulkowski MS, Stewart TG, Fenkel JM, El-Genaidi H, Mah’moud MA, Abraham GM, Stewart PW. Virological outcomes and treatment algorithms utilisation in observational study of patients with chronic hepatitis C treated with boceprevir or telaprevir. Aliment Pharmacol Ther. 2015;41:671-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Hulskotte EG, Feng HP, Xuan F, van Zutven MG, Treitel MA, Hughes EA, O’Mara E, Youngberg SP, Wagner JA, Butterton JR. Pharmacokinetic interactions between the hepatitis C virus protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, darunavir, and lopinavir. Clin Infect Dis. 2013;56:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |